Filed Pursuant to Rule 424(b)(4)
Registration Nos. 333-266223 and 333-266512

Applied DNA Sciences, Inc.
$12,000,000
3,000,000 Shares of Common Stock
and Accompanying Series A Warrants to Purchase 3,000,000 Shares of Common Stock and Series B Warrants to Purchase 3,000,000
Shares of Common Stock
or
3,000,000 Pre-Funded Warrants to Purchase 3,000,000 Shares of Common Stock and
Accompanying Series A Warrants to Purchase 3,000,000 Shares of Common Stock and Series B Warrants to Purchase 3,000,000 Shares of Common Stock
Shares of Common Stock Underlying the Pre-Funded
Warrants and Series Warrants
We are offering 3,000,000 shares of
our common stock together with Series A warrants to purchase 3,000,000 shares of our common stock (the “Series A Warrants”)
and Series B warrants to purchase 3,000,000 shares of our common stock (the “Series B Warrants” and, together with
the Series A Warrants, the “Series Warrants”) at a combined public offering price of 4.00 per share and Series Warrant (and
the shares of common stock that are issuable from time to time upon exercise of the Series Warrants) pursuant to this prospectus. The
shares of common stock and Series Warrants will be issued separately but must be purchased together and the Series Warrants will be issued
to purchasers in the ratio of one to one per share of common stock. The Series A Warrants will be exercisable beginning on the date of
issuance (the “Initial Exercise Date”), at an exercise price of $ 4.00 per share and will expire on the five-year anniversary
of the Initial Exercise Date. The Series B Warrants will be exercisable beginning on the Initial Exercise Date, at an exercise price of
$ 4.00 per share and will expire thirteen months from the Initial Exercise Date.
We are also offering to those purchasers, if any,
whose purchase of our common stock in this offering would otherwise result in such purchaser, together with its affiliates and certain
related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately
following the consummation of this offering, the opportunity, in lieu of purchasing common stock, to purchase 3,000,000 pre-funded
warrants to purchase shares of our common stock. Each pre-funded warrant is being issued together with the same Series Warrants described
above being issued with each share of common stock. The purchase price of each pre-funded warrant will equal the price per share at which
shares of our common stock are being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant
will equal $0.0001 per share of common stock. Each pre-funded warrant will be exercisable upon issuance and may be exercised at any time
until all of the pre-funded warrants are exercised in full. The pre-funded warrants and Series Warrants must be purchased together but
are immediately separable and will be issued separately in this offering. For each pre-funded warrant and accompanying Series Warrants
purchased in this offering in lieu of common stock, we will reduce the number of shares of common stock being sold in the offering by
one. Pursuant to this prospectus, we are also offering the shares of common stock issuable upon the exercise of the Series Warrants and
the pre-funded warrants.
Each pre-funded warrant is exercisable for one
share of our common stock (subject to adjustment as provided for therein) at any time at the option of the holder until such pre-funded
warrant is exercised in full, provided that the holder will be prohibited from exercising pre-funded warrants for shares of our common
stock if, as a result of such exercise, the holder, together with its affiliates, would own more than 4.99% of the total number of shares
of our common stock then issued and outstanding. However, any holder may increase such percentage to any other percentage not in excess
of 9.99%, provided that any increase in such percentage shall not be effective until 61 days after such notice to us.
We have engaged H.C. Wainwright & Co.,
LLC (the “Placement Agent”), to act as our exclusive placement agent in connection with the securities offered by this prospectus.
The Placement Agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus.
The Placement Agent is not purchasing or selling any of the securities we are offering, and the Placement Agent is not required to arrange
the purchase or sale of any specific number of securities or dollar amount.
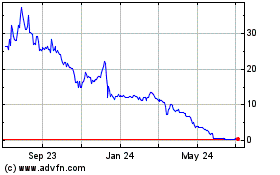
Our common stock is listed on The Nasdaq Capital
Market under the symbol “APDN.” The closing price of our common stock on August 3, 2022, as reported by The Nasdaq Capital
Market, was $4.10 per share.
There is no established public trading market for
the pre-funded warrants or Series Warrants, and we do not expect a market to develop. In addition, we do not intend to apply for the listing
of the pre-funded warrants or Series Warrants on any national securities exchange. Without an active trading market, the liquidity of
the Series Warrants and the pre-funded warrants will be limited.
We have agreed to pay the Placement Agent the Placement
Agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus. See “Plan
of Distribution” on page 39 of this prospectus for more information regarding these arrangements. There
is no minimum number of shares of common stock or pre-funded warrants or minimum aggregate amount of proceeds that is a condition for
this offering to close. We may sell fewer than all of the shares of common stock and
pre-funded warrants offered hereby, which may significantly reduce the amount of proceeds received by us, and investors in this
offering will not receive a refund if we do not sell all of the securities offered hereby. In addition, we have not specified a minimum
number of securities or amount of proceeds and we have not established an escrow account in connection with this offering. Because there
is no escrow account and no minimum number of securities or amount of proceeds, investors could be in a position where they have invested
in us, but we have not raised sufficient proceeds in this offering to adequately fund the intended uses of the proceeds as described in
this prospectus.
| |
|
Per (1)
Share and
Series Warrant |
|
|
Per Pre-Funded
Warrant and
Series Warrant |
|
|
Total |
|
| Public offering price |
|
$ |
4.00 |
|
|
$ |
3.9999 |
|
|
$ |
12,000,000 |
|
| Placement Agent fees(2) |
|
|
0.28 |
|
|
|
0.28 |
|
|
|
840,000 |
|
| Proceeds, before expenses, to us(3) |
|
$ |
3.72 |
|
|
$ |
3.72 |
|
|
$ |
11,160,000 |
|
(1) Based on a public offering price of $4.00 per
share of common stock.
(2) We have agreed to pay the Placement Agent a total
cash fee equal to 7.0% of the aggregate gross proceeds raised in this offering and to reimburse the Placement Agent for its legal fees
and expenses and other out-of-pocket expenses in an amount up to $50,000, and for its closing costs in an amount of up to $15,950. See
“Plan of Distribution” for a description of the compensation to be received by the Placement Agent.
(3) Because there is no minimum number of securities or
amount of proceeds required as a condition to closing in this offering, the actual public offering amount, Placement Agent fees, and proceeds
to us, if any, may be substantially less than the total maximum offering amounts set forth above. For
more information, see “Plan of Distribution.”
Investing
in our securities involves a high degree of risk. See “Risk Factors” beginning on page 11 of this prospectus and
elsewhere in this prospectus for a discussion of information that should be considered in connection with an investment in our securities.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus.
Any representation to the contrary is a criminal offense. The securities are not being offered in any jurisdiction where the offer is
not permitted.
Delivery of the securities offered hereby is expected
to be made on or about August 8, 2022, subject to satisfaction of certain customary closing conditions.
H.C. Wainwright &
Co.
The date of this Prospectus is August 4,
2022.
Table
of Contents
About
this Prospectus
The registration statement of which this prospectus
forms a part that we have filed with the Securities and Exchange Commission (the “SEC”) includes exhibits that provide more
detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together
with the additional information described under the headings “Where You Can Find More Information” and “Incorporation
by Reference” before making your investment decision.
You should rely only on the information provided
in or incorporated by reference in this prospectus, in any prospectus supplement or in a related free writing prospectus, or documents
to which we otherwise refer you. We have not authorized anyone else to provide you with different information.
We have not authorized any dealer, agent or other
person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus
and any accompanying prospectus supplement or any related free writing prospectus. You must not rely upon any information or representation
not contained or incorporated by reference in this prospectus or an accompanying prospectus supplement or any related free writing prospectus.
This prospectus and any accompanying prospectus supplement and any related free writing prospectus, if any, do not constitute an offer
to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus
and any accompanying prospectus supplement and any related free writing prospectus, if any, constitute an offer to sell or the solicitation
of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
You should not assume that the information contained in this prospectus and any accompanying prospectus supplement and any related free
writing prospectus, if any, is accurate on any date subsequent to the date set forth on the front of such document or that any information
we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though
this prospectus and any accompanying prospectus supplement and any related free writing prospectus is delivered or securities are sold
on a later date.
We have not done anything that would permit this
offering or possession or distribution of this prospectus or any free writing prospectus in any jurisdiction where action for that purpose
is required, other than in the United States. You are required to inform yourself about and to observe any restrictions relating as to
this offering and the distribution of this prospectus and any such free writing prospectus outside the United States.
We further note that the representations, warranties
and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference in this prospectus
were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among
the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations,
warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should
not be relied on as accurately representing the current state of our affairs.
You should also read and consider the information
in the documents to which we have referred you under the caption “Where You Can Find More Information” in this prospectus.
In addition, this prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference
is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents.
Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to
the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the
heading “Where You Can Find More Information.”
Unless
the context otherwise requires, references in this prospectus to “Applied DNA,” the “Company,” “we,”
“us” and “our” refer to Applied DNA Sciences, Inc., a Delaware corporation, and our subsidiaries. Our
trademarks currently used in the United States include Applied DNA Sciences®, SigNature® molecular tags, SigNature® T molecular
tags, fiberTyping®, DNAnet®, SigNify®, Beacon®, CertainT®, LinearDNA™, Linea™ COVID-19 Diagnostic Assay
Kit and safeCircleTM COVID-19
testing. Solely for convenience, trademarks and tradenames referred to in this prospectus may appear without the ® or ™
symbols, but such references are not intended to indicate in any way that we will not assert, to the fullest extent under applicable law,
our rights, or that the applicable owner will not assert its rights, to these trademarks and tradenames. We do not intend our use or display
of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
This prospectus contains and incorporates by reference
market data and industry statistics and forecasts that are based on our own internal estimates as well as independent industry publications
and other publicly-available information. Although we believe these sources are reliable, we do not guarantee the accuracy or completeness
of this information and we have not independently verified this information. Although we are not aware of any misstatements regarding
the market and industry data presented in this prospectus or the documents incorporated herein by reference, these estimates involve risks
and uncertainties and are subject to change based on various factors, including those discussed under the headings “Risk Factors”
in this prospectus, and under similar headings in the other documents that are incorporated herein by reference. Accordingly, investors
should not place undue reliance on this information.
Forward-Looking
Statements
This prospectus, the documents incorporated by
reference herein and any “free writing prospectus” we have authorized in connection with this offering contain “forward-looking
statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”)
and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are intended to qualify
for the “safe harbor” created by those sections. In addition, we may make forward-looking statements in other documents filed
with or furnished to the SEC, and our management and other representatives may make forward-looking statements orally or in writing to
analysts, investors, representatives of the media and others.
