As
filed with the Securities and Exchange Commission on September 13, 2019
Registration
No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
S-1
REGISTRATION
STATEMENT UNDER THE SECURITIES ACT OF 1933
|
CITIUS
PHARMACEUTICALS, INC.
|
|
(Exact
name of registrant as specified in its charter)
|
|
|
|
Nevada
|
|
8731
|
|
27-3425913
|
|
(State
or other jurisdiction of
|
|
(Primary
Standard Industrial
|
|
(I.R.S.
Employer
|
|
incorporation
or organization)
|
|
Classification
Code Number)
|
|
Identification
Number)
|
11
Commerce Drive, First Floor
Cranford,
New Jersey 07016
(908)
967-6677
(Address,
including zip code and telephone number, including area code, of registrant’s principal executive offices)
Myron
Holubiak
President
and Chief Executive Officer
11
Commerce Drive, First Floor
Cranford,
New Jersey 07016
(908)
967-6677
(Name,
address, including zip code and telephone number, including area code, of agent for service)
Copies
to:
|
Alexander
M. Donaldson
Lorna
A. Knick
Wyrick
Robbins Yates & Ponton LLP
4101
Lake Boone Trail, Suite 300
Raleigh,
North Carolina 27607
(919)
781-4000
|
|
Oded
Har-Even
Ron
Ben-Bassat
Zysman,
Aharoni, Gayer and Sullivan & Worcester LLP
1633
Broadway
New
York, NY 10019
(212)
660-5000
|
Approximate
date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If
any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under
the Securities Act of 1933, as amended (the “Securities Act”), check the following box. ☒
If
this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the
following box and list the Securities Act registration statement number of the earlier effective registration statement for the
same offering. ☐
If
this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list
the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
Large
accelerated filer ☐
|
Accelerated
filer ☐
|
|
|
Non-accelerated
filer ☒
|
Smaller
reporting company ☒
Emerging
growth company ☐
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act.
☐
CALCULATION
OF REGISTRATION FEE
|
Title of Each Class of Securities To Be Registered
|
|
Proposed Maximum Aggregate Offering Price (1)(2)
|
|
|
Amount of Registration Fee (2)
|
|
|
Units, each unit consisting of one share of common stock, par value $0.001 per share, and one common warrant to purchase one share of common stock (3)
|
|
$
|
9,2000,000
|
|
|
$
|
1,115.04
|
|
|
(i) Common stock included in the units (4)
|
|
|
--
|
|
|
|
--
|
|
|
(ii) Common warrants included in the units (4)
|
|
|
--
|
|
|
|
--
|
|
|
Pre-funded units, each pre-funded unit consisting of one pre-funded warrant to purchase one share of common stock and one common warrant to purchase one share of common stock (3)
|
|
$
|
9,108,000
|
|
|
$
|
1,103.89
|
|
|
(i) Pre-funded warrants included in the pre-funded units (4)
|
|
|
--
|
|
|
|
--
|
|
|
(ii) Common warrants included in the pre-funded units (4)
|
|
|
--
|
|
|
|
--
|
|
|
Shares of common stock underlying common warrants included in the units and the pre-funded units (3)
|
|
$
|
18,400,000
|
|
|
$
|
2,230.08
|
|
|
Shares of common stock underlying pre-funded warrants included in the pre-funded units (3)
|
|
$
|
92,000
|
|
|
$
|
11.15
|
|
|
Underwriter’s warrants to purchase common stock (5)
|
|
|
--
|
|
|
|
--
|
|
|
Common stock issuable upon exercise of the underwriter’s warrants (6)
|
|
$
|
805,000
|
|
|
$
|
97.57
|
|
|
Total
|
|
$
|
37,605,000
|
|
|
$
|
4,557.73
|
|
|
|
(1)
|
Estimated
solely for purposes of computing the amount of the registration fee pursuant to Rule 457(o)
under the Securities Act of 1933, as amended (the “Securities Act”). Includes
securities subject to the underwriter’s option to purchase additional securities.
|
|
|
(2)
|
Pursuant
to Rule 416 under the Securities Act, the shares of common stock registered hereby
also include an indeterminate number of additional shares of common stock as may, from
time to time, become issuable by reason of stock splits, stock dividends, recapitalizations
or other similar transactions.
|
|
|
(3)
|
The
proposed maximum aggregate offering price of the units proposed to be sold in the offering
will be reduced on a dollar-for-dollar basis based on the offering price of any pre-funded
units offered and sold in the offering, and as such the proposed maximum aggregate offering
price of the units and pre-funded units (including the common stock issuable upon exercise
of the pre-funded warrants included in the pre-funded units), if any, is $8,000,000 (or $9,200,000 if the underwriter’s
option to purchase additional securities is exercised in full).
|
|
|
(4)
|
No
additional registration fee is payable pursuant to Rule 457(i) under the Securities
Act.
|
|
|
(5)
|
No
additional registration fee is payable pursuant to Rule 457(g) under the Securities
Act.
|
|
|
(6)
|
Represents
warrants to purchase a number of shares of common stock equal to 7.0% of the number of
shares of common stock (i) included within the units and (ii) issuable upon the
exercise of the pre-funded warrants included within the pre-funded units placed in this
offering at an exercise price equal to 125% of the offering price per unit (excluding
any shares of common stock underlying the common warrants included in the units and the
pre-funded units sold in this offering).
|
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until
the registrant shall file a further amendment that specifically states that this registration statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act, or until the registration statement shall become effective on
such date as the Commission, acting pursuant to said Section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement
filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is
not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject
to Completion, Dated September 13, 2019
PRELIMINARY
PROSPECTUS

Up
to Units (each Unit contains one Share of Common Stock and one Common Warrant to
purchase
one Share of Common Stock)
Up
to Pre-funded Units (each Pre-funded Unit contains one Pre-funded Warrant to
Purchase
one Share of Common Stock and one Common Warrant to purchase one Share of Common Stock)
Shares
of Common Stock Underlying the Pre-funded Warrants and
Shares
of Common Stock Underlying the Common Warrants
We
are offering up to units (each unit consisting of one share of our common stock and one common warrant to purchase one share of
our common stock). Each common warrant contained in a unit has an exercise price of $ per
share. The common warrants contained in the units will be exercisable immediately and will expire five years from the date of
issuance. We are also offering the shares of our common stock that are issuable from time to time upon exercise of the common
warrants contained in the units.
We
are also offering to each purchaser whose purchase of units in this offering would otherwise result in the purchaser, together
with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately
following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, pre-funded units (each
pre-funded unit consisting of one pre-funded warrant to purchase one share of our common stock and one common warrant to purchase
one share of our common stock) in lieu of units that would otherwise result in the purchaser’s beneficial ownership exceeding
4.99% of our outstanding common stock (or at the election of the purchaser, 9.99%). Each pre-funded warrant contained in a pre-funded
unit will be exercisable for one share of our common stock. The purchase price of each pre-funded unit will equal the price per
unit being sold to the public in this offering minus $0.01, and the exercise price of each pre-funded warrant included in the
pre-funded unit will be $0.01 per share. The pre-funded warrants contained in the pre-funded units will be exercisable immediately
and may be exercised at any time until the pre-funded warrants are exercised in full. This offering also relates to the shares
of common stock issuable upon exercise of any pre-funded warrants contained in the pre-funded units sold in this offering. Each
common warrant contained in a pre-funded unit has an exercise price of $ per
share. The common warrants contained in the pre-funded units will be exercisable immediately and will expire five years from the
date of issuance. We are also offering the shares of our common stock that are issuable from time to time upon exercise of the
common warrants contained in the pre-funded units.
For
each pre-funded unit we sell, the number of units we are offering will be decreased on a one-for-one basis. Units and the pre-funded
units will not be issued or certificated. The shares of common stock or pre-funded warrants, as the case may be, and the common
warrants can only be purchased together in this offering, but the securities contained in the units or pre-funded units will be
issued separately.
At
least one of our directors has indicated an interest in purchasing up to approximately $ worth of units in this offering at the
public offering price and other directors may do so as well. However, because indications of interest are not binding agreements
or commitments to purchase, the underwriter may sell more, less or no units in this offering to any of these persons, or any of
these persons may determine to purchase more, less or no units in this offering. The underwriter will receive the same underwriting
discounts and commissions and other compensation on any units purchased by these persons as they will on any other units sold
to the public in this offering.
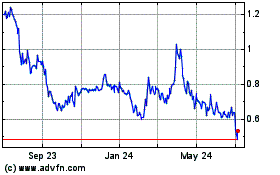
Our
common stock is listed on the Nasdaq Capital Market under the ticker symbol “CTXR”. On September 12, 2019, the
closing price of our common stock on the Nasdaq Capital Market was $0.9688. We do not intend to apply for listing of the
common warrants or pre-funded warrants on any securities exchange or other nationally recognized trading system. There is no
established public trading market for the common warrants or pre-funded warrants, and we do not expect a market to develop.
Without an active trading market, the liquidity of the common warrants and pre-funded warrants will be limited. We have
assumed a public offering price of $ per unit, the closing price for our
common stock as reported on the Nasdaq Capital Market on September , 2019, and
$ per pre-funded unit. The actual offering price per unit or pre-funded unit,
as the case may be, will be negotiated between us and the underwriter based on the trading of our common stock prior to the
offering, among other things, and may be at a discount to the current market price. Therefore, the assumed public offering
price used throughout this prospectus may not be indicative of the final offering price.
You
should read this prospectus, together with additional information described under the headings “Incorporation of Certain
Information by Reference” and “Where You Can Find More Information,” carefully before you invest in our securities.
|
|
|
Per Unit
|
|
|
Per Pre-Funded Unit
|
|
|
Total
|
|
|
Public offering price
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Underwriting discounts and commissions (1)
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
Proceeds to us, before expenses
|
|
$
|
|
|
|
$
|
|
|
|
$
|
|
|
|
|
(1)
|
We
have agreed to reimburse the underwriter for certain of its expenses and to issue the
underwriter warrants to purchase our common stock. See “Underwriting” on
page 23 of this prospectus for a description of the additional compensation to be
received by the underwriter.
|
We
have granted the underwriter the option to purchase up to additional shares of common stock at a purchase price of $ per
share and/or common warrants to purchase up to an aggregate of shares of common stock at a purchase price of $0.01 per common
warrant with an exercise price of $ per
share, less the underwriting discounts and commissions. The underwriter may exercise its option at any time within 30 days
from the date of this prospectus. If the underwriter exercises the option in full, the total underwriting discounts and commissions
payable by us will be $ , and the total
proceeds to us, before expenses, will be $ .
The
underwriter expects to deliver the securities to purchasers on or about , 2019.
Investing
in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the
heading “Risk Factors” beginning on page 8 of this prospectus.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Sole
Book-Running Manager
H.C.
Wainwright & Co.
The
date of this prospectus is ,
2019.
TABLE
OF CONTENTS
You
should read this prospectus, including the information incorporated by reference herein, and any related free writing prospectus
that we have authorized for use in connection with this offering.
You
should rely only on the information that we have included or incorporated by reference in this prospectus and any related free
writing prospectus that we may authorize to be provided to you. We have not authorized any underwriter, dealer or other person
to give any information or to make any representation other than those contained or incorporated by reference in this prospectus
or any related free writing prospectus that we may authorize to be provided to you. You must not rely upon any information or
representation not contained or incorporated by reference in this prospectus or any related free writing prospectus. This prospectus
and any related free writing prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities
other than the registered securities to which they relate, nor do this prospectus or any related free writing prospectus constitute
an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to
make such offer or solicitation in such jurisdiction.
You
should not assume that the information contained in this prospectus or any related free writing prospectus is accurate on any
date subsequent to the date set forth on the front of the document or that any information we have incorporated by reference herein
or therein is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus
or any related free writing prospectus is delivered, or securities are sold, on a later date.
This
prospectus contains or incorporates by reference summaries of certain provisions contained in some of the documents described
herein, but reference is made to the actual documents for complete information. Copies of some of the documents referred to herein
have been filed or have been incorporated by reference as exhibits to the registration statement of which this prospectus forms a
part, and you may obtain copies of those documents as described in this prospectus under the headings “Incorporation of
Certain Documents by Reference” and “Where You Can Find More Information.”
Unless
the context indicates otherwise, as used in this prospectus, the terms “Citius,” “we,” “us,”
“our,” “the Company,” “our company” and “our business” refer to Citius Pharmaceuticals,
Inc.
We
own or have rights to various U.S. federal trademark registrations and applications, and unregistered trademarks and servicemarks,
including Mino-Lok®. All other trade names, trademarks and service marks appearing in this prospectus are the property of
their respective owners. We have assumed that the reader understands that all such terms are source-indicating. Accordingly, such
terms, when first mentioned in this prospectus, appear with the trade name, trademark or service mark notice and then throughout
the remainder of this prospectus without trade name, trademark or service mark notices for convenience only and should not be
construed as being used in a descriptive or generic sense.
PROSPECTUS
SUMMARY
This
summary highlights certain information about us and this offering contained elsewhere in this prospectus or incorporated herein
by reference. Because it is only a summary, it does not contain all of the information that you should consider before investing
in our securities and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus
or incorporated herein by reference. Before you decide to invest in our securities, you should read the entire prospectus carefully,
including “Risk Factors” beginning on page 8, and the consolidated financial statements and related notes incorporated
by reference into this prospectus.
Company
Overview
Citius
Pharmaceuticals, Inc., headquartered in Cranford, New Jersey, is a specialty pharmaceutical company dedicated to the development
and commercialization of critical care products addressing important medical needs with a focus on anti-infective products in
adjunct cancer care and unique prescription products. Our goal is to achieve leading market positions by providing therapeutic
products that address unmet medical needs, yet have a lower development risk than new chemical entities usually have. New formulations
of previously approved drugs targeting new indications with substantial safety and efficacy data are a core focus. We seek to
reduce development and clinical risks associated with drug development, yet still focus on innovative applications. Our strategy
centers on products that have intellectual property and regulatory exclusivity protection, while providing competitive advantages
over other existing therapeutic approaches.
Since
its inception, the Company has devoted substantially all of its efforts to business planning, research and development, recruiting
management and technical staff, and raising capital. We are developing three proprietary products: Mino-Lok®, an antibiotic
lock solution used to treat patients with catheter related bloodstream infections (“CRBSIs”) by salvaging the infected
catheter and avoiding costly and discomforting catheter exchange; Mino-Wrap, a liquifying gel-based wrap for reducing tissue expander
infections following breast reconstructive surgeries; and Hydro-Lido, a corticosteroid-lidocaine topical formulation that is intended
to provide anti-inflammatory and anesthetic relief to persons suffering from hemorrhoids. We believe these unique markets for
our products are large, growing, and underserved by the current prescription products or procedures.
Mino-Lok®
Mino-Lok
is a patented solution containing minocycline, disodium ethylenediaminetetraacetic acid (edetate), and ethyl alcohol, all of which
act synergistically to treat biofilm encrusted pathogens and salvage infected central venous catheters (“CVCs”) in
patients with CRBSIs. Mino-Lok breaks down biofilm barriers formed by bacterial colonies, eradicates the bacteria, and provides
anti-clotting properties to maintain patency in CVCs.
The
administration of Mino-Lok consists of filling the lumen of the catheter with 0.8 ml to 2.0 ml of Mino-Lok solution. The catheter
is then “locked”, meaning that the solution remains in the catheter without flowing into the vein; the lock is maintained
for a dwell-time of two hours while the catheter is not in use. If the catheter has multiple lumens, all lumens may be locked
with the Mino-Lok solution either simultaneously or sequentially. If patients are receiving continuous infusion therapy, the catheters
alternate between being locked with the Mino-Lok solution and delivering therapy. The Mino-Lok therapy is designed to be administered
for two hours per day for at least five days, usually with two additional locks in the subsequent two weeks. After locking the
catheter for two hours, the Mino-Lok solution is aspirated, and the catheter is flushed with normal saline. At that time, either
the infusion will be continued, or will be locked with the standard-of-care lock solution until further use of the catheter is
required. In a clinical study conducted by MD Anderson Cancer Center (“MDACC”), there were no serum levels of either
minocycline or edetate detected in the sera of several patients who underwent daily catheter lock solution with minocycline and
edetate (“M-EDTA”) at the concentration level proposed in Mino-Lok treatment. Thus, it has been demonstrated that
the amount of either minocycline or edetate that leaks into the serum is very low or none at all.
