Leap Therapeutics Sees Positive Data for Trial of Cancer Treatment
September 13 2021 - 8:17AM
Dow Jones News
By Chris Wack
Leap Therapeutics Inc. said it saw positive initial data from
its first-line cohort of its Phase 2a clinical trial evaluating its
anti-Dickkopf-1 antibody, DKN-01, in combination with tislelizumab
and chemotherapy in patients with gastric or gastroesophageal
junction cancer.
The company said patients whose tumors had high levels of DKK1
expression showed the highest response rates.
The study is being conducted in the U.S. and South Korea.
Enrollment of part A has been completed with 25 first-line HER2-
G/GEJ cancer patients whose tumors express either high levels of
DKK1 or low levels of DKK1. Part B of the study will enroll up to
48 patients with second-line, DKK1-high G/GEJ cancer, the company
said.
Leap is conducting the combination study as part of an exclusive
option and license agreement with BeiGene Ltd. for the development
of DKN-01 in Asia, excluding Japan, Australia, and New Zealand.
The company said DKN-01 in combination with tislelizumab and
chemotherapy demonstrated compelling overall response rates as a
first-line treatment for advanced G/GEJ cancer. In the overall
intent to treat population, including those patients who didn't
receive a full cycle of therapy, the overall response rate was 60%,
with a 75% overall response rate in DKK1-high patients as compared
to a 56% ORR in DKK1-low patients
Leap Therapeutics shares were up 40% to $2.05 in premarket
trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
September 13, 2021 08:02 ET (12:02 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
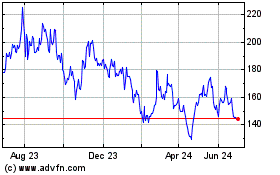
BeiGene (NASDAQ:BGNE)
Historical Stock Chart
From Mar 2024 to Apr 2024
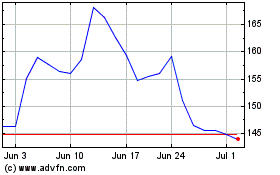
BeiGene (NASDAQ:BGNE)
Historical Stock Chart
From Apr 2023 to Apr 2024