Filed pursuant to General Instruction II.L. of Form F-10
File No. 333-257872
GREENBROOK TMS
INC.
The Offering is being made concurrently in each
of the provinces and territories of Canada (except Québec) under the terms of this Prospectus Supplement and in the United States
of America (the “United States” or the “U.S.”) pursuant to the Company’s registration statement
on Form F-10 (as amended, the “Registration Statement”) filed with the United States Securities and Exchange Commission
(the “SEC”), of which this Prospectus Supplement forms a part.
The Company intends to use net proceeds of the
Offering to fund the Acquisition (as defined below) and for working capital and general corporate purposes. See “Recent Developments”,
“Use of Proceeds” and “Risk Factors” in this Prospectus Supplement.
Operating through 132 Company-operated
treatment centers (149 upon completion of the Acquisition), Greenbrook is a leading provider of Transcranial Magnetic Stimulation
(“TMS”) therapy, an FDA-cleared, non-invasive therapy for the treatment of Major Depressive Disorder (“MDD”)
and other mental health disorders, in the United States. TMS therapy provides local electromagnetic stimulation to specific brain regions
known to be directly associated with mood regulation. Greenbrook has provided more than 675,000 TMS treatments to over 19,000 patients
struggling with depression. See “The Company” in this Prospectus Supplement.
The Offered Shares are being offered in Canada
by Stifel Nicolaus Canada Inc. and Bloom Burton Securities Inc. (collectively, the “Lead Underwriters” or the “Canadian
Underwriters”) and in the United States by Lake Street Capital Markets, LLC (the “United States Underwriter”
and, together with the Canadian Underwriters, the “Underwriters”). The Underwriters have severally agreed to purchase
the Offered Shares from the Company at a price of US$7.75 per Offered Share, subject to the terms and conditions of the Underwriting Agreement
(as defined below) described under “Plan of Distribution”. The Offering Price was determined by negotiation between the Company
and the Lead Underwriters, on behalf of the Underwriters.
The following table sets out the maximum number
of Over-Allotment Shares that may be issued pursuant to the Over-Allotment Option:
The Underwriters, as principals, conditionally
offer the Offered Shares, subject to prior sale, if, as and when issued by the Company and accepted by the Underwriters in accordance
with the conditions contained in the Underwriting Agreement described in this Prospectus Supplement under “Plan of Distribution”
and subject to the approval of certain legal matters on behalf of the Company by Torys LLP with respect to Canadian law and United States
law and on behalf of the Underwriters by Dentons Canada LLP with respect to Canadian law and Dentons US LLP with respect to United States
law.
The Company has been advised by the Underwriters
that, in connection with the Offering, the Underwriters may over-allot or effect transactions that stabilize or maintain the market price
of the Shares at levels other than those which otherwise might prevail on the open market. Such transactions, if commenced, may be discontinued
at any time. The Underwriters may offer the Offered Shares to the public at a price lower than that stated above. See “Plan of
Distribution” in this Prospectus Supplement.
Subscriptions will be received subject to rejection or allocation in
whole or in part and the Underwriters reserve the right to close the subscription books at any time without notice. Closing of the Offering
is expected to occur on or about September 27, 2021 or such other date as the Company and the Lead Underwriters, on behalf of the Underwriters,
may agree (the “Closing Date”). Registrations and transfers of the Offered Shares will be effected electronically through
the non-certificated inventory (“NCI”) system administered by CDS Clearing and Depository Services Inc. (“CDS”).
Beneficial owners of Offered Shares will not receive physical certificates evidencing their ownership of Offered Shares unless a request
for a certificate is made to the Company. See “Plan of Distribution” in this Prospectus Supplement.
The TSX has conditionally approved the
listing of the Offered Shares. Listing is subject to the Company fulfilling all of the listing requirements of the TSX on or before
November 2, 2021.
Each of MSS and 1315 Capital (each as defined
below) intends to exercise its Participation Right (as defined below) in connection with the Offering to purchase, directly or indirectly,
approximately 200,000 and 167,570 Offered Shares, respectively, at the Offering Price. See “Plan of Distribution” in this
Prospectus Supplement.
The principal, head and registered office of the
Company is located at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada, M4W 3P4. The Company’s United States corporate headquarters
is located at 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, United States, 22102.
BASE SHELF PROSPECTUS
ABOUT
THIS PROSPECTUS SUPPLEMENT
This document consists of
two parts. The first part is this Prospectus Supplement, which describes the specific terms of the Offering and adds to and supplements
information contained in the Shelf Prospectus and the documents incorporated by reference therein. The second part is the Shelf Prospectus,
which gives more general information, some of which may not apply to the Offering. This Prospectus Supplement is incorporated by reference
into the Shelf Prospectus solely for the purpose of the Offering.
A prospective purchaser of
Offered Shares should read this entire Prospectus Supplement and the Shelf Prospectus, including the documents incorporated herein and
therein by reference, and consult its own professional advisors to assess the income tax, legal, risks and other aspects of its investment
in the Offered Shares. A prospective purchaser of Offered Shares should rely only on the information contained in this Prospectus Supplement
and the Shelf Prospectus. The Company, Greybrook Health Inc. (“Greybrook Health” or the “Promoter”)
and the Underwriters have not authorized anyone to provide prospective purchasers of Offered Shares with additional or different information.
The information contained in this Prospectus Supplement and the Shelf Prospectus is accurate only as of their respective dates, regardless
of the time of delivery of this Prospectus Supplement and the accompanying Shelf Prospectus or any sale of the Offered Shares. The Company’s
business, financial condition, results of operations and prospects may have changed since those dates.
The Company has filed the
Registration Statement with the SEC under the United States Securities Act of 1933, as amended (the “U.S. Securities Act”),
relating to the Offered Shares being offered hereunder. This Prospectus Supplement forms a part of the Registration Statement. This Prospectus
Supplement does not contain all of the information set forth in the Registration Statement, certain items of which are contained in the
exhibits to the Registration Statement as permitted or required by the rules and regulations of the SEC. Items of information omitted
from this Prospectus Supplement but contained in the Registration Statement are available on EDGAR at www.sec.gov.
The Offered Shares may be
sold only in those jurisdictions where offers and sales are permitted. This Prospectus Supplement and the accompanying Shelf Prospectus
is not an offer to sell or a solicitation of an offer to buy the Offered Shares in any jurisdiction where it is unlawful.
If the information varies
between this Prospectus Supplement and the accompanying Shelf Prospectus, the information in this Prospectus Supplement supersedes the
information in the accompanying Shelf Prospectus.
Interpretation
Unless otherwise noted or
the context otherwise requires, the “Company”, “Greenbrook”, “we”, “us” or “our”
refer to Greenbrook TMS Inc. together with its subsidiaries.
Where the context requires,
all references in this Prospectus Supplement to the “Offering” include the Over-Allotment Option and all references in this
Prospectus Supplement to “Offered Shares” include the Over-Allotment Shares that may be issued pursuant to the Over-Allotment
Option.
All Share numbers in this
Prospectus Supplement and the accompanying Shelf Prospectus have been adjusted to give effect to the consolidation of all of the Company’s
issued and outstanding Shares effected on February 1, 2021 on the basis of one post-consolidation Share for every five pre-consolidation
Shares (the “Share Consolidation”).
Presentation of Financial Information
The financial statements of
the Company incorporated by reference in this Prospectus Supplement and the accompanying Shelf Prospectus are presented in United States
dollars and have been prepared in accordance with IFRS.
Certain calculations
included in tables and other figures in this Prospectus Supplement and the accompanying Shelf Prospectus have been rounded for clarity
of presentation.
EXCHANGE
RATE INFORMATION
All references to “C$”
or “Canadian dollars” included or incorporated by reference into this Prospectus Supplement and the accompanying Shelf Prospectus
refer to Canadian dollar values. All references to “US$” or “United States dollars” are used to indicate United
States dollar values.
The following table sets forth,
for each of the periods indicated, the high, low, average and period end spot rates of exchange for one United States dollar, expressed
in Canadian dollars, published by the Bank of Canada.
|
|
|
Year ended December 31,
|
|
|
Six months ended June 30,
|
|
|
|
|
|
|
|
|
|
|
2021
(C$)
|
|
|
2020(C$)
|
|
|
High
|
|
|
1.4496
|
|
|
|
1.3600
|
|
|
|
1.2828
|
|
|
|
1.4496
|
|
|
Low
|
|
|
1.2718
|
|
|
|
1.2988
|
|
|
|
1.2040
|
|
|
|
1.2970
|
|
|
Average
|
|
|
1.3415
|
|
|
|
1.3269
|
|
|
|
1.2470
|
|
|
|
1.3651
|
|
|
Period End
|
|
|
1.2732
|
|
|
|
1.2988
|
|
|
|
1.2394
|
|
|
|
1.3628
|
|
On September 21, 2021, the
rate of exchange posted by the Bank of Canada for conversion of United States dollars into Canadian dollars was US$1.00 = C$1.2801. The Company
makes no representation that Canadian dollars could be converted into United States dollars at that rate or any other rate.
FORWARD-LOOKING
STATEMENTS
Certain statements contained
in this Prospectus Supplement, the Shelf Prospectus and the documents incorporated by reference herein and therein constitute forward-looking
statements within the meaning of applicable securities laws in Canada and the United States, including the United States Private Securities
Litigation Reform Act of 1995. Forward-looking information may relate to the Company’s future financial outlook and anticipated
events or results and may include information regarding the Company’s business, financial position, results of operations, business
strategy, growth plans and strategies, technological development and implementation, budgets, operations, financial results, taxes, dividend
policy, plans and objectives. Particularly, information regarding the Acquisition and the Company’s expectations of future results,
performance, achievements, prospects or opportunities or the markets in which it operates is forward-looking information. In some cases,
forward-looking information can be identified by the use of forward-looking terminology such as “plans”, “targets”,
“expects” or “does not expect”, “is expected”, “an opportunity exists”, “budget”,
“scheduled”, “estimates”, “outlook”, “forecasts”, “projection”, “prospects”,
“strategy”, “intends”, “anticipates”, “does not anticipate”, “believes”, or
variations of such words and phrases or state that certain actions, events or results “may”, “should”, “could”,
“would”, “might”, “will”, “will be taken”, “occur” or “be achieved”.
In addition, any statements that refer to expectations, intentions, projections or other characterizations of future events or circumstances
contain forward-looking information. Statements containing forward-looking information are not facts but instead represent management’s
expectations, estimates and projections regarding future events or circumstances.
Forward-looking information
in this Prospectus Supplement, the Shelf Prospectus and the documents incorporated by reference herein and therein includes, among other
things, statements relating to: the timing and closing of the Offering; information relating to the Acquisition, including
the anticipated timing of completion of the Acquisition and the potential benefits and synergies to be derived therefrom, as more particularly
described in “Recent Developments” and “Use of Proceeds”; the intended use by the Company of the net proceeds
of the Offering as described under “Use of Proceeds”; the listing on the TSX of the Offered Shares; the Company’s expectations
regarding its revenue, expenses, cash flow and operations; changes in laws and regulations affecting the Company and the regulatory environments
in which it operates; the Company’s expectations regarding the potential market opportunity for the delivery of TMS therapy; the
Company’s expectations regarding its growth rates and growth plans and strategies, including expectations regarding future growth
of the TMS market; potential expansion of additional therapeutic indications approved for TMS therapy by the United States Food and Drug
Administration (“FDA”); the Company’s business plans and strategies; the Company’s expectations regarding
the implementation and expansion of the Spravato® pilot program; changes in reimbursement rates by insurance payors; the Company’s
expectations regarding the outcome of litigation and payment obligations in respect of prior settlements; the Company’s ability
to attract and retain medical practitioners and qualified technicians at the Company’s network of outpatient mental health service
centers that specialize in TMS treatment (each, a “TMS Center”); the Company’s competitive position in its industry
and its expectations regarding competition; anticipated trends and challenges in the Company’s business and the markets in which
it operates; access to capital and the terms relating thereto; technological changes in the Company’s industry; the Company’s
expectations regarding geographic expansions; the Company’s expectations regarding new TMS Center openings and the timing thereof;
the Company’s expectations regarding mergers and acquisitions; successful execution of internal plans; anticipated costs of capital
investments; the Company’s intentions with respect to the implementation of new accounting standards; and the impact of the novel
coronavirus, COVID-19 (“COVID-19”), pandemic on the Company.
This forward-looking information
and other forward-looking information are based on the Company’s opinions, estimates and assumptions in light of its experience
and perception of historical trends, current conditions and expected future developments, as well as other factors that the Company currently
believes are appropriate and reasonable in the circumstances. Despite a careful process to prepare and review the forward-looking information,
there can be no assurance that the underlying opinions, estimates and assumptions will prove to be correct.
The forward-looking information in this Prospectus Supplement, the
Shelf Prospectus and the documents incorporated by reference herein and therein is necessarily based on a number of opinions, estimates
and assumptions that the Company considered appropriate and reasonable as of the date such statements were made. It is also subject to
known and unknown risks, uncertainties, assumptions and other factors that may cause the actual results, level of activity, performance
or achievements to be materially different from those expressed or implied by such forward-looking information, including the following
risk factors: successful execution of the Company’s growth strategies; inability to attract key managerial and other non-medical
personnel; risks related to changes in reimbursement rates by commercial insurance plans, Medicare and other non-Medicare government insurance
plans; imposition of additional requirements related to the provision of TMS therapy by commercial insurance plans, Medicare and other
non-Medicare government insurance plans that increase the cost or complexity of furnishing TMS therapy; reduction in reimbursement rates
by higher-paying commercial insurance providers; dependency on referrals from physicians and failure to attract new patients; failure
to recruit and retain sufficient qualified psychiatrists; ability to obtain TMS devices from the Company’s suppliers on a timely
basis at competitive costs could suffer as a result of deterioration or changes in supplier relationships or events that adversely affect
the Company’s suppliers or cause disruption to their businesses; failure to reduce operating expenses and labor costs in a timely
manner; inability to achieve or sustain profitability in the future or an inability to secure additional financing to fund losses; risks
related to the use of partnerships and other management services frameworks; risks associated with leasing space and equipment for the
Company’s TMS Centers; inability to successfully open and operate new TMS Centers profitably or at all; risks associated with geographic
expansion in regions which may have lower awareness of the Company’s brand or TMS therapy in general; claims made by or against
the Company, which may result in litigation; risks associated with professional malpractice liability claims; reduction in demand for
the Company’s services as a result of new drug development and/or technological changes within the Company’s industry; impact
of uncertainty related to potential changes to U.S. healthcare laws and regulations; risks associated with anti-kickback, fraud and abuse
laws; risks associated with compliance with laws relating to the practice of medicine; the constantly evolving nature of the regulatory
framework in which the Company operates; costs associated with compliance with U.S. federal and state laws and regulations and risks associated
with failure to comply; assessments for additional taxes, which could affect the Company’s operating results; inability to manage
the Company’s operations at its current size; the Company’s competitive industry and the size and resources of some of its
competitors; the labor-intensive nature of the Company’s business being adversely affected if it is unable to maintain satisfactory
relations with its employees or the occurrence of union attempts to organize its employees; insurance-related risks; complications associated
with the Company’s billing and collections systems; material disruptions in or security breaches affecting the Company’s information
technology systems; natural disasters and unusual weather; disruptions to the operations at the Company’s head office locations;
upgrade or replacement of core information technology systems; changes in accounting standards and subjective assumptions, estimates and
judgments by management related to complex accounting matters; inability to maintain effective controls over financial reporting; the
possible failure to complete the Acquisition; risks related to the integration of Achieve TMS East/Central (as defined herein) into the
Company’s business; the possible failure to realize expected returns on the Acquisition; risks related to the assumption of liabilities
from the Acquisition; risks associated with dilution of equity ownership; volatility in the market price for the Shares; prolonged decline
in the price of the Shares reducing the Company’s ability to raise capital; significant influence of Greybrook Health; increases
to indebtedness levels causing a reduction in financial flexibility; future sales of the Company’s securities by existing shareholders
causing the market price for Shares to decline; impact of future offerings of debt securities on dividend and liquidation distributions;
no cash dividends for the foreseeable future; an active, liquid and orderly trading market for Shares failing to develop; different shareholder
protections in Canada as compared to the United States and elsewhere; treatment of the Company as a U.S. domestic corporation for U.S.
federal income tax purposes; the Company’s ability to continue as a going concern due to recurring losses from operations; any issuance
of preferred shares may hinder another person’s ability to acquire the Company; the Company’s trading price and volume could
decline if analysts do not publish research or publish inaccurate or unfavorable research about the Company or its business; increases
to costs as a result of operating as a U.S. public company; the Company’s potential to incur significant additional costs if it
were to lose its “foreign private issuer” status in the future; the COVID-19 pandemic having a material adverse impact on
the future results of the Company; and risks related to forward-looking information contained in this Prospectus Supplement, the Shelf
Prospectus and the documents incorporated by reference herein and therein.
If any of these risks or uncertainties
materialize, or if the opinions, estimates or assumptions underlying the forward-looking information prove incorrect, actual results or
future events might vary materially from those anticipated in the forward-looking information. The opinions, estimates or assumptions
referred to above should be considered carefully by readers. Additional information about these assumptions, risks and uncertainties is
contained in this Prospectus Supplement and the accompanying Shelf Prospectus under the heading “Risk Factors” and in the
Company’s filings with securities regulators, including the Annual Information Form, the Annual MD&A and the Interim MD&A
(each as defined below).
Various assumptions or factors
are typically applied in drawing conclusions or making the forecasts or projections set out in forward-looking information. Those assumptions
and factors are based on information currently available to the Company, including information obtained from third party industry analysts
and other third party sources. In some instances, material assumptions and factors are presented or discussed elsewhere in this Prospectus
Supplement, the Shelf Prospectus or the documents incorporated by reference herein or therein in connection with the statements or disclosure
containing the forward-looking information. Readers are cautioned that the following list of material factors and assumptions is not exhaustive.
The factors and assumptions include, but are not limited to: no unforeseen changes in the legislative and operating framework for the
Company’s business; no unforeseen changes in the prices for the Company’s services in markets where prices are regulated;
no unforeseen changes in the reimbursement rates of commercial, Medicare and other non-Medicare government insurance plans; no unforeseen
changes in the regulatory environment for the Company’s services; a stable competitive environment; and no significant event occurring
outside the ordinary course of business.
Although the Company has attempted
to identify important risk factors that could cause actual results or future events to differ materially from those contained in forward-looking
information, there may be other risk factors not presently known to the Company or that the Company presently believes are not material
that could also cause actual results or future events to differ materially from those expressed in such forward-looking information. There
can be no assurance that such information will prove to be accurate. Accordingly, readers should not place undue reliance on forward-looking
information, which speaks only to opinions, estimates and assumptions as of the date made. The forward-looking information contained in
this Prospectus Supplement, the Shelf Prospectus and the documents incorporated by reference herein and therein represents the Company’s
expectations as of the date of this Prospectus Supplement (or as of the date they are otherwise stated to be made) and are subject to
change after such date. The Company disclaims any intention or obligation or undertaking to update or revise any forward-looking information
whether as a result of new information, future events or otherwise, except as required under applicable securities laws.
All of the forward-looking
information contained in this Prospectus Supplement, the Shelf Prospectus or the documents incorporated by reference herein or therein
is expressly qualified by the foregoing cautionary statements.
DOCUMENTS
INCORPORATED BY REFERENCE
This Prospectus Supplement
is incorporated by reference into the Shelf Prospectus solely for the purposes of the Offering. Other documents are also incorporated,
or deemed to be incorporated, by reference into the Shelf Prospectus and reference should be made to the Shelf Prospectus for full particulars
thereof.
Information has been incorporated
by reference in this Prospectus Supplement from documents filed with the securities commissions or similar regulatory authorities in each
of the provinces and territories of Canada and which have been filed with the SEC in the United States as exhibits to the Registration
Statement. Copies of these documents may be obtained on request without charge from the General Counsel of Greenbrook at 890 Yonge
Street, 7th Floor, Toronto, Ontario, Canada M4W 3P4, by telephone at (416) 915-9100 or by accessing these documents through the Internet
on the Company’s website at www.greenbrooktms.com, on SEDAR at www.sedar.com or on EDGAR at www.sec.gov.
Except to the extent that
their contents are modified or superseded by a statement contained in this Prospectus Supplement or in any other subsequently filed document
that is also incorporated by reference in this Prospectus Supplement, the following documents of the Company filed with the securities
commissions or similar regulatory authorities in each of the provinces and territories of Canada and which have been filed with the SEC
in the United States as exhibits to the Registration Statement are specifically incorporated by reference into, and form an integral part
of, this Prospectus Supplement:
|
|
(k)
|
the template version of the term sheet for
the Offering dated September 21, 2021 (the “Marketing Materials”).
|
Documents referenced in any
of the documents incorporated by reference in this Prospectus Supplement or the accompanying Shelf Prospectus but not expressly incorporated
by reference therein or herein and not otherwise required to be incorporated by reference therein or in the Prospectus Supplement or the
accompanying Shelf Prospectus are not incorporated by reference in this Prospectus Supplement. Any documents of the type described in
Section 11.1 of Form 44-101F1 — Short Form Prospectus Distributions filed by the Company with the various securities commissions
or similar authorities in each of the provinces and territories of Canada pursuant to the requirements of applicable securities legislation
after the date of this Prospectus Supplement and prior to the termination of the distribution of the Offered Shares under this Prospectus
Supplement are deemed to be incorporated by reference into this Prospectus Supplement. All such documents shall be filed as exhibits to
the Registration Statement or otherwise submitted on Form 6-K or an annual report filed by the Company with the SEC and shall be deemed
to be incorporated by reference into the Registration Statement if and to the extent, in the case of any Form 6-K, expressly provided
in such document. The documents incorporated or deemed to be incorporated herein by reference contain meaningful and material information
relating to the Company and readers should review all information contained in this Prospectus Supplement, the Shelf Prospectus and the
documents incorporated or deemed to be incorporated by reference herein and therein.
Any statement
contained in this Prospectus Supplement, the Shelf Prospectus or in a document incorporated or deemed to be incorporated by
reference herein or therein will be deemed to be modified or superseded for the purposes of this Prospectus Supplement to the extent
that a statement contained herein, or in any other subsequently filed document which also is or is deemed to be incorporated by
reference herein, modifies or supersedes that prior statement. The modifying or superseding statement need not state that it has
modified or superseded a prior statement or include any other information set out in the document or statement that it modifies or
supersedes. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of
this Prospectus Supplement. The making of a modifying or superseding statement will not be deemed an admission for any purposes that
the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an
omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of
the circumstances in which it was made.