Forward-looking statements can generally be identified
by the fact that they do not relate strictly to historical or current facts and include, but are not limited to, statements using terminology
such as “can”, “may”, “could”, “should”, “assume”, “forecasts”,
“believe”, “designed to”, “will”, “expect”, “plan”, “anticipate”,
“estimate”, “potential”, “position”, “predicts”, “strategy”, “guidance”,
“intend”, “seek”, “budget”, “project” or “continue”, or the negative thereof
or other comparable terminology regarding beliefs, plans, expectations or intentions regarding the future. You should read statements
that contain these words carefully because they:
| • | discuss our future expectations; |
| • | contain projections of our future results of operations or of our financial condition; and |
| • | state other “forward-looking” information. |
We believe it is important to communicate our expectations.
Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus and incorporated by
reference into this prospectus, we caution you that these statements are based on our projections of the future that are subject to known
and unknown risks and uncertainties and other factors that may cause our actual results, level of activity, performance or achievements
expressed or implied by these forward-looking statements, to differ. Forward-looking statements involve risks and uncertainties and our
actual results and the timing of certain events could differ materially from those discussed in forward-looking statements as a result
of certain factors, including those set forth under “Risk Factors” and “Prospectus Summary – Our Company”
set forth in this prospectus and the documents incorporated herein by reference.
Accordingly, our actual results and the timing
of certain events may differ materially from those expressed or implied in such forward-looking statements due to a variety of factors
and risks, including, but not limited to, those set forth under “Risk Factors,” those set forth from time to time in our other
filings with the SEC, and the following factors and risks:
| • | our expectations of future revenues, expenditures, capital or other funding requirements; |
| • | the adequacy of our cash and working capital to fund present and planned operations and growth; |
| • | the substantial doubt relating to our ability to continue as a going concern; |
| • | our business strategy and the timing of our expansion plans; |
| • | demand for our Therapeutic DNA Production Services; |
| • | our expectations concerning existing or potential development and license agreements for third-party collaborations and joint ventures; |
| • | regulatory approval and compliance for our Therapeutic DNA Production Services; |
| • | the effect of governmental regulations generally; |
| • | our expectations of when regulatory submissions may be filed or when regulatory approvals may be received; |
| • | our expectations concerning product candidates for our technologies; and |
| • | our expectations of when or if we will become profitable. |
Any or all of our forward-looking statements may
turn out to be wrong. They may be affected by inaccurate assumptions that we might make or by known or unknown risks and uncertainties.
Actual outcomes and results may differ materially from what is expressed or implied in our forward-looking statements. Among the factors
that could affect future results are:
| • | the inherent uncertainties of product development based on our new and as yet not fully proven technologies; |
| • | the risks and uncertainties regarding the actual effect on humans of seemingly safe and efficacious formulations and treatments when
tested clinically; |
| • | the inherent uncertainties associated with clinical trials of product candidates; |
| • | the inherent uncertainties associated with the process of obtaining regulatory clearance or approval to market product candidates; |
| • | the inherent uncertainties associated with commercialization of products that have received regulatory clearance or approval; |
| • | economic and industry conditions generally and in our specific markets; |
| | | |
| | • | we may conduct a reverse stock split of our common stock to meet the
requirements of Nasdaq which may adversely impact the market price and liquidity of our common stock; |
| • | the volatility of, and decline in, our stock price; and |
| • | our current lack of financing for operations and our ability to obtain the necessary financing to fund our operations and effect our
strategic development plan. |
All forward-looking statements and risk factors
included in this prospectus are made as of the date hereof, or in the case of documents incorporated by reference, the original date of
any such documents, based on information available to us as of such date, and we assume no obligations to update any forward-looking statement
or risk factor, unless we are required to do so by law. If we do update one or more forward-looking statements, no inference should be
drawn that we will make updates with respect to other forward-looking statements or that we will make any further updates to those forward-looking
statements at any future time. All forward-looking statements are qualified in their entirety by this cautionary statement. Our forward-looking
statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may
make. You should read this prospectus and the documents that we have filed as exhibits to this prospectus and incorporated by reference
herein completely and with the understanding that our actual future results may be materially different from the plans, intentions and
expectations disclosed in the forward-looking statements we make.
Any of the assumptions underlying the forward-looking
statements contained in this prospectus could prove inaccurate and, therefore, we cannot assure you that the results or events contemplated
in any of such forward-looking statements will be realized. Based on the significant uncertainties inherent in these forward-looking statements,
the inclusion of any such statement should not be regarded as a representation or as a guarantee by us that our objectives or plans will
be achieved, and we caution you against relying on any of the forward-looking statements contained herein.
Prospectus
Summary
This
summary highlights certain information about us, this offering and in the documents we incorporate by reference in this prospectus. This
summary is not complete and does not contain all of the information that you should consider before investing in our securities. After
you carefully read this summary, to fully understand our Company and this offering and its consequences to you, you should read this
entire prospectus including the information referred to under the heading “Risk Factors” in this prospectus beginning on
page 11, as well as the other documents that we incorporate by reference into this prospectus, including our financial
statements and the notes to those financial statements, which are incorporated herein by reference from our Annual
Report on Form 10-K for the year ended September 30, 2021, filed on December 9, 2021, as
amended by Amendment No. 1 filed on December 14, 2021, as further amended
by Amendment No. 2 filed on January 28, 2022, our Quarterly
Report on Form 10-Q for the three month period ended December 31, 2021, filed on February 10, 2022, and our Quarterly
Report on Form 10-Q for the three and six- month periods ended March 31, 2022, filed on May 12, 2022. Please read
“Where You Can Find More Information” on page 41 of this prospectus.
Our Company
Business Overview
Applied DNA Sciences is a biotechnology company
developing technologies to produce and detect deoxyribonucleic acid (“DNA”). Using the polymerase chain reaction (“PCR”)
to enable both the production and detection of DNA, we operate in three primary business markets: (i) the manufacture of DNA for
use in nucleic acid-based therapeutics (“Therapeutic DNA Production Services”); (ii) the detection of DNA in molecular
diagnostics testing services (“MDx Testing Services”); and (iii) the manufacture and detection of DNA for industrial
supply chain security services (“DNA Tagging and Security Products and Services”).
Our growth strategy is to primarily focus our resources on the further
development, commercialization, and customer adoption of our Therapeutic DNA Production Services, including the expansion of our contract
development and manufacturing operation (“CDMO”) for the manufacture of DNA for nucleic acid-based therapies and the development
of our own product candidates in veterinary health. To offset these development costs, we plan to leverage our MDx Testing Services and
our DNA Tagging and Security Products and Services business to generate cashflows.
Therapeutic DNA Production Services
Through our LinearRx, Inc. (“LRx”)
subsidiary we are developing and commercializing the LinearDNA (“linDNA”) platform. The linDNA platform enables the rapid,
efficient, and large-scale cell-free manufacture of high-fidelity DNA sequences for use in nucleic acid-based therapeutics. The linDNA
platform enzymatically produces a linear form of DNA we call ‘linDNA’ that is an alternative to plasmid-based DNA manufacturing
technologies that have supplied the DNA used in biotherapeutics for the past 40 years.
We believe our enzymatic linDNA platform has numerous
advantages over existing cell-based plasmid DNA manufacturing platforms. Plasmid-based DNA manufacturing is based on the complex, costly
and time-consuming biological process of amplifying DNA in living cells. Once amplified, the DNA must be separated from the living cells
and other process contaminants via multiple rounds of purification, adding further complexity and costs. Unlike plasmid-based DNA manufacturing,
the linDNA platform does not require living cells and instead amplifies DNA via the enzymatic process of PCR. The linDNA platform is
simple, with only four ingredient inputs, and can rapidly produce very large quantities of DNA without the need for complex purification
steps.
We believe the key advantages of the linDNA platform
include:
| • | Speed –
Production of linDNA can be measured in terms of hours, not days and weeks as is the
case with plasmid-based DNA manufacturing platforms. |
| • | Scalability –
linDNA production takes place on efficient bench-top instruments, allowing for rapid scalability
in a minimal footprint. |
| • | Purity –
DNA produced via PCR is pure, resulting in only large quantities of only the target DNA sequence.
Unwanted DNA sequences such as plasmid backbone and antibiotic resistance genes, inherent
to plasmid DNA, are not present in linDNA. |
| • | Simplicity
– The production of linDNA is streamlined relative to plasmid-based DNA production.
linDNA requires only four ingredients, does not require living cells or complex fermentation
systems and does not require multiple rounds of purification. |
| • | Flexibility –
DNA produced via the linDNA platform can be easily chemically modified to suit specific customer
applications. In addition, the linDNA platform can produce a wide range of complex DNA sequences
that are difficult to produce via plasmid-based DNA production platforms. These complex sequences
include inverted terminal repeats (ITRs) and polyadenylation sequences (poly (A) tail)
important to gene therapy and mRNA therapies, respectively. |
Preclinical studies have shown that linDNA is
substitutable for plasmid DNA in numerous nucleic acid-based therapies, including:
| • | therapeutic
and prophylactic DNA vaccines; |
| • | DNA
templates for in vitro transcription to produce ribonucleic acid (“RNA”),
including messenger RNA (“mRNA”); and |
| • | adoptive
cell therapy manufacturing. |
Further, we believe that linDNA is also substitutable
for plasmid DNA in the following nucleic acid-based therapies:
| • | viral
vector manufacturing for in vivo and ex vivo gene editing; |
| • | CRISPR-mediated
homology-directed repair (“HDR”); and |
As of the fourth quarter of calendar 2021,
there were 3,483 gene, cell and RNA therapies in development from preclinical through pre-registration stages, almost all of which
use DNA in their manufacturing process. (Source: ASGCT Gene, Cell & RNA Therapy Landscape: Q4 2021 Quarterly Report). Due
to what we believe are the linDNA platform’s numerous advantages over legacy plasmid-based DNA manufacturing platforms, we
believe this large number of therapies under development represents a substantial market opportunity for linDNA to supplant plasmid
DNA in the manufacture of nucleic acid-based therapies.
Our linDNA is currently manufactured pursuant
to Good Laboratory Practices (“GLP”) that we believe are sufficient for pre-clinical discovery and development of
nucleic acid-based therapies. In addition, for indirect clinical use of linDNA (i.e., where linDNA is a starting material but is not
incorporated into the final therapeutic product, as is the case with the production of mRNA or certain viral vectors), we believe
that high-quality grade GLP linDNA is sufficient for clinical and commercial stage customers of our Therapeutic DNA Production
Services. For the direct clinical use of our linDNA (i.e., nucleic acid-based therapies where our linDNA is incorporated into the
final therapeutic product, as in the production of DNA vaccines, adoptive cell therapies and certain gene therapies) we believe
clinical and commercial stage customers of our Therapeutic DNA Production Services will generally require our manufacturing
facilities to meet current Good Manufacturing Practices (“cGMP”). We currently do not have any manufacturing facilities
that meet cGMP. We will need to develop and maintain manufacturing facilities that meet cGMP to support customers that wish to use
our linDNA for direct clinical use. In the longer term, we believe that the development and maintenance of a cGMP manufacturing
facility for linDNA will benefit the entirety of our Therapeutic DNA Production Services business, in both direct and indirect
clinical applications.