Phase
2b Results
From
April 2013 to July 2014, 30 patients with CVC-related bloodstream infection were enrolled at MDACC in a prospective Phase 2b study.
Patients received Mino-Lok therapy for two hours once daily for a minimum of five days within the first week followed by two additional
locks within the next two weeks. Patients were followed for one month post lock therapy. Demographic information, clinical characteristics,
laboratory data, therapy, as well as adverse events and outcome were collected for each patient. Median age at diagnosis was 56
years (range: 21-73 years). In all patients, prior to the use of lock therapy, systemic treatment with a culture-directed, first-line
intravenous antibiotic was started. Microbiological eradication was achieved at the end of therapy in all cases. None of the patients
experienced any serious adverse event related to the lock therapy.
The
active arm, which is the Mino-Lok treated group of patients, was then compared to 60 patients in a matched cohort that experienced
removal and replacement of their CVCs within the same contemporaneous timeframe. The patients were matched for cancer type, infecting
organism, and level of neutropenia. All patients were cancer patients and treated at the MDACC. The efficacy of Mino-Lok therapy
was 100% in salvaging CVCs, demonstrating equal effectiveness of a salvaged CVC to removing the infected CVC and replacing with
a new catheter.
The
main purpose of the study was to show that Mino-Lok therapy was at least as effective as the removal and replacement of CVCs when
CRBSIs are present, and that the safety was better, that is, the complications of removing an infected catheter and replacing
with a new one could be avoided. In addition to having a 100% efficacy rate with all CVCs being salvaged, Mino-Lok therapy had
no significant adverse events (“SAEs”), compared to an 18% SAE rate in the matched cohort where patients had the infected
CVCs removed and replaced (“R&R”) with a fresh catheter. There were no overall complication rates in the Mino-Lok
arm group compared to 11 patients with events (18%) in the control group. These events included bacterial relapse (5%) at four
(4) weeks post-intervention, and a number of complications associated with mechanical manipulation in the removal or replacement
procedure for the catheter (10%) or development of deep seated infections such as septic thrombophlebitis and osteomyelitis (8%).
As footnoted, six (6) patients had more than one (1) complication in the control arm group.
|
Parameter
|
|
Mino-Lok Arm
|
|
|
Control Arm
|
|
|
|
|
N
|
|
|
(%)
|
|
|
N
|
|
|
(%)
|
|
|
Patients
|
|
|
30
|
|
|
|
(100
|
%)
|
|
|
60
|
|
|
|
(100
|
%)
|
|
Cancer type
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Hematologic
|
|
|
20
|
|
|
|
(67
|
)
|
|
|
48
|
|
|
|
(80
|
)
|
|
- Solid tumor
|
|
|
10
|
|
|
|
(33
|
)
|
|
|
12
|
|
|
|
(20
|
)
|
|
ICU Admission
|
|
|
4
|
|
|
|
(13
|
)
|
|
|
4
|
|
|
|
(7
|
)
|
|
Mech.Ventilator
|
|
|
3
|
|
|
|
(10
|
)
|
|
|
0
|
|
|
|
(0
|
)
|
|
Bacteremia
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- Gram+
|
|
|
17
|
|
|
|
(57
|
)*
|
|
|
32
|
|
|
|
(53
|
)
|
|
- Gram-
|
|
|
14
|
|
|
|
(47
|
)*
|
|
|
28
|
|
|
|
(47
|
)
|
|
Neutropenia (<500)
|
|
|
19
|
|
|
|
(63
|
)
|
|
|
36
|
|
|
|
(60
|
)
|
|
Microbiologic Eradication
|
|
|
30
|
|
|
|
(100
|
)
|
|
|
60
|
|
|
|
(100
|
)
|
|
- Relapse
|
|
|
0
|
|
|
|
(0
|
)
|
|
|
3
|
|
|
|
(5
|
)
|
|
Complications
|
|
|
0
|
|
|
|
(0
|
)
|
|
|
8
|
|
|
|
(13
|
)
|
|
SAEs related R&R
|
|
|
0
|
|
|
|
(0
|
)
|
|
|
6
|
|
|
|
(10
|
)
|
|
Overall Complication Rate
|
|
|
0
|
|
|
|
(0
|
%)
|
|
|
11
|
**
|
|
|
(18
|
%)
|
|
|
*
|
1
Polymicrobial patient had a Gram+ and a Gram- organism cultured
|
|
|
**
|
6
Patients had > 1 complication
|
Source:
Dr. Issam Raad, Antimicrobial Agents and Chemotherapy, June 2016, Vol. 60 No. 6, Page 3429
Phase
3 Initiation
In
November 2016, the Company initiated site recruitment for Phase 3 clinical trials. From initiation through first quarter 2017,
the Company received input from several sites related to the control arm as being less than standard of care for some of the respective
institutions. The Company worked closely with the United States Food and Drug Administration (“FDA”) with respect
to the design of the Phase 3 trial, and received feedback on August 17, 2017. The FDA stated that they recognized that there is
an unmet medical need in salvaging infected catheters and agreed that an open label, superiority design would address the Company’s
concerns and would be acceptable to meet the requirements of a new drug application (“NDA”). The Company amended the
Phase 3 study design to remove the saline and heparin placebo control arm and to use an active control arm that conforms with
today’s current standard of care. Patient enrollment commenced in February 2018.
The
Mino-Lok Phase 3 Trial is planned to enroll 700 patients in 50 participating institutions, all located in the U.S. There will
be interim analyses at the 50% and 75% point of the trial as measured by the number of patients treated. As of August 31, 2019,
there are 27 active sites currently enrolling patients including such academic centers as MDACC, Henry Ford Health Center, Georgetown
University Medical Center, University of Chicago, and others. There are 9 additional well renowned medical centers in startup
mode. There are no remaining sites in feasibility.
In
September 2019, the Company announced that the FDA agreed to a new primary efficacy endpoint of “time to catheter failure”
in comparing Mino Lok to the antibiotic lock control arm. Additionally, the Company submitted a response to the FDA that it will
implement this change in the primary endpoint and expected it to result in less than 150 subjects needed in its Phase 3 trial,
which the FDA is reviewing.
Fast
Track Designation
In
October 2017, the Company received official notice from FDA that the investigational program for Mino-Lok was granted “Fast
Track” status. Fast Track is a designation that expedites FDA review to facilitate development of drugs which treat a serious
or life-threatening condition and fill an unmet medical need. A drug that receives Fast Track designation is eligible for the
following:
|
|
●
|
More
frequent meetings with FDA to discuss the drug’s development plan and ensure collection
of appropriate data needed to support drug approval;
|
|
|
●
|
More
frequent written correspondence from FDA about the design of the clinical trials;
|
|
|
●
|
Priority
review to shorten the FDA review process for a new drug from ten months to six months;
and,
|
|
|
●
|
Rolling
review, which means Citius can submit completed sections of its NDA for review by FDA,
rather than waiting until every section of the application is completed before the entire
application can be reviewed.
|
Mino-Lok
International Study
In
October 2017, data from an international study on Mino-Lok was presented at the Infectious Disease Conference, in San Diego, California.
The 44 patient study was conducted in Brazil, Lebanon, and Japan and showed Mino-Lok therapy was an effective intervention to
salvage long term, infected CVCs in CRBSI in patients who had cancer with limited vascular access. This study showed 95% effectiveness
for Mino-Lok therapy in achieving microbiological eradication of the CVCs as compared to 83% for the control. The single failure
in the Mino-Lok arm was due to a patient with Burkholderia cepacia that was resistant to all antibiotics tested.
Stability
Patent Application for Mino-Lok
In
July 2018, the Company received notice from the MDACC that the US Patent and Trademark Office (“USPTO”) has reviewed
and examined the patent application US 2017/051373 A1 and that it is allowed for issuance as a patent. The new invention overcomes
limitations in mixing antimicrobial solutions in which components have precipitated because of physical and/or chemical factors,
thus limiting the stability of the post-mix solutions.
In
September 2018, the Company reported that the European Patent Application (No. 16806326.1) for Mino-Lok with Enhanced Stability
was published (September 12, 2018) under serial number 3370794. This patent, which already received a Notice of Allowance from
the USPTO in July of 2018, and which patent was issued by the USPTO in September 2018, will provide and strengthen intellectual
property protection for Mino-Lok through November of 2036. The new invention overcomes limitations in mixing antimicrobial solutions,
in which components have precipitated because of physical and/or chemical factors, thus limiting the stability of the post-mix
solutions. The scientists and technologists at MDACC have been able to improve the stability of the post-mixed solutions through
adjustments of the post-mixed pH of the solution. This may allow for longer storage time of the ready-to-use solution.
In
October 2018, the USPTO issued U.S. patent 10/086,114, entitled “Antimicrobial Solutions with Enhanced Stability.”
The new invention overcomes limitations in mixing antimicrobial solutions in which components have precipitated because of physical
and/or chemical factors, thus limiting the stability of the post-mix solutions. The scientists and technologists at MDACC have
been able to improve the stability of the post-mixed solutions through adjustments of the post-mixed pH of the solution. This
may allow for longer storage time of the ready-to-use solution. Citius holds the exclusive worldwide license which provides access
to this patented technology for development and commercialization of Mino-Lok.
Mino-Wrap
On
January 2, 2019, we entered into a patent and technology license agreement with the Board of Regents of the University of Texas
System on behalf of the MDACC, whereby we in-licensed exclusive worldwide rights to the patented technology for any and all uses
relating to breast implants. We intend to develop a liquefying, gel-based wrap containing minocycline and rifampin for the
reduction of infections associated with breast implants following breast reconstructive surgeries (“Mino-Wrap”).
We are required to use commercially reasonable efforts to commercialize Mino-Wrap under several regulatory scenarios and achieve
milestones associated with these regulatory options leading to an approval from the FDA. Mino-Wrap will require pre-clinical development
prior to any regulatory pathway. In July 2019, we announced that we intend to pursue the FDA’s Investigational New Drug
(“IND”) regulatory pathway for the development of Mino-Wrap.
Hydro-Lido
Overview
Hydro-Lido
(“CITI-001”) is a topical formulation of hydrocortisone and lidocaine that is intended for the treatment of hemorrhoids.
To our knowledge, there are currently no FDA-approved prescription drug products for the treatment of hemorrhoids. Some physicians
are known to prescribe topical steroids for the treatment of hemorrhoids. In addition, there are various strengths of topical
combination prescription products containing hydrocortisone along with lidocaine or pramoxine, each a topical anesthetic, that
are prescribed by physicians for the treatment of hemorrhoids. These products contain drugs that were in use prior to the start
of the Drug Efficacy Study Implementation (“DESI”) program and are commonly referred to as DESI drugs. However, none
of these single-agent or combination prescription products have been clinically evaluated for safety and efficacy and approved
by the FDA for the treatment of hemorrhoids. Further, many hemorrhoid patients use over the counter (“OTC”) products
as their first line therapy. OTC products contain any one of several active ingredients including glycerin, phenylephrine, pramoxine,
white petrolatum, shark liver oil and/or witch hazel, for symptomatic relief.
Development
of Hemorrhoids Drugs
Hemorrhoids
are a common gastrointestinal disorder, characterized by anal itching, pain, swelling, tenderness, bleeding and difficulty defecating.
In the U.S., hemorrhoids affect nearly 5% of the population, with approximately 10 million persons annually admitting to having
symptoms of hemorrhoidal disease. Of these persons, approximately one third visit a physician for evaluation and treatment of
their hemorrhoids. The data also indicate that for both sexes a peak of prevalence occurs from age 45 to 65 years with a subsequent
decrease after age 65 years. Caucasian populations are affected significantly more frequently than African Americans, and increased
prevalence rates are associated with higher socioeconomic status in men but not women. Development of hemorrhoids before age 20
is unusual. In addition, between 50% and 90% of the general U.S., Canadian and European population will experience hemorrhoidal
disease at least once in life. Although hemorrhoids and other anorectal diseases are not life-threatening, individual patients
can suffer from agonizing symptoms which can limit social activities and have a negative impact on the quality of life.
Hemorrhoids
are defined as internal or external according to their position relative to the dentate line. Classification is important for
selecting the optimal treatment for an individual patient. Accordingly, physicians use the following grading system referred to
as the Goligher’s classification of internal hemorrhoids:
|
|
Grade
I
|
Hemorrhoids
not prolapsed but bleeding.
|
|
|
Grade
II
|
Hemorrhoids
prolapse and reduce spontaneously with or without bleeding.
|
|
|
Grade
III
|
Prolapsed
hemorrhoids that require reduction manually.
|
|
|
Grade
IV
|
Prolapsed
and cannot be reduced including both internal and external hemorrhoids that are confluent from skin tag to inner anal canal.
|
Development
Activities to Date
In
the fall of 2015, we completed dosing patients in a double-blind dose ranging placebo controlled Phase 2 study where six different
formulations containing hydrocortisone and lidocaine in various strengths were tested against the vehicle control. The objectives
of this study were to: 1) demonstrate the safety and efficacy of the formulations when applied twice daily for two weeks in subjects
with Grade I or II hemorrhoids and 2) assess the potential contribution of lidocaine hydrochloride and hydrocortisone acetate,
alone or in combination for the treatment of symptoms of Goligher’s Classification Grade I or II hemorrhoids.
In
March 2018, we announced that we are selecting a higher potency corticosteroid in our steroid/anesthetic topical formulation program
for the treatment of hemorrhoids. The original topical preparation, CITI-001, was a combination of hydrocortisone acetate and
lidocaine hydrochloride. The new formulation, CITI-002, which we refer to as Halo-Lido, will combine lidocaine with the higher
potency corticosteroid for symptomatic relief of the pain and discomfort of hemorrhoids.
We
held a Type C meeting with the FDA in December 2017 to discuss the results of the Phase 2a study and to obtain the FDA’s
view on development plans to support the potential formulation change for the planned Phase 2b study. We also requested the FDA’s
feedback on our Phase 2b study design, including target patient population, inclusion/exclusion criteria, and efficacy endpoints.
The pre-clinical and clinical development programs for CITI-002 are planned to be similar to those conducted for the development
of CITI-001 to support the design for a planned Phase 3 clinical trial.
Corporate
Information
The
Company was founded as Citius Pharmaceuticals, LLC, a Massachusetts limited liability company, on January 23, 2007. On September
12, 2014, Citius Pharmaceuticals, LLC entered into a Share Exchange and Reorganization Agreement, with Citius Pharmaceuticals,
Inc. (formerly Trail One, Inc.), a publicly traded company incorporated under the laws of the State of Nevada (the “Reverse
Acquisition”). Citius Pharmaceuticals, LLC became a wholly-owned subsidiary of Citius. On March 30, 2016, Citius acquired
Leonard-Meron Biosciences, Inc. (“LMB”) as a wholly-owned subsidiary. LMB was a pharmaceutical company focused on
the development and commercialization of critical care products with a concentration on anti-infectives.
The
Company’s principal executive offices are located at 11 Commerce Drive, First Floor, Cranford, New Jersey 07016 and its
telephone number is (908) 976-6677.