ENFORCEABILITY
OF CIVIL LIABILITIES IN THE UNITED STATES
The Company is a corporation
incorporated under and governed by the Business Corporations Act (Ontario) (“OBCA”). Some of the Company’s
directors and officers and some of the experts named in this Prospectus Supplement reside principally in Canada, and some of the Company’s
assets and all or a substantial portion of the assets of these persons is located outside the United States. In addition, some of the
Underwriters are not resident in the United States. The Company has appointed an agent for service of process in the United States, but
it may be difficult for investors who reside in the United States to effect service of process upon these persons in the United States,
or to enforce a U.S. court judgment predicated upon the civil liability provisions of the U.S. federal securities laws against the Company
or any of these persons. There is substantial doubt whether an action could be brought in Canada in the first instance predicated solely
upon U.S. federal securities laws.
The Company has filed with
the SEC, in connection with the Registration Statement, an appointment of agent for service of process on Form F-X. Under the Form F-X,
the Company appointed TMS NeuroHealth Centers Inc. as its agent for service of process in the United States in connection with any investigation
or administrative proceeding conducted by the SEC and any civil suit or action brought against or involving the Company in a United States
court arising out of or related to or concerning the offering of Offered Shares under this Prospectus Supplement.
MARKETING
MATERIALS
The Marketing Materials are
not part of this Prospectus Supplement or the accompanying Shelf Prospectus to the extent that the contents of the Marketing Materials
have been modified or superseded by a statement contained in this Prospectus Supplement or any amendment. Any “template version”
of “marketing materials” (each as defined in National Instrument 41-101 – General Prospectus Requirements) filed
with the securities commission or similar authority in each of the provinces and territories of Canada in connection with the Offering
after the date hereof but prior to the termination of the distribution of the Offered Shares under this Prospectus Supplement (including
any amendments to, or an amended version of, any marketing materials) is deemed to be incorporated by reference herein. However, such
“template version” of “marketing materials” will not form a part of this Prospectus Supplement to the extent that
the contents of the marketing materials or the template version of the marketing materials, as applicable, have been modified or superseded
by a statement contained in this Prospectus Supplement.
ELIGIBILITY
FOR INVESTMENT
In the opinion of Torys LLP,
counsel to the Company, and Dentons Canada LLP, Canadian counsel to the Underwriters, based on the current provisions of the Income
Tax Act (Canada) and the regulations thereunder (together, the “Tax Act”), the Offered Shares, if issued on the
date hereof, would be, on such date, “qualified investments” under the Tax Act for trusts governed by registered retirement
savings plans (“RRSPs”), registered retirement income funds (“RRIFs”), registered education savings
plans (“RESPs”), deferred profit sharing plans, registered disability savings plans (“RDSPs”) and
tax-free savings accounts (“TFSAs”), provided that the Offered Shares are listed on a “designated stock exchange”
(as defined in the Tax Act), which currently includes the TSX and NASDAQ.
Notwithstanding that the
Offered Shares may be qualified investments for a trust governed by a TFSA, RDSP, RRSP, RRIF or RESP (each, a
“Plan”), a holder of a TFSA or RDSP, an annuitant under an RRSP or RRIF or a subscriber of an RESP (each, a
“Plan Holder”) will be subject to a penalty tax if the Offered Shares are a “prohibited investment”
(as defined in subsection 207.01(1) of the Tax Act) for a Plan. The Offered Shares will generally not be a prohibited investment for
a Plan provided that the Plan Holder deals at arm’s length with the Company for purposes of the Tax Act and does not have a
“significant interest” (within the meaning of subsection 207.01(4) of the Tax Act) in the Company. In addition, the
Offered Shares will not be a “prohibited investment” for a Plan if the Offered Shares are “excluded
property” (as defined in subsection 207.01(1) of the Tax Act) for such Plan. Prospective purchasers who intend to hold the
Offered Shares in a Plan should consult their own tax advisors regarding the application of the foregoing prohibited investment
rules in their particular circumstances.
WHERE
YOU CAN FIND MORE INFORMATION
The Company has filed a Registration
Statement with the SEC with respect to the Offered Shares offered pursuant to this Prospectus Supplement. This Prospectus Supplement,
which constitutes a part of the Registration Statement, does not contain all of the information required to be contained in the Registration
Statement, certain items of which are contained in the exhibits to the Registration Statement as permitted by the rules and regulations
of the SEC.
The Company files certain
reports with certain securities regulatory authorities of Canada and the SEC pursuant to the United States Securities Exchange Act of
1934, as amended (the “U.S. Exchange Act”). Under the MJDS, such reports and other information may be prepared in accordance
with the disclosure requirements of the securities regulatory authorities of Canada, which requirements are different from those of the
United States. As a foreign private issuer, the Company is also exempt from the rules under the U.S. Exchange Act prescribing the furnishing
and content of proxy statements, and the Company’s officers and directors are exempt from the reporting and short swing profit liability
provisions contained in Section 16 of the U.S. Exchange Act. The Company’s reports and other information filed or furnished with
or to the SEC are available from EDGAR at www.sec.gov, as well as from commercial document retrieval services, and on the Company’s
website at www.greenbrooktms.com. The Company’s Canadian filings are available on SEDAR at www.sedar.com and on the
Company’s website at www.greenbrooktms.com.
THE
COMPANY
Operating through 132
Company-operated treatment centers (149 upon completion of the Acquisition), Greenbrook is a leading provider of TMS therapy,
an FDA-cleared, non-invasive therapy for the treatment of MDD and other mental health disorders, in the United States. TMS therapy provides
local electromagnetic stimulation to specific brain regions known to be directly associated with mood regulation. Greenbrook has provided
more than 675,000 TMS treatments to over 19,000 patients struggling with depression.
The principal, head and registered
office of the Company is located at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada M4W 3P4. The Company’s United States corporate
headquarters is located at 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, United States, 22102.
Further information regarding
the Company and its business is set out in the Annual Information Form, which is incorporated herein by reference.
RECENT
DEVELOPMENTS
Acquisition of Achieve
TMS East/Central
On
September 21, 2021, the Company entered into a purchase agreement (the “Purchase Agreement”) pursuant to which
the Company, through its wholly-owned subsidiary TMS NeuroHealth Centers Inc., will indirectly acquire all of the issued and outstanding
equity interests in Achieve TMS East, LLC (“Achieve TMS East”) and Achieve TMS Central, LLC (“Achieve TMS
Central”, and together with Achieve TMS East, “Achieve TMS East/Central”) for an aggregate initial
cash purchase price of US$8.0 million, net of Achieve TMS East/Central’s cash and debt and subject to customary working capital
adjustments (the “Acquisition”). In addition, a portion of the total purchase price payable in respect of the Acquisition
is subject to a capped earn-out of up to an additional US$2.5 million based on the financial performance of Achieve TMS East during the
twelve-month period following completion of the Acquisition.
Achieve TMS East was founded
in 2016, with a vision of increasing accessibility to TMS therapy in the New England area. Since founding its first TMS Center in Northampton,
Massachusetts, Achieve TMS East has grown to 14 locations in the States of Massachusetts and Connecticut. In 2019, Achieve TMS East’s
management team expanded its operations in the Midwest United States through the establishment of Achieve TMS Central which currently
operates 3 TMS Centers in the State of Iowa. The Acquisition is expected to enhance Greenbrook’s position as a leading provider
of TMS therapy in the United States and, following completion of the Acquisition, will add an additional 17 TMS Centers to the Company’s
existing service delivery platform, for a total of 149 TMS Centers.
Key highlights in respect
of the Acquisition include:
|
|
·
|
A Profitable Platform with Additional Capacity and Strategic Expansion Opportunities
|
o
Opportunity to increase capacity at Achieve TMS East’s existing TMS Centers and to utilize the 14 locations in Massachusetts
and Connecticut as a springboard for expansion throughout New England.
o
Opportunity to expand Achieve TMS Central operations into the State of South Dakota as well as in other states that are proximate
to Greenbrook’s current operations in Missouri and Illinois.
|
|
·
|
Well-Established Payor Contracting
|
o
Achieve TMS East/Central’s affiliated medical practices benefit from strong reimbursement from key commercial payors.
o
The Acquisition removes the need to establish new contractual relationships with payors, eliminating a process which is a key barrier
to expansion.
|
|
·
|
Access to Robust Physician Networks
|
o
Provides Greenbrook with a strong physician network in the States of Massachusetts, Connecticut and Iowa.
|
|
·
|
Provides Proven Regional Management Team and Potential Synergies
|
o
Achieve TMS East/Central has an experienced regional management and operations team and robust infrastructure.
o
Anticipated post-Acquisition synergies with Greenbrook’s established shared services function.
The
Acquisition is expected to close in the fourth quarter of 2021, subject to customary closing conditions. The Company intends
to satisfy the initial cash purchase price in respect of the Acquisition through a portion of the net proceeds of the Offering. See “Use
of Proceeds” and “Risk Factors”.
Completion of Private
Placement
On June 14, 2021, the Company
completed a non-brokered private placement (the “Private Placement”) of Shares in reliance upon Rule 506(c) under the
U.S. Securities Act. Pursuant to the Private Placement, an aggregate of 2,353,347 Shares were issued at a price of US$10.00 per Share,
for aggregate gross proceeds to the Company of approximately US$23.5 million. The financing was led by new investor Masters Special Situations,
LLC and affiliates thereof (“MSS”). Additional new investors, including BioStar Capital, Steward Capital Holdings,
LP and Avenaero Holdings, LLC, also participated in the financing, along with existing investors, Greybrook Health and 1315 Capital II,
L.P. (“1315 Capital”). The Company intends to use the net proceeds from the Private Placement to develop new TMS Centers
as well as for working capital and general corporate purposes.
In connection with the
Private Placement, MSS, Greybrook Health and 1315 Capital (collectively, the “Investors”) each received the right
to appoint a nominee to the board of directors of the Company (the “Board”) as well as rights to participate in
future equity issuances by the Company to maintain such investors’ pro rata ownership interest in the Company. In
addition, each of the subscribers in the Private Placement received customary resale, demand and “piggy-back”
registration rights. Immediately following the completion of the Private Placement, the Investors, collectively, held an aggregate
of 7,752,215 Shares, representing approximately 48.2% of the voting control of the Company.
On July 27, 2021, the Company
filed a prospectus supplement in each of the provinces and territories of Canada (except Québec) and with the SEC under the Registration
Statement registering the periodic resale, from time to time, of up to an aggregate of 2,353,347 Shares that the subscribers purchased
in the Private Placement (the “Resale Prospectus”). The participation of each subscriber in the filing of the Resale
Prospectus is not an indication of such subscriber’s intention to sell the Shares at any particular time or in any particular amount.
See “Risk Factors” in this Prospectus Supplement.
On August 3, 2021, the Company
appointed Robert Higgins to the Board following the exercise by MSS of its board nomination right granted in connection with the Private
Placement. Adele C. Oliva and Sasha Cucuz, current members of the Board, represent 1315 Capital’s and Greybrook Health’s board
nominees, respectively.
TMS Center Expansion Opportunities
In addition to the Acquisition, the Company is in various stages of
negotiations and due diligence in respect of other potential TMS Center expansion opportunities. There can be no assurance that these
negotiations will result in TMS Center expansion for the Company or, if they do, what the final terms or timing of such opportunities
would be. The Company expects to continue current negotiations and discussions and actively pursue other TMS Center expansion opportunities,
including through in-region growth and development, development of new regions and merger and acquisition opportunities.
CONSOLIDATED
CAPITALIZATION
The table below sets forth our capitalization as of June 30, 2021 (1)
on an actual basis and (2) as adjusted to give effect to the Offering (assuming no exercise of the Over-Allotment Option) and the expected
use of proceeds of the Offering as discussed under “Use of Proceeds”. You should read this table in conjunction with our Interim
Financial Statements which is incorporated by reference herein.
|
|
|
As of June 30, 2021
|
|
|
|
|
(Unaudited)
|
|
|
(US$)
|
|
(Actual)
|
|
|
(As adjusted)
|
|
|
Cash
|
|
$
|
18,980,884
|
|
|
$
|
27,701,384
|
(3)
|
|
|
|
|
|
|
|
|
|
|
|
Long-term portion of:
|
|
|
|
|
|
|
|
|
|
Loans payable
|
|
$
|
14,308,819
|
|
|
$
|
14,308,819
|
|
|
Deferred grant income(1)
|
|
|
108,681
|
|
|
|
108,681
|
|
|
Lease liabilities(2)
|
|
|
22,718,300
|
|
|
|
22,718,300
|
|
|
Total long-term debt
|
|
$
|
37,135,800
|
|
|
$
|
37,135,800
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
Shares
|
|
$
|
86,557,612
|
|
|
$
|
95,278,112
|
|
|
Contributed surplus
|
|
|
3,734,173
|
|
|
|
3,734,173
|
|
|
Deficit
|
|
|
(74,604,355
|
)
|
|
|
(74,604,355
|
)
|
|
Non-controlling interest
|
|
|
(1,072,836
|
)
|
|
|
(1,072,836
|
)
|
|
Total equity
|
|
$
|
14,614,594
|
|
|
$
|
23,335,094
|
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization (long-term debt and equity)
|
|
$
|
51,750,394
|
|
|
$
|
60,470,894
|
|
Notes:
|
|
(1)
|
The deferred grant income arises from the difference between the fair value of the Company’s unsecured
loan in the amount of US$3,080,760 made under the United States Paycheck Protection Program at inception and the loan proceeds received
therefrom in April 2020.
|
|
|
(2)
|
Under IFRS 16 – Leases, a lessee is required to recognize a right-of-use asset, representing
its right to use the underlying asset, and a lease liability, representing its obligation to make future lease payments for all leases
with a term of more than 12 months.
|
|
|
(3)
|
Net of estimated transaction related costs of approximately US$1,354,500 incurred in connection with the
Offering.
|
DESCRIPTION
OF SHARE CAPITAL
General
The following is a summary
of the rights, privileges, restrictions and conditions of or attaching to the Shares. The Company is authorized to issue an unlimited
number of Shares and an unlimited number of preferred shares, issuable in series. As at September 21, 2021, there were 16,094,135 Shares
and no preferred shares issued and outstanding.
Shares
Each Share entitles the holder
thereof to receive notice of any meetings of shareholders of the Company, to attend and to cast one vote at all such meetings. Holders
of Shares do not have cumulative voting rights with respect to the election of directors and, accordingly, holders of a majority of the
Shares entitled to vote in any election of directors may elect all directors standing for election. The holders of Shares are entitled
to receive if, as and when declared by the Board, dividends in such amounts as shall be determined by the Board in its discretion. The
holders of Shares have the right to receive the Company’s remaining property and assets after payment of debts and other liabilities
on a pro rata basis in the event of a liquidation, dissolution or winding-up, whether voluntary or involuntary. The Shares do not
carry any pre-emptive, subscription, redemption or conversion rights, nor do they contain any sinking or purchase fund provisions.
Further information relating
to the Shares is set out in the Annual Information Form, which is incorporated by reference herein.
PLAN
OF DISTRIBUTION
Pursuant to an underwriting
agreement dated September 22, 2021 between the Company and the Underwriters (the “Underwriting Agreement”), the Company
has agreed to sell and the Underwriters have severally agreed to purchase on the Closing Date, an aggregate of 1,300,000 Offered Shares
at a purchase price of US$7.75 per Offered Share, payable in cash to the Company by the Underwriters against delivery of the Offered
Shares for aggregate gross proceeds of US$10,075,000. The Underwriters will receive the Underwriters’ Fee of US$604,500 (or 6.0%
of the gross proceeds of the Offering), excluding any fees payable pursuant to the Over-Allotment Option. A portion of the proceeds of
the Offering may be settled in Canadian dollars.
In addition, the Company
has granted to the Underwriters the Over-Allotment Option, exercisable in whole or in part at any time for a period of 30 days from the
closing of the Offering to purchase up to 195,000 Over-Allotment Shares on the same terms as set forth above. The Underwriting Agreement
provides that the Company will pay the Underwriters the Underwriters’ Fee of US$0.465 per Over-Allotment Share with respect to
Over-Allotment Shares issued under the Over-Allotment Option. This Prospectus Supplement qualifies the grant of the Over-Allotment Option
and the issuance of Over-Allotment Shares on the exercise of the Over-Allotment Option. A purchaser who acquires Shares forming part
of the Underwriters’ over-allocation position acquires those Shares under this Prospectus Supplement, regardless of whether the
over-allocation position is ultimately filled through the exercise of the Over-Allotment Option or secondary market purchases. In the
event that the Over-Allotment Option is exercised in full, the aggregate Underwriters’ Fee shall be US$695,175.
The Offering is being made
concurrently in each of the provinces and territories of Canada (except Québec) and in the United States pursuant to the MJDS.
The Underwriters will offer the Offered Shares for sale in the United States and Canada either directly or through their respective broker-dealer
affiliates or agents registered in each jurisdiction. No securities will be sold in any jurisdiction except by a dealer appropriately
registered under the securities laws of that jurisdiction or pursuant to an exemption from the registered dealer requirements of the securities
laws of that jurisdiction. Subject to applicable law and the terms of the Underwriting Agreement, the Underwriters may offer the Offered
Shares outside the United States and Canada.
The Offering Price was
determined by negotiation between the Company and the Lead Underwriters, on behalf of the Underwriters. The Underwriters propose to
offer the Offered Shares initially at the Offering Price. After the Underwriters have made a reasonable effort to sell all of the
Offered Shares at the Offering Price, the Offering Price may be decreased and may be further changed from time to time to an amount
not greater than the Offering Price, and the compensation realized by the Underwriters will be decreased by the amount that the
aggregate price paid by purchasers to the Underwriters for the Offered Shares is less than the price paid by the Underwriters to the
Company.
The obligations of the Underwriters
under the Underwriting Agreement are several and may be terminated at their discretion pursuant to “regulatory out”, “material
adverse change out”, “disaster out” and “material breach out” provisions and upon the occurrence of certain
other stated events. The Underwriters are, however, severally obligated to take up and pay for all of the Offered Shares that they have
agreed to purchase if any of the Offered Shares are purchased under the Underwriting Agreement.
The TSX has conditionally
approved the listing of the Offered Shares. Listing is subject to the Company fulfilling all of the listing requirements of the TSX on
or before November 2, 2021.
In connection with the
Offering, each of MSS and 1315 Capital intends to exercise its Participation Right pursuant to the investor rights agreement among
the Company and the Investors dated June 14, 2021 (the “Investor Rights Agreement”), to purchase, directly or
indirectly, approximately 200,000 and 167,570 Offered Shares, respectively, at the Offering Price. Under the Investor Rights
Agreement, for so long as the applicable Investor (together with its affiliates) owns, controls or directs, directly or indirectly,
at least 5% of the outstanding Shares (on a partially-diluted basis), such Investor shall have the right, subject to certain
customary exceptions, to participate in any offering of equity or voting securities of the Company or securities convertible into or
exchangeable for equity or voting securities of the Company, or an option or other right to acquire any such securities to purchase
Shares (collectively, “Subject Securities”) on the same terms as the offering of the Subject Securities in order
to maintain such Investors’ percentage ownership interest in the Company prior to such offering (the “Participation
Right”).
The Company has engaged Clarus
Securities Inc. and Desjardins Securities Inc. to serve as independent financial advisors to advise the Company in connection with the
Offering.
Under the Underwriting Agreement,
the Company has agreed that it will not, without the prior written consent of the Lead Underwriters, on behalf of the Underwriters, such
consent not to be unreasonably withheld or delayed, issue, offer to sell or sell any Shares or other securities convertible into or exchangeable
for Shares for the period up to and including 90 days after the Closing Date; provided that such restriction shall not apply to
issuances of Shares upon exercise of the Company’s outstanding options or warrants or pursuant to certain other exceptions customary
for transactions similar to the Offering. In addition, each of the Company’s directors, executive officers and Greybrook Health
will agree, in a lock-up agreement to be executed on or prior to the Closing Date, that for a period of 90 days from the Closing Date,
without the consent of the Lead Underwriters, on behalf of the Underwriters, such consent not to be unreasonably withheld or delayed, that
they will not, directly or indirectly, offer, sell, transfer, pledge, assign, hypothecate or otherwise encumber, directly or indirectly,
any Shares or other securities convertible into or exchangeable for Shares for the period up to and including 90 days after the Closing
Date, except in respect of a bona fide take-over bid or any other similar transaction made generally to all of the shareholders
of the Company, provided that, in the event the change of control or other similar transaction is not completed, such securities shall
remain subject to such lock-up agreement, and except pursuant to certain other exceptions customary for transactions similar to the Offering.
Subscriptions
for the Offered Shares will be received subject to rejection or allotment in whole or in part and the right is reserved to close
the subscription books at any time without notice. The Offering will be conducted under the NCI system. Offered Shares registered in
the name of CDS or its nominee will be deposited electronically with CDS on an NCI basis at closing. A subscriber who purchases Offered
Shares will generally only receive a customer confirmation from the registered dealer from or through whom Offered Shares are purchased
and who is a CDS participant. Certificates evidencing the Offered Shares will not be issued unless a request for a certificate is made
to the Company.
In accordance with rules
and policy statements of certain Canadian securities regulators, the Underwriters may not, at any time during the period of
distribution, bid for or purchase Shares. The foregoing restriction is, however, subject to exceptions where the bid or purchase is
not made for the purpose of creating actual or apparent active trading in, or raising the price of, the Shares. These exceptions
include a bid or purchase permitted under the by-laws and rules of applicable regulatory authorities and the TSX, including the
Universal Market Integrity Rules for Canadian Marketplaces, relating to market stabilization and passive market making activities
and a bid or purchase made for and on behalf of a customer where the order was not solicited during the period of distribution. As a
result of these activities, the price of the Shares may be higher than the price that otherwise might exist in the open market. If
these activities are commenced, they may be discontinued by the Underwriters at any time.
