Enanta Pharmaceuticals Appoints Scott T. Rottinghaus, M.D., as Senior Vice President and Chief Medical Officer
August 08 2022 - 4:00PM
Business Wire
Enanta Pharmaceuticals, Inc. (NASDAQ: ENTA), a clinical-stage
biotechnology company dedicated to creating novel, small molecule
drugs for viral infections and liver diseases, today announced the
appointment of Scott T. Rottinghaus, M.D., as Senior Vice President
and Chief Medical Officer, effective today, August 8, 2022. With
over 20 years of experience in drug development across a broad
range of therapeutic areas, Dr. Rottinghaus will lead the
development, regulatory, clinical and medical functions in support
of Enanta’s pipeline.
This press release features multimedia. View
the full release here:
https://www.businesswire.com/news/home/20220808005491/en/
Scott T. Rottinghaus, M.D., Senior Vice
President and Chief Medical Officer, Enanta Pharmaceuticals (Photo:
Business Wire)
“We are delighted to welcome Scott Rottinghaus to our senior
management team. Scott’s extensive leadership experience in
industry clinical development, as well as his background as an
infectious disease trained physician, position him to be a strong
leader for our clinical team,” stated Jay Luly, Ph.D., President
and CEO, Enanta Pharmaceuticals. “We look forward to leveraging
Scott’s skillset and his experience with multiple regulatory
submissions and U.S. Food and Drug Administration Advisory
Committee participation to further drive our clinical programs,
particularly as we anticipate initiation of our Phase 2 COVID
study, as well as advancement of our respiratory syncytial virus
program.”
“I am impressed with the robust progress the Enanta team has
made as they remain dedicated to their vision of becoming a leader
in oral antiviral treatments for respiratory and liver viral
infections, and I look forward to joining the team at such an
exciting time for the company,” stated Dr. Rottinghaus. “Enanta has
deep drug discovery capabilities and I am eager to help lead the
continued advancement of the company’s pipeline with multiple study
initiations and advancements on the horizon for both RSV and
COVID-19.”
Dr. Rottinghaus brings over 20 years of clinical experience in
drug development, with expertise in a variety of therapeutic areas
including rare disease, hematology, nephrology, neurology,
dermatology, rheumatology, and infectious diseases. Prior to
joining Enanta, Dr. Rottinghaus was Vice President and Head of
Clinical Development for Hematology and Nephrology at Alexion,
AstraZeneca Rare Disease, where he led clinical development for
several assets, including ravulizumab, a humanized monoclonal
antibody complement inhibitor medication designed for the treatment
of paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic
uremic syndrome. Before his time at Alexion, Dr. Rottinghaus was a
senior director at Pfizer, driving the advancement of several drug
programs such as tofacitinib in rheumatology and dermatology, as
well as tigecycline, voriconazole, and anidulafungin in the
infectious disease field. Earlier in his Pfizer career, he worked
as a clinician on early stage clinical trials for influenza vaccine
development. Dr. Rottinghaus’ experience includes multiple NDA and
MAA submissions and FDA Advisory Committee participation. During
his industry career, Dr. Rottinghaus continued to practice as an
attending physician and assistant clinical professor in infectious
diseases at Yale School of Medicine. He has co-authored more than
30 scientific publications. Dr. Rottinghaus holds an M.D. from Mayo
Medical School, an M.Sc. in Biology from the University of
Cambridge where he studied as a Marshall Scholar, and a B.S. in
Biology as well as a B.A. in Latin and Greek from Kansas State
University.
About Enanta Pharmaceuticals, Inc. Enanta is using its
robust, chemistry-driven approach and drug discovery capabilities
to become a leader in the discovery and development of small
molecule drugs for the treatment of viral infections and liver
diseases. Enanta’s research and development programs include
clinical candidates currently in development for the following
disease targets: respiratory syncytial virus (RSV), SARS-CoV-2
(COVID-19) and hepatitis B virus (HBV). Enanta is also conducting
research in human metapneumovirus (hMPV).
Enanta’s research and development activities are funded by
royalties from hepatitis C virus (HCV) products developed under its
collaboration with AbbVie. Glecaprevir, a protease inhibitor
discovered by Enanta, is part of one of the leading treatment
regimens for curing chronic HCV infection and is sold by AbbVie in
numerous countries under the tradenames MAVYRET® (U.S.) and
MAVIRET® (ex-U.S.) (glecaprevir/pibrentasvir). Please visit
www.enanta.com for more information.
Forward Looking Statements Disclaimer This press release
contains forward-looking statements, including statements with
respect to the prospects for advancement of Enanta’s clinical
candidates. Statements that are not historical facts are based on
management’s current expectations, estimates, forecasts and
projections about Enanta’s business and the industry in which it
operates and management’s beliefs and assumptions. The statements
contained in this release are not guarantees of future performance
and involve certain risks, uncertainties and assumptions, which are
difficult to predict. Therefore, actual outcomes and results may
differ materially from what is expressed in such forward-looking
statements. Important factors and risks that may affect actual
results include: the impact of development, regulatory and
marketing efforts of others with respect to competitive treatments
for RSV, COVID-19 and HBV; the development risks of Enanta’s
clinical programs ; the competitive impact of development,
regulatory and marketing efforts of others in the disease areas of
those programs; any continuing impact of the COVID-19 pandemic on
business operations and clinical trials; Enanta’s lack of clinical
development experience; Enanta’s need to attract and retain senior
management and key research and development personnel; Enanta’s
need to obtain and maintain patent protection for its product
candidates and avoid potential infringement of the intellectual
property rights of others; and other risk factors described or
referred to in “Risk Factors” in Enanta’s Form 10-Q for the fiscal
quarter ended March 31, 2022, and any other periodic reports filed
more recently with the Securities and Exchange Commission. Enanta
cautions investors not to place undue reliance on the
forward-looking statements contained in this release. These
statements speak only as of the date of this release, and Enanta
undertakes no obligation to update or revise these statements,
except as may be required by law.
View source
version on businesswire.com: https://www.businesswire.com/news/home/20220808005491/en/
Media and Investor Jennifer Viera 617-744-3848
jviera@enanta.com
Enanta Pharmaceuticals (NASDAQ:ENTA)
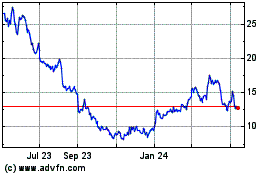
Historical Stock Chart
From Mar 2024 to Apr 2024
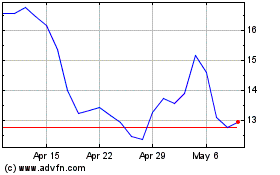
Enanta Pharmaceuticals (NASDAQ:ENTA)
Historical Stock Chart
From Apr 2023 to Apr 2024