THE
OFFERING
|
Units
offered by us
|
Up
to units, each consisting of one
share of our common stock and one common warrant to purchase one share of our common stock.
|
|
|
|
|
Pre-funded
units offered by us
|
We
are also offering to each purchaser whose purchase of units in this offering would otherwise result in the purchaser, together
with its affiliates and certain related parties, beneficially owning more than 4.99% of our outstanding common stock immediately
following the consummation of this offering, the opportunity to purchase, if the purchaser so chooses, pre-funded units (each
pre-funded unit consisting of one pre-funded warrant to purchase one share of our common stock and one common warrant to purchase
one share of our common stock) in lieu of units that would otherwise result in the purchaser’s beneficial ownership
exceeding 4.99% of our outstanding common stock (or, at the election of the purchaser, 9.99%). The purchase price of each
pre-funded unit will equal the price at which the units are being sold to the public in this offering, minus $0.01, and the
exercise price of each pre-funded warrant included in each pre-funded unit will be $0.01 per share. The pre-funded warrants
will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. This
offering also relates to the shares of common stock issuable upon exercise of any pre-funded warrants sold in this offering.
For each pre-funded unit we sell, the number of units we are offering will be decreased on a one-for-one basis. Because we
will issue a common warrant as part of each unit or pre-funded unit, the number of common warrants sold in this offering will
not change as a result of a change in the mix of the units and pre-funded units sold.
|
|
|
|
|
Common
warrants offered by us
|
Common
warrants to purchase an aggregate of shares
of our common stock. Each unit and each pre-funded unit includes a common warrant to purchase one share of our common stock.
Each common warrant will have an exercise price of $ per
share, will be immediately separable from the common stock or pre-funded warrant, as the case may be, will be immediately
exercisable and will expire on the fifth anniversary of the original issuance date, at which point it will automatically be
exercised on a cashless basis. This prospectus also relates to the offering of the shares of common stock issuable upon exercise
of the common warrants.
|
|
|
|
|
Option
to purchase additional securities
|
The
underwriter has the option to purchase up to additional
shares of common stock at a purchase price of $ per
share and/or common warrants to purchase up to an aggregate of shares
of common stock at a purchase price of $0.01 per common warrant with an exercise price of $ per
share, less underwriting discounts and commissions. The underwriter can exercise this option at any time within 30 days
from the date of this prospectus.
|
|
|
|
|
Common
stock to be outstanding immediately after this offering (1)
|
shares
(assuming no exercise of the underwriter’s option to purchase additional securities, assuming no sale of any pre-funded
units and assuming none of the common warrants issued in this offering are exercised).
|
|
|
|
|
Public
offering price
|
The
assumed public offering price is $ per
unit and $ per pre-funded unit,
which is based on the closing price for our common stock as reported on the Nasdaq Capital Market on September ,
2019. The actual offering price per each unit and pre-funded unit will be negotiated between us and the underwriter based
on the trading of our common stock prior to the offering, among other things, and may be at a discount to the current market
price.
|
|
|
|
|
Use
of proceeds
|
We
estimate that the net proceeds from this offering will be approximately $ ,
after deducting the underwriter discounts and commissions and estimated offering expenses payable by us. We intend to use
substantially all of the net proceeds from this offering primarily towards the research and development of our product candidates
and the remainder for capital expenditures, working capital and other general corporate purposes. See “Use of Proceeds”
for a more complete description of the intended use of proceeds from this offering.
|
|
|
|
|
Underwriter
warrants
|
The
registration statement of which this prospectus is a part also registers for sale warrants to purchase up to shares
of our common stock to the underwriter as a portion of its compensation payable in connection with this offering. The warrants
will be exercisable during a period commencing at six months from the effective date of the offering and ending five years
from the effective date of the offering at an exercise price equal to 125% of the public offering price of the common stock.
Please see “Underwriting – Underwriter Warrants” for a description of these warrants.
|
|
Risk
Factors
|
An
investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page 8 of
this prospectus and the similarly titled sections in the documents incorporated by reference into this prospectus.
|
|
|
|
|
NASDAQ
Capital Market trading symbol
|
Our
common stock is listed on the Nasdaq Capital Market under the symbol “CTXR.” We do not intend to apply for listing
of the common warrants or pre-funded warrants on any securities exchange or other nationally recognized trading system. There
is no established public trading market for the common warrants or pre-funded warrants, and we do not expect a market to develop.
Without an active trading market, the liquidity of the common warrants and pre-funded warrants will be limited.
|
|
|
(1)
|
The
number of shares of our common stock that will be outstanding immediately after this
offering is based on 22,075,781 shares of our common stock outstanding as of August 31,
2019 and excludes:
|
|
|
●
|
16,490,794
shares of common stock issuable upon the exercise of warrants outstanding, with a weighted
average price of $2.337 per share;
|
|
|
●
|
1,771,039
shares of our common stock issuable upon the exercise of options outstanding under our
2014 Stock Incentive Plan (“2014 Plan”) and 2018 Omnibus Stock Incentive
Plan (“2018 Plan”) with a weighted average price of $4.029 per share;
|
|
|
●
|
5,799
shares of our common stock reserved for future issuance under our 2014 Plan and 1,085,000
shares of our common stock reserved for future issuance under our 2018 Plan;
|
|
|
●
|
100,667
shares of common stock and warrants to purchase 100,667 shares of common stock, at an
exercise price of $9.00 per share, each issued or issuable pursuant to certain units,
in the form of a unit purchase option agreement, with a price of $9.00 per unit;
|
|
|
●
|
shares
of common stock issuable upon the exercise of common warrants to be issued to investors
in this offering at an exercise price of $
per share; and
|
|
|
●
|
shares
(or shares if the underwriter’s option to purchase additional securities is exercised
in full) of our common stock issuable upon exercise of the warrants being issued to the
underwriter in connection with this offering.
|
Except
as otherwise indicated herein, all information in this prospectus, including the number of shares that will be outstanding after
this offering, does not assume or give effect to the exercise of options or warrants outstanding as of June 30, 2019 and assumes
no sale of any pre-funded units in this offering.
Unless
otherwise indicated, all information contained in this prospectus assumes no exercise by the underwriter of its option to purchase
additional securities.
SPECIAL
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements
of historical facts, included in this prospectus or the documents incorporated herein by reference regarding our strategy, future
operations, future product research or development, future financial position, future revenues, projected costs, prospects, plans
and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “goals,”
“estimate,” “expect,” “intend,” “may,” “might,” “plan,”
“predict,” “project,” “target,” “potential,” “will,” “would,”
“could,” “should,” “continue” and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain these identifying words. Forward-looking statements in this prospectus
include, but are not limited to, statements about:
|
|
●
|
our
need for, and ability to raise, additional capital;
|
|
|
●
|
the
number, designs, results and timing of our clinical trials;
|
|
|
●
|
the
regulatory review process and any regulatory approvals that may be issued or denied by
the FDA or other regulatory agencies;
|
|
|
●
|
the
commercial success and market acceptance of any of our products and product candidates
that are approved for marketing in the United States or other countries;
|
|
|
●
|
the
accuracy of our estimates of the size and characteristics of the markets that may be
addressed by our products and product candidates;
|
|
|
●
|
our
ability to manufacture sufficient amounts of our product candidates for clinical trials
and our products for commercialization activities;
|
|
|
●
|
our
need to secure collaborators to license, manufacture, market and sell any products for
which we receive regulatory approval;
|
|
|
●
|
our
ability to protect our intellectual property and operate our business without infringing
upon the intellectual property rights of others;
|
|
|
●
|
the
medical benefits, effectiveness and safety of our products and product candidates;
|
|
|
●
|
the
safety and efficacy of medicines or treatments introduced by competitors that are targeted
to indications which our products and product candidates have been developed to treat;
|
|
|
●
|
our
current or prospective collaborators’ compliance or non-compliance with their obligations
under our agreements with them; and
|
|
|
●
|
other
factors discussed elsewhere in this prospectus.
|
We
may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not
place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions
and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements
included in this prospectus, particularly under “Risk Factors” on page 8 of this prospectus and the documents
incorporated herein that we believe could cause actual results or events to differ materially from the forward-looking statements
that we make.
You
should read this prospectus, the documents incorporated herein by reference and the documents that we have filed as exhibits to
this prospectus completely and with the understanding that our actual future results may be materially different from what we
expect.
Except
as required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or
future events or developments. You should therefore not rely on these forward-looking statements as representing our views as
of any date subsequent to the date of this prospectus. You also should not assume that our silence over time means that actual
events are bearing out as expressed or implied in such forward-looking statements. Before deciding to purchase our securities,
you should carefully consider the risk factors discussed and incorporated by reference in this prospectus and the documents incorporated
herein.
RISK
FACTORS
An
investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should carefully
consider the risks described below and those discussed under the section captioned “Risk Factors” contained in our
Annual Report on Form 10-K for the fiscal year ended September 30, 2018, filed with the Securities and Exchange Commission
(the “SEC”) on December 11, 2018, which is incorporated by reference in this prospectus, together with the information
included in this prospectus and documents incorporated by reference herein, and in any free writing prospectus that we have authorized
for use in connection with this offering. If any of these risks actually occurs, our business, financial condition, results of
operations or cash flow could be harmed. This could cause the trading price of our common stock to decline, resulting in a loss
of all or part of your investment.
Risk
Related to this Offering
We
may be required to raise additional financing by issuing new securities with terms or rights superior to those of our existing
securityholders, which could adversely affect the market price of shares of our common stock and our business.
We
will require additional financing to fund future operations, including our research, development, sales and marketing activities.
We may not be able to obtain financing on favorable terms, if at all. If we raise additional funds by issuing equity securities,
the percentage ownership of our current stockholders will be reduced, and the holders of the new equity securities may have rights
superior to those of our existing securityholders, which could adversely affect the market price of our common stock and the voting
power of shares of our common stock. If we raise additional funds by issuing debt securities, the holders of these debt securities
would similarly have some rights senior to those of our existing securityholders, and the terms of these debt securities could
impose restrictions on operations and create a significant interest expense for us which could have a materially adverse effect
on our business.
Issuances
of shares of our common stock or securities convertible into or exercisable for shares of our common stock following this offering,
as well as the exercise of outstanding options and warrants, will dilute your ownership interests and may adversely affect the
future market price of our common stock.
The
issuance of additional shares of our common stock or securities convertible into or exchangeable for our common stock could be
dilutive to stockholders if they do not invest in future offerings. We intend to use the net proceeds from this offering for the
continued clinical development of our product candidates, Mino-Lok, Mino-Wrap and Hydro-Lido, and for other general corporate
purposes, which may include working capital, research and development expenditures, the funding of in-licensing agreements for
product candidates, additional technologies or other forms of intellectual property, expenditures relating to manufacturing infrastructure
and other capital expenditures, and general and administrative expenses. We may seek additional capital through a combination
of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements, which
may cause your ownership interest to be diluted.
In
addition, we have a substantial number of options and warrants to purchase shares of our common stock outstanding, which will
be significantly increased by the number of pre-funded warrants and common warrants issued in the offering. If these securities
are converted or exercised, you may incur further dilution. Moreover, to the extent that we issue additional convertible notes,
convertible preferred stock, options or warrants to purchase, or securities convertible into or exchangeable for, shares of our
common stock in the future and those options, warrants or other securities are exercised, converted or exchanged, stockholders
may experience further dilution.
There
is no public market for the common warrants or the pre-funded warrants in this offering.
There
is no established public trading market for the common warrants or the pre-funded warrants in this offering, and we do not expect
a market to develop. In addition, we do not intend to apply to list the common warrants or the pre-funded warrants on any national
securities exchange or other nationally recognized trading system, including The Nasdaq Capital Market. Without an active market,
the liquidity of the common warrants and the pre-funded warrants will be limited.
The
common warrants and the pre-funded warrants in this offering are speculative in nature.
Neither
the common warrants nor the pre-funded warrants in this offering confer any rights of common stock ownership on its holders, such
as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at
a fixed price and, with respect to the common warrants, during a fixed period of time. Specifically, commencing on the date of
issuance, holders of the common warrants may exercise their right to acquire the common stock and pay an exercise price of $ per
share, subject to certain adjustments, prior to the expiration of the common warrants on the fifth anniversary of the original
issuance date, at which time the common warrants would be automatically exercised on a cashless basis. Commencing on the date
of issuance, holders of the pre-funded warrants may exercise their right to acquire the common stock and pay an exercise price
of $0.01 per share, subject to certain adjustments, at any time until the pre-funded warrants are exercised in full. Moreover,
following this offering, the market value of the common warrants and the pre-funded warrants, if any, is uncertain and there can
be no assurance that the market value of the common warrants or the pre-funded warrants will equal or exceed their imputed offering
price. Neither the common warrants nor the pre-funded warrants will be listed or quoted for trading on any market or exchange.
There can also be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the common
warrants, and consequently, whether it will ever be profitable for holders of the common warrants to exercise the common warrants.
You
will experience immediate and substantial dilution in the net tangible book value per share of the common stock included in the
units or issuable upon exercise of the common warrants or pre-funded warrants in this offering.
Since
the effective price per share of common stock included in the units or issuable upon exercise of the common warrants or the pre-funded
warrants being offered is substantially higher than the net tangible book deficit per share of our common stock outstanding prior
to this offering, you will suffer immediate and substantial dilution in the net tangible book value of the common stock included
in the units or issuable upon the exercise of the common warrants or the pre-funded warrants issued in this offering. See the
section titled “Dilution” on page 16 for a more detailed discussion of the dilution you will incur if you purchase
units in this offering.
A
significant portion of our total outstanding shares are eligible to be sold into the market, which could cause the market price
of our common stock to drop significantly, even if our business is doing well.
Sales
of a substantial number of shares of our common stock in the public market, either by us or by our current stockholders, or the
perception that these sales could occur, could cause a decline in the market price of our securities. Such sales, along with any
other market transactions, could adversely affect the market price of our common stock.
Upon
completion of this offering, based on our shares outstanding as of June 30, 2019, we will have shares of common stock outstanding
based on the issuance and sale of units in this offering, assuming no sale of any pre-funded units. Of these shares , are subject
to a contractual lock-up with the underwriter for this offering for a period of 90 days following this offering. These shares
can be sold, subject to any applicable volume limitations under federal securities laws, after the earlier of the expiration of,
or release from, the 90-day lock-up period. The balance of our outstanding shares of common stock, including any shares of common
stock included in units or issuable upon the exercise of the common warrants and pre-funded warrants purchased in this offering
other than shares acquired by our current stockholders who are also subject to the contractual lock-up, may be resold into the
public market immediately without restriction, unless owned or purchased by our affiliates.
As
of June 30, 2019, there were an aggregate of 16,490,794 shares subject to outstanding warrants, many of which shares we have registered
under the Securities Act of 1933, as amended (the “Securities Act”). These shares can be freely sold in the public
market upon issuance, subject to volume limitations applicable to affiliates to the extent applicable.
As
of June 30, 2019, there were 856,039 shares subject to outstanding options that are issuable under our 2014 Plan, all of which
shares we have registered under the Securities Act on a registration statement on Form S-8. These shares can be freely sold
in the public market upon issuance, subject to volume limitations applicable to affiliates, to the extent applicable. As of June
30, 2019, there were 915,000 shares subject to outstanding options that are issuable under our 2018 Plan, which shares have not
yet been registered under the Securities Act and therefore may not be freely sold in the public market upon issuance.
Our
management will have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our
management will have broad discretion in the application of the net proceeds from this offering, and our stockholders will not
have the opportunity as part of their investment decision to assess whether the net proceeds are being used appropriately. Because
of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use
may vary substantially from their currently intended use. The failure by our management to apply these funds effectively could
harm our business. See “Use of Proceeds” on page 10 of this prospectus for a description of our proposed use of
proceeds from this offering.
