
Immunotherapeutic PolyTope® from ImmunoPrecise (IPA)
Potently Neutralizes the SARS-CoV-2 Omicron Variant – Moves Towards
FDA/IND-Submission
-
During in vitro
pseudovirus assays, IPA's PolyTope® advanced immunotherapeutic
TATX-03 prevents infection of cells by SARS-CoV-2
variant-of-concern, Omicron, and all variants of concern tested to
date.
-
On January 27,
2022, Talem Therapeutic LLC, a wholly owned subsidiary of IPA,
filed a non-provisional US patent application and concurrent PCT
(Patent Cooperation Treaty) international and other national patent
applications, relating to the discovery of PolyTope TATX-03, it's
in vitro and in vivo effects, and therapeutic use.
VICTORIA, BRITISH COLUMBIA (CANADA), February 7th, 2022 –
InvestorsHub NewsWire -- IPA (IMMUNOPRECISE ANTIBODIES LTD.)
(the "Company") (NASDAQ: IPA) (TSXV: IPA) is pleased to announce the
release of data demonstrating strong neutralizing potency of its
PolyTope® TATX-03 antibody cocktail towards the SARS-CoV-2 Omicron
variant (B.1.1.529) in in
vitro pseudovirus assays. This first generation four
antibody cocktail against SARS-CoV-2 was rationally designed to
sustain efficacy against all SARS-CoV-2 strains and variants with
the goal of protecting and treating all individuals.
The Company believes that it possesses the
only first-generation cocktail therapy against SARS-CoV-2 (first
publicly announced 2020) that has been demonstrated to retain
efficacy against every variant of concern to date through
in vitro pseudovirus
assays conducted with respect to such variants of concern. PolyTope
TATX-03 is unique in its ability to engage multiple modes of
action, facilitated through simultaneously targeting various
non-overlapping epitopes on the spike trimer.
The Company expects that, upon completion of its ongoing
studies, the aforementioned data will enable the Company to file an
Investigational New Drug ("IND") application in accordance with its
internal schedule previously announced on November 30, 2021. The
approval of the U.S. Food and Drug Administration (FDA) with
respect to the IND application will be required prior to commencing
any first-in-human clinical studies.
With the ongoing threat of COVID-19 and rising concerns from
the frontlines about the effectiveness of existing vaccines and
antibody therapies, IPA's Polytope® antibody cocktail continues to
demonstrate promising efficacy during studies conducted by the
Company, even when tested against newly emerged variants. New data
from IPA reveals remarkable consistency in the ability of PolyTope
TATX-03 antibody cocktail to retain its potent and complete
in vitro neutralization
against all variants, as demonstrated with Omicron pseudovirus
reinforced with neutralization data against the wild-type parental
virus (Wuhan) and all predominant variants of concern (see Figure 1
below).
The Company's PolyTope TATX-03 antibody cocktail was
developed to target multiple, non-overlapping epitopes on the spike
trimer, reducing the risk of mutagenic escape, and facilitating
engagement of mechanisms of action that are collectively
distinctive from other SARS therapies obtainable to
date.
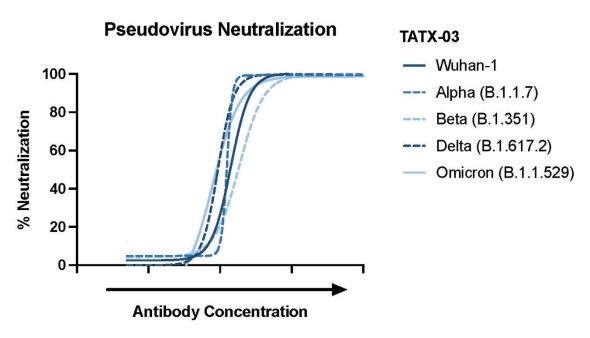
Figure 1. Demonstration of Potent
Neutralization Efficacy for IPA's PolyTope TATX-03 Against
Pseudoviruses of Prominent Variants of Concern, Including
Omicron.
The previously observed high viral clearance efficacy of
TATX-03 in SARS-CoV-2-D614G-challenged hamsters implied a concerted
action of the respective antibodies. Apart from anti-viral effects
resulting from blocking host cell infection via synergistic
effects, the demonstrated ability of TATX-03 to simultaneously bind
to four different regions of the spike trimer is anticipated to
promote viral clearance by the host's immune system. Recently
obtained in vitro
studies support this role of antibody-mediated clearance via
activation of the Fc?-receptors, cell surface receptors involved in
antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis
(ADCP). As Fc receptor engagement of the fully human antibodies of
PolyTope TATX-03 is expected to be even more efficient in humans
compared to hamsters, the previously demonstrated potent
in vivo efficacy in the
hamster SARS-CoV-2 challenge model may even be an
underestimation.
On January 27th, 2022, Talem Therapeutics LLC, a wholly owned
subsidiary of IPA, filed for patent protection of its PolyTope®
TATX-03 antibody cocktail via the PCT (Patent Cooperation Treaty)
system (which has 154 member states) as well as national filings in
the US, Taiwan, Argentina, and Paraguay, enabling IPA to pursue
patent protection of the PolyTope TATX-03 cocktail in all sizeable
potential markets. In the meantime, the PolyTope TATX-03 final
IND-enabling studies are expected to conclude as
scheduled.