Our business strategy for the linDNA platform
is (i) to utilize our current GLP linDNA production capacity to secure CDMO contracts to supply linDNA to pre-clinical therapy
developers, as well as clinical and commercial therapy developers and manufacturers that are pursuing therapeutics that require the
indirect clinical use of linDNA; and (ii) upon our development of cGMP linDNA production facilities, to secure CDMO contracts
with clinical stage therapy developers and commercial manufactures to supply linDNA for direct clinical use.
In addition, we plan to leverage our
Therapeutic DNA Production Services and deep knowledge of PCR to develop and monetize, ourselves or with strategic partners, one or
more linDNA-based therapeutic or prophylactic vaccines for the veterinary health market. Currently, we have in-licensed a
therapeutic DNA vaccine candidate against canine lymphoma, which accounts for up to 24% of all cancers in canines. Our lymphoma
vaccine candidate has been licensed from Takis S.R.L and EvviVax, S.R.L. for exclusive use by the Company in association with our
linDNA platform, and is subject to certain commercialization milestones. We believe the linDNA platform provides a
substantial advantage to the development and monetization of a therapeutic DNA vaccine against canine lymphoma.
MDx Testing Services
Through Applied DNA Clinical Labs, LLC
(“ADCL”), our clinical laboratory subsidiary, we leverage our expertise in DNA detection via PCR to provide and develop
clinical molecular diagnostics (“MDx”) testing services. ADCL is a New York State Department of Health (“NYSDOH”) Clinical
Laboratory Evaluation Program (“CLEP”) permitted, Clinical Laboratory Improvement Amendments
(“CLIA”)-certified laboratory which is currently permitted for virology. In providing MDx testing services, ADCL employs
its own or third-party molecular diagnostic tests.
Under our MDx testing services, ADCL provides COVID-19 testing for
large populations marketed under our safeCircleTM trademark. Leveraging ADCL’s customizable high-throughput robotic pooled
testing workflow and the Cleared4 digital health platform owned and operated by Cleared4 Inc. (the “Cleared4 Platform”), our
safeCircle testing service is an adaptable turnkey large population COVID-19 testing solution that provides for all aspects of COVID-19
testing, including test scheduling, sample collection and automated results reporting. Our safeCircle testing service utilizes high-sensitivity
robotically pooled real-time PCR (“RT-PCR”) testing to help prevent virus spread by quickly identifying COVID-19 infections within a community,
school, or workplace. Our safeCircle COVID-19 testing is performed using either the Company’s internally developed Linea 2.0 RT-PCR
Assay, a NYSDOH conditionally approved laboratory developed test (“LDT”) or third-party emergency use authorization (“EUA”)-authorized
RT-PCR COVID-19 assays. Our safeCircle testing service also incorporates the Cleared4 Platform to enable large-scale digital test scheduling,
in-field sample collection and registration, and results reporting. By leveraging the combination of our robotic pooled workflows and
the Cleared4 Platform, our safeCircle testing services typically return testing results within 24 to 48 hours. We provide safeCircle testing
services to primary/secondary/higher education institutions, private clients, and businesses and college athletic programs primarily located
in New York State.
In addition to our safeCircle testing
services, we are currently developing and validating pharmacogenetics (“PGx”) testing services. Our PGx testing services will utilize
a 120-target PGx panel test to evaluate the unique genotype of a specific patient to help guide individual drug therapy decisions.
Our PGx testing services are designed to interrogate DNA targets on over 35 genes and provide genotyping information relevant to
certain cardiac, mental health and pain management drug therapies. We believe the economics of complex MDx testing services such as
PGx are more favorable to the Company as compared to high volume, low complexity MDx tests such as COVID-19 testing. Our PGx testing
services will require NYSDOH approval prior to initiating our patient testing services. If approved, we plan to commercialize our
PGx testing services by offering PGx clinical reference laboratory testing services to other clinical laboratories and healthcare
facilities nationwide.
Going forward, our business strategy for ADCL
is to leverage our deep knowledge of PCR to develop and commercialize high complexity, high value and differentiated MDx testing services
that will be offered to other clinical laboratories and healthcare facilities as clinical reference laboratory testing services. We believe
operating as a clinical reference laboratory has several advantages when compared to operating as a typical clinical non-reference laboratory,
including:
| • | the ability to leverage our deep expertise in PCR to develop and perform
high-value esoteric MDx testing services not performed by conventional clinical non-reference laboratories; |
| • | reduced
sample acquisition costs; |
| • | reduced
marketing costs; and |
| • | a national customer base that may lead to a larger total addressable market. |
The clinical reference laboratory services market
is forecasted to have incremental growth of $26.0B between 2020 and 2025 with a 6.71% compound annual growth rate (“CAGR”).
We believe that the rapidly increasing number of specialized MDx tests for early disease detection, disease prognosis, disease risk, companion
diagnostics and personalized medicine will drive an increase in the demand for highly specialized MDx clinical reference laboratory services.
DNA Tagging and Security Products and Services
By leveraging our expertise in both the manufacture
and detection of DNA via PCR, our DNA Tagging and Security Products and Services allow our customers to use non-biologic DNA tags manufactured
on our linDNA platform to mark objects in a unique manner and then identify these objects by detecting the absence or presence of the
DNA tag. We believe our DNA tags are not economically feasible nor practical to replicate, and that our disruptive tracking platform
offers broad commercial relevance across many industry verticals. The Company’s core DNA Tagging and Security Products and Services,
which are marketed collectively as a platform under the trademark CertainT®, include:
| • | SigNature®
Molecular Tags, which are short non-biologic DNA taggants produced by the Company’s
linDNA platform, provide a methodology to authenticate goods within large and complex supply
chains for materials such as cotton, leather, pharmaceuticals, nutraceuticals and other products. |
| • | SigNify®
IF portable DNA readers and SigNify consumable reagent test kits provide definitive real-time
authentication of the Company’s DNA tags in the field, providing a front-line solution
for supply chain integrity backed with forensic-level molecular tag authentication. The Company’s
software platform enables customers to track materials throughout a supply chain or product
life. |
| • | fiberTyping®,
which uses PCR-based DNA detection to determine a cotton cultivar, and other product genotyping
services that utilize PCR-based DNA detection to detect a product’s naturally occurring
DNA sequences for the purposes of product provenance authentication and supply chain security. |
Our DNA Tagging and Security Products and
Services are fully developed, highly scalable, and currently used in several commercial applications. To date, our largest
commercial application for our DNA Tagging and Security Products and Services is in the tracking and provenance authentication of
cotton. Cotton home textile products utilizing our DNA Tagging and Security Products and Services are available in national
retail chains including Costco® and Bed Bath & Beyond®.
We believe that the Uyghur Forced Labor Prevention
Act (“UFLPA”), signed into law on December 23, 2021, may be helpful to increase demand for our DNA Tagging and Security
Products and Services. The UFLPA establishes a rebuttable presumption that any goods mined, produced, or manufactured wholly or in part
in the Xinjiang Uyghur Autonomous Region (“XUAR”) of the People’s Republic of China are not entitled to entry to the
United States. The presumption applies unless the importer of record has complied with specified conditions and, by clear and convincing
evidence, shown that the goods were not produced using forced labor. On June 17, 2022, an implementation strategy for the UFLPA
was published that listed DNA tagging as evidence that importers may present to potentially prove that a good did not originate in XUAR
or did not benefit from forced labor. Approximately 20% of the world’s cotton garments contain cotton that originated in the XUAR.
Our business plan is to leverage growing consumer
demand for product traceability and the newly enacted UFLPA to expand our existing partnerships and seek new partnerships for our DNA
Tagging and Security Products and Services with a focus on cotton and synthetic fibers.
Intellectual Property
The proprietary nature
of and protection for our various technologies and know-how are important to our business. Our success depends in part on our ability
to protect the proprietary nature of our technologies and know-how, to operate without infringing on the proprietary rights of others
and to prevent others from infringing our proprietary rights. We seek and maintain patent protection in the United States and internationally
for our various technologies associated with our three primary business markets. We endeavor to patent or in-license technology, inventions
and improvements that we consider important to the development of our business. We also rely on trade secrets, know-how and continuing
innovation to develop and maintain our competitive position.
Because the development
of our Therapeutic DNA Production Services and MDx Testing Services businesses are at an early stage, our intellectual property portfolio
with respect to certain technologies associated with these businesses are also at an early stage. As further described below, we have
filed or intend to file patent applications on certain technologies associated with these business markets, and as we continue the development
of our technologies, we intend to identify additional means of obtaining patent protection that would potentially enhance commercial
success.
We cannot be certain
that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed
by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially
useful in protecting our technology. Any of our intellectual property and proprietary rights could be challenged, invalidated, circumvented,
infringed or misappropriated, or such intellectual property and proprietary rights may not be sufficient to permit us to take advantage
of current market trends or otherwise to provide competitive advantages. For more information, see “Risk Factors — Risks
Related to Our Intellectual Property.”
As of July 1, 2022,
our patent portfolio included the following issued and pending patent applications applicable to each of our three primary business markets:
| • | Therapeutic
DNA Production Services |
| o | 5
issued patents and 10 pending patent applications in the United States |
| o | 11
issued foreign patents and 5 pending foreign patent applications |
| o | 5
issued patents and 1 pending patent applications in the United States |
| o | 4
issued foreign patents and 1 pending foreign patent applications |
| • | DNA
Tagging and Security Products and Services |
| o | 28
issued patents and 5 pending patent applications in the United States |
| o | 47
issued foreign patents and 14 pending foreign patent applications |
In addition to patent
protection, we also rely on trade secrets, know how, other proprietary information and continuing technological innovation to develop
and maintain our competitive position. In our Therapeutic DNA Production Services, we currently rely heavily on trade secret protection.
We seek to protect and maintain the confidentiality of proprietary information to protect aspects of our business that are not amenable
to, or that we do not consider appropriate for, patent protection. Although we take steps to protect our proprietary information and
trade secrets, including through contractual means with our employees and consultants, third parties may independently develop substantially
equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. Thus, we
may not be able to meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific
collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or
consulting relationships with us. These agreements provide that all confidential information concerning our business or financial affairs
developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential
and not disclosed to third parties except in specific circumstances. Our agreements with employees also provide that all inventions conceived
by the employee in the course of employment with us or from the employee’s use of our confidential information are our exclusive
property. However, such confidentiality agreements and invention assignment agreements can be breached and we may not have adequate remedies
for any such breach. For more information regarding the risks related to our intellectual property, see “Risk Factors — Risks
Related to Our Intellectual Property.”