USE
OF PROCEEDS
We
estimate that the net proceeds from this offering will be approximately $ , based on an assumed offering price of $
per unit, which was the closing price of our common stock on the Nasdaq Capital Market on September , 2019, and assuming the sale
of units and no sale of any pre-funded units in this offering, after deducting the underwriting discounts and commissions and
estimated offering expenses payable by us, and excluding the proceeds, if any, from the exercise of the common warrants issued
pursuant to this offering. If the underwriter exercises its option to purchase additional securities in full, we estimate that
the net proceeds will be approximately $ , assuming an offering price of $ per unit, and assuming no sale of any pre-funded units
in this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, and
excluding the proceeds, if any, from the exercise of the common warrants issued pursuant to this offering.
The
actual offering price per unit and pre-funded unit, as applicable, will be as determined between us and the underwriter at the
time of pricing, and may be at a discount to the current market price of our common stock. These estimates exclude the proceeds,
if any, from the exercise of common warrants in this offering. If all of the common warrants sold in this offering were to be
exercised in cash at an assumed exercise price of $
per unit, we would receive additional net proceeds of approximately $ million.
However, the common warrants contain a cashless exercise provision that permit exercise of the common warrants on a cashless basis
(i) at any time where there is no effective registration statement under the Securities Act covering the issuance of the underlying
shares or (ii) on the expiration date of the common warrant. We cannot predict when or if these common warrants will be exercised
or whether they will be exercised for cash. It is possible that these common warrants may be exercised solely on a cashless basis.
A
$0.25 increase or decrease in the assumed public offering price of per unit, which was the closing price of our common stock on
the Nasdaq Capital Market on September , 2019, would increase or decrease the net proceeds to us from this offering by $ ,
assuming that the number of units offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting
the estimated underwriter discounts and commissions and estimated offering expenses payable by us, assuming no sale of any pre-funded
units and excluding the proceeds, if any, from the exercise of the common warrants issued pursuant to this offering.
Similarly,
each increase or decrease of 500,000 units offered by us would increase or decrease the net proceeds to us by approximately $
, assuming the assumed public offering price of $ per unit remains the same, after deducting the underwriting discounts and commissions
and estimated offering expenses payable by us, assuming no sale of any pre-funded units and excluding the proceeds, if any, from
the exercise of the common warrants issued pursuant to this offering.
We
intend to use the net proceeds from the sale of our common stock by us under this prospectus for general corporate purposes, including
our Phase 3 clinical Mino-Lok trial for the treatment of catheter related bloodstream infections, the IND development pathway
for Mino-Wrap and our Phase 2b clinical trial of Hydro-Lido cream for the treatment of hemorrhoids, and working capital and capital
expenditures.
MARKET
FOR COMMON STOCK
Prior
to August 3, 2017, our common stock traded under the ticker symbol “CTXR.QB” on the OTCQB Venture Market operated
by OTC Markets Group, Inc. (the “OTCQB”). On August 3, 2017, our common stock began trading on the Nasdaq Capital
Market, under the symbol “CTXR”.
On
September 12, 2019, the closing price as reported on the Nasdaq Capital Market of our common stock was $0.9688. As of
August 31, 2019, there were 98 holders of record of our common stock.
DIVIDEND
POLICY
We
have not paid any cash dividends on our common stock and our Board of Directors presently intends to continue a policy of retaining
earnings, if any, for use in our operations.
CAPITALIZATION
The
following table sets forth our cash and cash equivalents and capitalization as of June 30, 2019:
|
|
●
|
on
an actual basis; and
|
|
|
●
|
on
an as adjusted basis to give effect to our sale of units in this offering at an assumed
public offering price of $ per unit, the closing price for our common stock on the Nasdaq
Capital Market on September , 2019, assuming no sale of any pre-funded units in this
offering, after deducting the underwriting discounts and commissions and estimated offering
expenses payable by us, and excluding the proceeds, if any, from the exercise of the
common warrants issued pursuant to this offering.
|
Our
capitalization following the closing of this offering will be adjusted based on the actual public offering price and other terms
of this offering determined at pricing. You should read this table together with our consolidated financial statements and the
related notes and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations”
section of our Quarterly Report on Form 10-Q for the quarter ended June 30, 2019, filed with the SEC on August 14, 2019,
which is incorporated by reference into this prospectus.
|
|
|
As of June 30, 2019
|
|
|
|
|
Actual
|
|
|
As
|
|
|
|
|
(Unaudited)
|
|
|
Adjusted
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
4,510,751
|
|
|
$
|
|
|
|
Preferred stock – $0.001 par value; 10,000,000 shares authorized; no shares issued and outstanding
|
|
|
--
|
|
|
|
|
|
|
Common stock - $0.001 par value; 200,000,000 shares authorized; 22,075,781 shares issued and outstanding at June 30, 2019; with _________ shares issued and outstanding as adjusted
|
|
|
22,076
|
|
|
|
|
|
|
Additional paid-in capital
|
|
|
73,655,109
|
|
|
|
|
|
|
Accumulated deficit
|
|
|
(52,170,180
|
)
|
|
(
|
|
)
|
|
Total Stockholders’ Equity
|
|
|
21,507,105
|
|
|
|
|
|
|
Total Capitalization
|
|
$
|
21,680,075
|
|
|
$
|
|
|
|
|
(1)
|
Each
$0.25 increase or decrease in the assumed public offering price per unit would increase
or decrease the amount of cash and cash equivalents, working capital, total assets, and
total stockholders’ equity by approximately $ , assuming the number of units offered
by us, as set forth on the cover page of this prospectus, remains the same and after
deducting underwriting discounts and commissions and estimated offering expenses payable
by us of $400,000. We may also increase or decrease the number of units offered in this
offering. Each increase or decrease of 500,000 units offered by us would increase or
decrease the as adjusted amount of cash and cash equivalents, working capital, total
assets and total stockholders’ equity by approximately $ assuming that the assumed
public offering price remains the same, and after deducting underwriting discounts and
commissions and estimated offering expenses payable by us. The as adjusted information
discussed above is illustrative only and will be adjusted based on the actual public
offering price and other terms of this offering as determined between us and the underwriter
at pricing, and assumes no sale of pre-funded units or exercise of the underwriter’s
over-allotment option.
|
The
foregoing table and calculations are based on 22,075,781 shares of our common stock outstanding as of June 30, 2019, and excludes:
|
|
●
|
16,490,794
shares of common stock issuable upon the exercise of warrants, with a weighted average
price of $2.337 per share;
|
|
|
●
|
1,771,039
shares of our common stock issuable upon the exercise of options outstanding under our
2014 Plan and 2018 Plan with a weighted average price of $4.029 per share.
|
|
|
●
|
5,799
shares of our common stock reserved for future issuance under our 2014 Plan and 1,085,000
shares of our common stock reserved for future issuance under our 2018 Plan;
|
|
|
●
|
100,667
shares of common stock and warrants to purchase 100,667 shares of common stock, at an
exercise price of $9.00 per share, each issued or issuable pursuant to certain units,
in the form of a unit purchase option agreement, with a price of $9.00 per unit;
|
|
|
●
|
shares
of common stock issuable upon the exercise of common warrants to be issued to investors
in this offering at an exercise price of $
per share; and
|
|
|
●
|
shares
of common stock (or shares if the underwriter’s option to purchase additional securities
is exercised in full) of our common stock issuable upon exercise of the warrants being
issued to the underwriter in connection with this offering.
|
FINANCIAL
STATEMENTS
Please
see Part II, Item 8 in our Annual Report on Form 10-K for the fiscal year ended September 30, 2018, filed with the SEC on December
11, 2018, which is incorporated herein by reference, for the following financial statements:
|
|
●
|
Report
of Independent Registered Public Accounting Firm
|
|
|
●
|
Consolidated
Balance Sheets as of September 30, 2018 and 2017
|
|
|
●
|
Consolidated
Statements of Operations for the years ended September 30, 2018, 2017 and 2016
|
|
|
●
|
Consolidated
Statements of Stockholders’ Equity (Deficit) for the years ended September 30,
2018, 2017 and 2016
|
|
|
●
|
Consolidated
Statements of Cash Flows for the years ended September 30, 2018, 2017 and 2016
|
|
|
●
|
Notes
to Consolidated Financial Statements
|
See
also Part I, Item 1 in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2019, filed with the SEC on August 14,
2019, which is incorporated herein by reference, for the following financial statements:
|
|
●
|
Condensed
Consolidated Balance Sheets as of June 30, 2019 and September 30, 2018 (Unaudited)
|
|
|
●
|
Condensed
Consolidated Statements of Operations for the three and nine months ended June 30, 2019
and 2018 (Unaudited)
|
|
|
●
|
Condensed
Consolidated Statement of Changes in Stockholders’ Equity for the nine months ended
June 30, 2019 (Unaudited)
|
|
|
●
|
Condensed
Consolidated Statements of Cash Flows for the nine months ended June 30, 2019 and 2018
(Unaudited)
|
|
|
●
|
Notes
to Condensed Consolidated Financial Statements (Unaudited)
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND
RESULTS OF OPERATIONS
Please
see Item 7 in our Annual Report on Form 10-K for the fiscal year ended September 30, 2018, filed with the SEC on December 11,
2018, and Item 2 in our Quarterly Report on Form 10-Q for the quarter ended June 30, 2019, filed with the SEC on August 14, 2019,
both of which are incorporated herein by reference, for our management’s discussion and analysis of financial condition
and results of operations for the respective periods.
BUSINESS
Please
see Item 1 in our Annual Report on Form 10-K for the fiscal year ended September 30, 2018 filed with the SEC on December 11, 2018,
which is incorporated herein by reference, for a discussion of our business.
Employees
As
of June 30, 2019, the Company had nine employees and various consultants providing support. Through our consulting and collaboration
arrangements, and including our Scientific Advisory Board, we have access to more than 30 additional professionals, who possess
significant expertise in business development, legal, accounting, regulatory affairs, clinical operations and manufacturing. We
also rely upon a network of consultants to support our clinical studies and manufacturing efforts.
Properties
We
maintain our offices at 11 Commerce Drive, First Floor, Cranford, NJ 07016. We do not intend to expand our operations for the
foreseeable future and do not intend to lease additional space.
Legal
Proceedings
We
are not involved in any litigation that we believe could have a material adverse effect on our financial position or results of
operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency,
self-regulatory organization or body pending or, to the knowledge of our executive officers, threatened against or affecting our
company or our officers or directors in their capacities as such.
MANAGEMENT
Please
see “Election of Directors” and “Information Regarding the Board and its Committees” in our proxy statement
on Schedule 14A for our 2019 Annual Meeting of Stockholders, filed with the SEC on December 20, 2018, which is incorporated herein
by reference, for information regarding our board and directors. Please see “Executive Officers of Citius” in Item
1 of our Annual Report on Form 10-K for the fiscal year ended September 30, 2018, filed with the SEC on December 11, 2018, which
is incorporated herein by reference, for information regarding our executive officers.
Participation
in this Offering
At
least one of our directors has indicated an interest in purchasing up to approximately $ worth of units in this offering at the
public offering price and other directors may do so as well. However, because indications of interest are not binding agreements
or commitments to purchase, the underwriter may sell more, less or no units in this offering to any of these persons, or any of
these persons may determine to purchase more, less or no units in this offering. The underwriter will receive the same underwriting
discounts and commissions and other compensation on any units purchased by these persons as they will on any other units sold
to the public in this offering.
EXECUTIVE
AND DIRECTOR COMPENSATION
Please
see the sections captioned “Election of Directors — Director Compensation,” “Compensation Discussion and
Analysis,” “Executive Compensation,” and “Election of Directors — Compensation Committee Interlocks
and Insider Participation” in our proxy statement on Schedule 14A for our 2019 Annual Meeting of Stockholders, filed with
the SEC on December 20, 2018, which is incorporated herein by reference, for a discussion of executive and director compensation.
TRANSACTIONS
WITH RELATED PERSONS
Please
see “Election of Directors — Transactions with Related Persons” in our proxy statement on Schedule 14A for our
2019 Annual Meeting of Stockholders, filed with the SEC on December 20, 2018, which is incorporated herein by reference.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The
following table shows the amount of our common stock beneficially owned as of August 31, 2019 by (i) each person or group as those
terms are used in Section 13(d)(3) of the Securities Exchange Act of 1934, as amended, (the “Exchange Act”) believed
by us to beneficially own more than 5% of our common stock, (ii) each of our directors, (iii) each of our named executive officers,
and (iv) all of our current directors and executive officers as a group. Except as otherwise noted, each person named in the table
has sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community
property laws.
|
Name of Beneficial Owner (1)
|
|
Common Stock
Beneficially Owned Prior to Offering
|
|
|
Common Stock
Beneficially Owned After the Offering
|
|
|
|
|
Shares (2)
|
|
|
%(3)
|
|
|
Shares
|
|
|
%
|
|
|
Directors and Named Executive Officers:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Myron Holubiak (4)
|
|
|
2,452,965
|
|
|
|
10.62
|
%
|
|
|
|
|
|
|
|
%
|
|
Leonard Mazur (5)
|
|
|
13,449,098
|
|
|
|
48.90
|
%
|
|
|
|
|
|
|
|
%
|
|
|
|
Jaime Bartushak (6)
|
|
|
150,009
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
Suren Dutia (7)
|
|
|
51,667
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
Dr. William Kane (8)
|
|
|
50,404
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
Howard Safir (8)
|
|
|
50,404
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
Carol Webb (8)
|
|
|
50,404
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
Eugene Holuka (9)
|
|
|
40,749
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
All current executive officers and directors as a group (8 persons)
|
|
|
16,295,700
|
|
|
|
56.47
|
%
|
|
|
|
|
|
|
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5% or Greater Stockholders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Armistice Capital Master Fund Ltd. (10)
|
|
|
2,213,078
|
(10)
|
|
|
9.99
|
%
|
|
|
|
|
|
|
|
%
|
|
|
|
Craig Drill Capital Corporation (11)
|
|
|
1,554,500
|
|
|
|
6.97
|
|
|
|
|
|
|
|
|
%
|
|
|
|
|
(1)
|
The
address for our officers and directors is c/o of the Company, 11 Commerce Drive, First
Floor, Cranford, New Jersey 07016.
|
|
|
(2)
|
Beneficial
ownership is determined in accordance with the rules of the SEC and generally includes
voting or investment power with respect to securities. Shares of common stock subject
to options or warrants currently exercisable or convertible, or exercisable or convertible
within 60 days of June 30, 2019 are deemed outstanding for computing the percentage of
the person holding such option or warrant but are not deemed outstanding for computing
the percentage of any other person.
|
|
|
(3)
|
Percentage
based on 22,075,781 shares of common stock issued and outstanding at August 31, 2019.
|
|
|
(4)
|
Consists
of (i) 1,433,646 shares of common stock, (ii) 105,555 shares of common stock issuable
upon exercise of options and (iii) warrants to purchase an aggregate of 913,764 shares
of common stock.
|
|
|
(5)
|
Consists
of (i) 8,020,643 shares of common stock, (ii) 298,888 shares of common stock issuable
upon exercise of options and (iii) warrants to purchase an aggregate of 5,129,567 shares
of common stock.
|
|
|
(6)
|
Consists
of (i) 60,353 shares of common stock and (ii) 89,656 shares of common stock issuable
upon exercise of options.
|
|
|
(7)
|
Consists
of 51,667 shares of common stock issuable upon exercise of options.
|
|
|
(8)
|
Consists
of 50,404 shares of common stock issuable upon exercise of options.
|
|
|
(9)
|
Consists
of 40,749 shares of common stock issuable upon exercise of options.
|
|
|
(10)
|
Based
on a Schedule 13G filed with the SEC on February 14, 2019 by Armistice Capital Master
Fund Ltd., Armistice Capital, LLC and Steven Boyd. Includes warrants to purchase an aggregate
of 6,057,492 shares of common stock. The warrants are subject to a beneficial ownership
limitation of either 9.99% or 4.99% (as specified in the individual warrant agreements),
which does not permit the exercise of that portion of the warrants that would result
in any of Armistice Capital Master Fund Ltd., Armistice Capital, LLC and Steven Boyd
and their affiliates owning, after exercise, a number of shares of common stock in excess
of the beneficial ownership limitation. Each of Armistice Capital Master Fund Ltd., Armistice
Capital, LLC and Steven Boyd share voting and dispositive power over the shares and may
be deemed to be the beneficial owner of these shares. Armistice Capital, LLC and Steve
Boyd each disclaims beneficial ownership of the securities, except to the extent of his
or its pecuniary interest therein. The amounts and percentages in the table give effect
to the beneficial ownership limitation. The business address of Armistice Capital Master
Fund Ltd. is 510 Madison Avenue, 7th Floor, New York, New York 10022.
|
|
|
(11)
|
Based
on a Schedule 13G filed with the SEC on May 28, 2019 by Craig A. Drill, Craig Drill Capital,
L.L.C. and Craig Drill Capital Corporation. Includes a warrant to purchase an aggregate
of 213,106 shares of common stock. The warrant is subject to a beneficial ownership limitation
of 9.99%, which does not permit the exercise of that portion of the warrants that would
result in the Drill entities and their affiliates owning, after exercise, a number of
shares of common stock in excess of the beneficial ownership limitation. The amounts
and percentages in the table give effect to the beneficial ownership limitation. All
of the securities are owned by advisory clients of Craig Drill Capital Corporation, none
of whom own more than 5% of the outstanding shares of common stock of Citius. Each of
Craig A. Drill, Craig Drill Capital, L.L.C. and Craig Drill Capital Corporation share
voting and dispositive power over the shares and may be deemed to be the beneficial owner
of these shares. The business address of each of Craig A. Drill, Craig Drill Capital,
L.L.C. and Craig Drill Capital Corporation is 724 Fifth Avenue, 9th Floor, New York New
York 10019.
|
DILUTION
If
you invest in our securities in this offering, your ownership interest will be diluted immediately to the extent of the difference
between the effective public offering price per share of our common stock included in the units or issuable upon exercise of the
pre-funded warrants and the as adjusted net tangible book deficit per share of our common stock after this offering.