At the request of the FDA, IPA has prepared a comprehensive
status update demonstrating the performance of PolyTope TATX-03
toward Omicron, and other variants of concern listed in Figure 1,
for FDA review. Meanwhile, the Company is also compiling a data
package to seek rapid scientific advice from the EMA (European
Medicines Agency). Finally, the latest data on Omicron and
alternative mechanisms of action of PolyTope TATX-03 has been
processed for inclusion in IPA's updated scientific paper
"Cornering an Ever-Evolving Coronavirus: TATX-03, a Fully Human,
Synergistic, Multi-Antibody Cocktail Targeting the SARS-CoV-2 Spike
Protein with in vivo
Efficacy" (https://doi.org/10.1101/2021.07.20.452858)
and the Company expects to publish an extended version on bioRxiv
in the coming weeks.
"Today's news is yet another exciting milestone as we
continue to validate the significance of generating sustainable and
meaningful therapies based on well comprehended scientific
principles. It is amazing to consider how far we have come from two
years ago, when we clarified why IPA was (and why others should be)
concerned about the potential for viral variants to emerge, to
today, owning what we believe is the only remaining first
generation therapy to have retained strong efficacy against
all tested variants of
concern", stated Dr. Jennifer Bath, IPA CEO and
President.
"In stark contrast to most companies, large and small, we
could replace today's quote with any PolyTope quote from the past
24 months and it would remain just as relevant. From day one, our
scientific team chose a path of rigorous investigation to arrive at
a diverse set of functional therapeutic antibodies and we did not
sacrifice quality", continued Dr. Bath, concluding "Our enduring
position is clearly supported today as the Omicron variant, with
its unusually large number of mutations, cannot escape the efficacy
of our well-conceived cocktail".
ImmunoPrecise Antibodies Ltd.
ImmunoPrecise Antibodies Ltd. is a biotherapeutic,
innovation-powered company that supports its business partners in
their quest to discover and develop novel antibodies against a
broad range of target classes and diseases. The Company offers a
hybrid of services and programs with advanced platforms and
technologies — dynamic scientists and business advisors — to
optimize antibody discovery and implementation, against rare and/or
challenging epitopes. For further information, visit
www.immunoprecise.com.
Investor contact:
LifeSci Advisors
John Mullaly
Email: jmullaly@lifesciadvisors.com
Forward Looking Information
This news release contains forward-looking statements within
the meaning of applicable United States securities laws and
Canadian securities laws. Forward-looking statements are often
identified by the use of words such as "potential", "plans",
"expects" or "does not expect", "is expected", "estimates",
"intends", "anticipates" or "does not anticipate", or "believes",
or variations of such words and phrases or state that certain
actions, events or results "may", "could", "would", "might" or
"will" be taken, occur or be achieved. Forward-looking information
contained in this news release include, but are not limited to,
statements regarding the Company's ability to complete its pre-IND
studies, the ability of the Company to successfully submit an IND
application with respect to PolyTope® TATX-03, statements regarding
regulatory approvals, statements regarding future publications of
scientific papers, statements regarding the potential of IPA's
PolyTope® monoclonal antibodies, including TATX-03, to promote
antibody-mediated clearance, to provide strong anti-viral effects
against SARS-CoV-2/COVID-19 disease or any variant of the virus as
either a prophylactic (preventative) or treatment (therapeutic), to
retain efficacy over time, to be more efficient in humans compared
to hamsters and to reduce or suppress the emergence of novel
variants as well as its potential to prevent the spread of
variants. In respect of the forward-looking information contained
herein, the Company has provided such statements and information in
reliance on certain assumptions that management believed to be
reasonable at the time.
Forward-looking information involves known and unknown risks,
uncertainties and other factors which may cause the actual results,
performance or achievements stated herein to be materially
different from any future results, performance or achievements
expressed or implied by the forward-looking information. Actual
results could differ materially from those currently anticipated
due to a number of factors and risks, including, without
limitation, the Company may not be successful in timely completing
its pre-IND studies or submitting an IND application to the FDA,
developing its PolyTope® monoclonal antibodies, including TATX-03,
or other vaccines or therapeutics against COVID-19 through the
successful and timely completion of preclinical assays, studies and
clinical trials, or may not receive all regulatory approvals to
commence and then continue clinical trials of its products,
including PolyTope® TATX-03 and, be successful in partnering or
commercializing its products related to COVID-19, the coverage and
applicability of the Company's intellectual property rights to its
PolyTope® antibody cocktails, as well as those risks discussed in
the Company's Annual Information Form dated July 27, 2021 (which
may be viewed on the Company's profile at www.sedar.com)
and the Company's Form 40-F, Amendment No, 1 dated September 28,
2021 (which may be viewed on the Company's profile at
www.sec.gov).
Furthermore, there can be no assurance that the pending patent
applications will issue as patents and that challenges will not be
instituted against the validity or enforceability of such patents.
Should one or more of these risks or uncertainties materialize, or
should assumptions underlying the forward-looking statements prove
incorrect, actual results, performance, or achievements may vary
materially from those expressed or implied by the forward-looking
statements contained in this news release. Accordingly, readers
should not place undue reliance on forward-looking information
contained in this news release.
The forward-looking statements contained in this news release
are made as of the date of this release and, accordingly, are
subject to change after such date. The Company does not assume any
obligation to update or revise any forward-looking statements,
whether written or oral, that may be made from time to time by us
or on our behalf, except as required by applicable law.
Neither the TSX Venture Exchange nor its Regulation Services
Provider (as that term is defined in policies of the TSX Venture
Exchange) accepts responsibility for the adequacy or accuracy of
this release.
SOURCE ImmunoPrecise Antibodies