Gamida Cell to Present Corporate Highlights at Multiple Investor Conferences in September
September 02 2021 - 7:00AM
Business Wire
Gamida Cell Ltd. (Nasdaq: GMDA), an advanced cell therapy
company committed to cures for blood cancers and serious blood
diseases, today announced that company management will present its
corporate highlights at the following virtual investor conferences
in September:
- H.C. Wainwright 23rd Annual Global Investment Conference,
September 13-15, 2021. Company presentation will be available to
view on-demand beginning on September 13 at 7:00 a.m. ET.
- Baird’s 2021 Global Healthcare Conference, September 14, 2021
with a presentation at 11:25 a.m. ET.
In the fourth quarter of 2021, Gamida Cell is targeting a BLA
submission for omidubicel, the first potential approval of a cell
therapy for blood cancer patients in need of an allogeneic bone
marrow transplant. This submission is expected to occur by the end
of the year, subject to a pre-BLA meeting with the U.S. Food and
Drug Administration (FDA) planned for the fourth quarter. In the
third quarter of 2021, the Company is planning an IND submission to
support the initiation of a Phase 1/2 clinical study of
cryopreserved, off-the-shelf GDA-201 in patients with follicular
and diffuse large b-cell lymphomas.
A webcast of both conference presentations will be available on
the “Investors & Media” section of Gamida Cell’s website at
www.gamida-cell.com, and will be available for at least 14 days
following the event.
About Gamida Cell
Gamida Cell is an advanced cell therapy company committed to
cures for patients with blood cancers and serious blood diseases.
We harness our cell expansion platform to create therapies with the
potential to redefine standards of care in areas of serious medical
need. For additional information, please visit www.gamida-cell.com
or follow Gamida Cell on LinkedIn or Twitter at @GamidaCellTx.
Cautionary Note Regarding Forward Looking Statements
This press release contains forward-looking statements as that
term is defined in the Private Securities Litigation Reform Act of
1995, including with respect to timing of initiation and progress
of, and data reported from, the clinical trials of Gamida Cell’s
product candidates, anticipated regulatory filings (including the
submission of the BLA for omidubicel to the FDA), commercialization
planning efforts, the potentially life-saving or curative
therapeutic and commercial potential of omidubicel, and Gamida
Cell’s expectations regarding its projected cash to be used for
operating activities and cash runway. Any statement describing
Gamida Cell’s goals, expectations, financial or other projections,
intentions or beliefs is a forward-looking statement and should be
considered an at-risk statement. Such statements are subject to a
number of risks, uncertainties and assumptions, including those
related to the impact that the COVID-19 pandemic could have on our
business, and including the scope, progress and expansion of Gamida
Cell’s clinical trials and ramifications for the cost thereof;
clinical, scientific, regulatory and technical developments; and
those inherent in the process of developing and commercializing
product candidates that are safe and effective for use as human
therapeutics, and in the endeavor of building a business around
such product candidates. In light of these risks and uncertainties,
and other risks and uncertainties that are described in the Risk
Factors section and other sections of Gamida Cell’s Annual Report
on Form 20-F, filed with the Securities and Exchange Commission
(SEC) on March 9, 2021, as amended, and other filings that Gamida
Cell makes with the SEC from time to time (which are available at
http://www.sec.gov), the events and circumstances discussed
in such forward-looking statements may not occur, and Gamida Cell’s
actual results could differ materially and adversely from those
anticipated or implied thereby. Although Gamida Cell’s
forward-looking statements reflect the good faith judgment of its
management, these statements are based only on facts and factors
currently known by Gamida Cell. As a result, you are cautioned not
to rely on these forward-looking statements.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20210902005027/en/
For investors: Stephanie Ascher Stern Investor Relations, Inc.
stephanie.ascher@sternir.com 1-212-362-1200
For media: Rhiannon Jeselonis Ten Bridge Communications
rhiannon@tenbridgecommunications.com 1-978-417-1946
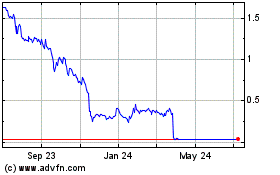
Gamida Cell (NASDAQ:GMDA)
Historical Stock Chart
From Mar 2024 to Apr 2024
Gamida Cell (NASDAQ:GMDA)
Historical Stock Chart
From Apr 2023 to Apr 2024