Reata Begins Rolling FDA Submission of Omaveloxolone in Friedreich's Ataxia
January 31 2022 - 7:43AM
Dow Jones News
By Colin Kellaher
Reata Pharmaceuticals Inc. on Monday said it has initiated a
rolling submission of a new-drug application seeking U.S. Food and
Drug Administration approval of omaveloxolone for the treatment of
patients with Friedreich's ataxia.
The Plano, Texas, clinical-stage biopharmaceutical company said
the rolling submission lets it submit portions of the application
to the FDA for review on an ongoing basis, adding that it expects
to complete the submission by the end of the first quarter of
2022.
Reata said omaveloxolone, if approved, would be the first
therapy indicated for the treatment of Friedreich's ataxia, a rare
neuromuscular disease.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
January 31, 2022 07:28 ET (12:28 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Reata Pharmaceuticals (NASDAQ:RETA)
Historical Stock Chart
From Mar 2024 to Apr 2024
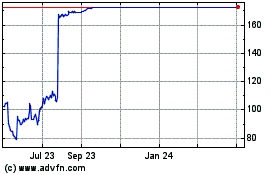
Reata Pharmaceuticals (NASDAQ:RETA)
Historical Stock Chart
From Apr 2023 to Apr 2024