Our
net tangible book value as of June 30, 2019 was $520,309 or approximately $0.0236 per share. Net tangible book value per share
represents our total tangible assets (excluding goodwill and in-process research and development) less total liabilities divided
by the number of shares of common stock outstanding as of June 30, 2019.
After
giving effect to our sale of units in this offering at an assumed public offering price of $ per unit, the closing price of our
common stock on the Nasdaq Capital Market on September , 2019, assuming no sale of any pre-funded units in this offering, and
after deducting the underwriting discounts and commissions and estimated offering expenses payable by us, and excluding the proceeds,
if any, from the exercise of the common warrants issued pursuant to this offering, our as adjusted net tangible book value as
of June 30, 2019 would have been $ or $ per share. This represents an immediate increase in net tangible book value per
share of $ to existing stockholders and immediate dilution of $ per share to new investors purchasing units in this offering.
Dilution per share to new investors is determined by subtracting as adjusted net tangible book value per share after this offering
from the public offering price per unit paid by new investors. The following table illustrates this dilution on a per share basis:
|
Assumed public offering price per unit
|
|
|
|
|
|
$
|
|
|
|
Net tangible book value per share as of June 30, 2019
|
|
$
|
0.0236
|
|
|
|
|
|
|
Increase per share attributable to new investors
|
|
$
|
|
|
|
|
|
|
|
As adjusted net tangible book value per share after this offering
|
|
|
|
|
|
$
|
|
|
|
Dilution per share to new investors in the offering
|
|
|
|
|
|
$
|
|
|
Each
$0.25 increase or decrease in the assumed public offering price of $ per unit, the closing price of our common stock on the Nasdaq
Capital Market on September , 2019, would increase (decrease) our as adjusted net tangible book value per share after this offering
by approximately $ , and the dilution per share to new investors purchasing units in this offering by $
or $ , respectively, assuming the number of units offered
by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions
and estimated offering expenses payable by us of $400,000. We may also increase or decrease the number of units to be offered
in this offering. Each increase or decrease of 500,000 units offered by us would increase (decrease) our as adjusted net tangible
book value per share by $ , and the dilution per share to new investors purchasing units in this offering by $ or $ , respectively,
assuming that the assumed public offering price remains the same, and after deducting underwriting discounts and commissions and
estimated offering expenses payable by us, and excluding the proceeds, if any, from the exercise of the common warrants issued
pursuant to this offering. The information discussed above is illustrative only and will be adjusted based on the actual public
offering price and other terms of this offering as determined between us and the underwriter at pricing.
If
the underwriter exercises its option to purchase additional securities in full, and assuming no sale of any pre-funded units in
this offering, the as adjusted net tangible book value per share after this offering would be $ per share, the increase in net
tangible book value per share to existing stockholders would be $ per share and the dilution to new investors purchasing units
in this offering would be $ per share.
The
foregoing table and calculations are based on 22,075,781 shares of our common stock outstanding as of June 30, 2019, and excludes:
|
|
●
|
16,490,794
shares of common stock issuable upon the exercise of warrants outstanding with a weighted
average price of $2.337 per share;
|
|
|
●
|
1,771,039
shares of our common stock issuable upon the exercise of options outstanding under our
2014 Stock Incentive Plan and our 2018 Omnibus Stock Incentive Plan with a weighted average
price of $4.029 per share;
|
|
|
●
|
5,799
shares of our common stock reserved for future issuance under our 2014 Stock Inventive
Plan and 1,085,000 shares of our common stock reserved for future issuance under our
2018 Omnibus Stock Incentive Plan;
|
|
|
●
|
100,667
shares of common stock and warrants to purchase 100,667 shares of common stock, at an
exercise price of $9.00 per share, each issued or issuable pursuant to certain units,
in the form of a unit purchase option agreement, with a price of $9.00 per unit;
|
|
|
●
|
shares
of common stock issuable upon the exercise of common warrants to be issued to investors
in this offering with an exercise price of $ per
share; and
|
|
|
●
|
shares
(or shares if the underwriter’s option to purchase additional securities is exercised
in full) of common stock issuable upon exercise of the warrants being issued to the underwriter
in connection with this offering.
|
DESCRIPTION
OF CAPITAL STOCK
The
following description summarizes the material terms of Citius capital stock as of the date of this prospectus. Because it is only
a summary, it does not contain all the information that may be important to you. For a complete description of our capital stock,
you should refer to our certificate of incorporation and our bylaws, and to the provisions of applicable Nevada law.
General
Our
authorized capital stock consists of 200,000,000 shares of common stock, par value $0.001, of which 22,075,781 shares were issued
and outstanding as of August 31, 2019, and 10,000,000 shares of preferred stock, none of which are issued and outstanding.
Our preferred stock and/or common stock may be issued from time to time without prior approval by our stockholders. Our preferred
stock and/or common stock may be issued for such consideration as may be fixed from time to time by our Board of Directors. Our
Board of Directors may issue such shares of our preferred stock and/or common stock in one or more series, with such voting powers,
designations, preferences and rights or qualifications, limitations or restrictions thereof as shall be stated in the resolution
or resolutions.
Common
Stock
The
Company, a Nevada corporation, is authorized to issue 200,000,000 shares of common stock, $0.001 par value per share. Each share
of common stock shall have one vote per share for all purposes. The holders of a majority of the shares entitled to vote, present
in person or represented by proxy shall constitute a quorum at all meetings of our stockholders. Our common stock does not provide
preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights. Our common stock
holders are not entitled to cumulative voting for election of the Board of Directors.
Holders
of common stock are entitled to receive ratably such dividends as may be declared by the Board of Directors out of funds legally
available therefore as well as any distributions to the security holder. We have never paid cash dividends on our common stock,
and do not expect to pay such dividends in the foreseeable future.
In
the event of a liquidation, dissolution or winding up of our company, holders of common stock are entitled to share ratably in
all of our assets remaining after payment of liabilities. Holders of common stock have no preemptive or other subscription or
conversion rights. There are no redemption or sinking fund provisions applicable to the common stock.
Preferred
Stock
Our
Board of Directors is authorized to cause us to issue, from our authorized but unissued shares of preferred stock, one or more
series of preferred stock, to establish from time to time the number of shares to be included in each such series, as well as
to fix the designation and any preferences, conversion and other rights and limitations of such series. These rights and limitations
may include voting powers, limitations as to dividends, and qualifications and terms and conditions of redemption of the shares
of each such series.
Options
On
September 12, 2014, our stockholders approved the Company’s 2014 Plan, which provides for the award of stock options, stock
appreciation rights, restricted stock and other equity awards for up to an aggregate of 866,667 shares of common stock. The shares
of common stock underlying any awards that are forfeited, canceled, reacquired by us prior to vesting, satisfied without any issuance
of stock, expire or are otherwise terminated (other than by exercise) under the 2014 Plan will be added back to the shares of
common stock available for issuance under the 2014 Plan.
On
February 7, 2018, our stockholders approved the Company’s 2018 Plan, which provides for the award of stock options, stock
appreciation rights, restricted stock and other equity awards for up to an aggregate of 2,000,000 shares of common stock. The
shares of common stock underlying any awards that are forfeited, canceled, reacquired by us prior to vesting, satisfied without
any issuance of stock, expire or are otherwise terminated (other than by exercise) under the 2018 Plan will be added back to the
shares of common stock available for issuance under the 2018 Plan.
The
2014 Plan and the 2018 Stock Plan are administered by the Compensation Committee of our Board of Directors, but the Board of Directors
may exercise any of the powers and authority of the Committee. The Committee has the authority to determine, within the limits
of the express provisions of the 2018 Stock Plan, the individuals to whom awards will be granted, the nature, amount and terms
of such awards and the objectives and conditions for earning such awards. The Committee may grant awards to any employee, director
or consultant providing services to our Company or any of our affiliates.
In
the event of any corporate event or transaction that results in a change in the capital structure of our Company, including a
change resulting from a stock dividend or stock split, or combination or reclassification of shares, the Committee is empowered
to make such equitable adjustments with respect to awards or any provisions of the 2018 Stock Plan as it deems necessary and appropriate,
including, if necessary, any adjustments in the kind of shares issuable under the 2018 Stock Plan, the maximum number of shares
of common stock subject to the 2018 Stock Plan, the number of shares of common stock subject to and the exercise price of an outstanding
award, or the maximum number of shares that may be subject to one or more awards granted to any one recipient during a calendar
year.
As
of August 31, 2019, we had outstanding options to purchase an aggregate of 1,771,039 shares of our common stock at a weighted
average exercise price of $4.029 per share. Of these, an aggregate of 794,714 are exercisable. The remainder has vesting requirements.
There are 5,799 shares reserved for future issuance under our 2014 Plan and 1,085,000 shares reserved for future issuance under
our 2018 Plan.
Unit
Purchase Options
On
April 7, 2017, the Company issued a three-year Unit Purchase Option Agreement for the purchase of 38,000 units at a purchase price
of $9.00 per unit. Each unit consists of one share of common stock and a warrant to purchase one share of common stock at an exercise
price of $9.00 per share which expires on the earlier of three years after exercise of the Unit Purchase Option Agreement or April
7, 2023.
On
June 29, 2017, the Company issued a three-year Unit Purchase Option Agreement for the purchase of 62,667 units at a purchase price
of $9.00 per unit. Each unit consists of one share of common stock and a warrant to purchase one share of common stock at an exercise
price of $9.00 per share which expires on the earlier of three years after exercise of the Unit Purchase Option Agreement or June
29, 2022.
Warrants
As
of August 31, 2019, we had outstanding warrants to purchase an aggregate of 16,490,794 shares of our common stock at a weighted
average price of $2.337 per share, with a weighted average remaining life of 3.09 years.
Trading
Market
The
shares of our common stock are currently quoted on the Nasdaq Capital Market under the symbol “CTXR”.
Transfer
Agent
The
transfer agent of our common stock is VStock Transfer, LLC. Their address is 18 Lafayette Place, Woodmere, NY 11598.
Nevada’s
Anti-Takeover Law and Provisions of Our Articles of Incorporation and Bylaws
Acquisition
of Controlling Interest Statutes. Nevada’s “acquisition of controlling interest” statutes contain provisions
governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide
generally that any person that acquires a “controlling interest” in certain Nevada corporations may be denied certain
voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These
statutes provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation
that, but for the application of these provisions of the Nevada Revised Statutes, would enable that person to exercise (1) one-fifth
or more, but less than one-third, (2) one-third or more, but less than a majority or (3) a majority or more, of all of the voting
power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired
in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person
acquired or offered to acquire a controlling interest become “control shares” to which the voting restrictions described
above apply. Our articles of incorporation and bylaws currently contain no provisions relating to these statutes, and unless our
articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest were to provide
otherwise, these laws would apply to us if we were to (i) have 200 or more stockholders of record (at least 100 of which have
addresses in the State of Nevada appearing on our stock ledger) and (ii) do business in the State of Nevada directly or through
an affiliated corporation. As of August 31, 2019, we have 98 record stockholders and do not have 100 stockholders of
record with Nevada addresses appearing on our stock ledger. If these laws were to apply to us, they might discourage companies
or persons interested in acquiring a significant interest in or control of the Company, regardless of whether such acquisition
may be in the interest of our stockholders.
Combination
with Interested Stockholders Statutes. Nevada’s “combinations with interested stockholders” statutes prohibit
certain business “combinations” between certain Nevada corporations and any person deemed to be an “interested
stockholder” for two years after such person first becomes an “interested stockholder” unless (i) the corporation’s
Board of Directors approves the combination (or the transaction by which such person becomes an “interested stockholder”)
in advance, or (ii) the combination is approved by the Board of Directors and sixty percent of the corporation’s voting
power not beneficially owned by the interested stockholder, its affiliates and associates. Furthermore, in the absence of prior
approval certain restrictions may apply even after such two-year period. For purposes of these statutes, an “interested
stockholder” is any person who is (x) the beneficial owner, directly or indirectly, of ten percent or more of the voting
power of the outstanding voting shares of the corporation, or (y) an affiliate or associate of the corporation and at any time
within the two previous years was the beneficial owner, directly or indirectly, of ten percent or more of the voting power of
the then outstanding shares of the corporation. The definition of the term “combination” is sufficiently broad to
cover most significant transactions between the corporation and an “interested stockholder”. Subject to certain timing
requirements set forth in the statutes, a corporation may elect not to be governed by these statutes. We have not included any
such provision in our articles of incorporation.
The
effect of these statutes may be to potentially discourage parties interested in taking control of the Company from doing so if
it cannot obtain the approval of our Board of Directors
Articles
of Incorporation and Bylaws. Provisions of our certificate of incorporation and bylaws may delay or discourage transactions
involving an actual or potential change of control or change in our management, including transactions in which stockholders might
otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests.
Therefore, these provisions could adversely affect the price of our common stock. Among other things, these provisions include:
|
|
●
|
the
authorization of 10,000,000 shares of “blank check” preferred stock, the
rights, preferences and privileges of which may be established and shares of which may
be issued by our Board of Directors at its discretion from time to time and without stockholder
approval;
|
|
|
●
|
limiting
the removal of directors by the stockholders;
|
|
|
●
|
allowing
for the creation of a staggered Board of Directors;
|
|
|
●
|
eliminating
the ability of stockholders to call a special meeting of stockholders; and
|
|
|
●
|
establishing
advance notice requirements for nominations for election to the Board of Directors or
for proposing matters that can be acted upon at stockholder meetings.
|
DESCRIPTION
OF SECURITIES WE ARE OFFERING
We
are offering (i) up to units, each unit consisting of one share of our common stock and one common warrant to purchase one
share of our common stock, or (ii) up to pre-funded units, each pre-funded unit consisting of one pre-funded warrant to purchase
one share of our common stock and one common warrant to purchase one share of our common stock. For each pre-funded unit we sell,
the number of units we are offering will be decreased on a one-for-one basis. The share of common stock and accompanying common
warrant included in each unit will be issued separately, but cannot be purchased separately, and the pre-funded warrant to purchase
one share of common stock and the accompanying common warrant included in each pre-funded unit will be issued separately, but
cannot be purchased separately. Units or pre-funded units will not be issued or certificated. We are also registering the shares
of common stock included in the units and the shares of common stock issuable from time to time upon exercise of the pre-funded
warrants included in pre-funded units and common warrants included in the units and the pre-funded units offered hereby.