USE
OF PROCEEDS
The estimated net proceeds
to the Company from the Offering, after deducting the Underwriters’ Fee and the expenses of the Offering (estimated to be US$750,000),
will be US$8,720,500 (or US$10,141,075 if the Over-Allotment Option is exercised in full). The Company intends to use the net proceeds
of the Offering as follows (assuming no exercise of the Over-Allotment Option):
|
Anticipated Use of Proceeds
|
|
|
Allocated Funds
|
|
|
The Acquisition:
|
|
US$
|
8,000,000
|
|
|
Working capital and general corporate purposes:
|
|
US$
|
720,500
|
|
|
Total:
|
|
US$
|
8,720,500
|
|
The Offering is not conditional
on the closing of the Acquisition. If the Acquisition does not take place as contemplated, the proceeds of the Offering will not be refunded
and the Company will use the proceeds that otherwise would have been used for the Acquisition for the development of new TMS Centers and
related working capital, potential future acquisitions, working capital and general corporate purposes. See “Risk Factors”
in this Prospectus Supplement.
To date, the Company has had
negative cash flow from operating activities. Although the Company believes it will have positive cash flow from operating activities
in the future, it may require additional financing in addition to the Offering to fund operating and investing activities. See “Risk
Factors” in the Shelf Prospectus and this Prospectus Supplement. While the New Credit Facility provides the Company with an option
of drawing up to an additional US$15 million in three US$5 million delayed-draw term loan tranches within the 24 months following closing
of the New Credit Facility, the ability to draw on such delayed-draw term loans is subject to the Company achieving specific financial
milestones. As of the date of this Prospectus Supplement, the Company does not currently meet these financial milestones and is, therefore,
unable to draw down on any of the delayed-draw term loan tranches under the New Credit Facility at this time.
The Company, as part of
its annual budgeting process, evaluates its estimated annual cash requirements to fund planned expansion activities and working
capital requirements of existing operations. Based on this cash budget and considering its anticipated cash flows from regional
operations, its holdings of cash, the New Credit Facility, the net proceeds from the Private Placement and, assuming the successful
completion of the Offering, the Company believes that it has sufficient capital to meet its future operating expenses, capital
expenditures and future debt service requirements for approximately the next 12 months. The Company’s cash balance and
working capital as at June 30, 2021 was approximately US$19.0 million and US$14.3 million, respectively.
In addition to the net
proceeds from the Offering, the Company had cash of approximately US$19.0 million as at June 30, 2021. The net proceeds of the
Offering will be spent on the Acquisition and expenditures incurred following closing of the Offering. Until applied, the net
proceeds of the Offering will be held as cash balances in the Company’s bank account or invested at the discretion of the
Chief Financial Officer.
If the Over-Allotment Option
is exercised in part or in full, the Company intends to use the additional net proceeds, after deducting the Underwriters’ Fee but
not the expenses of the Offering, of up to US$1,420,575, to fund operating activities and for other working capital and general corporate
purposes.
The Company intends to spend
the funds available as stated in this Prospectus Supplement. However, there may be circumstances where, for sound business reasons, a
reallocation of funds may be deemed prudent or necessary. See “Risk Factors” in this Prospectus Supplement.
PRIOR
SALES
The Company has not issued
any Shares, or securities convertible or exchangeable into Shares, during the 12-month period preceding the date of this Prospectus Supplement,
except as described below:
|
Date Issued
|
|
Type of Securities Issued
|
|
Number of Securities Issued
|
|
|
Issue/Exercise Price Per Share
|
|
|
Nature of Issuance
|
|
December 31, 2020
|
|
Warrants
|
|
|
51,307
|
|
|
|
C$11.20
|
|
|
Issuance of Lender Warrants
|
|
February 4, 2021
|
|
Shares
|
|
|
1,800
|
|
|
|
C$16.25
|
|
|
Exercise of Broker Warrants
|
|
February 17, 2021
|
|
Options
|
|
|
139,500
|
|
|
|
C$20.43
|
|
|
Stock Option Grant
|
|
March 26, 2021
|
|
Shares
|
|
|
231,011
|
|
|
|
C$16.70
|
|
|
Earn-Out Consideration(1)
|
|
April 27, 2021
|
|
Shares
|
|
|
500
|
|
|
|
US$7.50
|
|
|
Exercise of Stock Options
|
|
May 14, 2021
|
|
Options
|
|
|
5,000
|
|
|
|
US$10.70
|
|
|
Stock Option Grant
|
|
May 25, 2021
|
|
Shares
|
|
|
5,000
|
|
|
|
US$5.00
|
|
|
Exercise of Stock Options
|
|
June 14, 2021
|
|
Shares
|
|
|
2,353,347
|
|
|
|
US$10.00
|
|
|
Private Placement
|
|
August 5, 2021
|
|
Options
|
|
|
5,000
|
|
|
|
US$10.35
|
|
|
Stock Option Grant
|
Note:
|
|
(1)
|
A portion of the purchase price payable in respect of the acquisition of Achieve TMS Centers, LLC
and Achieve TMS Alaska, LLC (collectively, “Achieve TMS West”) is subject to an earn-out based on the earnings
before interest, tax, depreciation and amortization achieved by Achieve TMS West during the twelve-month period following September
26, 2019 (the “Earn-Out”). The Earn-Out was partially settled through the issuance of an aggregate of 231,011
Shares to the vendors on March 26, 2021.
|
TRADING
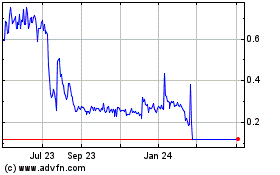
PRICE RANGE AND TRADING VOLUME OF THE SHARES
The Shares are listed for
trading on the TSX under the symbol “GTMS” and on NASDAQ under the symbol “GBNH”. The following tables show the
monthly range of high and low prices per Share at the close of market on the TSX and NASDAQ, as well as total monthly volumes of the Shares
traded on the TSX and NASDAQ for the periods indicated:
TSX(1)
|
|
|
High
(C$)
|
|
|
Low
(C$)
|
|
|
Volume
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
September
|
|
|
7.80
|
|
|
|
6.60
|
|
|
|
125,557
|
|
|
October
|
|
|
8.25
|
|
|
|
6.50
|
|
|
|
112,452
|
|
|
November
|
|
|
7.80
|
|
|
|
6.65
|
|
|
|
351,154
|
|
|
December
|
|
|
13.85
|
|
|
|
7.00
|
|
|
|
279,064
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January
|
|
|
20.45
|
|
|
|
11.15
|
|
|
|
330,406
|
|
|
February
|
|
|
22.40
|
|
|
|
17.50
|
|
|
|
519,725
|
|
|
March
|
|
|
19.80
|
|
|
|
14.24
|
|
|
|
446,792
|
|
|
April
|
|
|
17.58
|
|
|
|
13.00
|
|
|
|
101,864
|
|
|
May
|
|
|
14.69
|
|
|
|
11.80
|
|
|
|
117,680
|
|
|
June
|
|
|
16.63
|
|
|
|
11.70
|
|
|
|
149,677
|
|
|
July
|
|
|
16.49
|
|
|
|
13.80
|
|
|
|
82,806
|
|
|
August
|
|
|
14.67
|
|
|
|
12.58
|
|
|
|
74,774
|
|
|
September 1 – 21
|
|
|
13.39
|
|
|
|
10.62
|
|
|
|
96,903
|
|
Note:
|
|
(1)
|
The Shares commenced trading on the TSX on a post-Share Consolidation basis effective at the opening of
trading on February 4, 2021. The data presented in the table above is shown on a post-Share Consolidation basis for all periods.
|
NASDAQ(2)
|
|
|
High
(US$)
|
|
|
Low
(US$)
|
|
|
Volume
|
|
|
2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 16 – 31
|
|
|
16.00
|
|
|
|
11.55
|
|
|
|
32,680
|
|
|
April
|
|
|
14.00
|
|
|
|
10.79
|
|
|
|
25,965
|
|
|
May
|
|
|
12.01
|
|
|
|
9.23
|
|
|
|
55,331
|
|
|
June
|
|
|
13.89
|
|
|
|
9.55
|
|
|
|
171,350
|
|
|
July
|
|
|
13.01
|
|
|
|
11.09
|
|
|
|
83,050
|
|
|
August
|
|
|
12.00
|
|
|
|
10.12
|
|
|
|
86,719
|
|
|
September 1 – 21
|
|
|
11.13
|
|
|
|
8.26
|
|
|
|
60,202
|
|
Note:
|
|
(2)
|
The Shares commenced trading on NASDAQ on March 16, 2021.
|
CERTAIN
CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
In the opinion of Torys LLP,
counsel to the Company, and Dentons Canada LLP, Canadian counsel to the Underwriters, the following is a general summary, as of the date
hereof, of the principal Canadian federal income tax considerations under the Tax Act generally applicable to a holder of the Offered
Shares who acquires such shares pursuant to the Offering, and who at all relevant times, for purposes of the Tax Act holds the Offered
Shares as capital property and deals at arm’s length with the Company and the Underwriters and is not affiliated with the Company
or the Underwriters (a “Holder”). Generally, the Offered Shares will be capital property to a Holder unless they are
held or acquired in the course of carrying on a business or as part of an adventure or concern in the nature of trade. Certain Holders
who are residents of Canada and who might not otherwise be considered to hold their Offered Shares as capital property may, in certain
circumstances, be entitled to have them and every other “Canadian security” (as defined in the Tax Act) owned by such Holder
be treated as capital property by making an irrevocable election in accordance with subsection 39(4) of the Tax Act. Holders considering
making such election should first consult their own tax advisors.
This summary is not applicable
to a Holder: (a) that is a “financial institution”, as defined in the Tax Act for purposes of the mark-to-market rules; (b)
an interest in which is a “tax shelter investment”, as defined in the Tax Act; (c) that is a “specified financial institution”,
as defined in the Tax Act; (d) that has made an election under the Tax Act to determine its Canadian tax results in a currency of a country
other than Canada; (e) that has entered or will enter into a “derivative forward agreement” under the Tax Act with respect
to the Offered Shares; (f) that receives dividends on the Offered Shares under or as part of a “dividend rental arrangement”,
as defined in the Tax Act; or (g) that is a corporation resident in Canada and is, or becomes, or does not deal at arm’s length
for purposes of the Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or event or series of
transactions or events that includes the acquisition of the Offered Shares, controlled by a non-resident person, or a group of non-resident
persons not dealing with each other at arm’s length, for purposes of the “foreign affiliate dumping” rules in section
212.3 of the Tax Act. Such Holders should consult their own tax advisors.
This summary is based on the
facts set out in this Prospectus Supplement, the current provisions of the Tax Act and the regulations thereunder, all specific proposals
to amend the Tax Act and the regulations thereunder publicly announced by or on behalf of the Minister of Finance (Canada) prior to the
date of hereof (“Tax Proposals”) and counsel’s understanding of the current published administrative policies
and assessing practices of the Canada Revenue Agency. No assurance can be made that the Tax Proposals will be enacted in the form proposed
or at all. This summary is not exhaustive of all possible Canadian federal income tax considerations and, other than the Tax Proposals,
does not take into account or anticipate any changes in law or in administrative policy or assessing practice, whether by legislative,
regulatory, administrative or judicial decision or action, nor does it take into account provincial, territorial or foreign income tax
legislation or considerations, which may differ significantly from the Canadian federal income tax considerations discussed herein.
This summary is of a general
nature only and is not exhaustive of all possible Canadian federal income tax considerations applicable to an investment in the Offered
Shares. The income and other tax consequences of acquiring, holding or disposing of the Offered Shares will vary depending on a Holder’s
particular status and circumstances, including the province or territory in which the Holder resides or carries on business. This summary
is not intended to be, nor should it construed to be, legal or tax advice to any particular Holder. Holders should consult their own tax
advisors with respect to an investment in the Offered Shares having regard to their particular circumstances.
Holders Resident in Canada
The following portion of this
summary applies to Holders who, at all relevant times, for purposes of the Tax Act and any applicable income tax treaty or convention,
are or are deemed to be resident in Canada (“Resident Holders”).
Dividends on the Offered Shares
In the case of a Resident
Holder who is an individual (other than certain trusts), dividends received or deemed to be received by such Resident Holder on the Offered
Shares will be included in computing the Resident Holder’s income and will be subject to the gross-up and dividend tax credit rules
that apply to “taxable dividends” received from “taxable Canadian corporations” (each as defined in the Tax Act).
Provided that appropriate designations are made by the Company, such dividends will be treated as an “eligible dividend” for
the purposes of the Tax Act and a Resident Holder who is an individual will be entitled to an enhanced dividend tax credit in respect
of such dividend. There may be limitations on the Company’s ability to designate dividends as eligible dividends.
Dividends received by a Resident
Holder who is an individual (other than certain trusts) may result in such Resident Holder being liable for alternative minimum tax under
the Tax Act. Such Resident Holders should consult their own tax advisors in this regard.
Dividends received or deemed
to be received on the Offered Shares by a Resident Holder that is a corporation will be required to be included in computing the corporation’s
income for the taxation year in which such dividends are received, but such dividends will generally be deductible in computing the corporation’s
taxable income. In certain circumstances, subsection 55(2) of the Tax Act will treat a taxable dividend received by a Resident Holder
that is a corporation as proceeds of disposition or a capital gain. Resident Holders that are corporations should consult their own tax
advisors having regard to their own circumstances.
A Resident Holder that is
a “private corporation” or a “subject corporation” (each as defined in the Tax Act) may be liable under Part IV
of the Tax Act to pay a refundable tax on dividends received or deemed to be received on the Offered Shares to the extent that such dividends
are deductible in computing the Resident Holder’s taxable income for the taxation year.
A Resident Holder may be subject
to U.S. withholding tax on dividends received on the Offered Shares (see “Certain United States Federal Income Tax Considerations”
in this Prospectus Supplement). Any U.S. withholding tax paid by or on behalf of a Resident Holder in respect of dividends received on
the Offered Shares generally will not be eligible for a foreign tax credit under the Tax Act. However, the Company does not currently
anticipate paying dividends on the Offered Shares.
Dispositions of the Offered Shares
On a disposition or deemed
disposition of the Offered Shares by a Resident Holder, the Resident Holder will generally realize a capital gain (or a capital loss)
equal to the amount by which the proceeds of disposition are greater (or less) than the aggregate of the Resident Holder’s adjusted
cost base of the Offered Shares immediately before the disposition and any reasonable costs of disposition. The adjusted cost base to
a Resident Holder of the Offered Shares acquired pursuant to the Offering will be determined by averaging the cost of such Offered Shares
with the adjusted cost base of all other Shares (if any) held by the Resident Holder as capital property at that time.
One-half of a capital gain
(a “taxable capital gain”) realized by a Resident Holder on the disposition of an Offered Share must be included in
the Resident Holder’s income under the Tax Act. One-half of a capital loss (an “allowable capital loss”) realized
on the disposition of an Offered Share will generally be deducted against taxable capital gains realized by the Resident Holder in that
same year, and allowable capital losses in excess of taxable capital gains for such year may be carried back and deducted in any of the
three preceding taxation years or carried forward and deducted in any subsequent year against net taxable capital gains realized in such
years, to the extent and under the circumstances described in the Tax Act.
The amount of any capital
loss realized by a Resident Holder that is a corporation on the disposition of an Offered Share may be reduced by the amount of dividends
received or deemed to be received by it on such Offered Share (or on a share for which the Offered Share has been substituted) to the
extent and under the circumstances described in the Tax Act. Similar rules may apply where a Resident Holder that is a corporation is
a member of a partnership or a beneficiary of a trust that owns the Offered Shares, directly or indirectly, through a partnership or a
trust. Resident Holders to whom these rules may be relevant should consult their own tax advisors.
Taxable capital gains realized
by a Resident Holder who is an individual (other than certain trusts) may give rise to alternative minimum tax depending on the Resident
Holder’s circumstances. Such Resident Holders should consult their own tax advisors in this regard.
A Resident Holder that is,
throughout the relevant taxation year, a “Canadian-controlled private corporation” (as defined in the Tax Act) may be liable
to pay a refundable tax on its “aggregate investment income” (as defined in the Tax Act), including amounts in respect of
net taxable capital gains.
Holders Not Resident in Canada
The following portion of this
summary applies to a Holder that, at all relevant times, for purposes of the Tax Act and any applicable income tax treaty or convention,
(i) is not, and is not deemed to be, resident in Canada for purposes of the Tax Act and any applicable income tax treaty or convention,
and (ii) holds the Offered Shares as capital property for purposes of the Tax Act and does not use or hold, and is not deemed to use or
hold, the Offered Shares in the course of carrying on, or otherwise in connection with, a business in Canada (a “Non-Resident
Holder”). Special rules, which are not discussed in this summary, may apply to a Non-Resident Holder that is an insurer carrying
on an insurance business in Canada and elsewhere or an “authorized foreign bank” (as defined in the Tax Act).
Dividends on the Offered Shares
Dividends paid or
credited, or deemed to be paid or credited, on the Offered Shares to a Non-Resident Holder will be subject to Canadian withholding
tax at the rate of 25% of the gross amount of the dividend unless the rate is reduced under the provisions of an applicable tax
treaty or convention between Canada and the country in which the Non-Resident Holder is resident. For example, under the
Canada-United States Tax Convention (1980), as amended (the “Treaty”), the rate of withholding tax on dividends
paid or credited to a Non-Resident Holder who is a resident of the United States for purposes of the Treaty, is the beneficial owner
of the dividends, and is entitled to full benefits under the Treaty (a “U.S. Resident Holder”), is generally
reduced to 15% or, in the case of a U.S. Resident Holder that is a corporation that owns at least 10% of the voting shares of the
corporation paying the dividend, the rate is reduced to 5%. Non-Resident Holders should consult their own tax advisors in this
regard.
Dispositions of the Offered Shares
A Non-Resident Holder generally
will not be subject to tax under the Tax Act in respect of a disposition or deemed disposition of an Offered Share, unless the Offered
Share constitutes “taxable Canadian property” (as defined in the Tax Act) of the Non- Resident Holder at the time of disposition
and the Non- Resident Holder is not entitled to relief under an applicable tax treaty or convention between Canada and the country in
which the Non-Resident Holder is resident.
Generally, an Offered Share
will not constitute “taxable Canadian property” of a Non-Resident Holder at a particular time provided that the Offered Share
is listed on a “designated stock exchange” (as defined in the Tax Act, which currently includes the TSX and NASDAQ) at that
time, unless, at any time during the 60-month period that ends at that time, both: (i) one or any combination of (a) the Non- Resident
Holder, (b) persons with whom the Non-Resident Holder does not deal at arm’s length for purposes of the Tax Act and (c) partnerships
in which the Non-Resident Holder or a person described in (b) holds a membership interest (directly or indirectly through one or more
partnerships), owns 25% or more of the issued shares of any class or series of the Company, and (ii) more than 50% of the fair market
value of the Offered Shares was derived directly or indirectly from one or any combination of: (a) real or immovable property situated
in Canada, (b) “timber resource properties” (as defined in the Tax Act), (c) “Canadian resource properties” (as
defined in the Tax Act) or (d) options in respect of, or interests in, or for civil law rights in, any of the foregoing, whether or not
the property exists. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, an Offered Share may be deemed to
be “taxable Canadian property”.
In the case of a U.S. Resident
Holder for whom the Offered Shares constitute “taxable Canadian property”, no Canadian taxes will generally be payable on
a capital gain realized on the disposition or deemed disposition of such shares by reason of the Treaty, unless the value of such shares
is derived principally from “real property situated in Canada” for purposes of the Treaty at the time of the disposition.
U.S. Resident Holders for whom Offered Shares may constitute “taxable Canadian property” should consult their own tax advisor.
In the case where a Non-Resident
Holder disposes, or is deemed to dispose, of an Offered Share, that is “taxable Canadian property” of that Non-Resident Holder,
and the Non-Resident Holder is not entitled to an exemption from tax under the Tax Act or pursuant to the terms of an applicable income
tax treaty or convention, the consequences under the heading “Holders Resident in Canada – Dispositions of the Offered Shares”
will generally be applicable to such disposition. Non-Resident Holders for whom Offered Shares may constitute “taxable Canadian
property” should consult their own tax advisor.
CERTAIN
UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a discussion
of certain U.S. federal income tax considerations relating to the ownership and disposition of Offered Shares. This summary is based upon
the U.S. Internal Revenue Code of 1986, as amended (the “Code”), the Treasury Regulations promulgated thereunder (the
“Treasury Regulations”), judicial authorities, published positions of the Internal Revenue Service (the “IRS”),
and other applicable authorities, all as in effect on the date hereof and all of which are subject to change or differing interpretations,
possibly with retroactive effect. There can be no assurance that the IRS will not challenge any of the tax considerations described in
this summary, and the Company has not obtained, nor does it intend to obtain, a ruling from the IRS or an opinion from legal counsel with
respect to the U.S. federal income tax considerations discussed herein. This summary addresses only certain considerations arising under
U.S. federal income tax law, and it does not address the tax on net investment income or the alternative minimum tax, any other federal
tax considerations (such as estate or gift taxation), or any tax considerations arising under the laws of any state, locality, or non-U.S.
taxing jurisdiction.
This summary is of a
general nature only and does not address all of the U.S. federal income tax considerations that may be relevant to a holder of
Offered Shares in light of such holder’s circumstances. In particular, this discussion applies only to holders of Offered
Shares that hold such shares as “capital assets” (generally, property held for investment purposes), and it does not
address the special tax rules that may apply to special categories of taxpayers, including, without limitation:
|
|
·
|
securities broker-dealers;
|
|
|
·
|
persons that hold Offered Shares as part of a hedging or integrated financial transaction or straddle;
|
|
|
·
|
individuals that have ceased to be United States citizens or lawful permanent residents;
|
|
|
·
|
U.S. Holders (as defined below) whose functional currency is not the U.S. dollar;
|
|
|
·
|
persons that are owners of an interest in a partnership or other passthrough entity that holds Offered
Shares;
|
|
|
·
|
partnerships or other passthrough entities;
|
|
|
·
|
real estate investment trusts;
|
|
|
·
|
regulated investment companies;
|
|
|
·
|
pension plans, retirement plans, retirement accounts, and other tax-deferred accounts;
|
|
|
·
|
financial institutions;
|
|
|
·
|
traders that have elected a mark-to-market method of accounting;
|
|
|
·
|
tax-exempt organizations;
|
|
|
·
|
persons that own or have owned, directly, indirectly, or constructively, more than 5% of the Offered Shares;
|
|
|
·
|
passive foreign investment companies, controlled foreign corporations, and corporations that accumulate
earnings to avoid U.S. federal income tax; and
|
|
|
·
|
persons who received their Offered Shares upon the exercise of employee stock options or otherwise as
compensation.
|
For purposes of this summary,
a “U.S. Holder” is a beneficial owner of Offered Shares acquired pursuant to the Offering and who is, for U.S. federal
income tax purposes:
|
|
·
|
an individual who is a citizen or a resident of the United States;
|
|
|
·
|
a corporation (or other entity classified as a corporation for U.S. federal income tax purposes) created
or organized in or under the laws of the United States, any state thereof, or the District of Columbia;
|
|
|
·
|
an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
|
|
|
·
|
a trust (i) that has validly elected to be treated as a U.S. person for
U.S. federal income tax purposes or (ii) the administration over which a U.S. court can exercise primary supervision and all of the substantial
decisions of which one or more U.S. persons have the authority to control.
|
For purposes of this summary,
a “Non-U.S. Holder” is any person who is a beneficial owner of Offered Shares acquired pursuant to the Offering who
is not a U.S. Holder and is not a partnership or other entity or arrangement that is classified as a partnership for U.S. federal income
tax purposes.