The patent positions of biotechnology companies like ours are generally uncertain and involve complex legal, scientific and factual questions.
Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. It is uncertain whether
the issuance of any third party patent would require us to alter our development or commercial strategies, or our manufacturing processes,
obtain licenses or cease certain activities. Our breach of any license agreements or our failure to obtain a license to proprietary rights
required to develop or commercialize our future products or services may have a material adverse impact on us. If third parties prepare
and file patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference
or derivation proceedings in the United States Patent and Trademark Office, or USPTO, to determine priority of invention. For more information,
see “Risk Factors — Risks Related to Our Intellectual Property.”
Risk Factor Summary
This summary does not address all of the risks
that we face. Additional discussions of the risks summarized in this risk factor summary, and other risks that we face, can be found
below and should be carefully considered, together with other information in this prospectus before making investment decisions.
| • | There
is substantial doubt relating to our ability to continue as a going concern. |
| • | We
have produced limited revenues. This makes it difficult to evaluate our future prospects
and increases the risk that we will not be successful. |
| • | Our
new emphasis on Therapeutic DNA Production Services and MDx Testing Services may reduce our
ability to maintain and expand our existing DNA Tagging and Security Products and Services
businesses. |
| • | We
may encounter difficulties in managing our growth, and these difficulties could impair our
profitability. |
| • | If
we are unable to expand our DNA manufacturing capacity, we could lose revenue and our business
could suffer. |
| • | Rapidly
changing technology and extensive competition in synthetic biology could make the services
or products we are developing obsolete or non-competitive unless we continue to develop new
and improved services or products and pursue new market opportunities. |
| • | Our
operating results could be adversely affected by a reduction in business with our significant
customers. |
| • | Pharmaceutical
and biologic products are highly complex, and if we or our collaborators and customers are
unable to provide quality and timely offerings to our respective customers, our business
could suffer. |
| • | Pharmaceutical
and biologic-related revenue will be dependent on our collaborators’ and customers’
demand for our manufacturing services. |
| • | Our
safeCircleTM COVID-19 testing service could become obsolete or its utility could
be significantly diminished. |
| • | We
may be unable to consistently manufacture or source our products to the necessary specifications
or in quantities necessary to meet demand on a timely basis and at acceptable performance
and cost levels. |
| • | We
will need to develop and maintain manufacturing facilities that meet current Good Manufacturing
Practices. |
| • | If
we fail to successfully identify, finance and develop our linDNA platform, our commercial
opportunities in pharmaceuticals and biologics may be limited. |
| • | The
markets for our drug and biologic candidates and synthetic DNA are very competitive, and
we may be unable to continue to compete effectively in these industries in the future. |
| • | The
markets for our supply chain security and product authentication solutions are very competitive,
and we may be unable to continue to compete effectively in these industries in the future. |
| • | We
compete with life science, pharmaceutical and biotechnology companies, some of whom are our
customers, who are substantially larger than we are and potentially capable of developing
new approaches that could make our products and technology obsolete or develop their own
internal capabilities that compete with our products. |
| • | Our
intellectual property rights are valuable, and any inability to protect them could reduce
the value of our products, services and brand. |
| • | Pharmaceutical
and biologic-related revenue is generally dependent on regulatory approval, oversight and
compliance. |
| • | Our
product candidates or the product candidates of our collaborators or customers may cause
undesirable side effects or have other properties that could halt their clinical development,
prevent their regulatory approval, limit their commercial potential, or result in significant
negative consequences. |
| • | If
the FDA were to begin to enforce regulation of LDTs, we could incur substantial costs and
delays associated with trying to obtain pre-market clearance or approval and costs associated
with complying with post-market requirements. |
| • | If
we fail to comply with laboratory licensing requirements, we could lose the ability to offer
our clinical testing services or experience disruptions to our business. |
| • | If
we fail to comply with healthcare laws, we could face substantial penalties and our business,
operations and financial conditions could be adversely affected. |
| • | If
we are unable to continue to retain the services of Dr. Hayward, we may not be able
to continue our operations. |
| • | There
are a large number of shares of common stock underlying our outstanding options and warrants
and the sale of these shares may depress the market price of our common stock and cause immediate
and substantial dilution to our existing stockholders. |
| • | If
we fail to comply with the continuing listing standards of Nasdaq, our securities could be
delisted, which could limit investors’ ability to make transactions in our common stock
and subject us to additional trading restrictions. |
| • | If
we are unable to obtain additional financing our business operations may be harmed or discontinued. |
| • | Management
will have broad discretion as to the use of proceeds from this offering and we may use the
net proceeds in ways with which you may disagree. |
| • | The
public offering price will be set by our board of directors and does not necessarily indicate
the actual or market value of our common stock. |
| • | If
you purchase the common stock or pre-funded warrants sold in this offering, you will experience
immediate dilution as a result of this offering and future equity issuances. |
| |
• |
There is no public market
for the pre-funded warrants or Series Warrants being offered in this offering. |
| |
• |
Holders of pre-funded warrants
or Series Warrants purchased in this offering will have no rights as common stockholders until such holders exercise their pre-funded
warrants or Series Warrants and acquire our common stock. |
| |
• |
The sale of our common stock
and the Series Warrants in this offering could result in the reset of the exercise price of certain outstanding warrants. |
| | • | We may have conflicts of interest with our affiliates and related parties,
and in the past we have engaged in transactions and entered into agreements with affiliates that were not negotiated at arms’ length. |
Corporate History
We are a Delaware corporation, which was initially
formed in 1983 under the laws of the State of Florida as Datalink Systems, Inc. In 1998, we reincorporated in the State of Nevada,
and in 2002, we changed our name to our current name, Applied DNA Sciences, Inc. On December 17, 2008, we reincorporated from
the State of Nevada to the State of Delaware.
Our corporate headquarters are located at the
Long Island High Technology Incubator at Stony Brook University in Stony Brook, New York, where we have established laboratories for
the manufacture and detection of DNA to support our various business units. In addition, this location also houses our NYSDOH CLEP-permitted,
CLIA-certified clinical laboratory where we perform MDx testing. The mailing address of our corporate headquarters is 50 Health Sciences
Drive, Stony Brook, New York 11790, and our telephone number is (631) 240-8800.
To date, we have produced limited recurring revenues
from our products and services, have incurred substantial expenses and have sustained significant losses. Moreover, we have concluded
that there is substantial doubt as to our ability to continue as a going concern and our auditors have included an explanatory paragraph
to that effect in their report for the year ended September 30, 2021. Consequently, our operations are subject to all the risks
inherent in the establishment and development of a biotechnology company.
Recent Developments
Preliminary Financial Information
The Company expects
estimated revenues for the third quarter of fiscal year 2022 ended June 30, 2022, to be in the range of $4.0 million to $4.3 million
compared to revenues of approximately $1.7 million in the third quarter of fiscal year 2021. For the nine-month period ended June 30,
2022, we expect estimated revenues to be in the range of $14.3 to $14.6 million compared to revenues of approximately $6.0 million for
the first nine months of fiscal year 2021. We estimate our loss from operations for the three-month period ended June 30, 2022 to be
in the range of $2.8 million to $3.1 million, compared to a loss from operations of $3.4 million for the three-month period ended June
30, 2021. We estimate our loss from operations for the nine-month period ended June 30, 2022 to be in the range of $9.6 million to $9.9
million, compared to a loss from operations of $8.8 million for the nine-month period ended June 30, 2021. Our cash balance as of June
30, 2022 was approximately $4.7 million.
The preliminary financial
information included in this prospectus reflects management’s estimates based solely upon information available to us as of the
date of this prospectus and is the responsibility of management. The preliminary financial results presented above are not a comprehensive
statement of our financial results for the quarter ended June 30, 2022 and have not been audited, reviewed or compiled by our independent
registered public accounting firm, Marcum, LLP. Accordingly, Marcum, LLP does not express an opinion and assumes no responsibility for
and disclaims any association with such preliminary financial results. The preliminary financial results presented above are subject
to the completion of our financial closing procedures, which have not yet been completed. Our actual results for the quarter ended June 30,
2022 will not be available until after this offering is completed and may differ from these estimates. Accordingly, you should not place
undue reliance upon these preliminary financial results. For example, during the course of the preparation of the respective financial
statements and related notes, additional items that would require material adjustments to be made to the preliminary estimated financial
results presented above may be identified. There can be no assurance that these estimates will be realized, and estimates are subject
to risks and uncertainties, many of which are not within our control. See “Risk Factors” and “Special Note Regarding
Forward-Looking Statements.” The Company expects to issue full financial results for the third fiscal quarter of 2022 in mid-August.
Successful Expression of linDNA Encapsulated
by Lipid Nanoparticles (“LNPs”)
The
Company, via its LRx subsidiary, has recently demonstrated the successful expression of linDNA encapsulated by LNPs in vitro and
in in vivo (mice studies). The LNP-encapsulated linDNA encoded generic reporter proteins. For the mice studies, successful expression
of the LNP-encapsulated linDNA was achieved via intramuscular (“IM”) injection administered without the use of concurrent
electroporation. This is the Company’s first successful animal study using LNP-mediated IM delivery, and its first successful animal
study to achieve in vivo expression without the use of concurrent electroporation.
Development of Monkeypox MDx Testing
Services
Via its ADCL subsidiary,
the Company is developing PCR-based MDx testing services for the Monkeypox virus. The testing services are being developed as a NYSDOH
LDT. Analytical validation of our Monkeypox testing services are currently underway. Once validation is complete, the Company will submit
its validation data to NYSDOH. Our Monkeypox testing services will require NYSDOH approval prior to initiating our testing services.
Available Information
Because we are subject to the information and
reporting requirements of the Exchange Act, we file or furnish, as applicable, annual, quarterly and current reports, proxy statements
and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements, and other information
regarding issuers that file electronically with the SEC. The address of that website is www.sec.gov. We make available on our website
at www.adnas.com, free of charge, copies of these reports, as soon as reasonably practicable after we electronically file such material
with, or furnish it to, the SEC. The information in or accessible through the websites referred to above are not incorporated into, and
are not considered part of, this prospectus. Further, our references to the URLs for these websites are intended to be inactive textual
references only.