Common
Stock
The
material terms and provisions of our common stock and each other class of our securities which qualifies or limits our common
stock are described under the caption “Description of Capital Stock” on page 17 of this prospectus.
Common
Warrants
The
following is a summary of all material terms and provisions of the common warrants that are being offered hereby, the form of
which has been filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should
carefully review the terms and provisions of the form of common warrant for a complete description of the terms and conditions
of the common warrants.
Duration
and Exercise Price
Each
common warrant offered hereby will have an exercise price equal to $ .
The common warrants will be immediately exercisable and may be exercised until the fifth anniversary of the issuance date, at
which time they will be automatically exercised on a cashless basis. The exercise price and number of shares of common stock issuable
upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events
affecting our common stock and the exercise price. The common warrants will be issued separately from the common stock or pre-funded
warrants sold as part of the units or pre-funded units, respectively, and may be transferred separately immediately thereafter.
Common warrants will be issued in certificated form only.
Exercisability
The
common warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise
notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the
case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such
holder’s common warrants to the extent that the holder would own more than 4.99% of the outstanding common stock immediately
after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the
amount of ownership of outstanding stock after exercising the holder’s common warrants up to 9.99% of the number of shares
of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in
accordance with the terms of the common warrants.
Cashless
Exercise
If,
at the time a holder exercises its common warrants, a registration statement registering the issuance of the shares of common
stock underlying the common warrants under the Securities Act is not then effective or available for the issuance of such shares,
then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate
exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares
of common stock determined according to a formula set forth in the common warrant. The common warrants will be automatically exercised
on a cashless basis on the expiration date.
Fundamental
Transactions
In
the event of any fundamental transaction, as described in the common warrants and generally including any merger with or into
another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common
stock, then upon any subsequent exercise of a common warrant, the holder will have the right to receive as alternative consideration,
for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such
fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if
it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder
of the number of shares of our common stock for which the common warrant is exercisable immediately prior to such event. In addition,
in certain circumstances, upon a fundamental transaction, the holder will have the right to require us to repurchase its common
warrants at their fair value using the Black Scholes option pricing formula; provided, however, such holder may not require us
or our successor entity to repurchase the common warrants for the Black Scholes value solely in connection with a fundamental
transaction that is not approved by our board of directors, and therefore not within our control.
Transferability
Subject
to applicable laws and a standard legend with regard to restriction on transfer only in compliance with a public offering or an
available exemption therefrom, the common warrant may be transferred at the option of the holder upon surrender of the common
warrant to us together with the appropriate instruments of transfer.
Fractional
Shares
No
fractional shares of common stock will be issued upon the exercise of the common warrants. Rather, the number of shares of common
stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment in
respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading
Market
There
is no established trading market for the common warrants, and we do not expect an active trading market to develop. We do not
intend to apply to list the common warrants on any securities exchange or other trading market. Without a trading market, the
liquidity of the common warrants will be extremely limited.
Right
as a Stockholder
Except
as otherwise provided in the common warrants or by virtue of the holder’s ownership of shares of our common stock, such
holder of common warrants does not have the rights or privileges of a holder of our common stock, including any voting rights,
until such holder exercises such holder’s common warrants.
Waivers
and Amendments
No
term of the common warrants may be amended or waived without the written consent of the holder of such warrant.
Pre-Funded
Warrants
The
following is a summary of all material terms and provisions of the pre-funded warrants that are being offered hereby, the form
of which has been filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors
should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and
conditions of the pre-funded warrants.
Pre-funded
warrants provide any purchaser in this offering with the ability to purchase pre-funded units (each pre-funded unit consisting
of one pre-funded warrant to purchase one share of our common stock and one common warrant to purchase one share of our common
stock) in lieu of units that would otherwise result in the purchaser’s beneficial ownership exceeding 4.99% of our outstanding
common stock (or, at the election of the purchaser, 9.99%). This is accomplished through purchasing pre-funded warrants at a price
equal to the purchase price for units, less $0.01, which $0.01 is the exercise price for the pre-funded warrants. Each pre-funded
warrant is exercisable into one share of our common stock as offered hereunder. Thus, the purchaser is paying essentially the
purchase price for a unit at closing of the offering but is not deemed to beneficially own the shares of common stock included
in the units until the purchaser exercises the pre-funded warrant. Once purchased, the purchase price of the pre-funded warrants
is not refundable. While the pre-funded warrants permit waiver of provisions by us and the holder of the pre-funded warrants,
this would not affect the pre-funding as that is the purchase price of the instrument which is paid at the time of closing and
becomes part of our proceeds received from this offering. In addition, the pre-funded warrants are perpetual and do not have an
expiration date.
Duration
and Exercise Price
Each
pre-funded warrant will have an outstanding exercise price per share equal to $0.01. The pre-funded warrants will be immediately
exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price and number
of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits,
reorganizations or similar events affecting our common stock and the exercise price. The pre-funded warrants will be issued separately
from the accompanying common warrants included in the pre-funded units, and may be transferred separately immediately thereafter.
Exercisability
The
pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed
exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except
in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of
the pre-funded warrant to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after
exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount
of ownership of outstanding common stock after exercising the holder’s pre-funded warrants up to 9.99% of the number of
shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined
in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded units in this offering may also elect prior
to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock.
Cashless
Exercise
If,
at the time a holder exercises its pre-funded warrants, there is no effective registration statement registering, or the prospectus
contained therein is not available for an issuance of the shares underlying the pre-funded warrants to the holder, then in lieu
of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price,
the holder may elect instead to exercise its pre-funded warrants on a cashless basis and receive upon such exercise (either in
whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrant.
Fundamental
Transactions
In
the event of any fundamental transaction, as described in the pre-funded warrants and generally including any merger with or into
another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our common
stock, then upon any subsequent exercise of a pre-funded warrant, the holder will have the right to receive as alternative consideration,
for each share of our common stock that would have been issuable upon such exercise immediately prior to the occurrence of such
fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if
it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder
of the number of shares of our common stock for which the pre-funded warrant is exercisable immediately prior to such event.
Transferability
Subject
to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant
to us together with the appropriate instruments of transfer.
Fractional
Shares
No
fractional shares of common stock will be issued upon the exercise of the pre-funded warrants. Rather, the number of shares of
common stock to be issued will, at our election, either be rounded up to the nearest whole number or we will pay a cash adjustment
in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading
Market
There
is no established trading market for the pre-funded warrants on any securities exchange or nationally recognized trading system,
and we do not expect an active trading market to develop. We do not intend to list the pre-funded warrants on any securities exchange
or other trading market. Without a trading market, the liquidity of the pre-funded warrants will be extremely limited.
Right
as a Stockholder
Except
as otherwise provided in the pre-funded warrants or by virtue of the holder’s ownership of shares of our common stock, such
holder of pre-funded warrants does not have the rights or privileges of a holder of our common stock, including any voting rights,
until such holder exercises such holder’s pre-funded warrants.
UNDERWRITING
We
have entered into an underwriting agreement dated ,
2019, with H.C. Wainwright & Co., LLC, as underwriter of this offering. Subject to the terms and conditions
of the underwriting agreement, we have agreed to sell to the underwriter and the underwriter has agreed to purchase from us, at
the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, units
and pre-funded
units.
A
copy of the form of underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus
is a part. The units and pre-funded units we are offering are being offered by the underwriter subject to certain conditions specified
in the underwriting agreement.
We
have been advised by the underwriter that it proposes to offer the units and pre-funded units directly to the public at the public
offering price set forth on the cover page of this prospectus. Any units or pre-funded units sold by the underwriter to securities
dealers will be sold at the public offering price less a selling concession not in excess of $ per
unit or pre-funded unit. At least one of our directors has indicated an interest in purchasing units in this offering and other
directors may so as well, which would reduce the number of units sold to the general public. However, because indications of interest
are not binding agreements or commitments to purchase, these persons may determine not to purchase any units in this offering.
The
underwriting agreement provides that the underwriter’s obligation to purchase the securities we are offering is subject
to conditions contained in the underwriting agreement. The underwriter is obligated to purchase and pay for all of the units and/or
pre-funded units offered by this prospectus if any of these units and/or pre-funded units are purchased, other than those shares
of common stock and/or common warrants covered by the option to purchase additional securities described below.
No
action has been taken by us or the underwriter that would permit a public offering of units or pre-funded units in any jurisdiction
where action for that purpose is required. None of the units or pre-funded units included in this offering may be offered or sold,
directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer
and sales of any of the units or pre-funded units be distributed or published in any jurisdiction, except under circumstances
that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus
are advised to inform themselves about and to observe any restrictions relating to this offering of the units or pre-funded units
and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the
units or pre-funded units in any jurisdiction where that would not be permitted or legal.
The
underwriter has advised us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
Underwriting
Discounts, Commissions and Expenses
We
have agreed to pay an underwriter discount equal to 7% of the aggregate gross proceeds raised in this offering.
The
following table shows the public offering price, underwriting discounts and commissions and proceeds, before expenses to us. These
amounts are shown assuming both no exercise and full exercise of the underwriter’s option to purchase additional securities.
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
Per
Unit
|
|
|
Per
Pre-funded
Unit
|
|
|
Without
Option
Exercise
|
|
|
With
Full Option
Exercise
|
|
|
Public
offering price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Underwriting
discounts and commissions
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds,
before expenses, to us
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We
estimate the total expenses payable by us for this offering to be approximately $ , assuming no sale of any pre-funded units,
which amount includes (i) an assumed underwriting discount of $ ($ if the underwriter’s option to purchase additional
securities is exercised in full) based upon the assumed public offering price of $ per unit (the closing price of our common stock
on the Nasdaq Capital Market on September , 2019), (ii) $35,000 non-accountable expense allowance payable to the underwriter,
(iii) reimbursement of the accountable expenses of the underwriter equal to $100,000, including the legal fees of the underwriter
being paid by us, as well as $10,000 in clearing agent expenses, (iv) a management fee payable to the underwriter equal to 1%
of the gross proceeds of the offering, and (v) other estimated expenses of approximately $160,000 which include legal, accounting,
printing costs and various fees associated with the registration and listing of our shares.
Underwriter
Warrants
We
have agreed to issue to the underwriter warrants to purchase a number of shares of our common stock equal to 7.0% of the aggregate
number of shares of common stock (i) included within the units, (ii) issuable upon the exercise of the pre-funded warrants
included within the pre-funded units sold in this offering and (iii) included in the option to purchase additional securities.
The underwriter warrants will have a term of five years from the effective date of this prospectus and an exercise price per share
equal to 125% of the public offering price for the shares sold in this offering. Pursuant to FINRA Rule 5110(g), the underwriter
warrants and any shares issued upon exercise of the underwriter warrants shall not be sold, transferred, assigned, pledged, or
hypothecated, or be the subject of any hedging, short sale, derivative, put or call transaction that would result in the effective
economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness
or commencement of sales of this offering, except the transfer of any security: (i) by operation of law or by reason of our
reorganization; (ii) to any FINRA member firm participating in the offering and the officers or partners thereof, if all
securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period; (iii) if
the aggregate amount of our securities held by the underwriter or related persons do not exceed 1% of the securities being offered;
(iv) that is beneficially owned on a pro rata basis by all equity owners of an investment fund, provided that no participating
member manages or otherwise directs investments by the fund and the participating members in the aggregate do not own more than
10% of the equity in the fund; or (v) the exercise or conversion of any security, if all securities remain subject to the
lock-up restriction set forth above for the remainder of the time period.
Right
of First Refusal
We
have also granted the underwriter, for a period of 12 months from the closing date of this offering, a right of first refusal
to act as sole book-running manager, sole underwriter, or sole placement agent for each and every future public or private equity
or debt offering by us or any of our successors or subsidiaries. We have also agreed to a tail fee equal to the cash and warrant
compensation in this offering if any investor to which the underwriter introduced us with respect to this offering during the
term of its engagement provides us with further capital in a public or private offering or capital raising transaction, with certain
exceptions, during the 12-month period following termination of our engagement of the underwriter.
Option
to Purchase Additional Securities
We
have granted the underwriter the option to purchase up to additional shares of common stock at a purchase price of $
per share and/or common warrants to purchase up to an aggregate of shares of common stock at a purchase price of $0.01 per common
warrant with an exercise price of $ per share,
less the underwriting discounts and commissions. The underwriter may exercise its option at any time, and from time to time, within
30 days from the date of this prospectus. If any additional securities are purchased pursuant to the option to purchase additional
shares of common stock and/or common warrants, the underwriter will offer these securities on the same terms as those on which
the other securities are being offered hereby.
Nasdaq
Capital Market Listing
Our
stock is currently traded on the Nasdaq Capital Market under the symbol “CTXR.” On September 12, 2019, the closing
price of our common stock was $0.9688 per share.
Lock-up
Agreements
Our
officers and directors have agreed with the underwriter to be subject to a lock-up period of 90 days following the date of
this prospectus. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to sell,
sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly,
any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock.
Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have
also agreed in the underwriting agreement, subject to certain exceptions, to similar lock-up restrictions on the issuance and
sale of our securities for 90 days following the closing of this offering. The underwriter may, in its sole discretion and
without notice, waive the terms of any of these lock-up agreements.
Stabilization,
Short Positions and Penalty Bids
The
underwriter may engage in syndicate covering transactions, stabilizing transactions and penalty bids or purchases for the purpose
of pegging, fixing or maintaining the price of our common stock:
|
|
●
|
Syndicate
covering transactions involve purchases of securities in the open market after the distribution
has been completed in order to cover syndicate short positions. Such a naked short position
would be closed out by buying securities in the open market. A naked short position is
more likely to be created if the underwriter is concerned that there could be downward
pressure on the price of the securities in the open market after pricing that could adversely
affect investors who purchase in the offering.
|
|
|
●
|
Stabilizing
transactions permit bids to purchase the underlying security so long as the stabilizing
bids do not exceed a specific maximum.
|
|
|
●
|
Penalty
bids permit the underwriter to reclaim a selling concession from a syndicate member when
the securities originally sold by the syndicate member are purchased in a stabilizing
or syndicate covering transaction to cover syndicate short positions.
|
These
syndicate covering transactions, stabilizing transactions and penalty bids may have the effect of raising or maintaining the market
prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result, the price of
our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make
any representation or prediction as to the effect that the transactions described above may have on the price of our common stock.
These transactions may be effected on the Nasdaq Capital Market, in the over-the-counter market or on any other trading market
and, if commenced, may be discontinued at any time.
In
connection with this offering, the underwriter also may engage in passive market making transactions in our common stock in accordance
with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering
and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not
in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market
maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize
the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may
be discontinued at any time.
Neither
we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions
described above may have on the prices of our securities. In addition, neither we nor the underwriter make any representation
that the underwriter will engage in these transactions or that any transactions, once commenced, will not be discontinued without
notice.
Indemnification
We
have agreed to indemnify the underwriter against certain liabilities, including certain liabilities arising under the Securities
Act, or to contribute to payments that the underwriter may be required to make for these liabilities.