If a partnership (or other
entity or arrangement classified as a partnership for U.S. federal income tax purposes) holds Offered Shares, the tax treatment of a partner
of such partnership generally will depend upon the status of such partner and the activities of the partnership. Partners of partnerships
holding Offered Shares are urged to consult their own tax advisers regarding the tax consequences relating to the ownership and disposition
of Offered Shares.
This summary is not intended
to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any particular shareholder. Prospective
investors are urged to consult their own tax advisers regarding the tax consequences of the ownership and disposition of Offered Shares
in light of their particular circumstances, as well as the tax consequences under state, local, and non-U.S. tax law and the possible
effect of changes in tax law.
Tax Residence of the Company for U.S. Federal
Income Tax Purposes
Although the Company is organized
as a corporation under the OBCA, the Company takes the position that it is treated as a U.S. domestic corporation for all U.S. federal
income tax purposes under Section 7874 of the Code and the Treasury Regulations thereunder. Accordingly, the Company generally will be
subject to U.S. federal income tax on its worldwide income. The remainder of this summary assumes that the Company will be treated as
a U.S. domestic corporation for U.S. federal income tax purposes.
Taxation of U.S. Holders
Distributions on Offered Shares
The Company does not currently
anticipate paying dividends on the Offered Shares. In the event that the Company makes a distribution on Offered Shares, the gross amount
of such distribution (including any amount withheld to pay Canadian withholding taxes) generally will be treated as a dividend to the
extent paid out of the Company’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles).
A distribution on Offered Shares in excess of current and accumulated earnings and profits will be treated as a tax-free return of capital
to the extent of a U.S. Holder’s adjusted tax basis in such Offered Shares and, to the extent in excess of adjusted basis, as capital
gain, which will be subject to the rules discussed below under “—Sale or Other Taxable Disposition of Offered Shares”.
Dividends received by individuals and other non-corporate U.S. Holders generally will be subject to tax at preferential rates applicable
to long-term capital gains, provided that such holders meet certain holding period and other requirements. Dividends received by corporate
U.S. Holders may qualify for the dividends-received deduction, subject to various limitations.
As discussed above under the
heading “Certain Canadian Federal Income Tax Considerations”, dividends paid by the Company to U.S. Holders generally will
be subject to Canadian withholding tax, subject to reduction under an applicable income tax treaty. For U.S. foreign tax credit purposes,
such dividends generally will not constitute foreign-source income for U.S. foreign tax credit purposes, based on the treatment of the
Company as a U.S. domestic corporation under Section 7874 of the Code, as described above. Therefore, a U.S. Holder may not be permitted
to claim a U.S. foreign tax credit for any such Canadian withholding tax, unless such U.S. Holder has sufficient foreign-source income
from other sources and certain other conditions are met. However, the U.S. Holder may be entitled to a deduction for the U.S. Holder’s
Canadian tax paid, provided the U.S. Holder has not elected to credit other foreign taxes with respect to the same taxable year.
The foreign tax credit rules
are complex, and U.S. Holders are urged to consult their own tax advisers regarding the availability of foreign tax credits and the U.S.
federal income tax treatment of distributions on Offered Shares in light of their particular circumstances.
Sale or Other Taxable Disposition of Offered Shares
A U.S. Holder who sells,
exchanges, or otherwise disposes of Offered Shares in a taxable transaction will recognize a gain or loss equal to the difference,
if any, between the U.S. dollar value of the amount realized on such sale or other taxable disposition and the U.S. Holder’s
adjusted tax basis in such shares. Any such gain or loss will be capital gain or loss, and will be long-term capital gain or loss if
the U.S. Holder held the Offered Shares for more than one year at the time of the sale or other taxable disposition. For
non-corporate U.S. Holders, long-term capital gains recognized in connection with a sale or other taxable disposition of Offered
Shares generally will be taxed at preferential capital gain rates. The deductibility of capital losses is subject to limitations.
Any such gain or loss recognized by a U.S. Holder generally will be treated as U.S.-source gain or loss for foreign tax credit
limitation purposes. U.S. Holders are urged to consult their own tax advisers regarding the U.S. federal income tax consequences of
the sale, exchange, or other taxable disposition of Offered Shares in light of their particular circumstances.
Receipt of Foreign Currency
The U.S. dollar value of any
cash payment in Canadian dollars to a U.S. Holder will be translated into U.S. dollars calculated by reference to the exchange rate prevailing
on the date of actual or constructive receipt of the payment, regardless of whether the Canadian dollars are converted into U.S. dollars
at that time. A U.S. Holder generally will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt.
Any U.S. Holder that receives payment in Canadian dollars and converts or disposes of the Canadian dollars after the date of receipt may
have a foreign currency exchange gain or loss that would be treated as ordinary income or loss and that generally would be U.S.-source
income or loss for foreign tax credit purposes. U.S. Holders are urged to consult their own tax advisers regarding the U.S. federal income
tax consequences of receiving, owning, and disposing of Canadian dollars.
Information Reporting and Backup Withholding
Dividend payments with respect
to Offered Shares and proceeds from the sale or other taxable disposition of Offered Shares generally will be subject to information reporting
to the IRS and possible backup withholding. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer
identification number and makes other required certifications, or who is otherwise exempt from backup withholding and establishes such
exempt status. Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s
U.S. federal income tax liability, and a U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding
rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information.
Taxation of Non-U.S. Holders
Distributions on Offered Shares
The Company does not currently
anticipate paying dividends on the Offered Shares. In the event that the Company makes a distribution on Offered Shares, the gross amount
of such distribution generally will be treated as a dividend to the extent paid out of the Company’s current or accumulated earnings
and profits (as determined under U.S. federal income tax principles). A distribution on Offered Shares in excess of current and accumulated
earnings and profits will be treated as a tax-free return of capital to the extent of a Non-U.S. Holder’s adjusted tax basis in
such Offered Shares and, to the extent in excess of adjusted basis, as capital gain, which will be subject to the rules discussed below
under “—Sale or Other Taxable Disposition of Offered Shares”.
Subject to the discussion
below regarding effectively connected income, dividends paid to a Non-U.S. Holder will be subject to U.S. federal withholding tax at a
rate of 30% of the gross amount of the dividends or such lower rate as is specified by an applicable income tax treaty, provided the Non-U.S.
Holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty
rate. A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain
a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders are urged to consult
their own tax advisers regarding their entitlement to benefits under any applicable income tax treaty.
If dividends paid to a
Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States
(and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States
to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above.
To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying
that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United
States. Any such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular
graduated rates. A Non-U.S. Holder that is a corporation may also be subject to a branch profits tax at a rate of 30% (or such lower
rate as is specified by an applicable income tax treaty) on such effectively connected dividends, as adjusted for certain items.
Non-U.S. Holders are urged to consult their own tax advisers regarding any applicable income tax treaties that may provide for
different rules.
Sale or Other Taxable Disposition of Offered Shares
A Non-U.S. Holder generally
will not be subject to U.S. federal income tax or withholding tax on any gain realized upon a sale, exchange, or other taxable disposition
of Offered Shares unless:
|
|
·
|
the gain is effectively connected with the holder’s conduct of a trade or business within the United
States and, if an applicable income tax treaty so provides, is attributable to a permanent establishment or a fixed base maintained by
the holder in the United States, in which case, the holder generally will be taxed on a net income basis at the graduated U.S. federal
income tax rates applicable to U.S. persons (and a corporate holder may be subject to the additional branch profits tax described above);
|
|
|
·
|
the holder is an individual that is present in the United States for 183 days or more in the taxable year
of the disposition and certain other conditions are met, in which case, the holder generally will be subject to a 30% tax on the net gain
derived from the disposition, which may be offset by U.S.-source capital losses, if any, realized during the same taxable year; or
|
|
|
·
|
the Company is or was a “United States real property holding corporation”
for U.S. federal income tax purposes at any time during the shorter of the five-year period preceding the disposition or the Non-U.S.
Holder’s holding period for the Offered Shares.
|
Generally, a corporation is
a United States real property holding corporation if the fair market value of its “United States real property interests”
equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held
for use in a trade or business. The Company believes that it is not currently, and it does not anticipate becoming in the future, a United
States real property holding corporation. However, because the determination of whether the Company is a United States real property holding
corporation is made from time to time and depends on the relative fair market values of its assets, there can be no assurance in this
regard. If the Company were a United States real property holding corporation, the tax relating to disposition of stock in a United States
real property holding corporation generally would not apply to a Non-U.S. Holder whose holdings, directly, indirectly, and constructively,
constituted 5% or less of our Offered Shares at all times during the applicable period, provided that our Offered Shares are “regularly
traded on an established securities market” (as provided in applicable Treasury Regulations) at any time during the calendar year
in which the disposition occurs. However, no assurance can be provided that our Offered Shares will be regularly traded on an established
securities market for purposes of the rules described above. If a Non-U.S. Holder is subject to U.S. federal income tax pursuant to these
rules, any gains on the sale or other disposition of such Offered Shares would be taxed on a net income basis at the graduated rates applicable
to U.S. persons, and the holder would be required to file a U.S. tax return with respect to such gains. Non-U.S. Holders are urged to
consult their own tax advisers regarding the possible adverse U.S. federal income tax consequences to them if the Company is, or were
to become, a United States real property holding corporation.
Information Reporting and Backup Withholding
Backup withholding will not
apply to payments of dividends on our Offered Shares to a Non-U.S. Holder, provided the applicable withholding agent does not have actual
knowledge or reason to know the holder is a U.S. person and the Non-U.S. Holder either provides to the applicable withholding agent a
properly executed IRS Form W-8BEN or W-8BEN-E (or other applicable form) certifying under penalties of perjury that the Non-U.S. Holder
is not a U.S. person or otherwise qualifies for an exemption. However, the applicable withholding agent generally will be required to
report to the IRS and to such Non-U.S. Holder payments of dividends on our Offered Shares and the amount of U.S. federal income tax, if
any, withheld with respect to those payments. Copies of the information returns reporting such dividends and any withholding may also
be made available to the tax authorities in the country in which the Non-U.S. Holder resides under the provisions of a treaty or agreement.
The gross proceeds from
the sale or other disposition of our Offered Shares may be subject, in certain circumstances discussed below, to U.S. backup
withholding and information reporting. If a Non-U.S. Holder sells or otherwise disposes of our Offered Shares outside the United
States through a non-U.S. office of a non-U.S. broker and the sale or disposition proceeds are paid to the Non-U.S. Holder outside
the United States, then the U.S. backup withholding and information reporting requirements generally will not apply to that payment.
However, U.S. information reporting, but not U.S. backup withholding, will apply to a payment of sale or disposition proceeds, even
if that payment is made outside the United States, if a Non-U.S. Holder sells our Offered Shares through a non-U.S. office of a
broker that is a U.S. person or has certain enumerated connections with the United States, unless the broker has documentary
evidence in its files that the Non-U.S. Holder is not a U.S. person and certain other conditions are met or the Non-U.S. Holder
otherwise qualifies for an exemption.
If a Non-U.S. Holder receives
payment of the proceeds of a sale or other disposition of our Offered Shares to or through a U.S. office of a broker, the payment will
be subject to both U.S. backup withholding and information reporting unless the Non-U.S. Holder provides to the applicable withholding
agent a properly executed IRS Form W-8BEN or W-8BEN-E (or other applicable form) certifying under penalties of perjury that the Non-U.S.
Holder is not a U.S. person (and the applicable withholding agent does not have actual knowledge or reason to know that such holder is
a U.S. person) or otherwise qualifies for an exemption. Backup withholding is not an additional tax. Any amounts withheld under the backup
withholding rules may be credited against the Non-U.S. Holder’s U.S. federal income tax liability (which may result in the Non-U.S.
Holder being entitled to a refund), provided that the required information is timely furnished to the IRS.
Additional Withholding Requirements Under FATCA
Under Sections 1471 through
1474 of the Code, and the Treasury Regulations and administrative guidance thereunder (“FATCA”), withholding tax may
apply to certain types of payments made to “foreign financial institutions” (as defined in the Code) and certain other non-U.S.
entities. Specifically, a 30% withholding tax may be imposed on dividends on the Offered Shares paid to a foreign financial institution
or to a non-financial foreign entity, unless (i) in the case of a foreign financial institution, such institution enters into an agreement
with the U.S. government to withhold on certain payments, and to collect and provide to the U.S. tax authorities substantial information
regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain
account holders that are non-U.S. entities with U.S. owners); (ii) in the case of a non-financial foreign entity, such entity certifies
that it does not have any “substantial United States owners” (as defined in the Code) or provides the applicable withholding
agent with a certification identifying the direct and indirect substantial United States owners of the entity (in either case, generally
on IRS Form W-8BEN-E); or (iii) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption
from these rules and provides appropriate documentation (such as IRS Form W-8BEN-E). Foreign financial institutions located in jurisdictions
that have an intergovernmental agreement with the United States may be subject to different rules. Under certain circumstances, a shareholder
might be eligible for refunds or credits of such taxes. Proposed Treasury Regulations would eliminate the requirement to withhold tax
under FATCA on gross proceeds from the sale or disposition of property that can produce U.S.-source interest or dividends. The IRS has
announced that taxpayers are permitted to rely on the proposed regulations until final Treasury Regulations are issued. Non-U.S. Holders
are encouraged to consult their own tax advisers regarding the effect of FATCA on their investment in Offered Shares in light of their
particular circumstances.
RISK
FACTORS
An investment in the Offered
Shares is subject to a number of risks, including those set forth herein and in the accompanying Shelf Prospectus, as well as in the Annual
Information Form, the Annual MD&A and the Interim MD&A, all of which are incorporated by reference herein. Prospective investors
should carefully consider these risks, in addition to information contained in this Prospectus Supplement, the Shelf Prospectus and the
information incorporated by reference herein and therein, before purchasing Offered Shares.
Risks Related to the Offering
Possible Failure to Complete the Acquisition
The Company expects to
complete the Acquisition in the fourth quarter of 2021, subject to satisfactory completion of customary closing conditions. The
Company, however, has no control over whether or not all of the conditions will be met and there can be no assurance that all
conditions will be satisfied or waived or that the Acquisition will be consummated on the specified timeframe or at all.
The
Offering is not conditional on the closing of the Acquisition and only a portion of the net proceeds of the Offering will be used
to fund the purchase price in respect of the Acquisition. If the Acquisition does not take place as contemplated, the proceeds of the
Offering will not be refunded and the Company will use the proceeds that otherwise would have been used for the Acquisition for the development
of new TMS Centers and related working capital, potential future acquisitions, working capital and general corporate purposes. If the
Acquisition is not consummated, the Company will not realize the benefits of the Acquisition and could suffer adverse consequences, including
the payment of a break-up fee. The price of the Shares may decline to the extent that the relevant current market price reflects a market
assumption that the Acquisition will be completed and certain costs related to the Acquisition, such as legal, accounting and consulting
fees, must be paid even if the Acquisition is not completed. The Company may be unable to identify other investments offering financial
returns comparable to those of the Acquisition.
Risks
Related to the Integration of Achieve TMS East/Central into the Company’s Business
In
order to achieve the anticipated benefits of the Acquisition, the Company will rely upon its ability to successfully retain staff,
consolidate functions and integrate operations, procedures and personnel in a timely and efficient manner and to realize the anticipated
growth opportunities from combining Achieve TMS East/Central and related operations with those of the Company. The integration of Achieve
TMS East/Central and related operations requires the dedication of management effort, time and resources, which may divert management’s
focus and resources away from other strategic opportunities and from operational matters during the integration process. The integration
process may result in the disruption of ongoing business that may materially adversely affect the Company’s ability to achieve the
anticipated benefits of the Acquisition. The challenges involved in the integration may include, among other things: the necessity of
coordinating both geographically disparate and geographically overlapping organizations; retaining key personnel during the period between
execution of the Purchase Agreement and the closing of the Acquisition, including addressing the uncertainties of key employees regarding
their future; integration of information technology systems and resources; integrating Achieve TMS East/Central into the Company’s
accounting system and adjusting the Company’s internal control environment to cover Achieve TMS East/Central’s operations;
and performance shortfalls relative to expectations at one or both of the businesses as a result of the diversion of management’s
attention to the Acquisition and integration.
Possible Failure to Realize Expected Returns
on the Acquisition
Business
combinations such as the Acquisition involve risks that could materially and adversely affect the Company’s business plan,
including the failure of the Acquisition to realize the results the Company expects. There can be no assurance that management of the
Company will be able to fully realize some or all of the expected benefits of the Acquisition, including, among other things, those described
under “Recent Developments – Acquisition of Achieve TMS East/Central”. The ability to realize these anticipated benefits
will depend in part on successfully consolidating functions and integrating operations, procedures and personnel in a timely and efficient
manner, as well as on the ability to realize growth opportunities and potential synergies from integrating Achieve TMS East/Central with
the Company’s existing business following closing of the Acquisition. There is a risk that some or all of the expected benefits
will fail to materialize, or may not occur within the time periods anticipated by management. The realization of some or all of such benefits
may be affected by a number of factors, many of which are beyond the control of the Company.
Assumption of Liabilities
Under
the terms of the Purchase Agreement, the Company will effectively assume all of Achieve TMS East/Central’s liabilities post-closing.
The Company may assume unknown liabilities that could be significant. There may be liabilities that the Company failed to discover or
was unable to quantify during its pre-Acquisition due diligence and the Company may not be indemnified for any of these liabilities under
the Purchase Agreement. The subsequent discovery or quantification of material liabilities could have a material adverse effect on the
Company’s business, financial condition or future prospects. The representations and warranties contained in the Purchase Agreement,
and related indemnification, may not apply or be sufficient so as to fully indemnify the Company for such liabilities.
Return on Investment is Not Guaranteed
There can be no
assurance regarding the amount of income to be generated by the Company. The Offered Shares are equity securities of the Company and
are not fixed income securities. Unlike fixed income securities, there is no obligation of the Company to distribute to shareholders
a fixed amount or any amount at all, or to return the initial purchase price of an Offered Share on any date in the future. The
market value of the Shares may deteriorate if the Company is unable to generate sufficient positive returns, and that deterioration
may be significant.
Dilution
The number of Shares that
the Company is authorized to issue is unlimited. The Company may, in its sole discretion, issue additional Shares from time to time subject
to the rules of any applicable stock exchange on which the Shares are then listed and applicable securities law. The issuance of any additional
Shares may have a dilutive effect on the interests of holders of Offered Shares. To the extent that any of the net proceeds of the Offering
remain uninvested pending their use, or are used to pay down existing indebtedness with a low interest rate, the Offering may result in
substantial dilution on a per Share basis to the Company’s net income and certain other financial measures used by the Company.
Market Discount
The price of the Shares will
fluctuate with market conditions and other factors. If a holder of Offered Shares sells his, her or its Offered Shares, the price received
may be more or less than the original investment. The Shares may trade at a discount from their book value. The Offered Shares may trade
at a price that is less than the Offering Price. This risk may be greater for investors who sell their Offered Shares relatively shortly
after closing of the Offering.
Use of Proceeds
The Company intends to use
the net proceeds from the Offering as described under “Use of Proceeds”. However, management will have discretion in the actual
application of the proceeds, and may elect to allocate proceeds differently from that described under “Use of Proceeds” if
the Acquisition does not close and we believe that it would be in the best interests of the Company to allocate proceeds otherwise or
if circumstances change. The failure by management to apply these funds effectively could have a material adverse effect on the business
of the Company.
Treatment of the Company as a U.S. Domestic
Corporation for U.S. Federal Income Tax Purposes
Although the Company is organized
as a corporation under the OBCA, the Company takes the position that it is treated as a U.S. domestic corporation for all U.S. federal
income tax purposes under Section 7874 of the Code. As a result, the Company generally is taxable on its worldwide income in both Canada
and the United States. This treatment is expected to continue indefinitely, which could have a material adverse effect on the Company’s
financial condition and results of operations. In addition, dividends received by Non-U.S. Holders (as defined above) generally will be
subject to U.S. federal withholding tax at the rate of 30%, except as reduced by an applicable income tax treaty. Shareholders who are
residents of Canada generally will not be permitted to claim a foreign tax credit under the Tax Act for any such U.S. withholding tax.
Investors are urged to consult their own tax advisers regarding the U.S. tax treatment of the Company and the tax consequences of owning
Offered Shares in light of their particular circumstances.
There is doubt about our ability to continue
as a going concern due to recurring losses from operations, accumulated deficit and insufficient cash resources to meet our business objectives
As discussed in Note 2(a)
to our financial statements, we have generated operating losses since inception, and we had negative cash flow from operating activities
for the year ended December 31, 2020, which together raises doubt about our ability to continue as a going concern. Our independent auditors
therefore have added an explanatory paragraph to their audit opinion issued in connection with our consolidated financial statements for
the years ended December 31, 2020 and December 31, 2019 with respect to their doubt about our ability to continue as a going concern.