Summary
of the Offering
| Common stock
to be offered |
|
3,000,000 shares of common stock on a “best efforts” basis. |
| |
|
|
| Pre-funded warrants
offered by us in this offering |
|
We are also offering
to each purchaser whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with
its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of
our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if the purchaser
so chooses, pre-funded warrants, in lieu of shares of common stock that would otherwise result in the purchaser’s beneficial
ownership exceeding 4.99% of our outstanding common stock. Subject to limited exceptions, a holder of pre-funded warrants will not
have the right to exercise any portion of its pre-funded warrants if the holder, together with its affiliates, would beneficially
own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of common stock outstanding immediately
after giving effect to such exercise. Each pre-funded warrant will be exercisable for one share of our common stock. The purchase
price of each pre-funded warrant will equal the price per share at which the shares of common stock are being sold to the public
in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. This offering also
relates to the shares of common stock issuable upon exercise of any pre-funded warrants sold in this offering. For each pre-funded
warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. |
| |
|
|
| Description of Series Warrants |
|
We are issuing to purchasers of shares of our common stock and/or pre-funded
warrants in this offering a Series A Warrant to purchase 1 share of our common stock and a Series B Warrant to purchase 1 share of
our common stock for each share and/or pre-funded warrant purchased in this offering for a combined purchase price of 4.00. The Series
A Warrants and the Series B Warrants are referred to herein together as the “Series Warrants”. Because a Series Warrant
to purchase share(s) of our common stock is being sold together in this offering with each share of common stock and, in the alternative,
each pre-funded warrant to purchase one share of common stock, the number of Series Warrants sold in this offering will not change as
a result of a change in the mix of the shares of our common stock and pre-funded warrants sold. Each Series A Warrant will have an
exercise price of $4.00 per share, will be exercisable upon issuance and will expire five years from the date of issuance. Each Series
B Warrant will have an exercise price of $4.00 per share, will be exercisable upon issuance and will expire thirteen months from the date
of issuance. No fractional shares of common stock will be issued in connection with the exercise of a Series Warrant. In lieu of fractional
shares, we will round up to the next whole share. See “Description of Securities — Series Warrants.” This prospectus
also relates to the offering of the shares of common stock issuable upon exercise of the Series Warrants. |
| Common stock outstanding prior to this offering |
|
8,982,520 shares. |
| |
|
|
| Common stock to be outstanding after this offering |
|
11,982,520 shares (assuming no sale of any pre-funded warrants and assuming none of the Series Warrants issued in this
offering are exercised). |
| Use of proceeds |
|
We estimate that the net proceeds to us from this offering will be approximately $10,900,696, after deducting the Placement Agent fees and estimated offering expenses payable by us and assuming no exercise of the Series Warrants. We intend to use the net proceeds from the sale of the securities for the further development of our Therapeutic DNA Production and MDx Testing Services, as well as general corporate purposes, which may include research and development expenses, capital expenditures, working capital and general and administrative expenses, and potential acquisitions of or investments in businesses, products and technologies that complement our business, although we have no present commitments or agreements to make any such acquisitions or investments as of the date of this prospectus. Pending these uses, we intend to invest the funds in short-term, investment grade, interest-bearing securities. It is possible that, pending their use, we may invest the net proceeds in a way that does not yield a favorable, or any, return for us. See “Use of Proceeds” on page 28 of this prospectus. |
| |
|
|
| Risk factors |
|
You should carefully read and consider the information set forth under “Risk Factors” on page 11 of this prospectus and the documents incorporated by reference herein before deciding to invest in our securities. |
| |
|
|
| Lock-up agreements |
|
We and all of our
executive officers and directors have entered into lock-up agreements with the Placement Agent. Under these agreements, we and
each of these persons may not, without the prior written approval of the Placement Agent, offer, sell, contract to sell or otherwise
dispose of or hedge common stock or securities convertible into or exchangeable for common stock, subject to certain exceptions. The
restrictions contained in these agreements will be in effect for a period of 90 days after the date of the closing of this offering.
For more information, see “Plan of Distribution” on page 39 of this prospectus. |
| |
|
|
| Market for common stock |
|
Our common stock is listed on The Nasdaq Capital Market under the symbol “APDN.” |
| |
|
|
| Listing of pre-funded warrants and Series Warrants |
|
We do not intend to list the pre-funded warrants or the Series Warrants on any securities exchange or nationally recognized trading system. Without a trading market, the liquidity of the pre-funded warrants and Series Warrants will be extremely limited. |
The discussion and tables above are based on 8,982,520
shares of our common stock outstanding as of July 27, 2022, which excludes shares of our common stock that may be issued upon exercise
of pre-funded warrants and Series Warrants issued in this offering, 1,063,143 shares of common stock issuable upon exercise of outstanding
options, 2,239,963 shares of common stock issuable upon exercise of outstanding warrants, 2,766,248 shares available for grant under our
Equity Incentive Stock Plans, as of such date, and shares of common stock initially issuable upon the exercise of the Series Warrants
to be issued pursuant to this prospectus.
Risk
Factors
Investment
in our securities, including our common stock, Series Warrants, and pre-funded warrants, involves a high degree of risk. In addition
to the risks and investment considerations discussed elsewhere in this prospectus, any document incorporated by reference herein or any
“free writing prospectus” we have authorized in connection with this offering, the following factors should be carefully
considered by anyone purchasing the securities offered by this prospectus. The risks and uncertainties described below are not the only
ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business
operations. We also update risk factors from time to time in our periodic reports on Forms
10-K, 10-Q
and 8-K
which will be incorporated by reference in this prospectus. If any of the following risks actually occur, our business could be harmed.
In such case, the trading price of our common stock could decline and investors could lose all or a part of their investment. All of
these risks could adversely affect our business, business prospects, results of operations, financial condition and cash flows.
See also the statements contained under the
heading “Forward-Looking Statements.”
Risks Related to Our Business:
There is substantial doubt relating to our ability to continue
as a going concern.
We have recurring net losses, which have resulted
in an accumulated deficit of $290,712,648 as of March 31, 2022 and $284,122,092 as of September 30, 2021. We have incurred
a net loss of $14,278,439 for the fiscal year ended September 30, 2021 and $6,480,708 for the six-month period ended March 31,
2022. At March 31, 2022 and September 30, 2021, we had cash and cash equivalents of $6,512,784 and $6,554,948, respectively.
We have concluded that these factors raise substantial doubt about our ability to continue as a going concern for one year from the issuance
of the financial statements.
In addition, the report from our independent registered
public accounting firm for the year ended September 30, 2021 includes an explanatory paragraph stating that our significant losses
and need to raise additional funds to meet our obligations and sustain operations raise substantial doubt about our ability to continue
as a going concern. We will continue to seek to raise additional working capital through public equity, private equity or debt financings.
If we fail to raise additional working capital, or do so on commercially unfavorable terms, it would materially and adversely affect
our business, prospects, financial condition and results of operations, and we may be unable to continue as a going concern. Future reports
from our independent registered public accounting firm may also contain statements expressing substantial doubt about our ability to
continue as a going concern. If we seek additional financing to fund our business activities in the future and there remains substantial
doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding
to us on commercially reasonable terms, if at all.
We have produced only limited revenues.
This makes it difficult to evaluate our future prospects and increases the risk that we will not be successful.
Our operations since
inception have produced limited revenues and may not produce significant revenues in the near term, or at all, which may harm our ability
to obtain additional financing and may require us to reduce or discontinue our operations. You must consider our business and prospects
in light of the risks and difficulties we will encounter as a company operating in a rapidly evolving industry. We may not be able to
successfully address these risks and difficulties, which could significantly harm our business, operating results, and financial condition.
Our opportunities in pharmaceuticals
and biologics will require substantial additional funding. We may not be successful in our efforts to create a pipeline of product candidates,
to develop commercially successful products, or to develop commercially successful biologic production. If we fail to successfully identify,
finance and develop product candidates and/or fail to develop commercially successful biologic production, our commercial opportunities
in pharmaceuticals and biologics may be limited.
We have no pharmaceutical
or biologic products approved for commercial sale and have not generated any revenue from pharmaceutical or biologic product sales. Identifying,
developing, obtaining regulatory approval and commercializing pharmaceutical and biologic product candidates and biologic production
will require substantial additional funding beyond our current available resources and is prone to the risks of failure inherent in drug
or biologic development. Developing product candidates and biologic production is expensive, and we expect to spend substantial amounts
as we fund our early-stage research projects, engage in preclinical development of early-stage programs and, in particular, advance program
candidates through preclinical development and clinical trials.
Investment in pharmaceutical
and biologic product development involves significant risk that any product candidate will fail to demonstrate adequate efficacy or an
acceptable safety profile, gain regulatory approval, and become commercially viable. We cannot provide any assurance that we will be
able to successfully advance any product candidates through the development process or, if approved, successfully commercialize any product
candidates.
Even if we receive regulatory
approval to market any of our product candidates, we cannot assure you that any such product candidate will be successfully commercialized,
widely accepted in the marketplace or be more effective than other commercially available alternatives.
Even if we are able to
generate revenue from the sale of any approved pharmaceutical and biologic products, we may not become profitable and may need to obtain
additional funding to continue operations. Our failure to become and remain profitable would decrease the value of our Company and could
impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our pipeline of product
candidates or continue our operations, and cause a decline in the value of our common stock, all or any of which may adversely affect
our viability.
Our operating results could be adversely
affected by a reduction in business with our significant customers.
Our revenue earned from
the sale of product and services for the six-month period ended March 31, 2022 included an aggregate of 51% of our total revenue
from one customer. At March 31, 2022, two customers accounted for an aggregate of 74% of our total accounts receivable. Our revenue
earned from the sale of products and services for the fiscal year ended September 30, 2021 included an aggregate of 31% of
our total revenues from two customers. At September 30, 2021, two customers accounted for an aggregate of 67% of our total accounts
receivable. Our revenue earned from the sale of products and services for the fiscal year ended September 30, 2020 included
an aggregate of 46% of our total revenues from four customers. At September 30, 2020, four customers accounted for an aggregate
of 74% of our total accounts receivable. Generally, our customers do not have an obligation to make purchases from us and may stop ordering
our products and services or may terminate existing orders or contracts at any time with little or no financial penalty. The loss of
any of our significant customers, any substantial decline in sales to these customers, or any significant change in the timing or volume
of purchases by our customers could result in lower revenues and could harm our business, financial condition or results of operations.
Fluctuations in quarterly results may
cause a decline in the price of our common stock.
Our revenues and profitability
are difficult to predict due to the nature of the markets in which we compete, as well as our recent entry into new markets and products,
fluctuating user demand, the uncertainty of current and future global economic conditions, and for many other reasons, including that
our operating results are highly dependent on the volume and timing of orders received during a quarter, which are difficult to forecast.
Customers generally order on an as-needed basis and we typically do not obtain firm, long-term purchase commitments from our customers.
The quarterly fluctuations in operating results described above may cause a decline in the price of our common stock.
The ongoing military conflict between
Russia and Ukraine has caused geopolitical instability, economic uncertainty, financial markets volatility and capital markets disruption.
Our business, financial condition and results of operations may be materially adversely affected by any negative impact on the capital
markets resulting from the conflict in Ukraine or any other geopolitical tensions.