Determination
of Offering Price
The
actual offering price of the securities we are offering will be negotiated between us and the underwriter based on the trading
of our common stock prior to the offering, among other things, and may be at a discount to the current market price.
Electronic
Offer, Sale and Distribution of Securities
A
prospectus in electronic format may be made available on the websites maintained by the underwriter, if any, participating in
this offering and the underwriter may distribute prospectuses electronically. Other than the prospectus in electronic format,
the information on these websites is not part of this prospectus or the registration statement of which this prospectus form a
part, has not been approved or endorsed by us or the underwriter, and should not be relied upon by investors.
Other
Relationships
The
underwriter acted as our placement agent in connection with our registered direct offerings and concurrent private placements
we consummated in December 2017, March 2018, August 2018 and April 2019, for which it received compensation.
The
underwriter and its respective affiliates have engaged in, and may in the future engage in, investment banking and other commercial
dealings in the ordinary course of business with us or our affiliates. The underwriter has received, or may in the future receive,
customary fees and commissions for these transactions.
LEGAL
MATTERS
The
validity of the shares of common stock being offered by this prospectus will be passed upon for us by Wyrick Robbins Yates &
Ponton, LLP, Raleigh, North Carolina. Certain legal matters in connection with this offering will be passed upon for
the underwriter by Zysman, Aharoni, Gayer and Sullivan & Worcester LLP, New York, New York.
EXPERTS
The
consolidated financial statements incorporated by reference in this prospectus from our Annual Report on Form 10-K for the year
ended September 30, 2018 have been audited by Wolf & Company, P.C., an independent registered public accounting firm, as stated
in their report, which is incorporated by reference and it has been so incorporated in reliance upon the report of such firm given
upon their authority as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus, which constitutes a part of the registration statement on Form S-1 that we have filed with the SEC under the Securities
Act, does not contain all of the information in the registration statement and its exhibits. For further information with respect
to us and the common stock offered by this prospectus, you should refer to the registration statement and the exhibits filed as
part of that document. Statements contained in this prospectus as to the contents of any contract or any other document referred
to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an
exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We
are subject to the reporting requirements of the Exchange Act and file annual, quarterly and current reports, proxy statements
and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the
SEC’s website at http://www.sec.gov. We also maintain a website at http://www.citiuspharma.com, at which you
may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished
to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus. You may
request a copy of these filings, at no cost, by writing or telephoning us at: 11 Commerce Drive, First Floor, Cranford, New Jersey
07016, (908) 967-6677.
INCORPORATION
OF CERTAIN DOCUMENTS BY REFERENCE
The
SEC allows us to “incorporate by reference” information that we file with them. Incorporation by reference allows
us to disclose important information to you by referring you to those other documents. The information incorporated by reference is
an important part of this prospectus, and information that we file later with the SEC will automatically update and supersede
this information. We filed a registration statement on Form S-1 under the Securities Act with the SEC with respect to the securities
being offered pursuant to this prospectus. This prospectus omits certain information contained in the registration statement,
as permitted by the SEC. You should refer to the registration statement, including the exhibits, for further information about
us and the securities being offered pursuant to this prospectus. Statements in this prospectus regarding the provisions of certain
documents filed with, or incorporated by reference in, the registration statement are not necessarily complete and each statement
is qualified in all respects by that reference. Copies of all or any part of the registration statement, including the documents
incorporated by reference or the exhibits, may be obtained upon payment of the prescribed rates at the offices of the SEC
listed above in “Where You Can Find More Information.” We are incorporating by reference the documents listed below,
which we have already filed with the SEC, and all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or
15(d) of the Exchange Act, except as to any portion of any future report or document that is not deemed filed under such provisions,
prior to the termination of the offering:
|
|
●
|
our
Annual Report on Form 10-K for the fiscal year ended September 30, 2018, filed with the
SEC on December 11, 2018;
|
|
|
●
|
our
Quarterly Report on Form 10-Q for the quarter ended December 31, 2018, filed with the
SEC on February 14, 2019;
|
|
|
●
|
our
Quarterly Report on Form 10-Q for the quarter ended March 31, 2019, filed with the SEC
on May 15, 2019;
|
|
|
●
|
our
Quarterly Report on Form 10-Q for the quarter ended June 30, 2019, filed with the SEC
on August 14, 2019;
|
|
|
●
|
our
proxy statement on Schedule 14A for our 2019 Annual Meeting of Stockholders, filed with
the SEC on December 20, 2018; and
|
|
|
●
|
the
description of our common stock contained in our registration statement on Form 8-A filed
with the SEC on July 28, 2017.
|
Any
statement contained in this prospectus or in a document incorporated or deemed to be incorporated by reference into this
prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained
in this prospectus or other subsequently filed document that also is or is deemed to be incorporated by reference herein modifies
or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded,
to constitute a part of this prospectus.
We
will furnish without charge to you, on written or oral request, a copy of any filing or report incorporated by reference, including
exhibits to the document. You should direct any requests for documents to Citius Pharmaceuticals, Inc., 11 Commerce Drive, First
Floor, Cranford, New Jersey 07016, (908) 967-6677, Attention: Jaime Bartushak.
Citius
Pharmaceuticals, Inc.
Up
to Units (each Unit contains one Share of Common Stock and one Common Warrant to
purchase
one Share of Common Stock)
Up
to Pre-funded Units (each Pre-funded Unit contains one Pre-funded Warrant to
Purchase
one Share of Common Stock and one Common Warrant to purchase one Share of Common Stock)
Shares
of Common Stock Underlying the Pre-funded Warrants and
Shares
of Common Stock Underlying the Common Warrants
PRELIMINARY
PROSPECTUS
Sole
Book-Running Manager
H.C.
Wainwright & Co.
,
2019
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution.
The
following table sets forth all costs and expenses paid or payable by us in connection with the sale of the common stock being
registered. All amounts shown are estimates except for the Securities and Exchange Commission, or SEC, registration fee and FINRA
filing fee.
|
Expense
|
|
Amount
Paid
or to be Paid
|
|
|
SEC
registration fee
|
|
$
|
4,558
|
|
|
FINRA
filing fee
|
|
$
|
*
|
|
|
Printing
expenses
|
|
$
|
10,000
|
|
|
Legal
fees and expenses
|
|
$
|
175,000
|
|
|
Accounting
fees and expenses
|
|
$
|
40,000
|
|
|
Miscellaneous
expenses
|
|
$
|
165,000
|
|
|
Total
|
|
$
|
*
|
|
|
|
*
|
To
be completed by amendment.
|
Item
14. Indemnification of Directors and Officers.
Neither
our Articles of Incorporation nor Bylaws prevent us from indemnifying our officers, directors and agents to the extent permitted
under the Nevada Revised Statute (“NRS”). NRS Section 78.7502 provides that a corporation shall indemnify any director,
officer, employee or agent of a corporation against expenses, including attorneys’ fees, actually and reasonably incurred
by him or her in connection with any the defense to the extent that a director, officer, employee or agent of a corporation has
been successful on the merits or otherwise in defense of any action, suit or proceeding referred to Section 78.7502(1) or 78.7502(2),
or in defense of any claim, issue or matter therein.
NRS
78.7502(1) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party to any
threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except
an action by or in the right of the corporation, by reason of the fact that he or she is or was a director, officer, employee
or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent
of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees,
judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with the action,
suit or proceeding if he: (a) is not liable pursuant to NRS 78.138; or (b) acted in good faith and in a manner which he or she
reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action
or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
NRS
Section 78.7502(2) provides that a corporation may indemnify any person who was or is a party or is threatened to be made a party
to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor
by reason of the fact that he or she is or was a director, officer, employee or agent of the corporation, or is or was serving
at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture,
trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably
incurred by him or her in connection with the defense or settlement of the action or suit if he: (a) is not liable pursuant to
NRS 78.138; or (b) acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best
interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been
adjudged by a court of competent jurisdiction, after exhaustion of all appeals there from, to be liable to the corporation or
for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was
brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case,
the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
NRS
Section 78.747 provides that except as otherwise provided by specific statute, no director or officer of a corporation is individually
liable for a debt or liability of the corporation, unless the director or officer acts as the alter ego of the corporation. The
court as a matter of law must determine the question of whether a director or officer acts as the alter ego of a corporation.
Insofar
as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling
us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC, such indemnification is against
public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling
person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or
controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter
has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by us is against public policy as expressed hereby in the Securities Act and we will be governed by the final adjudication of
such issue.
Item
15. Recent Sales of Unregistered Securities.
During
the year ended September 30, 2016, the Company sold 290,000 units for a purchase price of $8.10 per unit and 17,778 units
for a purchase price of $9.00 per unit for gross proceeds of $2,509,000. Each unit consists of one share of common stock
and one five-year warrant (the “Investor Warrant”) to purchase one share of common stock at an exercise price of
$9.00. The Investor Warrants will be redeemable by the Company at a price of $0.015 per Investor Warrant at any time subject to
the conditions that (i) the common stock has traded for 20 consecutive trading days with a closing price of at least $22.50 per
share with an average trading volume of 3,333 shares per day; (ii) the Company provides 20 trading days prior notice of the redemption
and the closing price of the common stock is not less than $17.55 for more than any three days during such notice period; and
(iii) the underlying shares of common stock are registered.
On
March 22, 2016, the Company sold 333,333 shares of common stock at $9.00 per share to its Chairman of the Board, Leonard Mazur,
for gross proceeds of $3,000,000.
In
February 2017, the Company completed a private placement offering (the “2016 Offering”) and sold 128,017 units at
$6.00 per unit for gross proceeds of $768,100. Each unit consisted of one share of common stock and a five-year warrant to purchase
one share of common stock at an exercise price of $8.25 per share.
On
June 7, 2017, the Company entered into a release agreement with the placement agent for the 2016 Offering. The placement agent
consented to future financings and waived certain covenants contained in the 2016 Offering agreements. As consideration for the
release, the Company issued 6,668 shares of common stock to the placement agent.
On
June 8, 2017, the Company entered into release agreements with the investors in the 2016 Offering where each investor released
the Company from the restrictions included in the unit purchase agreements. In exchange, the Company agreed that (i) in the event
that a financing is conducted at a price per share or price per unit lower than $6.00, then the Company will issue additional
shares to each investor sufficient to effectively reprice the sale of the 2016 Offering units to the lower price and in the event
that the financing is conducted at a price per share or price per unit less than the $8.25 exercise price of the warrants issued
in the 2016 Offering then the exercise price of the warrants shall be reduced to the lower price. On August 8, 2017, the Company
completed an underwritten public offering (the “2017 Offering) and issued 58,191 shares of common stock to the investors
in the 2016 Offering to reprice the sale of the 2016 Offering units to $4.125 per unit and repriced the 2016 Offering Warrants
to an exercise price of $4.125 per share.
Mr.
Mazur has also loaned the Company $4,710,000 pursuant to convertible promissory notes. On August 8, 2017, these notes and accrued
interest of $76,240 were converted into 1,547,067 shares of common stock at a price of $3.09 per share as part of the 2017 Offering.
On
December 19, 2017, we sold warrants to purchase up to an aggregate of 640,180 shares of our common stock. Subject to certain ownership
limitations, the warrants are exercisable immediately upon issuance at an exercise price equal to $4.63 per share of common stock,
subject to adjustments as provided under the terms of the warrants. The warrants are exercisable for five and a half years from
the issuance date. The Company paid the placement agent for the offering a fee of 7% of the gross proceeds totaling $420,566 and
issued the placement agent 89,625 immediately exercisable five-year warrants at $5.8656 per share. The Company also reimbursed
the placement agent for $85,000 in expenses and incurred $20,000 in other expenses. Net proceeds from the offering were $5,482,523.
On
March 29, 2018, we sold warrants to purchase up to an aggregate of 669,504 shares of our common stock. Subject to certain ownership
limitations, the warrants are exercisable immediately upon issuance at an exercise price equal to $2.86 per share of common stock,
subject to adjustments as provided under the terms of the warrants. The warrants are exercisable for five and a half years from
the issuance date. The Company paid the placement agent for the offering a fee of 7% of the gross proceeds totaling $139,893 and
issued the placement agent 46,866 immediately exercisable five-year warrants at $3.73125 per share. The Company also reimbursed
the placement agent for $85,000 in expenses and incurred $10,000 in other expenses. Net proceeds from the offering were $1,763,576.
On
April 3, 2019, we sold an aggregate of 3,430,421 shares of our common stock at a purchase price of $1.545 per share, pursuant
to a shelf registration statement. In a concurrent private placement, we sold warrants to purchase up to an aggregate of 3,430,421
shares of our common stock. Subject to certain ownership limitations, the warrants are exercisable immediately upon issuance at
an exercise price equal to $1.42 per share of common stock, subject to adjustments as provided under the terms of the warrants.
The warrants are exercisable for two years from the issuance date. The Company paid the placement agent for the offering a fee
of 7% of the gross proceeds totaling $371,000 and issued the placement agent 240,130 two-year warrants at $1.93125 per share.
The Company also reimbursed the placement agent for $85,000 in expenses and $10,000 in other expenses. Net proceeds from the offering
were $4,779,000.
The
transactions described above were exempt from registration under Section 4(a)(2) of the Securities Act and/or Rule 506 promulgated
under the Securities Act.
Item
16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
|
Exhibit
Number
|
|
Description
of Document
|
|
Registrant’s
Form
|
|
Dated
|
|
Exhibit
Number
|
|
Filed
Herewith
|
|
1.1*
|
|
Form
of Underwriting Agreement
|
|
|
|
|
|
|
|
|
|
2.1
|
|
Share
Exchange and Reorganization Agreement, among Citius Pharmaceuticals, LLC, Trail One, Inc. and the beneficial holders of the
membership interests of Citius Pharmaceuticals, LLC identified in the Agreement, dated as of September 12, 2014.
|
|
8-K
|
|
9/18/2014
|
|
2.1
|
|
|
|
2.2
|
|
Agreement
and Plan of Merger among Citius LMB Acquisition Corporation, Leonard-Meron Biosciences, Inc. and Citius Pharmaceuticals Holdings,
Inc., dated March 30, 2016.
|
|
8-K
|
|
4/5/2016
|
|
2.1
|
|
|
|
3.1
|
|
Amended
and Restated Articles of Incorporation of Citius Pharmaceuticals, Inc.
|
|
8-K
|
|
9/18/2014
|
|
3.1
|
|
|
|
3.2
|
|
Certificate
of Amendment to the Amended and Restated Articles of Incorporation of Citius Pharmaceuticals, Inc., effective September 16,
2016.
|
|
8-K
|
|
9/21/2016
|
|
3.1
|
|
|
|
3.3
|
|
Certificate
of Amendment to the Amended and Restated Articles of Incorporation of Citius Pharmaceuticals, Inc., effective June 9, 2017.
|
|
8-K
|
|
6/8/2017
|
|
3.1
|
|
|
|
3.4
|
|
Amended
and Restated Bylaws of Citius Pharmaceuticals, Inc.
|
|
8-K
|
|
2/9/2018
|
|
3.1
|
|
|
|
4.1
|
|
Form
of Registration Rights Agreement between the Purchasers named therein and Citius Pharmaceuticals Holdings, Inc., dated September
12, 2014.
|
|
8-K
|
|
9/18/2014
|
|
10.2
|
|
|
|
4.2
|
|
Placement
Agent’s Unit Warrant in favor of Merriman Capital, Inc., dated September 12, 2014.
|
|
S-1/A
|
|
12/29/2015
|
|
10.12
|
|
|
|
4.3
|
|
Form
of Investor Warrant, dated September 12, 2014.
|
|
8-K
|
|
9/18/2014
|
|
10.3
|
|
|
|
4.4
|
|
Form
of Common Stock Purchase Warrant, dated May 10, 2017.
|
|
10-Q
|
|
5/15/2017
|
|
10.4
|
|
|
|
4.5
|
|
Form
of Representative’s Warrant, dated August 3, 2017.