Risks Related to Ownership of the Shares
There are unexercised options and warrants
outstanding and which may be issued from time to time. If these are exercised or converted, an investor’s interest in Shares will
be diluted
Following completion of
the Offering there will be 17,394,135 Shares issued and outstanding (17,589,135 Shares if the Over-Allotment Option is
exercised in full). If all of the Company’s options that were issued and outstanding as of September 21, 2021, including
options that are not yet exercisable, were to be exercised, the Company would be required to issue up to an additional 860,500
Shares, or approximately 4.9% of the issued and outstanding Shares following
completion of the Offering on a non-diluted basis (approximately 4.9% if the
Over-Allotment Option is exercised in full). There will also be 51,307 warrants of the Company issued and outstanding following
completion of the Offering that are exercisable into Shares on a one-for-one basis. If all of these warrants were to be exercised,
the Company would be required to issue up to an additional 51,307 Shares, or approximately 0.3% of the issued and outstanding Shares following completion of the Offering
on a non-diluted basis (0.3% if the Over-Allotment Option is exercised in
full). In addition, to the extent that the Company draws down additional financing under the New Credit Facility, the Company will
be required to issue additional common share purchase warrants to Oxford Finance LLC (“Lender Warrants”) in
connection with the New Credit Facility in an amount equal to 3% of the amounts drawn divided by the lesser of (i) the closing price
of the Shares on the day prior to the issuance of such Lender Warrants and (ii) the average closing price of the Shares on the TSX
for the 10 days prior to the issuance of such additional Lender Warrants, in either case subject to approval by the TSX.
These issuances, to the extent
they occur, would decrease the proportionate ownership and voting power of all shareholders. This dilution could cause the price of the
Shares to decline and it could result in the creation of new control persons. In addition, the Company’s shareholders could suffer
dilution in the net book value per Share.
Greybrook Health continues to have significant
influence over us, including control over decisions that require the approval of shareholders, which could limit your ability to influence
the outcome of matters submitted to shareholders for a vote
Following completion of the
Offering and assuming no Offered Shares are issued to Greybrook Health, Greybrook Health will beneficially own, control or direct approximately
26.0% of the issued and outstanding Shares (25.7% if the Over-Allotment Option is exercised in full). As long as Greybrook Health
owns or controls a significant number of the outstanding Shares, they will have the ability to exercise substantial control over all corporate
actions requiring shareholder approval, irrespective of how our other shareholders may vote, including the election and removal of directors
and the size of our Board, any amendments to our Articles, or the approval of any merger, acquisition or other significant corporate transaction,
including a sale of all or substantially all of our assets.
In addition, Greybrook Health’s
interests may not align with the interests of our other shareholders. Greybrook Health is in the business of making investments in companies
and may acquire and hold, from time to time, interests in businesses that compete directly or indirectly with us. Greybrook Health may
also pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may
not be available to us.
Future sales of our securities by existing
shareholders or by us could cause the market price for the Shares to decline
Sales of a substantial number
of the Shares in the public market could occur at any time. These sales, or the market perception that the holders of a large number of
Shares intend to sell their Shares, could significantly reduce the market price of the Shares. We cannot predict the effect, if any, that
future public sales of these securities or the availability of these securities for sale will have on the market price of the Shares.
If the market price of the Shares was to drop as a result, this might impede our ability to raise additional capital and might cause remaining
shareholders to lose all or part of their investment.
It is a condition of
closing of the Offering that the Company’s directors and executive officers and Greybrook Health enter into 90 day lock-up
agreements. Upon the expiry of such lock-up agreements, those Shares will be available for sale in the public markets subject to
restrictions under applicable securities laws. In addition, as of the date hereof, there are 16,094,135 Shares outstanding. There
are also options to acquire 860,500 Shares currently outstanding and, on December 31, 2020, we issued the Lender Warrants to
acquire 51,307 Shares. In addition, to the extent that the Company draws down additional financing under the New Credit Facility,
the Company will be required to issue additional Lender Warrants to Oxford Finance LLC. The Shares issuable upon the exercise of
these options and Lender Warrants, will, to the extent permitted by any applicable vesting requirements, lock-up restrictions and
restrictions under applicable securities laws, also become eligible for sale in the public market.
Further,
we cannot predict the size of future issuances of Shares or the effect, if any, that future issuances and sales of Shares will have on
the market price of the Shares. In addition, the Resale Prospectus registers the resale, from time to time, of up to 2,353,347
of our Shares in the public market by investors in the Private Placement. See “Recent Developments” in this Prospectus Supplement.
Sales of substantial amounts of Shares, or the perception that such sales could occur, may adversely affect prevailing market prices for
the Shares.
We do not expect to pay any cash dividends for the foreseeable
future
We currently expect to retain
all available funds and future earnings, if any, for use in the operation and growth of our business and do not anticipate paying any
cash dividends for the foreseeable future. Any future determination to pay dividends will be at the discretion of our Board, subject to
compliance with applicable law and any contractual provisions, including under any existing or future agreements for indebtedness we may
incur, that restrict or limit our ability to pay dividends, and will depend upon, among other factors, our results of operations, financial
condition, earnings, capital requirements and other factors that our Board deems relevant. Accordingly, realization of a gain on your
investment will depend on the appreciation of the price of the Shares, which may never occur. Investors seeking cash dividends in the
foreseeable future should not invest in Shares.
The forward-looking
statements contained in this Prospectus Supplement may prove to be incorrect
There can be no assurance
that any estimates and assumptions contained in this Prospectus Supplement will prove to be correct (including, but not limited to, our
expectations regarding the Acquisition). Our actual results in the future may vary significantly from the historical and estimated results
and those variations may be material. There is no representation by us that actual results achieved by the Company in the future will
be the same, in whole or in part, as those included in this Prospectus Supplement. See “Forward-Looking Statements” in the
Shelf Prospectus and this Prospectus Supplement.
PROMOTER
Greybrook Health may be considered
a promoter of the Company within the meaning of securities legislation of certain provinces and territories of Canada. Greybrook Health
currently holds an approximate 28.1% ownership interest in the Company through ownership of 4,527,697 Shares. See “Risk Factors
– Risks Related to Ownership of the Shares” in this Prospectus Supplement.
LEGAL
MATTERS
Certain legal matters relating
to Canadian law with respect to the Offering will be passed upon on behalf of the Company by Torys LLP and on behalf of the Underwriters
by Dentons Canada LLP. Certain legal matters relating to United States law with respect to the Offering will be passed upon on behalf
Company by Torys LLP and on behalf of the Underwriters by Dentons US LLP. As of the date hereof, the “designated professionals”
(as such term is defined in Form 51-102F2 – Annual Information Form) of each of Torys LLP, as a group, and Dentons Canada
LLP, as a group, respectively, beneficially own, directly or indirectly, less than 1% of the outstanding securities of the Company.
AUDITORS
AND TRANSFER AGENT AND REGISTRAR
KPMG LLP are the auditors
of the Company and have confirmed that they are independent with respect to the Company within the meaning of the relevant rules and related
interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulation and also that they
are independent accountants with respect to the Company under all relevant U.S. professional and regulatory standards.
The transfer agent and registrar
for the Offered Shares is Computershare Investor Services Inc. at its principal office in Toronto, Ontario. Computershare Trust Company,
N.A. serves as the U.S. transfer agent for the Offered Shares.
DOCUMENTS
FILED AS PART OF THE REGISTRATION STATEMENT
The following documents have
been or will be filed with the SEC as part of the Registration Statement of which this Prospectus Supplement forms a part: (a) the documents
listed under the heading “Documents Incorporated by Reference”; (b) powers of attorney from the Company’s directors
or officers, as applicable; (c) the consent of KPMG LLP; (d) the consent of Torys LLP; (e) the consent of Dentons Canada LLP; and (f)
the Underwriting Agreement.
This short form
base shelf prospectus has been filed under legislation in each of the provinces and territories of Canada that permits certain information
about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that
information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within
a specified period of time after agreeing to purchase any of these securities.
No securities
regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form base shelf
prospectus constitutes an offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein
only by persons permitted to sell such securities in those jurisdictions.
Information
has been incorporated by reference in this short form base shelf prospectus from documents filed with securities commissions or similar
authorities in Canada and with the U.S. Securities and Exchange Commission. Copies of the documents incorporated herein by
reference may be obtained on request without charge from the General Counsel of Greenbrook TMS Inc. at 890 Yonge Street, 7th Floor, Toronto,
Ontario, Canada M4W 3P4 (telephone: (416) 915-9100), and are also available electronically at www.sedar.com and www.sec.gov.
SHORT FORM BASE SHELF
PROSPECTUS
|
New Issue and/or Secondary Offering
|
|
|
July
22, 2021
|

GREENBROOK TMS
INC.
Common
Shares
Preferred Shares
Warrants
Subscription Receipts
Units
US$150,000,000
Greenbrook TMS Inc.
(“Greenbrook” or the “Company”) may offer and issue from time to time common shares of the Company
(“Common Shares”), preferred shares of the Company (“Preferred Shares”), warrants to purchase Common
Shares or Preferred Shares (“Warrants”), subscription receipts (“Subscription Receipts”), or units
(“Units”) comprised of one or more of the other securities described in this short form base shelf prospectus (the
“Prospectus”) (all of the foregoing, collectively, the “Securities”) or any combination thereof
for up to an aggregate initial offering price of US$150,000,000 (or the equivalent thereof in other currencies) during the 25-month period
that this Prospectus, including any amendments hereto, remains effective. Securities may be offered separately or together, in amounts,
at prices and on terms to be determined based on market conditions at the time of sale and set forth in an accompanying prospectus supplement
(a “Prospectus Supplement”). In addition, the Securities may be offered and issued in consideration for the acquisition
of other businesses, assets or securities by the Company or one of its subsidiaries. The consideration for any such acquisition may consist
of the Securities separately, a combination of Securities or any combination of, among other things, Securities, cash and assumption of
liabilities. One or more securityholders of the Company (each,a “Selling Shareholder”) may also offer and sell Securities
under this Prospectus. See “Selling Shareholders”. The Company is filing this Prospectus in connection with the concurrent
filing of a U.S. registration statement on Form F-10, of which this Prospectus forms a part (the “Registration Statement”),
pursuant to the United States Securities Act of 1933, as amended (the “U.S. Securities Act”). See “Available
Information”.
The
specific terms of the Securities with respect to a particular offering will be set out in the applicable Prospectus Supplement and
may include, where applicable (i) in the case of Common Shares, the number of Common Shares offered, the offering price, whether the
Common Shares are being offered for cash, the persons offering the Common Shares and any other terms specific to the Common Shares
being offered, (ii) in the case of Preferred Shares, the number of Preferred Shares offered, the designation of a particular class
or series, if applicable, the offering price, whether the Preferred Shares are being offered for cash, the dividend rate, if any,
any terms for redemption or retraction, any conversion rights, and any other terms specific to the Preferred Shares being offered,
(iii) in the case of Warrants, the offering price, whether the Warrants are being offered for cash, the designation, the number and
the terms of the Common Shares or Preferred Shares purchasable upon exercise of the Warrants, any procedures that will result in the
adjustment of these numbers, the exercise price, the dates and periods of exercise, and any other terms specific to the Warrants
being offered, (iv) in the case of Subscription Receipts, the number of Subscription Receipts being offered, the offering price,
whether the Subscription Receipts are being offered for cash, the procedures for the exchange of the Subscription Receipts for
Common Shares, Preferred Shares or Warrants, as the case may be, and any other terms specific to the Subscription Receipts being
offered, and (v) in the case of Units, the designation and terms of the Units and of the securities comprising the Units and any
other specific terms. Where required by statute, regulation or policy, and where Securities are offered in currencies other than
Canadian dollars, appropriate disclosure of foreign exchange rates applicable to the Securities will be included in the Prospectus
Supplement describing the Securities.
All shelf information
permitted under applicable law to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be
delivered to purchasers together with this Prospectus to the extent required under applicable securities laws. Each Prospectus Supplement
will be incorporated by reference into this Prospectus for the purposes of securities legislation as of the date of the Prospectus Supplement
and only for the purposes of the distribution of the Securities to which the Prospectus Supplement pertains.
(continued on
next page)
(continued from cover)
This Prospectus
constitutes a public offering of the Securities only in those jurisdictions where they may be lawfully offered for sale and only by persons
permitted to sell the Securities in those jurisdictions. The Company and the Selling Shareholder(s) may offer and sell the Securities
to, or through, underwriters or dealers purchasing as principals and may also sell directly to one or more purchasers or through agents
or pursuant to applicable statutory exemptions. See “Plan of Distribution”. The Prospectus Supplement relating to each issue
of Securities offered thereby will set forth the names of any underwriters, dealers or agents involved in the offering and sale of the
Securities and will identify, if applicable, each underwriter, dealer or agent, as the case may be, engaged by the Company or the Selling
Shareholder(s) in connection with the offering and sale of the Securities, and will set forth the terms of the offering of such Securities,
including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters, dealers or agents in connection
with the offering, the method of distribution of the Securities, the identity of the Selling Shareholder(s), if any, the initial issue
price (in the event that the offering is a fixed price distribution), the proceeds that the Company or the Selling Shareholder(s) will,
or expects to receive and any other material terms of the plan of distribution.
The Securities may
be sold from time to time in one or more transactions at a fixed price or prices or at non-fixed prices. If offered on a non-fixed price
basis, the Securities may be offered at market prices prevailing at the time of sale (including, in the case of the Company but not the
Selling Shareholders, sales in transactions that are deemed to be “at-the-market distributions” as defined in National Instrument
44-102 — Shelf Distributions of the Canadian Securities Administrators (“NI 44-102”), at prices determined
by reference to the prevailing price of a specified security in a specified market or at prices to be negotiated with purchasers, in which
case the compensation payable to an underwriter, dealer or agent in connection with any such sale will be decreased by the amount, if
any, by which the aggregate price paid for Securities by the purchasers is less than the gross proceeds paid by the underwriter, dealer
or agent to the Company and/or the Selling Shareholder(s). The price at which the Securities will be offered and sold may vary from purchaser
to purchaser and during the period of distribution.
In connection with
any offering of Securities, other than an “at-the-market distribution” (as defined under applicable Canadian securities legislation),
unless otherwise specified in a Prospectus Supplement, the underwriters, dealers or agents, as the case may be, may over-allot or effect
transactions which stabilize, maintain or otherwise affect the market price of the Securities at a level other than those which otherwise
might prevail on the open market. Such transactions may be commenced, interrupted or discontinued at any time. A purchaser who acquires
Securities forming part of the underwriters’, dealers’ or agents’ over-allocation position acquires those securities
under this Prospectus and the Prospectus Supplement relating to the particular offering of Securities, regardless of whether the over-allocation
position is ultimately filled through the exercise of the over-allotment option or secondary market purchases. See “Plan of Distribution”.
No underwriter or dealer involved in an “at-the-market distribution” under this Prospectus, no affiliate of such an underwriter
or dealer and no person or company acting jointly or in concert with such underwriter or dealer will over-allot Securities in connection
with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the Securities.
The outstanding
Common Shares are listed and posted for trading on the Toronto Stock Exchange (the “TSX”) under the symbol “GTMS”
and on the Nasdaq Capital Market of The NASDAQ Stock Market LLC (“NASDAQ”) under the symbol “GBNH”. Unless
otherwise specified in the applicable Prospectus Supplement, no Securities, other than Common Shares, will be listed on any securities
exchange.
The principal,
head and registered office of the Company is located at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada, M4W 3P4. The Company’s
United States corporate headquarters is located at 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, United States, 22102.
The Company
is permitted, under a multijurisdictional disclosure system adopted by the securities regulatory authorities in Canada and the United
States (“MJDS”), to prepare this Prospectus in accordance with the disclosure requirements of Canada. Prospective purchasers
in the United States should be aware that such requirements are different from those of the United States. The financial statements included
or incorporated by reference herein have been prepared in accordance with International Financial Reporting Standards as issued by the
International Accounting Standards Board (“IFRS”) and may not be comparable to financial statements of United States companies.
The Company’s financial statements are audited in accordance with the Public Company Accounting Oversight Board (United States)
auditing standards and Canadian and United States auditor independence standards.
The enforcement
by investors of civil liabilities under United States federal securities laws may be affected adversely by the fact that the Companyis
incorporated under the laws ofthe Province of Ontario, Canada, that certain of its officers and directors and some of the experts named
in the Prospectus are residentsof Canada, that some or all of theexperts, underwriters, dealers or agents named in any Prospectus Supplement
may be residents of Canada, and that some of the Company’s assets and all or a substantial portion of the assets of these persons
are located outside of the United States. See “Enforceability of Civil Liabilities in the United States”.
THESE SECURITIES
HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) NOR ANY STATE OR
CANADIAN SECURITIES COMMISSION OR REGULATORY AUTHORITY NOR HAS THE SEC OR ANY STATE OR CANADIAN SECURITIES COMMISSION PASSED UPON THE
ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
Prospective purchasers
should be aware that the acquisition of the Securities may have tax consequences in Canada and the United States. Such consequences for
purchasers who are resident in, or citizens of, the United States may not be described fully herein or in any applicable Prospectus Supplement.
Prospective purchasers should read the tax discussion contained in this Prospectus under the heading “Certain Income Tax Considerations”
as well as the tax discussion, if any, contained in the applicable Prospectus Supplement with respect to a particular offering of Securities.
No underwriter has
been involved in the preparation of this Prospectus nor has any underwriter performed any review of the contents of this Prospectus.
Investing in
the Securities involves certain risks. Prospective purchasers of the Securities should carefully consider all the information in this
Prospectus and in the documents incorporated by reference in this Prospectus.
TABLE OF CONTENTS
GENERAL MATTERS
Unless
otherwise noted or the context otherwise requires, the “Company”, “Greenbrook”, “we”,
“us” or “our” refer to Greenbrook TMS Inc. together with its subsidiaries.
This
Prospectus provides a general description of the Securities that we or a Selling Shareholder may offer. Each time we or a Selling Shareholder
sells Securities under this Prospectus, we will provide you with a Prospectus Supplement that will contain specific information about
the terms of that offering. The Prospectus Supplement may also add, update or change information contained in this Prospectus. Before
investing in any Securities, you should read both this Prospectus and any applicable Prospectus Supplement, together with the additional
information described below and in the applicable Prospectus Supplement under “Documents Incorporated by Reference”.
Investors
should rely only on information contained or incorporated by reference in this Prospectus and any applicable Prospectus Supplement. The
Company has not authorized anyone to provide the investor with different information. The Company is not making an offer of the Securities
in any jurisdiction where the offer is not permitted. Investors should not assume that the information contained in this Prospectus is
accurate as of any date other than the date on the front of this Prospectus, unless otherwise noted herein or as required by law. It should
be assumed that the information appearing in this Prospectus and the documents incorporated herein by reference are accurate only as of
their respective dates. The business, financial condition, results of operations and prospects of the Company may have changed since those
dates.
All
Common Share numbers in this Prospectus have been adjusted to give effect to the consolidation of all of the Company’s issued and
outstanding Common Shares effected on February 1, 2021 on the basis of one post-consolidation Common Share for every five pre-consolidation
Common Shares (the “Share Consolidation”).
FORWARD-LOOKING
STATEMENTS
Certain
statements contained in this Prospectus and the documents incorporated by reference herein constitute forward-looking statements within
the meaning of applicable securities laws in Canada and the United States, including the United States Private Securities Litigation Reform
Act of 1995. Forward-looking information may relate to the Company’s future financial outlook and anticipated events or results
and may include information regarding the Company’s business, financial position, results of operations, business strategy, growth
plans and strategies, technological development and implementation, budgets, operations, financial results, taxes, dividend policy, plans
and objectives. Particularly, information regarding the Company’s expectations of future results, performance, achievements, prospects
or opportunities or the markets in which it operates is forward-looking information. In some cases, forward-looking information can be
identified by the use of forward-looking terminology such as “plans”, “targets”, “expects” or “does
not expect”, “is expected”, “an opportunity exists”, “budget”, “scheduled”, “estimates”,
“outlook”, “forecasts”, “projection”, “prospects”, “strategy”, “intends”,
“anticipates”, “does not anticipate”, “believes”, or variations of such words and phrases or state
that certain actions, events or results “may”, “could”, “would”, “might”, “will”,
“will be taken”, “occur” or “be achieved”. In addition, any statements that refer to expectations,
intentions, projections or other characterizations of future events or circumstances contain forward-looking information. Statements containing
forward-looking information are not facts but instead represent management’s expectations, estimates and projections regarding future
events or circumstances.
Forward-looking
information in this Prospectus and the documents incorporated by reference herein includes, among other things, statements relating
to: the Company’s expectations regarding its revenue, expenses, cash flow and operations; changes in laws and regulations
affecting the Company and the regulatory environments in which it operates; the Company’s expectations regarding the potential
market opportunity for the delivery of TMS (as defined below) therapy; the Company’s expectations regarding its growth rates
and growth plans and strategies, including expectations regarding future growth of the TMS market; potential expansion of additional
therapeutic indications approved for TMS therapy by the United States Food and Drug Administration (“FDA”); the
Company’s business plans and strategies; the Company’s expectations regarding the implementation and expansion of the
Spravato® pilot program; changes in reimbursement rates by insurance payors; the Company’s expectations regarding the
outcome of litigation and payment obligations in respect of prior settlements; the Company’s ability to attract and retain
medical practitioners and qualified technicians at the Company’s network of outpatient mental health service centers that
specialize in TMS treatment (each, a “TMS Center”); the Company’s competitive position in its industry and
its expectations regarding competition; anticipated trends and challenges in the Company’s business and the markets in which
it operates; access to capital and the terms relating thereto; technological changes in the Company’s industry; the
Company’s expectations regarding geographic expansions; the Company’s expectations regarding new TMS Center openings and
the timing thereof; the Company’s expectations regarding mergers and acquisitions; successful execution of internal plans;
anticipated costs of capital investments; the Company’s intentions with respect to the implementation of new accounting
standards; and the impact of the novel coronavirus, COVID-19 (“COVID-19”), pandemic on the Company.
This
forward-looking information and other forward-looking information are based on the Company’s opinions, estimates and assumptions
in light of its experience and perception of historical trends, current conditions and expected future developments, as well as other
factors that the Company currently believes are appropriate and reasonable in the circumstances. Despite a careful process to prepare
and review the forward- looking information, there can be no assurance that the underlying opinions, estimates and assumptions will prove
to be correct.