In late February 2022,
Russia invaded Ukraine, significantly amplifying already existing geopolitical tensions among Russia and other countries in the region
and in the west, including the United States. Russia’s invasion, the responses of countries and political bodies to Russia’s
actions, the larger overarching tensions, and Ukraine’s military response and the potential for wider conflict have resulted in
inflation, financial market volatility and capital markets disruption, potentially increasing in magnitude, and could have severe adverse
effects on regional and global economic markets and international relations. The extent and duration of the military action, sanctions
and resulting market disruptions are impossible to predict, but could be substantial.
Third
parties may use our products in ways that could damage our reputation.
After
our customers have received our products, we do not have any control over their use and our customers may use them in ways that are harmful
to our reputation as a supplier of synthetic DNA products. In addition, while we plan to establish a biosecurity program designed to
ensure that third parties do not obtain our products for malevolent purposes, we cannot guarantee that these preventative measures, once
instituted, will eliminate or reduce the risk of the domestic and global opportunities for the misuse of our products. Accordingly, in
the event of such misuse, our reputation, future revenue and operating results may suffer.
Our business could be adversely impacted
by inflation.
Increases
in inflation may have an adverse effect on our business. Current and future inflationary effects may be driven by, among other things,
supply chain disruptions and governmental stimulus or fiscal policies as well as the ongoing military conflict between Russia and Ukraine.
Continuing increases in inflation could impact the overall demand for our products, our costs for labor, material and services, and the
margins we are able to realize on our products, all of which could have an adverse impact on our business, financial position, results
of operations and cash flows.
We may encounter difficulties in managing
our growth, and these difficulties could impair our profitability.
Currently, we are working
simultaneously on multiple projects, expanding our DNA manufacturing capacity as well as targeting several market sectors, including
activities in the diagnostics, therapeutics, and the product security sectors. These diversified operations and activities place significant
demands on our limited resources and require us to substantially expand the capabilities of our technical, administrative, and operational
resources.
If we are unable to manage
this growth effectively, our shipments to our customers could be impacted, our time and resources could be diverted from other products
and offerings and our business and operating results could suffer. Our ability to manage our operations and costs, including research
and development, costs of components, manufacturing, sales and marketing, requires us to continue to enhance our operational, financial
and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented employees. Failure
to attract and retain sufficient numbers of talented employees will further strain our human resources and could impede our growth.
Our new emphasis on Therapeutic DNA
Production Services and MDx Testing Services may reduce our ability to maintain and expand our existing DNA Tagging and Security Products
and Services businesses.
Our new emphasis on Therapeutic DNA Production
Services and MDx Testing Services may divert funding and our limited managerial and other resources from our existing DNA Tagging and
Security Products and Services businesses. This may have the effect of reducing opportunities to grow or maintain revenues in our existing
businesses while at the same time we may fail to achieve the revenues and growth we seek in our Therapeutic DNA Production Services and
MDx Testing Services business.
Risks Relating to Manufacturing, Development,
and Industries:
If we are unable to expand our DNA manufacturing capacity, we
could lose revenue and our business could suffer.
In order to expand our manufacturing capacity
for our DNA production, including our linDNA platform, we need to either build additional internal manufacturing capacity, contract with
one or more partners, or both. Our technology and the production process for our DNA production are complex, involving specialized parts,
and we may encounter unexpected difficulties in the manufacture, improvement or increasing the capacity of our DNA production, and addressing
these difficulties may cause us to divert our time and resources from our other product offerings. There is no assurance that we will
be able to continue to increase manufacturing capacity internally or that we will find one or more suitable partners to help us towards
this objective, in order to meet the volume and quality requirements necessary for success in our existing and potential markets. Manufacturing
and product quality issues may arise as we continue to increase the scale of our production. If our DNA manufacturing equipment and tools
do not consistently produce DNA products that meet our customers’ performance expectations, our reputation may be harmed, and we
may be unable to generate sufficient revenue to become profitable. Any delay or inability in expanding our manufacturing capacity could
diminish our ability to develop or sell our DNA products, which could result in lost revenue and materially harm our business, financial
condition and results of operations.
Rapidly changing technology and extensive competition in synthetic
DNA could make the services or products we are developing obsolete or non-competitive unless we continue to develop and manufacture new
and improved services or products and pursue new market opportunities.
The synthetic DNA industry is characterized by
rapid and significant technological changes, frequent new product introductions and enhancements and evolving industry demands and standards.
Our future success will depend on our ability to continually improve the services we are developing and producing, to develop and introduce
new services that address the evolving needs of our customers on a timely and cost-effective basis and to pursue new market opportunities
that develop as a result of technological and scientific advances. These new market opportunities may be outside the scope of our proven
expertise or in areas which have unproven market demand, and the utility and value of new products and services developed by us may not
be accepted in the markets served by the new services. Our inability to gain market acceptance of existing products and services in new
markets or market acceptance of new products and services could harm our future operating results. Our future success also depends on
our ability to manufacture these new and improved products and services to meet customer demand in a timely and cost-effective manner,
including our ability to resolve manufacturing issues that may arise as we commence production of any new products and services we develop.
In addition, there is
extensive competition in the synthetic DNA industry, and our future success will depend on our ability to maintain a competitive position
with respect to technological advances. Technological development by others may result in our technologies, as well as products developed
using our technologies, becoming obsolete. Our ability to compete successfully will depend on our ability to develop proprietary technologies
and services that are technologically superior to and/or are less expensive than our competitors’ technologies and products. Our
competitors may be able to develop competing and/or superior technologies and processes and compete more aggressively and sustain that
competition over a longer period of time.
Pharmaceutical and biologic products
and services are highly complex, and if we or our collaborators and customers are unable to provide quality and timely offerings to our
respective customers, our business could suffer.
The process of manufacturing
pharmaceutical and biologics and their components is complex, highly-regulated and subject to multiple risks.
Manufacturing biologics
is highly susceptible to product loss due to contamination, equipment failure, improper installation or operation of equipment, vendor
or operator error, inconsistency in yields, variability in product characteristics and difficulties in scaling the production process.
Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply
disruptions.
Our ability to generate
revenue in the pharmaceutical and biologic market depends on our ability to manufacture products that meet exacting quality and safety
standards. If we are unable to manufacture these products to the required levels, it could have an adverse effect on our business, financial
condition, and results of operations and may subject us to regulatory actions, including product recalls, product seizures, injunctions
to halt manufacture or distribution, restrictions on our operations, or civil sanctions, including monetary sanctions and criminal actions.
In addition, we could be subject to costly litigation, including claims from our collaborators and customers for reimbursement for the
cost of our products or other related losses, the cost of which could be significant.
We will need to develop and maintain manufacturing facilities
that meet current Good Manufacturing Practices.
Since a primary focus of our business will be
contract manufacturing of synthetic DNA, it will be critical for us to be able to produce sufficient quantities of materials required
for the manufacture of our product candidates or the product candidates of our collaborators or customers for preclinical testing and
clinical trials, in compliance with applicable regulatory and quality standards. If we are unable to provide such manufacturing supplies
or fail to do so on commercially-reasonable terms, we may not be able to successfully produce sufficient supply of product candidate(s) or
we may be delayed in doing so. Such failure or substantial delay could materially harm our business.
Our customers will rely on us for synthetic DNA
and other biological materials that are used in their discovery and development programs. These materials can be difficult to produce
and occasionally have variability from the product specifications. Any disruption in the supply of these biological materials consistent
with our product specifications could materially adversely affect our business. Although we have control processes and screening procedures,
biological materials are susceptible to damage and contamination and may contain active pathogens. We may also have lower yields in manufacturing
batches, which can increase our costs and slow our development timelines. Improper storage of these materials, by us or any third-party
storage facilities, may require us to destroy some of our biological raw materials or product candidates.
We also face risks that we may fail to synthesize
and manufacture our customers’ product candidates in accordance with their product specifications, and the possibility of termination
or nonrenewal of the agreement by our customers at a time that is costly or damaging to us.
In addition, the FDA and other regulatory authorities
require that our products be manufactured according to cGMP and similar foreign standards relating to methods, facilities, and controls
used in the manufacturing, processing, and packing of the product, which are intended to ensure that biological products are safe and
that they consistently meet applicable requirements and specifications.
Pharmaceutical manufacturers are required to register
their facilities and list their products manufactured after beginning drug manufacturing and then annually thereafter with the FDA and
certain state and foreign agencies. If the FDA or a comparable foreign regulatory authority does not approve our customers’ product
candidates at any of our proposed contract manufacturer’s facilities, or if we fail to maintain a compliance status acceptable
to the FDA or a comparable foreign authority, our customers may need to find alternative manufacturing facilities, which would significantly
impact our ability to supply our customers’ product candidates, if approved. Any discovery of problems with a product, or a manufacturing
or laboratory facility used by us or our strategic partners, may result in restrictions on the product or on the manufacturing or laboratory
facility, including marketed product recall, suspension of manufacturing, product seizure, or a voluntary withdrawal of the drug from
the market. We may have little to no control regarding the occurrence of such incidents.
If we were unable to provide a solution in time,
our customers’ clinical trials could be delayed, thereby limiting our commercial activities associated with those products. The
sale of our customers’ products could contain other defects could adversely affect our business, financial condition, and results
of operations. Any failure by us or another third-party manufacturers to comply with cGMP or failure to scale up manufacturing processes,
including any failure to deliver sufficient quantities of product candidates in a timely manner, could lead to a delay in, or failure
to obtain, regulatory approval of any of our customers’ candidates and, therefore, affect our business.
Pharmaceutical manufacturers are also subject
to extensive pre- and post-marketing oversight by the FDA and comparable regulatory authorities in the jurisdictions where the product
is being studied or marketed, which include periodic unannounced and announced inspections by the FDA to assess compliance with cGMP
requirements. If an FDA inspection of our facilities reveals conditions that the FDA determines not to comply with applicable regulatory
requirements, the FDA may issue observations through a Notice of Inspectional Observations or a “Form FDA 483”. If observations
in the Form FDA 483 are not addressed in a timely manner and to the FDA’s satisfaction, the FDA may issue a Warning Letter
or pursue other forms of enforcement action. Any failure by us or another contract manufacturers to comply with cGMP or to provide adequate
and timely corrective actions in response to deficiencies identified in a regulatory inspection could result in enforcement action that
could impact our ability to attract and maintain other contract manufacturing arrangements or lead to a shortage of our customers’
products and harm our business, including withdrawal of approvals previously granted, seizure, injunction or other civil or criminal
penalties. The failure of us or another manufacturer to address any concerns raised by the FDA or foreign regulators could also lead
to plant shutdown or the delay or withholding of product approval by the FDA in additional indications, or by foreign regulators in any
indication. Certain countries may impose additional requirements on the manufacturing of drug products or drug substances, on us as contract
manufacturers, as part of the regulatory approval process for products in such countries. The failure by us or other third-party manufacturers
to satisfy such requirements could impact our ability to obtain or maintain contract manufacturing arrangements with our customers in
one or more countries.