|
|
8-K
|
|
8/4/2017
|
|
4.2
|
|
|
|
4.6
|
|
Form
of Investor Warrant, dated December 15, 2017.
|
|
8-K
|
|
12/19/2017
|
|
4.1
|
|
|
|
4.7
|
|
Form
of Placement Agent Warrant, dated December 15, 2017.
|
|
8-K
|
|
12/19/2017
|
|
4.2
|
|
|
|
4.8
|
|
Form
of Investor Warrant, dated March 28, 2018.
|
|
8-K
|
|
3/29/2018
|
|
4.1
|
|
|
|
4.9
|
|
Form
of Placement Agent Warrant, dated March 28, 2018.
|
|
8-K
|
|
3/29/2018
|
|
4.2
|
|
|
|
4.10
|
|
Form
of Common Stock Purchase Warrant, dated August 13, 2018.
|
|
8-K
|
|
8/13/2018
|
|
4.1
|
|
|
|
4.11
|
|
Form
of Pre-Funded Common Stock Purchase Warrant, dated August 13, 2018.
|
|
8-K
|
|
8/13/2018
|
|
4.2
|
|
|
|
4.12
|
|
Form
of Underwriter’s Common Stock Purchase Warrant, dated August 13, 2018.
|
|
8-K
|
|
8/13/2018
|
|
4.3
|
|
|
|
4.13
|
|
Form
of Investor Warrant, dated April 3, 2019.
|
|
8-K
|
|
4/3/2019
|
|
4.1
|
|
|
|
4.14
|
|
Form
of Placement Agent Warrant, dated April 3, 2019.
|
|
8-K
|
|
4/3/2019
|
|
4.2
|
|
|
|
4.15*
|
|
Form
of Common Stock Purchase Warrant
|
|
|
|
|
|
|
|
|
|
4.16*
|
|
Form
of Pre-Funded Common Stock Purchase Warrant
|
|
|
|
|
|
|
|
|
|
4.17*
|
|
Form
of Underwriter’s Common Stock Purchase Warrant
|
|
|
|
|
|
|
|
|
|
5.1*
|
|
Opinion
of Wyrick Robbins Yates & Ponton LLP
|
|
|
|
|
|
|
|
|
|
10.1
|
|
Collaboration and License Agreement between Alpex Pharma S.A. and Citius Pharmaceuticals, LLC, dated June 12, 2008.
|
|
S-1/A
|
|
10/16/2015
|
|
10.6
|
|
|
|
10.2
|
|
Product Development and Pilot Lot Manufacturing Proposal Version 01 between IGI, Inc. and Citius Pharmaceuticals, Inc., dated July 21, 2010.
|
|
S-1/A
|
|
10/16/2015
|
|
10.9
|
|
|
|
10.3
|
|
Exclusive License Agreement between Prenzamax, LLC and Citius Pharmaceuticals, Inc., dated November 15, 2011.
|
|
S-1/A
|
|
10/16/2015
|
|
10.8
|
|
|
|
10.4
|
|
Amendment and Coordination Agreement among Prenzamax LLC, Akrimax Pharmaceuticals, LLC, Citius Pharmaceuticals LLC and Alpex Pharma S.A., dated November 15, 2011.
|
|
S-1/A
|
|
10/16/2015
|
|
10.5
|
|
|
|
10.5
|
|
Supply Agreement between Prenzamax, LLC and Alpex Pharma S.A., dated November 15, 2011.
|
|
S-1/A
|
|
10/16/2015
|
|
10.10
|
|
|
|
Exhibit
Number
|
|
Description
of Document
|
|
Registrant’s
Form
|
|
Dated
|
|
Exhibit
Number
|
|
Filed
Herewith
|
|
10.6
|
|
Technical and Quality Agreement among Citius Pharmaceuticals LLC, Alpex Pharma S.A. and Akrimax Pharmaceuticals, LLC, dated November 15, 2011.
|
|
S-1/A
|
|
10/16/2015
|
|
10.11
|
|
|
|
10.7
|
|
Consultant Services Agreement between Neeta Wadekar and Citius Pharmaceuticals, Inc., dated September 1, 2014.
|
|
S-1/A
|
|
10/16/2015
|
|
10.7
|
|
|
|
10.8
|
|
Citius Pharmaceuticals, Inc. 2014 Stock Incentive Plan.
|
|
10-Q
|
|
8/15/2016
|
|
10.1
|
|
|
|
10.9
|
|
Form of Citius Pharmaceuticals, Inc. 2014 Stock Incentive Plan Nonqualified Stock Option.
|
|
10-Q
|
|
8/15/2016
|
|
10.2
|
|
|
|
10.10
|
|
Employment Agreement between Myron Holubiak and Citius Pharmaceuticals, Inc., executed March 30, 2016, effective March 1, 2016.
|
|
8-K
|
|
4/5/2016
|
|
10.2
|
|
|
|
10.11
|
|
Voting
Agreement among Citius Pharmaceuticals, Inc., Leonard Mazur and certain other stockholders of the Company, dated March 30,
2016.
|
|
8-K
|
|
4/5/2016
|
|
10.3
|
|
|
|
10.12
|
|
Form
of Unit Purchase Agreement, between each investor and Citius Pharmaceuticals, Inc., dated September 27, 2016.
|
|
10-Q
|
|
5/15/2017
|
|
10.5
|
|
|
|
10.13
|
|
Placement
Agency Agreement between Garden State Securities, Inc. and Citius Pharmaceuticals, Inc., dated September 27, 2016.
|
|
10-Q
|
|
5/15/2017
|
|
10.6
|
|
|
|
10.14
|
|
Amendment
to Placement Agency Agreement between Garden State Securities, Inc. and Citius Pharmaceuticals, Inc., dated November 23, 2016.
|
|
10-Q
|
|
5/15/2017
|
|
10.7
|
|
|
|
10.15
|
|
Second
Amendment to the Patent and Technology License Agreement between Novel Anti-Infective Technologies, LLC and Leonard-Meron
Biosciences, Inc., dated March 20, 2017.
|
|
10-Q
|
|
5/15/2017
|
|
10.8
|
|
|
|
10.16
|
|
Future
Advance Convertible Promissory Note between Leonard Mazur and Citius Pharmaceuticals, Inc., dated May 10, 2017.
|
|
10-Q
|
|
5/15/2017
|
|
10.1
|
|
|
|
10.17
|
|
Conversion
Agreement between Leonard Mazur and Citius Pharmaceuticals, Inc., dated May 10, 2017.
|
|
10-Q
|
|
5/15/2017
|
|
10.2
|
|
|
|
10.18
|
|
Amended
and Restated Demand Convertible Promissory Note between Leonard Mazur and Citius Pharmaceuticals, Inc., dated May 10, 2017.
|
|
10-Q
|
|
5/15/2017
|
|
10.3
|
|
|
|
10.19
|
|
Release Agreement between Garden State Securities, Inc. and Citius Pharmaceuticals, Inc., dated June 7, 2017.
|
|
8-K
|
|
6/13/2017
|
|
10.1
|
|
|
|
10.20
|
|
Form of Release Agreement between Citius Pharmaceuticals, Inc. and each investor, dated June 8, 2017.
|
|
8-K
|
|
6/13/2017
|
|
10.2
|
|
|
|
10.21
|
|
Warrant
Agent Agreement between VStock Transfer, LLC and Citius Pharmaceuticals, Inc., dated August 3, 2017.
|
|
8-K
|
|
8/4/2017
|
|
4.1
|
|
|
|
10.22
|
|
Amended and Restated Employment Agreement between Leonard Mazur and Citius Pharmaceuticals, Inc., dated October 19, 2017.
|
|
10-K
|
|
12/11/2018
|
|
10.23
|
|
|
|
10.23
|
|
Release Agreement between Aegis Capital Corp. and Citius Pharmaceuticals, Inc., dated November 7, 2017.
|
|
10-Q
|
|
2/14/2018
|
|
10.1
|
|
|
|
10.24
|
|
Employment Agreement between Jaime Bartushak and Citius Pharmaceuticals, Inc., dated November 27, 2017.
|
|
8-K
|
|
12/1/2017
|
|
10.1
|
|
|
|
10.25
|
|
Form
of Securities Purchase Agreement between Citius Pharmaceuticals, Inc. and the purchasers named therein, dated December 15,
2017.
|
|
8-K
|
|
12/19/2017
|
|
10.1
|
|
|
|
10.26
|
|
Engagement
Letter between H.C. Wainwright & Co., LLC and Citius Pharmaceuticals, Inc., dated December 15, 2017.
|
|
8-K
|
|
12/19/2017
|
|
10.2
|
|
|
|
10.27
|
|
Citius Pharmaceuticals, Inc. 2018 Omnibus Stock Incentive Plan
|
|
10-Q
|
|
2/14/2018
|
|
10.2
|
|
|
|
10.28
|
|
Form
of Securities Purchase Agreement between Citius Pharmaceuticals, Inc. and the purchasers named therein, dated March 28, 2018.
|
|
8-K
|
|
3/29/2018
|
|
10.1
|
|
|
|
10.29
|
|
Engagement
Letter between H.C. Wainwright & Co., LLC and Citius Pharmaceuticals, Inc., dated March 28, 2018.
|
|
8-K
|
|
3/29/2018
|
|
10.2
|
|
|
|
10.30
|
|
Patent and Technology License Agreement, dated January 2, 2019, between the Board of Regents of the University of Texas System on behalf of the University of Texas M. D. Anderson Cancer Center and Citius Pharmaceuticals, Inc.#
|
|
10-Q
|
|
2/14/2019
|
|
10.1
|
|
|
|
10.31
|
|
First Amendment, dated October 15, 2015, to Patent and Technology License Agreement, dated May 14, 2014, between Novel Anti-Infective Technologies, LLC and Leonard-Meron Biosciences, Inc.
|
|
10-Q
|
|
2/14/2019
|
|
10.2
|
|
|
|
Exhibit
Number
|
|
Description
of Document
|
|
Registrant’s
Form
|
|
Dated
|
|
Exhibit
Number
|
|
Filed
Herewith
|
|
10.32
|
|
Patent and Technology License Agreement, dated May 14, 2014, between Novel Anti-Infective Technologies, LLC and Leonard-Meron Biosciences, Inc. #
|
|
10-Q
|
|
2/14/2019
|
|
10.3
|
|
|
|
10.33
|
|
Form of Securities Purchase Agreement, dated April 1, 2019, by and between Citius Pharmaceuticals, Inc. and the purchasers named therein
|
|
8-K
|
|
4/3/2019
|
|
10.1
|
|
|
|
10.34
|
|
Engagement letter, dated March 22, 2019, between Citius Pharmaceuticals, Inc. and H.C. Wainwright & Co., LLC
|
|
8-K
|
|
4/3/2019
|
|
10.2
|
|
|
|
21
|
|
Subsidiaries
|
|
10-K
|
|
12/13/2017
|
|
21
|
|
|
|
23.1
|
|
Consent of Wolf & Company, P.C.
|
|
|
|
|
|
|
|
X
|
|
23.2*
|
|
Consent
of Wyrick Robbins Yates & Ponton LLP (included in Exhibit 5.1)
|
|
|
|
|
|
|
|
|
|
24.1
|
|
Power of Attorney (included on signature page)
|
|
|
|
|
|
|
|
X
|
*To
be filed by amendment.
#The
company has received confidential treatment of certain portions of this agreement. These portions have been omitted and filed
separately with the Securities and Exchange Commission pursuant to a confidential treatment request.
(b)
Financial statement schedule.
None.
Item
17. Undertakings.
(a)
The undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high
end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule
424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering
price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement;
provided,
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to
be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission
by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference
in the registration statement.
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall
be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at
that time shall be deemed to be the initial bona fide offering thereof.
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold
at the termination of the offering.
(6)
That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial
distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned
registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser,
if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant
will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i)
Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant
to Rule 424;
(ii)
Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred
to by the undersigned registrant;
(iii)
The portion of any other free writing prospectus relating to the offering containing material information about the undersigned
registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv)
Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
|
|
(b)
|
The
undersigned registrant hereby undertakes that, for purposes of determining any liability
under the Securities Act of 1933, each filing of the registrant’s annual report
pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and,
where applicable, each filing of an employee benefit plan’s annual report pursuant
to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference
in the registration statement shall be deemed to be a new registration statement relating
to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
|
|
|
(h)
|
Insofar
as indemnification for liabilities arising under the Act may be permitted to directors,
officers and controlling persons of the registrant pursuant to the foregoing provisions,
or otherwise, the registrant has been advised that in the opinion of the Securities and
Exchange Commission, or SEC, such indemnification is against public policy as expressed
in the Act and is, therefore, unenforceable. In the event that a claim for indemnification
against such liabilities (other than the payment by the registrant of expenses incurred
or paid by a director, officer or controlling person of the registrant in the successful
defense of any action, suit or proceeding) is asserted by such director, officer or controlling
person in connection with the securities being registered, the registrant will, unless
in the opinion of its counsel the matter has been settled by controlling precedent, submit
to a court of appropriate jurisdiction the question whether such indemnification by it
is against public policy as expressed in the Act and will be governed by the final adjudication
of such issue.
|
|
|
(i)
|
The
undersigned registrant hereby undertakes that:
|
(1)
For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus
filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant
pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement
as of the time it was declared effective.
(2)
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form
of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering
of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement on Form
S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Cranford, State of New Jersey, on
September 13, 2019.
|
|
CITIUS
PHARMACEUTICALS, INC.
|
|
|
|
|
|
|
By:
|
/s/
Myron Holubiak
|
|
|
Name:
|
Myron
Holubiak
|
|
|
Title:
|
Chief
Executive Officer
|
|
|
|
(Principal
Executive Officer)
|
POWER
OF ATTORNEY
KNOW
ALL BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Myron Holubiak and Leonard Mazur
as his true and lawful attorneys-in-fact and agents, each with the full power of substitution, for him and in his name, place
or stead, in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments),
and any subsequent registration statement filed by the registrant pursuant to Rule 462(b) of the Securities Act of 1933, as amended,
which relates to this registration statement, and to file the same, with exhibits thereto and other documents in connection therewith,
with the SEC, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each
and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as
he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their or his substitute
or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons
in the capacities and on the dates indicated.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/
Myron Holubiak
|
|
President
and Chief Executive Officer
|
|
September
13, 2019
|
|
Myron
Holubiak
|
|
(Principal
Executive Officer)
|
|
|
|
|
|
|
|
|
|
/s/
Jaime Bartushak
|
|
Chief
Financial Officer and Chief Accounting Officer
|
|
September
13, 2019
|
|
Jaime
Bartushak
|
|
(Principal
Financial Officer and Principal Accounting Officer)
|
|
|
|
|
|
|
|
|
|
/s/
Leonard Mazur
|
|
Executive
Chairman, Board of Directors
|
|
September
13, 2019
|
|
Leonard
Mazur
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Suren Dutia
|
|
Director
|
|
September
13, 2019
|
|
Suren
Dutia
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Carol Webb
|
|
Director
|
|
September
13, 2019
|
|
Carol
Webb
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Dr. William Kane
|
|
Director
|
|
September
13, 2019
|
|
Dr.
William Kane
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Howard Safir
|
|
Director
|
|
September
13, 2019
|
|
Howard
Safir
|
|
|
|
|
|
|
|
|
|
|
|
/s/
Dr. Eugene Holuka
|
|
Director
|
|
September
13, 2019
|
|
Dr.
Eugene Holuka
|
|
|
|
|
II-7
Citius Pharmaceuticals (NASDAQ:CTXR)
Historical Stock Chart
From Mar 2024 to Apr 2024
Citius Pharmaceuticals (NASDAQ:CTXR)
Historical Stock Chart
From Apr 2023 to Apr 2024