The
forward-looking information in this Prospectus and the documents incorporated by reference herein is necessarily based on a number
of opinions, estimates and assumptions that the Company considered appropriate and reasonable as of the date such statements were
made. It is also subject to known and unknown risks, uncertainties, assumptions and other factors that may cause the actual results,
level of activity, performance or achievements to be materially different from those expressed or implied by such forward- looking
information, including the following risk factors: successful execution of the Company’s growth strategies; inability to
attract key managerial and other non-medical personnel; risks related to changes in reimbursement rates by commercial insurance
plans, Medicare and other non-Medicare government insurance plans; imposition of additional requirements related to the provision of
TMS therapy by commercial insurance plans, Medicare and other non-Medicare government insurance plans that increase the cost or
complexity of furnishing TMS therapy; reduction in reimbursement rates by higher-paying commercial insurance providers; dependency
on referrals from physicians and failure to attract new patients; failure to recruit and retain sufficient qualified psychiatrists;
ability to obtain TMS devices (“TMS Devices”) from the Company’s suppliers on a timely basis at competitive
costs could suffer as a result of deterioration or changes in supplier relationships or events that adversely affect the
Company’s suppliers or cause disruption to their businesses; failure to reduce operating expenses and labor costs in a timely
manner; inability to achieve or sustain profitability in the future or an inability to secure additional financing to fund losses;
risks related to the use of partnerships and other management services frameworks; risks associated with leasing space and equipment
for the Company’s TMS Centers; inability to successfully open and operate new TMS Centers profitably or at all; risks
associated with geographic expansion in regions which may have lower awareness of the Company’s brand or TMS therapy in
general; claims made by or against the Company, which may result in litigation; risks associated with professional malpractice
liability claims; reduction in demand for the Company’s services as a result of new drug development and/or technological
changes within the Company’s industry; impact of uncertainty related to potential changes to U.S. healthcare laws and
regulations; risks associated with anti- kickback, fraud and abuse laws; risks associated with compliance with laws relating to the
practice of medicine; the constantly evolving nature of the regulatory framework in which the Company operates; costs associated
with compliance with U.S. federal and state laws and regulations and risks associated with failure to comply; assessments for
additional taxes which could affect the Company’s operating results; inability to manage the Company’s operations at its
current size; the Company’s competitive industry and the size and resources of some of its competitors; the labor-intensive
nature of the Company’s business being adversely affected if it is unable to maintain satisfactory relations with its
employees or the occurrence of union attempts to organize its employees; insurance-related risks; complications associated with the
Company’s billing and collections systems; material disruptions in or security breaches affecting the Company’s
information technology systems; natural disasters and unusual weather; disruptions to the operations at the Company’s head
office locations; upgrade or replacement of core information technology systems; changes in accounting standards and subjective
assumptions, estimates and judgments by management related to complex accounting matters; inability to maintain effective controls
over financial reporting; risks associated with dilution of equity ownership; volatility in the market price for the Shares;
prolonged decline in the price of the Shares reducing the Company’s ability to raise capital; significant influence of
Greybrook Health Inc. (“Greybrook Health” or the “Promoter”); increases to indebtedness levels
causing a reduction in financial flexibility; future sales of the Company’s securities by existing shareholders causing the
market price for the Common Shares to decline; impact of future offerings of debt securities on dividend and liquidation
distributions; no cash dividends for the foreseeable future; an active, liquid and orderly trading market for the Securities failing
to develop; different shareholder protections in Canada as compared to the United States and elsewhere; treatment of the Company as
a U.S. domestic corporation for U.S. federal income tax purposes; any issuance of Preferred Shares may hinder another person’s
ability to acquire the Company; the Company’s trading price and volume could decline if analysts do not publish research or
publish inaccurate or unfavorable research about the Company or its business; increases to costs as a result of operating as a U.S.
public company; the Company’s potential to incur significant additional costs if it were to lose its “foreign private
issuer” status in the future; the COVID-19 pandemic having a material adverse impact on the future results of the Company; and
risks related to forward- looking information contained in this Prospectus and the documents incorporated by reference herein.
If
any of these risks or uncertainties materialize, or if the opinions, estimates or assumptions underlying the forward-looking information
prove incorrect, actual results or future events might vary materially from those anticipated in the forward-looking information. The
opinions, estimates or assumptions referred to above should be considered carefully by readers. Additional information about these assumptions,
risks and uncertainties is contained in this Prospectus under the heading “Risk Factors” and in the Company’s filings
with securities regulators, including the Annual Information Form, the Annual MD&A and the Interim MD&A (each as defined below).
Various
assumptions or factors are typically applied in drawing conclusions or making the forecasts or projections set out in forward-looking
information. Those assumptions and factors are based on information currently available to the Company, including information obtained
from third party industry analysts and other third-party sources. In some instances, material assumptions and factors are presented or
discussed elsewhere in this Prospectus or the documents incorporated by reference herein in connection with the statements or disclosure
containing the forward-looking information. Readers are cautioned that the following list of material factors and assumptions is not exhaustive.
The factors and assumptions include, but are not limited to: no unforeseen changes in the legislative and operating framework for the
Company’s business; no unforeseen changes in the prices for the Company’s services in markets where prices are regulated;
no unforeseen changes in the reimbursement rates of commercial, Medicare and other non-Medicare government insurance plans; no unforeseen
changes in the regulatory environment for the Company’s services; a stable competitive environment; and no significant event occurring
outside the ordinary course of business.
Although
the Company has attempted to identify important risk factors that could cause actual results or future events to differ materially from
those contained in forward-looking information, there may be other risk factors not presently known to the Company or that the Company
presently believes are not material that could also cause actual results or future events to differ materially from those expressed in
such forward- looking information. There can be no assurance that such information will prove to be accurate. Accordingly, readers should
not place undue reliance on forward-looking information, which speaks only to opinions, estimates and assumptions as of the date made.
The forward-looking information contained in this Prospectus and the documents incorporated by reference herein represents the Company’s
expectations as of the date of this Prospectus (or as of the date they are otherwise stated to be made) and are subject to change after
such date. The Company disclaims any intention or obligation or undertaking to update or revise any forward- looking information whether
as a result of new information, future events or otherwise, except as required under applicable securities laws.
All
of the forward-looking information contained in this Prospectus or the documents incorporated by reference herein is expressly qualified
by the foregoing cautionary statements.
DOCUMENTS FILED
AS PART OF THE REGISTRATION STATEMENT
The following
documents have been, or will be filed with the SEC as part of the Registration Statement: (1) the
documents listed under the heading “Documents Incorporated by Reference”; (2) the consents of KPMG LLP and Torys LLP;
and (3) the powers of attorney from the directors and certain officers of the Company. A copy of the form of warrant indenture or
subscription receipt agreement, as applicable, will be filed by post-effective amendment or by incorporation by reference to
documents filed with, or furnished to, the SEC under the U.S. Securities Exchange Act of 1934, as amended (the “U.S.
Exchange Act”).
AVAILABLE INFORMATION
The
Company files reports and other information with the securities commissions and similar regulatory authorities in each of the provinces
and territories of Canada. These reports and information are available to the public free of charge on SEDAR at www.sedar.com.
The
Company has filed with the SEC the Registration Statement relating to the Securities. This Prospectus, which constitutes a part of the
Registration Statement, does not contain all of the information contained in the Registration Statement, certain items of which are contained
in the exhibits to the Registration Statement as permitted by the rules and regulations of the SEC. Statements included in this Prospectus
or incorporated herein by reference about the contents of any contract, agreement or other documents referred to are not necessarily complete,
and in each instance investors should refer to the exhibits for a more complete description of the matter involved. Each such statement
is qualified in its entirety by such reference.
The
Company is subject to the information requirements of the U.S. Exchange Act and applicable Canadian securities legislation and, in accordance
therewith, files reports and other information with the SEC and with the securities regulatory authorities in Canada. Under the multijurisdictional
disclosure system adopted by the United States and Canada, documents and other information that the Company files with the SEC may be
prepared in accordance with the disclosure requirements of Canada, which are different from those of the United States. As a foreign private
issuer, the Company is exempt from the rules under the U.S. Exchange Act prescribing the furnishing and content of proxy statements, and
its officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained
in Section 16 of the U.S. Exchange Act. In addition, the Company is not required to publish financial statements as promptly as U.S. companies
subject to the applicable provisions of the U.S. Exchange Act.
Investors
may read any document that the Company has filed with, or furnished to, the SEC at the SEC’s website at www.sec.gov for further
information about the public reference rooms. Investors may read and download some of the documents the Company has filed with the SEC’s
Electronic Data Gathering, Analysis and Retrieval system at www.sec.gov.
PRESENTATION OF
FINANCIAL INFORMATION
The
financial statements of the Company incorporated herein by reference and in any Prospectus Supplement are reported in U.S. dollars and
have been prepared in accordance with IFRS. IFRS differs in some significant respects from generally accepted accounting principles in
the United States, and thus the financial statements may not be comparable to financial statements of U.S. companies.
All
dollar amounts in this Prospectus are expressed in United States dollars, except as otherwise indicated. References to “$”,
“US$” or “dollars” are to United States dollars and references to “C$” are to Canadian dollars.
Certain
calculations included in tables and other figures in this Prospectus have been rounded for clarity of presentation.
EXCHANGE RATE INFORMATION
The
following table sets forth, for each of the periods indicated, the high, low, average and period end spot rates of exchange for one United
States dollar, expressed in Canadian dollars, published by the Bank of Canada.
|
|
|
Year ended December 31,
|
|
|
Three months ended March 31,
|
|
|
|
|
2020
(C$)
|
|
|
2019
(C$)
|
|
|
2021
(C$)
|
|
|
2020
(C$)
|
|
|
High
|
|
|
1.4496
|
|
|
|
1.3600
|
|
|
|
1.2828
|
|
|
|
1.4496
|
|
|
Low
|
|
|
1.2718
|
|
|
|
1.2988
|
|
|
|
1.2455
|
|
|
|
1.2970
|
|
|
Average
|
|
|
1.3415
|
|
|
|
1.3269
|
|
|
|
1.2660
|
|
|
|
1.3449
|
|
|
Period End
|
|
|
1.2732
|
|
|
|
1.2988
|
|
|
|
1.2575
|
|
|
|
1.4187
|
|
On
July 21, 2021, the rate of exchange posted by the Bank of Canada for conversion of United States dollars into Canadian dollars was US$1.00
= C$1.2589. The Company makes no representation that Canadian dollars could be converted into United States dollars at that rate or any
other rate.
THE COMPANY
Operating
through 130 Company-operated treatment centers, Greenbrook is a leading provider of Transcranial Magnetic Stimulation (“TMS”)
therapy, an FDA-cleared, non-invasive therapy for the treatment of Major Depressive Disorder (“MDD”) and other mental
health disorders, in the United States. TMS therapy provides local electromagnetic stimulation to specific brain regions known to be directly
associated with mood regulation. Greenbrook has provided more than 620,000 TMS treatments to over 17,000 patients struggling with depression.
The
principal, head and registered office of the Company is located at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada M4W 3P4. The
Company’s United States corporate headquarters is located at 8401 Greensboro Drive, Suite 425, Tysons Corner, Virginia, United States,
22102.
Further
information regarding the Company and its business is set out in the Annual Information Form, which is incorporated herein by reference.
RECENT DEVELOPMENTS
Completion of Private Placement
On
June 14, 2021, the Company completed a non-brokered private placement (the “Private Placement”) of Common Shares in
reliance upon rule 506(c) under the U.S. Securities Act. Pursuant to the Private Placement, an aggregate of 2,353,347 Common Shares were
issued at a price of US$10.00 per share, for aggregate gross proceeds to the Company of approximately US$23.5 million. The financing was
led by new investor Masters Special Situations, LLC and affiliates thereof (“MSS”). Additional new investors, including
BioStar Capital, also participated in the financing, along with existing investors, Greybrook Health and 1315 Capital II, L.P. (“1315
Capital”). The Company intends to use the proceeds from the Private Placement for the development of new TMS Centers as well
as working capital and general corporate purposes.
In
connection with the Private Placement, MSS, Greybrook Health and 1315 Capital each received the right to appoint a nominee to the board
of directors of the Company as well as rights to participate in future equity issuances by the Company in order to maintain such investors’
pro rata ownership interest in the Company. In addition, all of the Subscribers (as defined below) received customary resale, demand
and “piggy- back” registration rights. See “Selling Shareholders” and “Material Contracts”.
COVID-19 Business Update
Our
active TMS Centers are expected to remain open despite the COVID-19 pandemic to both current and new patients, including as “essential
businesses” under local health protocols.
Our
entire team continues to work tirelessly to deliver the highest quality of care at all of our TMS Centers, while at the same time taking
all possible steps to safeguard the health and well-being of our patients, employees and physician partners. We see these challenging
operating conditions as temporary and we are starting to see a positive change in sentiment.
The
COVID-19 pandemic has negatively impacted payor processes, causing a slowdown in collections and payors’ responsiveness to billing
related communications throughout fiscal 2020 and the three months ended March 31, 2021. We also experienced further delays in both credentialling
and re-credentialling processes completed as part of our billing enhancements across our payor population. See “Risk Factors —
The COVID-19 pandemic may have a material adverse effect on our business, financial condition and future growth opportunities”.
Supplemental Information Relating
to Revenue and Accounts Receivable
The Company
wishes to provide the following supplemental and clarifying information relating to the adjustment to its variable consideration
estimate, which we refer to as a “provision” from time to time, recognized by the Company against revenue in the fourth
quarter of the year ended December 31, 2020, as disclosed in the Annual MD&A incorporated by reference herein.
Due
to the nature of our industry and complexity of our revenue arrangements, where price lists are subject to the discretion of payors, variable
consideration exists that may result in price concessions and constraints to the transaction price for the services rendered.
In
estimating this variable consideration, we consider various factors including, but not limited to, the following:
|
|
•
|
commercial payors and the administrators of federally-funded healthcare programs
exercise discretion over pricing and may establish a base fee schedule for TMS (which is subject to change prior to final settlement)
or negotiate a specific reimbursement rate with an individual TMS provider;
|
|
|
•
|
average of previous net service fees received by the applicable payor and fees
received by other patients for similar services;
|
|
|
•
|
management’s best estimate, leveraging industry knowledge and expectations
of third-party payors’ fee schedules;
|
|
|
•
|
factors that would influence the contractual rate and the related
benefit coverage, such as obtaining pre-authorization of services and determining whether the procedure is medically necessary;
|
|
|
•
|
probability of failure in obtaining timely proper provider credentialing (including
re-credentialling) and documentation, in order to bill various payors which may result in enhanced price concessions; and
|
|
|
•
|
variation in coverage for similar services among various payors and various payor benefit plans.
|
The
Company updates the estimated transaction price (including updating its assessment of whether an estimate of variable consideration is
constrained) to represent faithfully the circumstances present at the end of the reporting period and the changes in circumstances during
the reporting period in which such variances become known.
The
above factors are not related to the creditworthiness of the large medical insurance companies and government-backed health plans encompassing
the significant majority of our payors. The payors (large insurers and government agencies) have the ability and intent to pay, but price
lists for our services are subject to the discretion of payors. As a result, the adjustment to reduce the transaction price and constrain
the variable consideration is a price concession and not indicative of credit risk on the payors (i.e. not a bad debt expense).
The
COVID-19 pandemic negatively impacted payor processes through a lack of timely and accurate communication resulting in a greater chance
of price concession. As a result, the following factors involved in estimating variable consideration further constrained the transaction
price for services rendered:
|
|
•
|
management’s best estimate, leveraging industry knowledge and expectations
of third-party payors’ fee schedules;
|
|
|
•
|
probability of failure in obtaining timely and accurate benefits information;
|
|
|
•
|
probability of failure in obtaining timely and accurate pre-authorization of services; and
|
|
|
•
|
probability of failure in obtaining timely proper provider credentialing (including
re-credentialling) and documentation, in order to bill various payors.
|
During
the initial onset of the COVID-19 pandemic, we expected a temporary impact to the payor processes, as described above, including the impact
of our billing enhancements. However, when operations began to normalize in the fourth quarter of 2020, management determined that additional
price concessions were necessary based on the continued nature of the impact to payor processes at that time.
A
quantitative reconciliation of accounts receivable in respect of the years ended December 31, 2020 and 2019 and the three-month
periods ended March 31, 2021 and 2020 are provided below, which includes a quantification of the provision for the adjustment to
variable consideration estimate resulting from the additional price concessions which were deemed necessary:
|
(US$)
|
|
December 31,
2020
|
|
|
December 31,
2019
|
|
|
March 31,
2021
|
|
|
March 31,
2020
|
|
|
Opening accounts receivable balance as at January 1,
|
|
|
10,091,087
|
|
|
|
7,131,661
|
|
|
|
10,708,062
|
|
|
|
10,091,087
|
|
|
Revenue recognized based on expected value .
|
|
|
46,284,419
|
|
|
|
35,685,531
|
|
|
|
12,482,512
|
|
|
|
11,420,502
|
|
|
Provision for adjustment to variable consideration estimate
|
|
|
(3,155,240
|
)
|
|
|
—
|
|
|
|
(1,169,337
|
)
|
|
|
—
|
|
|
Bad debt expense(1)
|
|
|
—
|
|
|
|
(2,894,989
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Payments received
|
|
|
(42,512,204
|
)
|
|
|
(29,831,116
|
)
|
|
|
(11,285,025
|
)
|
|
|
(10,555,114
|
)
|
|
Ending accounts receivable balance as at end of period
|
|
$
|
10,708,062
|
|
|
$
|
10,091,087
|
|
|
$
|
10,736,212
|
|
|
$
|
10,956,475
|
|
Note:
|
|
(1)
|
As described in the Company’s audited consolidated financial statements for
the financial year ended December 31, 2019, bad debt expense related to the write-off of accounts receivable that were identified during
the migration to a scalable billing and reimbursement platform completed during the year ended December 31, 2019.
|
The
Company’s aging schedule in respect of its accounts receivable balance as at December 31, 2020 and 2019, and as at March 31, 2021,
are provided below:
|
|
|
As at
|
|
|
As at
|
|
|
As at
|
|
|
|
|
March 31,
|
|
|
December 31,
|
|
|
December 31,
|
|
|
Days since service delivered
|
|
2021
|
|
|
2020
|
|
|
2019
|
|
|
0-90
|
|
$
|
4,959,069
|
|
|
$
|
5,009,224
|
|
|
$
|
5,208,231
|
|
|
91-180
|
|
$
|
1,890,888
|
|
|
$
|
2,317,030
|
|
|
$
|
1,904,727
|
|
|
181-270
|
|
$
|
1,700,847
|
|
|
$
|
1,746,512
|
|
|
$
|
1,354,368
|
|
|
270+
|
|
$
|
2,185,408
|
|
|
$
|
1,635,296
|
|
|
$
|
1,623,761
|
|
|
Total accounts receivable
|
|
$
|
10,736,212
|
|
|
$
|
10,708,062
|
|
|
$
|
10,091,087
|
|
Based
on the Company’s industry, none of the accounts receivable in the table above are considered “past due”. Furthermore,
the payors have the ability and intent to pay, but price lists for the Company’s services are subject to the discretion of payors.
As such, the timing of collections is not linked to increased credit risk. The Company continues to collect on services rendered in excess
of 24 months from the date such services were rendered.
TMS Center Expansion Opportunities
The Company
is in various stages of negotiations and due diligence in respect of other potential TMS Center expansion opportunities. There can
be no assurance that these negotiations will result in TMS Center expansion for the Company or, if they do, what the final terms or
timing of such opportunities would be. The Company expects to continue current negotiations and discussions and actively pursue
other TMS Center expansion opportunities, including through in-region growth and development, development of new regions and merger
and acquisition opportunities. Candidate TMS practices that are considered for potential acquisitions are those with, among other
potential attributes, well-established operations and regional footprints, experienced leadership and management teams and favorable
payor contract terms. None of these potential acquisitions, if consummated, would constitute a “significant acquisition”
for the Company under Part 8 of National Instrument 51-102 — Continuous Disclosure Obligations. Furthermore, the
Company’s current cash balance and working capital as at the date hereof will not be utilized to fund any cash consideration
necessary to fund the purchase price in respect of any merger and acquisition opportunities. Instead, management plans to secure any
necessary additional financing through the issuance of new public or private equity or debt instruments, and no merger and
acquisition opportunities will be consummated until such additional financing has been completed. There can be no assurance that
additional future funding will be available to the Company, or that it will be available on terms which are acceptable to
management. See “Risk Factors”.
DOCUMENTS INCORPORATED
BY REFERENCE
Information
has been incorporated by reference in this Prospectus from documents filed with the securities commissions or similar regulatory authorities
in Canada and which have been filed with the SEC in the United States as exhibits to the Registration Statement. Copies of these documents
may be obtained on request without charge from the General Counsel of Greenbrook at 890 Yonge Street, 7th Floor, Toronto, Ontario, Canada
M4W 3P4, by telephone at (416) 915-9100 or by accessing these documents through the Internet on the Company’s website at www.greenbrooktms.com,
on SEDAR at www.sedar.com or on EDGAR at www.sec.gov.
Except
to the extent that their contents are modified or superseded by a statement contained in this Prospectus or in any other subsequently
filed document that is also incorporated by reference in this Prospectus, the following documents of the Company filed with the securities
commissions or similar regulatory authorities in Canada and which have been filed with the SEC in the United States as exhibits to the
Registration Statement are specifically incorporated by reference into, and form an integral part of, this Prospectus:
Any
document of the type referred to in section 11.1 of Form 44-101F1 of National Instrument 44-101 — Short Form Prospectus
Distributions filed by the Company with the securities commissions or similar regulatory authorities in Canada and the United
States after the date of this Prospectus and all Prospectus Supplements, disclosing additional or updated information filed pursuant
to the requirements of applicable securities legislation in Canada and the United States and during the period that this Prospectus
is effective, shall be deemed to be incorporated by reference in this Prospectus. In addition, any “template version” of
“marketing materials” (as defined in National Instrument 41-101 — General Prospectus Requirements) filed
after the date of a Prospectus Supplement and prior to the termination of the offering of Securities to which such Prospectus
Supplement relates, shall be deemed to be incorporated by reference into such Prospectus Supplement. In addition, to the extent that
any document or information incorporated by reference into this Prospectus is included in any report on Form 6-K, Form 40-F, Form
20-F, Form 10-K, Form 10-Q or Form 8-K (or any respective successor form) that is filed with or furnished to the SEC after the date
of this Prospectus, such document or information shall be deemed to be incorporated by reference as an exhibit to the Registration
Statement of which this Prospectus forms a part. In addition, the Company may incorporate by reference as an exhibit to the
Registration Statement or into the Prospectus which forms a part of the Registration Statement, information from documents that the
Company files with or furnishes to the SEC pursuant to Section 13(a) or 15(d) of the U.S. Exchange Act. The documents incorporated
or deemed to be incorporated herein by reference contain meaningful and material information relating to the Company and prospective
purchasers of Securities should review all information contained in this Prospectus and the documents incorporated or deemed to be
incorporated herein by reference.