Our business also depends
on the ability of our collaborators and customers to manufacture the pharmaceutical or biologic products that incorporate our products.
If the FDA determines that our collaborators and customers are not in compliance with FDA laws and regulations, including those governing
cGMP regulations, the FDA may deny New Drug Application (“NDA”) or Biologics License Application (“BLA”) approval
until the deficiencies are corrected. Even if our collaborators or customers obtain regulatory approval for any of their product candidates,
there is no assurance that they will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory
authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product or to meet potential
future demand. If our collaborators or customers are unable to produce sufficient quantities for clinical trials or for commercialization,
commercialization efforts would be impaired, which would have an adverse effect on our business, financial condition, results of operations
and growth prospects.
Pharmaceutical and biologic-related
revenue will be dependent on our collaborators’ and customers’ demand for our manufacturing services.
The amount of customer
spending on pharmaceutical and biologic development and manufacturing will have an impact on our sales and profitability in the pharmaceutical
and biologic market. Our collaborators and customers determine the amounts that they will spend based upon, among other things, available
resources, access to capital, and their need to develop new products, which, in turn, are dependent upon a number of factors, including
their competitors’ research, development and product initiatives and the anticipated market uptake, and clinical and reimbursement
scenarios for specific products and therapeutic areas. Consolidation in the pharmaceutical and biologic industry may impact such spending
as customers integrate acquired operations, including R&D departments and manufacturing operations. Any reduction in spending on
pharmaceutical and biotechnology development and related services as a result of these and other factors could have a material adverse
effect on our business, results of operations and financial condition.
Our
safeCircleTM COVID-19 testing service could become obsolete
or its utility could be significantly diminished.
Surveillance
testing is not regulated by the FDA and Centers for Medicare & Medicaid Services (“CMS”) has stated that CLIA certification
is not required to conduct surveillance testing. ADCL is offering its safeCircleTM surveillance testing in compliance with
current Centers for Disease Control and Prevention (“CDC”), FDA, CMS and NYSDOH recommendations. The regulatory framework
or recommendations regarding COVID-19 Surveillance Testing could change at any time. In addition, our pooled COVID-19 screening testing
is conducted via a NYSDOH conditionally approved LDT. In the event that NYSDOH revokes the conditional approval or declines to fully
approve the LDT, ADCL will be required to utilize a third-party EUA-authorized COVID-19 assay and potentially stop utilizing pooled testing.
Further, our COVID-19
testing may become obsolete for a variety of reasons, including an end to the current pandemic, mutations in the genome of the SARS-CoV-2
virus, or the development and widespread distribution of a vaccine, including the vaccines developed by Pfizer-BioNTech, Moderna, and
Johnson & Johnson for which the FDA has granted emergency use authorization or approval. In addition, the utility of these services
will also diminish if positivity rates reach levels high enough to render surveillance testing ineffective or inefficient.
We have limited experience producing
and supplying our products. We may be unable to consistently manufacture or source our products to the necessary specifications or in
quantities necessary to meet demand on a timely basis and at acceptable performance and cost levels.
As we continue to scale
commercially and develop new products, and as our products incorporate increasingly sophisticated technology, it will become more difficult
to ensure our products are produced in the necessary quantities while maintaining quality. There is no assurance that we or our third-party
manufacturers will be able to continue to manufacture our products so that our technology consistently achieves the product specifications
and produces results with acceptable quality. Any future design issues, unforeseen manufacturing problems, such as contamination of our
or our manufacturers’ facilities, equipment malfunctions, aging components, quality issues with components and materials sourced
from third-party suppliers, or failures to strictly follow procedures or meet specifications, may have a material adverse effect on our
brand, business, reputation, results of operations and financial condition and could result in us or our third-party manufacturers losing
International Organization for Standardization (ISO) or quality management certifications. If our third-party manufacturers fail to maintain
ISO quality management certifications, our customers might choose not to purchase products from us.
In addition, as we scale
our commercial operations, we will also need to make corresponding improvements to other operational functions, such as our customer
support, service and billing systems, compliance programs and internal quality assurance programs. We cannot assure you that any increases
in scale, related improvements and quality assurance will be successfully implemented or that appropriate personnel will be available.
As we develop additional products, we may need to bring new equipment online, implement new systems, technology, controls and procedures
and hire personnel with different qualifications.
An inability to manufacture
products and components that consistently meet specifications, in necessary quantities, at commercially acceptable costs and without
significant delays, may have a material adverse effect on our business, results of operations, financial condition and prospects.
We must continue to secure and maintain
sufficient and stable supplies of components and raw materials.
Certain disruptions in
supply of, and changes in the competitive environment for, components and raw materials integral to the manufacturing of our products
may adversely affect our profitability. We use a broad range of materials and supplies in our products. A significant disruption in the
supply of these materials could decrease production and shipping levels, materially increase our operating costs and materially and adversely
affect our revenues and profit margins. Shortages of materials or interruptions in transportation systems, labor strikes, work stoppages,
war, acts of terrorism or other interruptions to or difficulties in the employment of labor or transportation in the markets in which
we purchase materials, components and supplies for the production of our products, in each case, may adversely affect our ability to
maintain production of our products and achieve profitability. Unforeseen discontinuation or unavailability of certain components, such
as enzymes or nucleotides, each of which we currently primarily source from single supplier, could cause backorders as we modify our
product specifications to accommodate replacement components. If we were to experience a significant or prolonged shortage of critical
components from any of our suppliers and could not procure the components from other sources, we would be unable to manufacture our products
and ship them to our customers in a timely fashion, or at all, which would adversely affect our sales, margins and customer relations.
The markets for
our drug and biologic candidates and synthetic DNA are very competitive, and we may be unable to continue to compete effectively in these
industries in the future.
The
principal markets for our drug and biologic candidates and synthetic DNA are intensely competitive. We compete with many existing suppliers
and new competitors continue to enter the market. Many of our competitors, both in the United States and elsewhere, are major pharmaceutical,
chemical and biotechnology companies, or have strategic alliances with such companies, and many of them have substantially greater capital
resources, marketing experience, research and development staff, and facilities than we do. Any of these companies could succeed in developing
products that are more effective than the product candidates that we have or may develop and may be more successful than us in producing
and marketing their existing products. Some of our competitors that operate in the nucleic-acid based therapeutic, biologics and DNA
manufacturing markets include: Precigen, Inc., Aldevron, LLC, Cobra Biologics, Limited, Integrated DNA Technologies, Inc.,
4basebio PLC, Ziopharm Oncology, Inc., MaxCyte, Inc., Touchlight Genetics Ltd., Generation Bio, Co., Novartis AG, Kite
Pharma, Inc., Juno Therapeutics, Inc., Promega Corporation, OriGene Technologies, Inc., Blue Heron Biotech, LLC, Gene
Art, GenScript Biotech Corporation, and others.
We expect this competition
to continue and intensify in the future. Our competitors also compete with us in recruiting and retaining qualified scientific and management
personnel, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunities could
be reduced or eliminated if our competitors develop and commercialize drug and biologic candidates or other forms of therapeutic DNA
that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any drug and
biologic candidates and linearDNA that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products
more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before
we are able to enter the market. Additionally, drug and biologic candidates and other forms of therapeutic DNA developed by our competitors
may render our potential drug and biologic candidates and linear DNA uneconomical or obsolete, and we may not be successful in marketing
any drug and biologic candidates and linearDNA we may develop against competitors.
If any of these risks
occur, our business, financial condition and results of operations could be significantly harmed.
The markets for our supply chain security
and product authentication solutions are very competitive, and we may be unable to continue to compete effectively in these industries
in the future.
The principal markets
for our supply chain security and product authentication offerings are intensely competitive. We compete with many existing suppliers
and new competitors continue to enter the market. Many of our competitors, both in the United States and elsewhere, are major pharmaceutical,
chemical and biotechnology companies, or have strategic alliances with such companies, and many of them have substantially greater capital
resources, marketing experience, research and development staff, and facilities than we do. Any of these companies could succeed in developing
products that are more effective than the products that we have or may develop and may be more successful than us in producing and marketing
their existing products. Some of our competitors that operate in the supply chain security and product authentication markets include:
AlpVision Sa, Authentix, Inc., Brandwatch Technologies, Inc., Chromologic LLC, Collectors Universe, Inc., DataDot Technology
Limited, De La Rue Plc., Digimarc Corporation, DNA Technologies, Inc., Haelixa Ltd., ICA Bremen GmbH, IEH Corporation, Informium
AG, opSec Security Group plc., MicroTag Temed Ltd., Nanotech Security Corp., Nokomis, Inc., Oritain Global Limited, SafeTraces, Inc.,
Selectamark Security Systems plc, SmartWater Technology, Inc., Sun Chemical Corporation, TraceTag International Ltd., TruTag
Technologies, Inc., Tailorlux gmbH and YottaMark, Inc.
We expect this competition
to continue and intensify in the future.
We
compete with life science, pharmaceutical and biotechnology companies, some of whom are our customers, who are substantially larger than
we are and potentially capable of developing new approaches that could make our products and technology obsolete or develop their own
internal capabilities that compete with our products.
The
market for biologics components products and services in the biopharmaceutical development, life science research, and diagnostics space
is intensely competitive, rapidly evolving, significantly affected by new product introductions and other market activities by industry
participants and subject to rapid technological change. We also expect increased competition as additional companies enter our market
and as more advanced technologies become available. We compete with other providers of outsourced biologics components products and services.
We also compete with the in-house discovery, development and commercial manufacturing functions of pharmaceutical and biotechnology companies.
Many of our competitors, which in some cases are also our customers, are large, well-capitalized companies with significantly greater
resources and market share than we have. They may undertake their own development of products that are substantially similar to or compete
with our products and they may succeed in developing products that are more effective or less costly than any that we may develop. These
competitors may be able to spend more aggressively on product and service development, marketing, sales and other initiatives than we
can. Many of these competitors also have:
| • | broader
name recognition; |
| • | longer
operating histories and the benefits derived from greater economies of scale; |
| • | larger
and more established distribution networks; |
| • | additional
product and service lines and the ability to bundle products and services to offer higher
discounts or other incentives to gain a competitive advantage; |
| • | more
experience in conducting research and development, manufacturing and marketing; |
| • | more
experience in entering into collaborations or other strategic partnership arrangements; and |
| • | more
financial, manufacturing and human resources to support product development, sales and marketing
and patent and other intellectual property litigation. |
These
factors, among others, may enable our competitors to market their products and services at lower prices or on terms more advantageous
to customers than we can offer. Competition may result in price reductions, reduced gross margins and loss of market share, any
of which could have a material adverse effect on our business, financial condition, results of operations, cash flows and prospects.