Upon
a new annual information form and related annual consolidated financial statements being filed by the Company with the applicable securities
regulatory authorities during the duration that this Prospectus is effective, the previous annual information form, the previous annual
consolidated financial statements and all interim consolidated financial statements, and in each case the accompanying management’s
discussion and analysis, information circulars (to the extent the disclosure is inconsistent) and material change reports filed prior
to the commencement of the financial year of the Company in which the new annual information form is filed shall be deemed no longer to
be incorporated into this Prospectus for purposes of future offers and sales of Securities under this Prospectus. Upon interim consolidated
financial statements and the accompanying management’s discussion and analysis being filed by the Company with the applicable securities
regulatory authorities during the duration that this Prospectus is effective, all interim consolidated financial statements and the accompanying
management’s discussion and analysis filed prior to the new interim consolidated financial statements shall be deemed no longer
to be incorporated by reference into this Prospectus for purposes of future offers and sales of Securities under this Prospectus.
A Prospectus
Supplement containing the specific terms of an offering of Securities and other information relating to the Securities will be delivered
to prospective purchasers of such Securities together with this Prospectus and will be deemed to be incorporated into this Prospectus
as of the date of such Prospectus Supplement but only for the purpose of the offering of the Securities covered by that Prospectus Supplement.
Any
statement contained in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded
for the purposes of this Prospectus to the extent that a statement contained herein, or in any other subsequently filed document which
also is or is deemed to be incorporated by reference herein, modifies or supersedes such statement. Any statement so modified or superseded
shall not constitute a part of this Prospectus, except as so modified or superseded. The modifying or superseding statement need not state
that it has modified or superseded a prior statement or include any other information set forth in the document that it modifies or supersedes.
The making of such a modifying or superseding statement shall not be deemed an admission for any purpose that the modified or superseded
statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact
that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
CONSOLIDATED CAPITALIZATION
The
table below sets forth our capitalization as of March 31, 2021 (1) on an actual basis and (2) as adjusted to give effect to the Private
Placement. You should read this table in conjunction with our Interim Financial Statements which is incorporated by reference herein.
|
|
|
As of March 31, 2021
|
|
|
|
|
(Actual)
|
|
|
(As adjusted)
|
|
|
(US$)
|
|
(Unaudited)
|
|
|
Cash
|
|
$
|
5,933,799
|
|
|
$
|
29,467,269
|
|
|
Long-term portion of:
|
|
|
|
|
|
|
|
|
|
Loans payable
|
|
$
|
14,762,928
|
|
|
$
|
14,762,928
|
|
|
Deferred grant income(1)
|
|
|
155,239
|
|
|
|
155,239
|
|
|
Lease liabilities(2)
|
|
|
22,572,241
|
|
|
|
22,572,241
|
|
|
Total long-term debt
|
|
$
|
37,490,408
|
|
|
$
|
37,490,408
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
Shares
|
|
$
|
63,254,170
|
|
|
$
|
86,787,640
|
|
|
Contributed surplus
|
|
|
3,548,936
|
|
|
|
3,548,936
|
|
|
Deficit
|
|
|
(67,828,530
|
)
|
|
|
(67,828,530
|
)
|
|
Non-controlling interest
|
|
|
(1,110,172
|
)
|
|
|
(1,110,172
|
)
|
|
Total equity
|
|
$
|
(2,135,596
|
)
|
|
$
|
21,397,874
|
|
|
Total capitalization (long-term debt and equity)
|
|
$
|
35,354,812
|
|
|
$
|
58,888,282
|
|
Notes:
|
|
(1)
|
The deferred grant income arises from the difference between the fair value of
the Company’s unsecured loan in the amount of US$3,080,760 made under the United States Paycheck Protection Program at inception
and the loan proceeds received therefrom in April 2020.
|
|
|
(2)
|
Under IFRS 16 — Leases, a lessee is required to recognize a right-of-use
asset, representing its right to use the underlying asset, and a lease liability, representing its obligation to make future lease payments
for all leases with a term of more than 12 months.
|
USE OF PROCEEDS
The
use of the net proceeds from the sale of Securities will be described in a Prospectus Supplement relating to the specific issuance of
such Securities. All expenses relating to an offering of Securities and any compensation paid to underwriters, dealers or agents, as the
case may be, will be paid out of the proceeds of the offering or from the Company’s general funds, unless otherwise stated in the
applicable Prospectus Supplement.
The
Company may, from time to time, issue securities (including Securities) other than pursuant to this Prospectus.
The
Company will not receive any proceeds from any sale of Securities by a Selling Shareholder.
SELLING SHAREHOLDERS
This
Prospectus may also, from time to time, relate to the offering of the Securities by way of a secondary offering (each, a “Secondary
Offering”) by one or more Selling Shareholders. As of the date of this Prospectus, the potential Selling Shareholders include
MSS, 1315 Capital, Greybrook Health, BioStar Capital, Steward Capital Holdings, LP, and Avenaero Holdings, LLC (collectively, the “Subscribers”).
Each such Subscriber has certain customary registrations rights, granted by us under certain registration rights agreements, for the future
resale in the United States of the Common Shares purchased by such Subscribers, including in connection with the Private Placement. See
“Recent Developments – Completion of Private Placement”. Other shareholders of the Company may, however, become a Selling
Shareholder under this Prospectus during the 25-month period that this Prospectus, including any amendments thereto, remains effective.
The terms
under which the Securities may be offered by Selling Shareholders will be described in the applicable Prospectus Supplement. The
Prospectus Supplement for or including any Secondary Offering will include, without limitation, where applicable: (i) the names of
the Selling Shareholders; (ii) the number and type of Securities owned, controlled or directed by each Selling Shareholder; (iii)
the number of Securitiesbeing distributed for the accounts of each Selling Shareholder; (iv) the number of Securities to be owned,
controlled or directed by each Selling Shareholder after the distribution and the percentage that number or amount represents out of
the total number of outstanding Securities of the class or series; (v) whether the Securities are owned by the Selling Shareholders,
both of record and beneficially, of record only or beneficially only; (vi) if a Selling Shareholder purchased any of the Securities
held by such Selling Shareholder in the 12 months preceding the date of the Prospectus Supplement, the date or dates the Selling
Shareholder acquired the Securities; (vii) if a Selling Shareholder acquired the Securities held by such Selling Shareholder in the
12 months preceding the date of the Prospectus Supplement, the cost thereof to the Selling Shareholder in the aggregate and on a per
security basis; and (viii) the disclosure required by Item 1.11 of Form 44-101F1 — Short Form Prospectus, and, if
applicable, each Selling Shareholder will file a non-issuer’s submission to jurisdiction form with the applicable Prospectus
Supplement. No Selling Shareholder may distribute Securities pursuant to an “at-the-market distribution” in Canada.
PLAN OF DISTRIBUTION
The
Company and the Selling Shareholders may from time to time during the 25-month period that this Prospectus, including any amendments thereto,
remains effective, offer for sale and issue up to an aggregate of US$150,000,000 (or the equivalent in other currencies based on the applicable
exchange rate at the time of the offering) in Securities hereunder. To the extent there are any Secondary Offerings, the aggregate amount
of Securities that may be offered and sold by the Company hereunder shall be reduced by the aggregate amount of such Secondary Offerings.
The
Company and the Selling Shareholders may offer and sell the Securities to or through underwriters or dealers purchasing as principals,
and may also sell directly to one or more purchasers or through agents or pursuant to applicable statutory exemptions. The Prospectus
Supplement relating to a particular offering of Securities will identify each underwriter, dealer or agent, as the case may be, engaged
by the Company or any Selling Shareholder in connection with the offering and sale of the Securities, and will set forth the terms of
the offering of such Securities, including, to the extent applicable, any fees, discounts or any other compensation payable to underwriters,
dealers or agents in connection with the offering, the method of distribution of the Securities, the identity of the Selling Shareholders,
if any, the initial issue price, the proceeds that the Company or the Selling Shareholder, as applicable, will receive and any other material
terms of the plan of distribution. Any initial offering price and discounts, concessions or commissions allowed or re- allowed or paid
to dealers may be changed from time to time.
In
addition, the Securities may be offered and issued in consideration for the acquisition of other businesses, assets or securities by the
Company or one of its subsidiaries. The consideration for any such acquisition may consist of the Securities separately, a combination
of Securities or any combination of, among other things, Securities, cash and assumption of liabilities. In addition, one or more Selling
Shareholders may sell Securities to or through underwriters or dealers purchasing as principals and may also sell Securities to one or
more purchasers directly, through statutory exemptions, or through agents designated from time to time.
The
Securities may be sold from time to time in one or more transactions at a fixed price or prices or at prices which may be changed or at
market prices prevailing at the time of sale, at prices related to such prevailing prices or at negotiated prices, including sales in
transactions that are deemed to be “at-the-market distributions” as defined in NI 44-102, including sales made directly on
the TSX, NASDAQ or other existing trading markets for the Common Shares. The price at which the Securities will be offered and sold may
vary from purchaser to purchaser and during the period of distribution.
In
connection with the sale of the Securities, underwriters, dealers or agents may receive compensation from the Company, any Selling
Shareholder or from other parties, including in the form of underwriters’, dealers’ or agents’ fees, commissions
or concessions. Underwriters, dealers and agents that participate in the distribution of the Securities may be deemed to be
underwriters for the purposes of applicable Canadian and/or U.S. securities legislation and any such compensation received by them
from the Company or any Selling Shareholder and any profit on the resale of the Securities by them may be deemed to be underwriting
commissions.
In
connection with any offering of Securities, except as otherwise set out in a Prospectus Supplement relating to a particular offering of
Securities and other than in relation to an “at-the-market distribution”, the underwriters, dealers or agents, as the case
may be, may over-allot or effect transactions intended to fix, stabilize, maintain or otherwise affect the market price of the Securities
at a level other than those which otherwise might prevail on the open market. Such transactions may be commenced, interrupted or discontinued
at any time.
Underwriters,
dealers or agents who participate in the distribution of the Securities may be entitled, under agreements to be entered into with the
Company and/or any Selling Shareholder, to indemnification by the Company and/or any Selling Shareholder against certain liabilities,
including liabilities under Canadian and/or U.S. securities legislation, or to contribution with respect to payments which such underwriters,
dealers or agents may be required to make in respect thereof. Such underwriters, dealers and agents may be customers of, engage in transactions
with, or perform services for, the Company in the ordinary course of business.
Unless
otherwise specified in the applicable Prospectus Supplement, each series or issue of Securities (other than Common Shares) will be a new
issue of Securities with no established trading market. Accordingly, there is currently no market through which the Securities (other
than Common Shares) may be sold and purchasers may not be able to resell such Securities purchased under this Prospectus. This may affect
the pricing of such Securities in the secondary market, the transparency and availability of trading prices, the liquidity of such Securities
and the extent of issuer regulation.
This
Prospectus constitutes a public offering of these Securities only in those jurisdictions where they may be lawfully offered for sale and
therein only by persons permitted to sell such Securities.
DESCRIPTION OF
SHARE CAPITAL
The
following is a summary of the rights, privileges, restrictions and conditions of or attaching to the Common Shares. The Company is authorized
to issue an unlimited number of Common Shares and an unlimited number of preferred shares, issuable in series. As at July 21, 2021, there
were 16,094,135 Common Shares and no Preferred Shares issued and outstanding.
Common Shares
Each
Common Share entitles the holder thereof to receive notice of any meetings of shareholders of the Company, to attend and to cast one vote
at all such meetings. Holders of Common Shares do not have cumulative voting rights with respect to the election of directors and, accordingly,
holders of a majority of the Common Shares entitled to vote in any election of directors may elect all directors standing for election.
The holders of Common Shares are entitled to receive if, as and when declared by the board of directors of the Company (the “Board”),
dividends in such amounts as shall be determined by the Board in its discretion. The holders of Common Shares have the right to receive
the Company’s remaining property and assets after payment of debts and other liabilities on a pro rata basis in the event
of a liquidation, dissolution or winding- up, whether voluntary or involuntary. The Common Shares do not carry any pre-emptive, subscription,
redemption or conversion rights, nor do they contain any sinking or purchase fund provisions.
Further
information relating to the Common Shares is set out in the Annual Information Form, which is incorporated by reference herein.
Preferred Shares
The
Preferred Shares may be issued in one or more series, each series to consist of such number of shares as may, before the issue thereof,
be fixed by resolution of the Board. The directors shall determine before the issue thereof the designations, rights, privileges, restrictions
and conditions attaching to the Preferred Shares of each series including the rate or amount of dividends or the method of calculating
dividends, the dates of payment thereof, the redemption and/or purchase prices and terms and conditions of redemption and/or purchase,
any voting rights, any conversion rights and any sinking fund or other provisions.
The
Preferred Shares of each series will, with respect to payment of dividends and the distribution of assets in the event of liquidation,
dissolution or winding up, rank on a parity with the Preferred Shares of every other series and be entitled to preference over the Common
Shares and over any other shares ranking junior to the Preferred Shares. The Preferred Shares of any series may also be given such other
preferences over the Common Shares and over any other shares ranking junior to the Preferred Shares as may be fixed by the directors.
TRADING PRICE AND
VOLUME
The
Common Shares are listed for trading on the TSX under the symbol “GTMS” and on NASDAQ under the symbol “GBNH”.
The following tables show the monthly range of high and low prices per Common Share at the close of market on the TSX and NASDAQ, as well
as total monthly volumes of the Common Shares traded on the TSX and NASDAQ for the periods indicated:
TSX(1)
|
|
|
High
|
|
|
Low
|
|
|
|
|
|
|
|
(C$)
|
|
|
(C$)
|
|
|
Volume
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
July
|
|
|
8.00
|
|
|
|
7.00
|
|
|
|
27,426
|
|
|
August
|
|
|
7.80
|
|
|
|
6.75
|
|
|
|
75,217
|
|
|
September
|
|
|
7.80
|
|
|
|
6.60
|
|
|
|
125,557
|
|
|
October
|
|
|
8.25
|
|
|
|
6.50
|
|
|
|
112,452
|
|
|
November
|
|
|
7.80
|
|
|
|
6.65
|
|
|
|
351,154
|
|
|
December
|
|
|
13.85
|
|
|
|
7.00
|
|
|
|
279,064
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January
|
|
|
20.45
|
|
|
|
11.15
|
|
|
|
330,406
|
|
|
February
|
|
|
22.40
|
|
|
|
17.50
|
|
|
|
519,725
|
|
|
March
|
|
|
19.80
|
|
|
|
14.24
|
|
|
|
446,792
|
|
|
April
|
|
|
17.58
|
|
|
|
13.00
|
|
|
|
101,864
|
|
|
May
|
|
|
14.69
|
|
|
|
11.80
|
|
|
|
117,680
|
|
|
June
|
|
|
16.63
|
|
|
|
11.70
|
|
|
|
149,677
|
|
|
July 1-21
|
|
|
16.49
|
|
|
|
14.06
|
|
|
|
36,460
|
|
Note:
|
|
(1)
|
The Shares commenced trading on the TSX on a post-Share Consolidation basis effective at the opening
of trading on February 4, 2021. The data presented in the table above is shown on a post-Share Consolidation basis for all periods.
|
NASDAQ(2)
|
|
|
High
|
|
|
Low
|
|
|
|
|
|
|
|
(US$)
|
|
|
(US$)
|
|
|
Volume
|
|
|
2021
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 16-31
|
|
|
16.00
|
|
|
|
11.55
|
|
|
|
32,680
|
|
|
April
|
|
|
14.00
|
|
|
|
10.79
|
|
|
|
25,965
|
|
|
May
|
|
|
12.01
|
|
|
|
9.23
|
|
|
|
55,331
|
|
|
June
|
|
|
13.89
|
|
|
|
9.55
|
|
|
|
171,350
|
|
|
July 1-21
|
|
|
13.01
|
|
|
|
11.13
|
|
|
|
63,809
|
|
Note:
|
|
(2)
|
The Shares commenced
trading on NASDAQ on March 16, 2021.
|
PRIOR SALES
The
Company has not issued any Common Shares, or securities convertible or exchangeable into Common Shares, during the 12-month period preceding
the date of this Prospectus, except as described below:
|
|
|
Type of Securities
|
|
Number of Securities
|
|
|
Issue/Exercise Price
|
|
|
|
|
Date Issued
|
|
Issued
|
|
Issued
|
|
|
Per Share
|
|
|
Nature of Issuance
|
|
December 31, 2020
|
|
Warrants
|
|
|
51,307
|
|
|
|
C$11.20
|
|
|
Issuance of Lender Warrants
|
|
February 4, 2021
|
|
Common Shares
|
|
|
1,800
|
|
|
|
C$16.25
|
|
|
Exercise of Broker Warrants
|
|
February 17, 2021
|
|
Stock Options
|
|
|
139,500
|
|
|
|
C$20.43
|
|
|
Stock Option Grant
|
|
March 26, 2021
|
|
Common Shares
|
|
|
231,011
|
|
|
|
C$16.70
|
|
|
Earn-Out Consideration(1)
|
|
April 27, 2021
|
|
Common Shares
|
|
|
500
|
|
|
|
US$7.50
|
|
|
Exercise of Stock Options
|
|
May 14, 2021
|
|
Stock Options
|
|
|
5,000
|
|
|
|
US$10.70
|
|
|
Stock Option Grant
|
|
May 25, 2021
|
|
Common Shares
|
|
|
5,000
|
|
|
|
US$5.00
|
|
|
Exercise of Stock Options
|
|
June 14, 2021
|
|
Common Shares
|
|
|
2,353,347
|
|
|
|
US$10.00
|
|
|
Private Placement
|
Note:
|
|
(1)
|
A portion of the purchase price payable in respect of the acquisition of Achieve
TMS Centers, LLC and Achieve TMS Alaska, LLC (collectively, “Achieve TMS”) is subject to an earn-out based on the earnings
before interest, tax, depreciation and amortization achieved by Achieve TMS during the twelve-month period following September 26, 2019
(the “Earn-Out”). The Earn-Out was partially settled through the issuance of an aggregate of 231,011 Common Shares
to the vendors on March 26, 2021.
|
DESCRIPTION OF
WARRANTS
The
Company may issue Warrants to purchase Common Shares or Preferred Shares. This section describes the general terms that will apply to
any Warrants issued pursuant to this Prospectus. Warrants may be offered separately or together with other Securities and may be attached
to or separate from any other Securities.
Unless
the applicable Prospectus Supplement otherwise indicates, each series of Warrants will be issued under a separate warrant indenture to
be entered into between the Company and one or more banks or trust companies acting as Warrant agent. The Warrant agent will act solely
as the agent of the Company and will not assume a relationship of agency with any holders of Warrant certificates or beneficial owners
of Warrants. A copy of each warrant indenture relating to any offering of Warrants will be filed by the Company with the securities regulatory
authorities in Canada and the United States after it has been entered into by the Company, and will be incorporated by reference as an
exhibit to the Registration Statement.
The
applicable Prospectus Supplement will include details of the warrant indentures, if any, governing the Warrants being offered. The specific
terms of the Warrants, and the extent to which the general terms described in this section apply to those Warrants, will be set out in
the applicable Prospectus Supplement. The Prospectus Supplement relating to any Warrants the Company offers will describe the Warrants
and the specific terms relating to the offering. The description will include, where applicable:
|
|
•
|
the designation and aggregate number of Warrants;
|
|
|
•
|
the price at which the Warrants will be offered;
|
|
|
•
|
the currency or currencies in which the Warrants will be offered;
|
|
|
•
|
the date on which the right to exercise the Warrants will commence and the date
on which the right will expire;
|
|
|
•
|
the designation, number and terms of the Common Shares or Preferred
Shares, as applicable, that may be purchased upon exercise of the Warrants, and the procedures that will result in the adjustment of
those numbers;
|
|
|
•
|
the exercise price of the Warrants;
|
|
|
•
|
the designation and terms of the Securities, if any, with which the Warrants will
be offered, and the number of Warrants that will be offered with each Security;
|
|
|
•
|
if the Warrants are issued as a unit with another Security,
the date, if any, on and after which the Warrants and the other Security will be separately transferable;
|
|
|
•
|
any minimum or maximum amount of Warrants that may be exercised at any one time;
|
|
|
•
|
any terms, procedures and limitations relating to the transferability,
exchange or exercise of the Warrants;
|
|
|
•
|
whether the Warrants will be subject to redemption or call and,
if so, the terms of such redemption or call provisions;
|
|
|
•
|
material U.S. and Canadian federal income tax consequences of owning the Warrants; and
|
|
|
•
|
any other material terms or conditions of the Warrants.
|
Warrant
certificates will be exchangeable for new Warrant certificates of different denominations at the office indicated in the Prospectus Supplement.
Prior to the exercise of their Warrants, holders of Warrants will not have any of the rights of holders of the securities subject to the
Warrants. The Company may amend the warrant indenture(s) and the Warrants, without the consent of the holders of the Warrants, to cure
any ambiguity, to cure, correct or supplement any defective or inconsistent provision, or in any other manner that will not prejudice
the rights of the holders of outstanding Warrants, as a group.
DESCRIPTION OF SUBSCRIPTION
RECEIPTS
The
Company may issue Subscription Receipts separately or together with Common Shares, Preferred Shares or Warrants, as the case may be. The
Subscription Receipts will be issued under a subscription receipt agreement. This section describes the general terms that will apply
to any Subscription Receipts that may be offered by the Company pursuant to this Prospectus.
The
applicable Prospectus Supplement will include details of the subscription receipt agreement covering the Subscription Receipts being offered.
A copy of each subscription receipt agreement relating to any offering of Subscription Receipts will be filed by the Company with the
securities regulatory authorities in Canada and the United States after it has been entered into by the Company, and will be incorporated
by reference as an exhibit to the Registration Statement. The specific terms of the Subscription Receipts, and the extent to which the
general terms described in this section apply to those Subscription Receipts, will be set forth in the applicable Prospectus Supplement.
This description will include, where applicable:
|
|
•
|
the number of Subscription Receipts;
|
|
|
•
|
the price at which the Subscription Receipts will be offered;
|
|
|
•
|
conditions to the exchange of Subscription Receipts into Common
Shares, Preferred Shares or Warrants, as the case may be, and the consequences of such conditions not being satisfied;
|
|
|
•
|
the procedures for the exchange of the Subscription Receipts
into Common Shares, Preferred Shares or Warrants;
|
|
|
•
|
the number of Common Shares, Preferred Shares or Warrants that
may be exchanged upon exercise of each Subscription Receipt;
|
|
|
•
|
the designation and terms of any other Securities with which
the Subscription Receipts will be offered, if any, and the number of Subscription Receipts that will be offered with each Security;
|
|
|
•
|
the dates or periods during which the Subscription Receipts
may be exchanged into Common Shares, Preferred Shares or Warrants;
|
|
|
•
|
terms applicable to the gross or net proceeds from the sale
of the Subscription Receipts plus any interest earned thereon;
|
|
|
•
|
material U.S. and Canadian federal income tax consequences of owning the Subscription Receipts;
|
|
|
•
|
any other rights, privileges, restrictions and conditions attaching to the Subscription Receipts; and
|
|
|
•
|
any other material terms and conditions of the Subscription Receipts.
|
Subscription
Receipt certificates will be exchangeable for new Subscription Receipt certificates of different denominations at the office indicated
in the Prospectus Supplement. Prior to the exchange of their Subscription Receipts, holders of Subscription Receipts will not have any
of the rights of holders of the securities subject to the Subscription Receipts.
DESCRIPTION OF
UNITS
The
Company may issue Units comprised of one or more of the other Securities described in this Prospectus in any combination. Each Unit will
be issued so that the holder of the Unit is also the holder of each Security included in the Unit. Thus, the holder of a Unit will have
the rights and obligations of a holder of each included Security. A unit agreement, if any, under which a Unit is issued may provide that
the Securities included in the Unit may not be held or transferred separately, at any time or at any time before a specified date. The
applicable Prospectus Supplement will describe the terms of the Units. The description will include, where applicable:
|
|
•
|
the designation of the Units and of the Securities comprising the Units;
|
|
|
•
|
the aggregated number of Units offered;
|
|
|
•
|
the price at which the Units will be offered;
|
|
|
•
|
the currency or currencies in which the Units will be offered;
|
|
|
•
|
any provisions for the issuance, payment, settlement, transfer
or exchange of the Units or of the Securities comprising the Units;
|
|
|
•
|
whether the Units and the securities comprising the Units are to be issued in
registered form, “book- entry only” form, non-certificated inventory system form, bearer form or in the form of temporary
or permanent global securities and the basis of exchange, transfer and ownership thereof; and
|
|
|
•
|
any other material terms or conditions of the Units.
|
Unit
certificates, if any, will be exchangeable for new Unit certificates of different denominations at the office indicated in the Prospectus
Supplement. Prior to the exchange of their Units, holders of Units will not have any of the rights of holders of the securities subject
to the Units.
CERTAIN INCOME TAX
CONSIDERATIONS
The
applicable Prospectus Supplement will describe certain material Canadian federal income tax consequences to an investor of the acquisition,
ownership and disposition of any Securities offered thereunder. The applicable Prospectus Supplement may also describe certain United
States federal income tax considerations generally applicable to the acquisition, ownership and disposition of any Securities offered
thereunder by an investor who is a United States person.
RISK FACTORS
Prospective
purchasers of Securities should carefully consider the risk factors described in this Prospectus, those described in a document incorporated
by reference in this Prospectus (including subsequently filed documents incorporated by reference) and those described in a Prospectus
Supplement relating to a specific offering of Securities. Discussions of certain risks affecting the Company in connection with its business
are provided in the Company’s disclosure documents filed with the various securities regulatory authorities which are incorporated
by reference in this Prospectus.
The COVID-19 pandemic
may have a material adverse effect on our business, financial condition and future growth opportunities
On
January 30, 2020, the World Health Organization declared a global emergency with respect to the outbreak of COVID-19 and then characterized
it as a pandemic on March 11, 2020. The outbreak has spread globally, causing public health authorities to impose restrictions, such as
quarantines, closures, cancellations and travel restrictions. While these effects are expected to be temporary and may be relaxed or rolled
back if and when the COVID-19 pandemic abates, the actions may be reinstated as the pandemic continues to evolve and in response to actual
or potential resurgences. The duration of the resulting business disruptions and related financial impact cannot be reasonably estimated
at this time. While all of the Company’s TMS Centers remain open, and are expected to remain open, during the pandemic, the Company
experienced a temporary decline in both patient visits/treatments and new patient treatment starts during the year ended December 31,
2020 as a result of the various “stay at home”, “shelter in place” and/or other restrictions imposed in response
to the COVID-19 pandemic. This decline negatively impacted the Company’s business, and in particular has negatively impacted the
Company’s cash flows during the year.
As
a result of our lower than expected cash flows during 2020, the Company was required to obtain additional financing through the 2020 Equity
Offering (as defined below) and under the New Credit Facility, the first $15 million tranche of which closed on December 31, 2020. However,
it is possible that our consolidated results in future periods will be negatively impacted by the COVID-19 pandemic. In addition, following
the initial closing under the New Credit Facility and the successful completion of the Private Placement, the Company expects to have
available liquidity for approximately the next 12 to 14 months. Although we believe we will become cash flow positive in the future, we
may require additional financing to fund operating and investing activities, and we can provide no assurance that such financing will
be available on acceptable terms, or at all. These conditions indicate the existence of a material uncertainty that may cast substantial
doubt as to our ability to continue as a going concern, as our independent auditors have indicated in an explanatory paragraph to their
audit opinion issued in connection with, and as discussed in Note 2(a) to, our Annual Financial Statements. The failure to raise such
capital when required could result in the delay or indefinite postponement of current business objectives and additional financing may
not be available on favorable terms or at all.
The
Company relies on third-party suppliers and manufacturers for its TMS Devices. This outbreak has resulted in the extended shutdown of
certain businesses around the globe, which may in turn result in disruptions or delays to the Company’s supply chain. These may
include disruptions from the temporary closure of third-party supplier and manufacturer facilities, interruptions in TMS Device supply
or restrictions on the export or shipment of TMS Devices. Any disruption to the Company’s suppliers and their contract manufacturers
will likely impact the Company’s revenue and operating results. The outbreak of COVID-19 may also impact the availability of key
TMS Device components, logistics flows and the availability of other resources to support critical operations.
The
Company also relies on payors to make timely payments to it for services provided to their beneficiaries. If payors are negatively impacted
by a decline in the economy, including as a result of the COVID-19 pandemic, the Company may experience slowdowns in collections and a
reduction in the amounts it expects to collect.
A local,
regional, national or international outbreak of a contagious disease, including, but not limited to, COVID-19, Middle East
Respiratory Syndrome, Severe Acute Respiratory Syndrome, H1N1 influenza virus, avian flu or any other similar illness, or a fear of
any of the foregoing, could adversely impact the Company by causing operating delays and disruptions, labor shortages and shutdowns
(including as a result of government regulation and prevention measures). If the Company is unable to mitigate the impacts of the
COVID-19 pandemic on operations, its costs may increase and revenue could decrease. It is unknown how the Company may be affected if
such an epidemic persists for an extended period of time. A widespread health crisis could adversely affect the global economy,
resulting in an economic downturn that could impact demand for the services the Company provides.
The
future impact of the outbreak is highly uncertain and cannot be predicted, and there is no assurance that the outbreak will not have a
material adverse impact on the Company’s future results. The extent of the impact will depend on future developments, including
actions taken to contain COVID-19.
We
have identified material weaknesses in our internal controls over financial reporting, and an inability to maintain effective internal
controls over financial reporting could increase the risk of an error in our financial statements and/or call into question the reliability
of our financial statements
We
are responsible for establishing and maintaining adequate internal controls over financial reporting, which is a process designed to provide
reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes
in accordance with IFRS. Because of our inherent limitations and the fact that we are a relatively new public company and are implementing
new financial control and management systems, internal controls over financial reporting may not prevent or detect misstatements. Also,
projections of any evaluation of effectiveness to future periods are subject to risk that controls may become inadequate because of changes
in conditions, or that the degree of compliance with the policies or procedures may deteriorate. A failure to prevent or detect errors
or misstatements may result in a decline in the market price of the Shares and harm our ability to raise capital in the future.
In
connection with the audit of our consolidated financial statements that were prepared in accordance with IFRS, and audited in accordance
with the standards of the Public Company Accounting Oversight Board (United States), our management identified material weaknesses in
our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control
over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements
will not be prevented or detected on a timely basis.
The
Company discovered that, as an emerging growth company, it did not have the formalized internal control environment necessary to satisfy
the accounting and financial reporting requirements, including a lack of documentation of its existing internal control environment. The
control breakdown that gave rise to the material audit adjustment to revenue for the estimate for variable consideration identified by
the external auditors was inadequate review and scrutiny of judgement involved in the application of IFRS 15, Revenue from Contracts
with Customers to changes to variable consideration estimates at December 31, 2020. The material weaknesses that our management identified
related to the following:
|
|
•
|
the Company did not have an effective risk assessment process
that successfully identified and assessed risks of misstatement to ensure controls were designed and implemented to respond to those
risks;
|
|
|
•
|
the Company did not have an effective monitoring process to
assess the consistent operation of internal control over financial reporting and to remediate known control deficiencies; and
|
|
|
•
|
the Company did not effectively design and maintain appropriate
segregation of duties and controls over the effective preparation, review and approval, and associated documentation of journal entries.
|
These
control deficiencies are pervasive in impact and resulted in certain material misstatements to the Company’s financial statements
identified through the audit, and which were corrected by management. Identified errors resulted in certain adjustments to the amounts
or disclosures included revenue, share-based compensation, contributed surplus, cash, accounts receivable, accounts payable and accrued
liabilities, loans payable, lender warrants, and professional and legal fees. These errors were corrected prior to the release of the
Annual Financial Statements.
The
existence of these material weaknesses creates a reasonable possibility that an error may not be prevented or detected in the Company’s
annual or interim financial statements on a timely basis.
We
have established a remediation plan which includes the following specific remedial actions undertaken by management:
|
|
•
|
implementing a system to manage and automate our internal control
over financial reporting processes and procedures;
|
|
|
•
|
hiring additional accounting and finance resources and personnel
with expertise in internal control over financial reporting;
|
|
|
•
|
implementing processes and controls to better identify and manage
the consistent operation of internal control over financial reporting and remediate known control deficiencies, including maintaining
appropriate segregation of duties;
|
|
|
•
|
implementing journal entry approval workflow within our key financial system; and
|
|
|
•
|
retaining an international accounting firm to conduct a comprehensive
assessment of our internal control over financial reporting processes and procedures and make recommendations for additional improvements
to such processes and procedures.
|
We
will take all measures necessary to address and cure the underlying causes of the material weaknesses. Once implemented, our remediation
plan may take significant time and expense to be fully implemented and may require significant management attention, and our efforts may
not prove to be successful in remediating the material weakness and do not guarantee that we will not suffer additional material weaknesses
and/or significant deficiencies in the future. At this time, management estimates that it will have remediated the material weaknesses
described above by the end of fiscal 2021.
Despite
the material weaknesses, and based on management’s assessment, management has concluded that the Company’s control environment
continued to result in financial statements in prior periods that presented fairly, in all material respects, the Company’s financial
position, results of operations, changes in equity (deficit) and cash flows in accordance with IFRS. As a result, no changes to the required
conclusions made by the Company’s certifying officers were necessary. Furthermore, management has concluded that the Annual Financial
Statements present fairly, in all material respects, the Company’sfinancial position, results of operations, changes in equity (deficit)
and cash flows in accordance with IFRS.
If
our management is unable to certify the effectiveness of our internal controls or if additional material weaknesses in our internal controls
are identified, we could be subject to regulatory scrutiny and a loss of public confidence, which could harm our business and cause a
decline in the price of the Common Shares. In addition, if we do not maintain adequate financial and management personnel, processes and
controls, we may not be able to accurately report our financial performance on a timely basis, which could cause a decline in the market
price of the Common Shares and harm our ability to raise capital.
We
do not expect that our disclosure controls and procedures and internal controls over financial reporting will prevent all error or fraud.
A control system, no matter how well-designed and implemented, can provide only reasonable, not absolute, assurance that the control system’s
objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits
of controls must be considered relative to their costs. Due to the inherent limitations in all control systems, no evaluation of controls
can provide absolute assurance that all control issues within an organization are detected. The inherent limitations include the realities
that judgments in decision making can be faulty, and that breakdowns can occur because of simple errors or mistakes. Controls can also
be circumvented by individual acts of certain persons, by collusion of two or more people or by management override of the controls. Due
to the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected
in a timely manner or at all. If we cannot provide reliable financial reports or prevent fraud, our reputation and operating results could
be materially adversely affected, which could also cause investors to lose confidence in our reported financial information, which in
turn could result in a reduction in the trading price of the Common Shares.
As a result
of our NASDAQ listing, we are now subject to the requirements of the Sarbanes-Oxley Act of 2002
(“Sarbanes-Oxley”). Section 404 of Sarbanes-Oxley (“Section 404”) requires companies subject
to the reporting requirements of the U.S. securities laws to complete a comprehensive evaluation of our internal controls over
financial reporting. To comply with this statute, we will be required to document and test our internal control procedures and our
management will be required to assess and issue a report concerning our internal controls over financial reporting. Pursuant to the
Jumpstart Our Business Startups Act (“JOBS Act”), we are classified as an “emerging growth company”.
Under the JOBS Act, emerging growth companies are exempt from certain reporting requirements, including the independent auditor
attestation requirements of Section 404(b) of Sarbanes-Oxley. Under this exemption, our independent auditor will not be required to
attest to and report on management’s assessment of our internal control over financial reporting during a transition period of
up to five years from our initial registration in the United States. We will need to prepare for compliance with Section 404 by
strengthening, assessing and testing our system of internal controls to provide the basis for our report. However, the continuous
process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore, we
believe that our business will grow in the United States, in which case our internal controls will become more complex and will
require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of
our testing, our management may identify additional material weaknesses or significant deficiencies, which may not be remedied in a
timely manner. If our management cannot favorably assess the effectiveness of our internal controls over financial reporting, or our
independent registered public accounting firm identifies additional material weaknesses in our internal controls, investor
confidence in our financial results may weaken, and the market price of our securities may suffer.
Cash flow
from operations and need for additional financing
To
date, we have had negative cash flow from operating activities. Although we believe that we will have positive cash flow from operating
activities in the future, we may require additional financing to fund operating and investing activities. To the extent that we have negative
cash flows in future periods, certain of the proceeds of any offering may need to be allocated to funding this negative cash flow in addition
to our operational expenses or other activities. While the New Credit Facility provides the Company with an option of drawing up to an
additional US$15 million in three US$5 million delayed-draw term loan tranches within the 24 months following closing of the New Credit
Facility, the ability to draw on such delayed-draw term loans is subject to the Company achieving specific financial milestones relating
to the achievement of certain tiered EBITDA and revenue targets; debt-to-EBITDA ratio and debt-to-enterprise value ratio targets; and
minimum unrestricted cash and daily average unrestricted cash requirements. As of the date of this Prospectus, the Company does not currently
meet these financial milestones and is, therefore, unable to draw down on any of the delayed-draw term loan tranches under the New Credit
Facility at this time.
The
Company, as part of its annual budgeting process, evaluates its estimated annual cash requirements to fund planned expansion activities
and working capital requirements of existing operations. Based on this cash budget and considering its anticipated cash flows from regional
operations, its holdings of cash, the New Credit Facility and the net proceeds from the Private Placement, the Company believes that it
has sufficient capital to meet its future operating expenses, capital expenditures and future debt service requirements for approximately
the next 12 to 14 months. The Company’s cash balance and working capital as at June 30, 2021 was approximately US$19.0 million and
US$14.3 million, respectively.
If
additional liquidity is required, management plans to secure the necessary financing through the issuance of new public or private equity
or debt instruments. There is no assurance that additional future funding will be available to the Company, or that it will be available
on terms which are acceptable to management. The failure to raise such capital could result in the delay or indefinite postponement of
all or any of the Company’s current contemplated or future business objectives and expansion plans and impede its continued development
and growth. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available,
the terms of such financing will be favorable to the Company. If additional funds are raised through issuances of equity, existing shareholders
could suffer significant dilution, and any new equity securities issued could have rights, preferences and privileges superior to those
of holders of Common Shares.
In
connection with the Company’s C$15 million (approximately $10.8 million) public offering of Common Shares completed on May 21,
2020 (the “2020 Equity Offering”), the Company noted that, basedon its cash budget and considering its
anticipated cash flows from regional operations, the cost containment measures implemented by the Company as a result of the
COVID-19 pandemic, and its holdings of cash and assuming the net proceeds from the 2020 Equity Offering, the Company believed that
it had sufficient capital to meet its future operating expenses, capital expenditures and future debt service requirements for
approximately 18 months. As a result of a number of underlying factors, the Company reduced this estimate in its management’s
discussion and analysis for the financial year ended December 31, 2020 incorporated by reference herein to three months (as of March
30, 2021). The underlying factors that were primarily responsible for the reduction from the May 2020 budget/forecast included:
|
|
•
|
US$15.7 million of increased operational cash burn as compared
to the Company’s original estimate in connection with the 2020 Equity Offering, primarily due to (i) a decrease in revenue (as
a result of lower patient volumes than anticipated in the original estimate) and collections (as a result of lower than anticipated cash
collections due to the negative impact that the COVID-19 pandemic had on payor processes) and (ii) higher than anticipated employee compensation
costs as a result of furloughed staff returning earlier than anticipated. This figure is distinct from the $3,155,240 provision for adjustment
to variable consideration estimate as described under “Recent Developments — Supplemental Information Relating to Revenue
and Accounts Receivable” in this Prospectus;
|
|
|
•
|
US$7.2 million of additional unforeseen expenses due to the out-performance of
Achieve TMS which resulted in an earn-out payable to the vendors that was higher than anticipated; and
|
|
|
•
|
US$1.0 million of additional unforeseen expenses associated with the preparation
and ultimate listing of the Common Shares on NASDAQ.
|
MATERIAL CONTRACTS
Except
for material contracts entered into in the ordinary course of business, the only material contracts entered into by the Company within
the most recently completed financial year and through to the date of this Prospectus, or prior thereto and that are still in effect as
of the date hereof, are set forth below:
|
|
(a)
|
The credit and security agreement dated December 31, 2020 between the Company and
Oxford Finance LLC;
|
|
|
(b)
|
The securities purchase agreement dated June 14, 2021 among the Company and the Subscribers
entered into in connection with the Private Placement;
|
|
|
(c)
|
The registration rights agreement dated June 14, 2021 among the Company and the Subscribers
entered into in connection with the Private Placement; and
|
|
|
(d)
|
The investor rights agreement dated June 14, 2021 among the Company, MSS, Greybrook
Health and 1315 Capital entered into in connection with the Private Placement.
|
Additional
details with respect to the terms of these contracts are included elsewhere in this Prospectus or in the documents incorporated by reference
herein. Copies of the material contracts noted above are available under the Company’s SEDAR profile at www.sedar.com or
in the United States through EDGAR at the website of the SEC at www.sec.gov.
PROMOTER
Greybrook
Health may be considered a promoter of the Company within the meaning of securities legislation of certain provinces and territories of
Canada. GreybrookHealth currently holds an approximate 28.1% ownership interest in the Company through ownership of 4,527,697 Shares.
EXEMPTIONS
Pursuant
to a decision of the Autorité des marchés financiers dated June 29, 2021, the Company was granted a permanent exemption
from the requirement to translate into French this Prospectus as well as the documents incorporated by reference therein and any Prospectus
Supplement to be filed in relation to an “at-the-market distribution”. This exemption is granted on the condition that this
Prospectus and any Prospectus Supplement (other than in relation to an “at-the-market distribution”) be translated into French
if the Company offers Securities to Québec purchasers in connection with an offering other than in relation to an “at-the-market
distribution”.
LEGAL MATTERS
Certain
legal matters relating to the offering of Securities hereunder will be passed upon on behalf of the Company by Torys LLP with respect
to Canadian and U.S. legal matters. At the date hereof, the partners and associates of Torys LLP, as a group, beneficially own, directly
or indirectly, less than one per cent of any outstanding securities of the Company or any associate or affiliate of the Company.
AUDITORS, TRANSFER
AGENT AND REGISTRAR
KPMG
LLP are the auditors of the Company and have confirmed that they are independent with respect to the Company within the meaning of the
relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or
regulation and also that they are independent accountants with respect to the Company under all relevant U.S. professional and regulatory
standards.
The
transfer agent and registrar for the Common Shares is Computershare Investor Services Inc. at its principal office in Toronto, Ontario.
Computershare Trust Company, N.A. serves as the U.S. transfer agent for the Common Shares.
ENFORCEABILITY OF
CIVIL LIABILITIES IN THE UNITED STATES
The
Company is a corporation incorporated under and governed by the Business Corporations Act (Ontario). Some of the Company’s
directors and officers and some of the experts named in this Prospectus reside principally in Canada, and some of the Company’s
assets and all or a substantial portion of the assets of these persons is located outside the United States. The Company has appointed
an agent for service of process in the United States, but it may be difficult for investors who reside in the United States to effect
service of process upon these persons in the United States, or to enforce a U.S. court judgment predicated upon the civil liability provisions
of the U.S. federal securities laws against the Company or any of these persons. There is substantial doubt whether an action could be
brought in Canada in the first instance predicated solely upon U.S. federal securities laws.
The
Company has filed with the SEC, concurrently with the initial filing of the Registration Statement, an appointment of agent for service
of process on Form F-X. Under the Form F-X, the Company appointed TMS NeuroHealth Centers Inc. as its agent for service of process in
the United States in connection with any investigation or administrative proceeding conducted by the SEC and any civil suit or action
brought against or involving the Company in a United States court arising out of or related to or concerning the offering of Securities
under this Prospectus.
1,300,000 Common Shares

PROSPECTUS
September
22, 2021
Stifel
Bloom Burton
Lake
Street
Greenbrook TMS (NASDAQ:GBNH)
Historical Stock Chart
From Mar 2024 to Apr 2024
Greenbrook TMS (NASDAQ:GBNH)
Historical Stock Chart
From Apr 2023 to Apr 2024