Additionally, our current and future competitors, including certain of our customers, may at any time develop additional products and
services that compete with our products and new approaches by these competitors may make our products, technologies and methodologies
obsolete or noncompetitive. We may not be able to compete effectively against these organizations.
In addition, to develop
and market our new products, services, technologies and methodologies successfully, we must accurately assess and meet customers’
needs, make significant capital expenditures, optimize our development and manufacturing processes to predict and control costs, hire,
train and retain the necessary personnel, increase customer awareness and acceptance of such services, provide high quality services in
a timely manner, price our products and services competitively and effectively integrate customer feedback into our business planning.
If we fail to create demand for our new products, services or technologies, our future business could be harmed.
The animal health industry is highly
competitive.
The animal health industry
is highly competitive. Our competitors include standalone animal health businesses, the animal health businesses of large pharmaceutical
companies, specialty animal health businesses and companies that mainly produce generic products. We believe many of our competitors are
conducting R&D activities in areas in which we are developing products. Several new start-up companies also compete in the animal
health industry. We also face competition from manufacturers of drugs globally, as well as producers of nutritional health products. These
competitors may have access to greater financial, marketing, technical and other resources. As a result, they may be able to devote more
resources to developing, manufacturing, marketing and selling their products, initiating or withstanding substantial price competition
or more readily taking advantage of acquisitions or other opportunities. Further, consolidation in the animal health industry could result
in existing competitors realizing additional efficiencies or improving portfolio bundling opportunities, thereby potentially increasing
their market share and pricing power, which could lead to a decrease in our revenue and profitability and an increase in competition.
For example, many of our competitors have relationships with key distributors and, because of their size, the ability to offer attractive
pricing incentives, which may negatively impact or hinder our relationships with these distributors. In addition to competition from established
market participants, new entrants to the animal health medicines and vaccines industry could substantially reduce our market share, render
our products obsolete or disrupt our business model.
To the extent that any
of our competitors are more successful with respect to any key competitive factor, or we are forced to reduce, or are unable to raise,
the price of any of our products in order to remain competitive, our business, financial condition and results of operations could be
materially adversely affected. Competitive pressure could arise from, among other things, more favorable safety and efficacy product profiles,
limited demand growth or a significant number of additional competitive products being introduced into a particular market, price reductions
by competitors, the ability of competitors to capitalize on their economies of scale, the ability of competitors to produce or otherwise
procure animal health products at lower costs than us and the ability of competitors to access more or newer technology than us.
Our research and development efforts
for new products may be unsuccessful.
We incur research and
development expenses to develop new products and technologies in an effort to maintain our competitive position in a market characterized
by rapid rates of technological advancement. Our research and development efforts are subject to unanticipated delays, expenses and technical
problems. There can be no assurance that any of these products or technologies will be successfully developed or that, if developed, will
be commercially successful. In the event that we are unable to develop commercialized products from our research and development efforts
or we are unable or unwilling to allocate amounts beyond our currently anticipated research and development investment, we could lose
our entire investment in these new products and technologies. Any failure to translate research and development expenditures into successful
new product introduction could have an adverse effect on our business.
In addition, research,
development, and commercialization of pharmaceutical and biologic products is inherently risky. We cannot give any assurance that any
of our pharmaceutical and biologic product candidates will receive regulatory approval, which is necessary before they can be commercialized.
Risks Related to Our Intellectual Property:
Our intellectual property rights are
valuable, and any inability to protect them could reduce the value of our products, services and brand.
Our patents, trademarks,
trade secrets, copyrights and all of our other intellectual property rights are important assets for us. There are events that are outside
of our control that pose a threat to our intellectual property rights as well as to our products and services. For example, effective
intellectual property protection may not be available in every country in which our products and services are distributed. The efforts
we have taken to protect our proprietary rights may not be sufficient or effective. Any significant impairment of our intellectual property
rights could harm our business or our ability to compete. Protecting our intellectual property rights is costly and time consuming. Any
increase in the unauthorized use of our intellectual property could make it more expensive to do business and harm our operating results.
Although we seek to obtain patent protection for our innovations, it is possible we may not be able to protect all or some of these innovations.
Given the costs of obtaining patent protection, we may choose not to protect certain innovations that later turn out to be important.
There is always the possibility that the scope of the protection gained from one of our issued patents will be insufficient or deemed
invalid or unenforceable. We also seek to maintain certain intellectual property as trade secrets. The secrecy could be developed independently,
compromised by third parties, or disclosed, intentionally or accidentally, by our employees which would cause us to lose the competitive
advantage resulting from these trade secrets.
Intellectual property litigation could
harm our business, financial condition and results of operations.
Litigation regarding patents
and other intellectual property rights is extensive in the drug and biotechnology industry. In the event of an intellectual property dispute,
we may be forced to litigate. This litigation could involve proceedings instituted by the U.S. Patent and Trademark Office or the International
Trade Commission, as well as proceedings brought directly by affected third parties. Intellectual property litigation can be extremely
expensive, and these expenses, as well as the consequences should we not prevail, could seriously harm our business.
If a third party claims
an intellectual property right to technology we use, we might need to discontinue an important product or product line, alter our products
and processes, pay license fees or cease our affected business activities. Although we might under these circumstances attempt to obtain
a license to this intellectual property, we may not be able to do so on favorable terms, or at all. Furthermore, a third party may claim
that we are using inventions covered by the third party’s patent rights and may go to court to stop us from engaging in our normal
operations and activities, including making or selling our products. These lawsuits are costly and could affect our results of operations
and divert the attention of managerial and technical personnel. A court may decide that we are infringing the third party’s patents
and would order us to stop the activities covered by the patents. In addition, a court may order us to pay the other party damages for
having violated the other party’s patents. The drug and biotechnology industry has produced a proliferation of patents, and it is
not always clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage
of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement,
we would need to demonstrate that our products or methods of use either do not infringe the patent claims of the relevant patent and/or
that the patent claims are invalid, and we may not be able to do this. Proving invalidity, in particular, is difficult since it requires
a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents.
Because some patent applications
in the United States may be maintained in secrecy until the patents are issued, because patent applications in the United States and many
foreign jurisdictions are typically not published until eighteen months after filing, and because publications in the scientific
literature often lag behind actual discoveries, we cannot be certain that others have not filed patent applications for technology covered
by our or our licensor’s issued patents or pending applications or that we or our licensors were the first to invent the technology.
During the ordinary course of our business, we do not conduct “prior art” searches before filing a patent application. Our
competitors may have filed, and may in the future file, patent applications covering technology similar to ours. Any such patent application
may have priority over our or our licensors’ patent applications and could further require us to obtain rights to issued patents
covering such technologies. If another party has filed a United States patent application on inventions similar to ours, we may have to
participate in an interference proceeding declared by the U.S. Patent and Trademark Office to determine priority of invention in the United
States. The costs of these proceedings could be substantial, and it is possible that such efforts would be unsuccessful, resulting in
a loss of our United States patent position with respect to such inventions.
Some of our competitors
may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources.
In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on
our ability to raise the funds necessary to continue our operations.
A cybersecurity incident and other technology
disruptions could negatively affect our business and our relationships with customers.
We use technology in substantially
all aspects of our business operations. The widespread use of technology, including mobile devices, cloud computing, and the internet,
give rise to cybersecurity risks, including security breach, espionage, system disruption, theft and inadvertent release of information.
Our business involves the storage and transmission of numerous classes of sensitive and/or confidential information and intellectual property,
including information relating to customers and suppliers, private information about employees, and financial and strategic information
about us and our business partners. If we fail to effectively assess and identify cybersecurity risks associated with the use of technology
in our business operations, we may become increasingly vulnerable to such risks. Additionally, while we have implemented measures to prevent
security breaches and cyber incidents, our preventative measures and incident response efforts may not be entirely effective. The theft,
destruction, loss, misappropriation, or release of sensitive and/or confidential information or intellectual property, or interference
with our information technology systems or the technology systems of third parties on which we rely, could result in business disruption,
negative publicity, brand damage, violation of privacy laws, loss of customers, potential liability and competitive disadvantage.
Risks Related to Regulatory Approval of
Our Customer and Collaborator’s Pharmaceutical and Biotherapeutic Product Candidates and Other Legal Compliance Matters:
Pharmaceutical and biologic-related
revenue is generally dependent on regulatory approval, oversight and compliance.
The sale and use of our
products and services in the pharmaceutical and biologic markets will generally be subject to regulatory approval and oversight, potentially
including approval and/or oversight in various foreign jurisdictions. In addition, our pharmaceutical and biologic products and services
may be incorporated into products that cannot be marketed in the United States or in many other jurisdictions without approval by the
FDA or comparable agencies of other countries or regions. Obtaining such regulatory approvals is costly, time-consuming, uncertain, and
subject to unanticipated delays. When, if ever, such approvals will be obtained is unknown. Our revenue in the pharmaceutical and biologic
markets is highly dependent upon obtaining such approval.
Federal
agencies, including the FDA and Federal Trade Commission, as well as state, local, and foreign authorities, also exercise ongoing review
and control of the manufacturing, packaging, labeling, advertising, sale, distribution, and monitoring of pharmaceutical and biologic
products. If our or our customers’ pharmaceutical or biologic product candidates or pharmaceutical or biologic products incorporating
our products are ever approved, failure to comply with any of these regulations or other requirements could also have an adverse effect
on our revenue in the pharmaceutical and biologic markets.
In addition, veterinary
vaccines in the United States are subject to review and regulatory approval by the United States Department of Agriculture (“USDA”).
The USDA’s Center for Veterinary Biologics is responsible for the regulation of animal health vaccines, including certain immunotherapeutics.
All manufacturers of animal health biologicals must show their products to be pure, safe, effective and produced by a consistent method
of manufacture as defined under the Virus Serum Toxin Act. Post-approval monitoring of products is required. Reports of product quality
defects, adverse events or unexpected results are submitted in accordance with the agency requirements.
Pharmaceutical and biologic-related
revenue will be highly dependent on our collaborators’ and customers’ success in obtaining regulatory approval and commercializing
their products.
Some of our products will
be incorporated into our customers’ products in the pharmaceutical and biologic market that are subject to comprehensive regulation
by the FDA and other regulatory agencies in the United States and by comparable authorities in other countries. In the United States,
to obtain approval from the FDA to market any future pharmaceutical or biologic product that incorporates our technology, our collaborators
or customers will be required to submit an New Drug Application (“NDA”) or Biologics License Application (“BLA”).
Ordinarily, the FDA requires a company to support an NDA or BLA with substantial evidence of the product candidate’s safety and
efficacy in treating the targeted indication based on data derived from adequate and well-controlled clinical trials, including Phase
III safety and efficacy trials conducted in patients with the disease or condition being targeted. The process of obtaining such regulatory
approvals is expensive, often takes many years if approval is obtained at all, and can vary substantially based upon the type, complexity
and novelty of the product candidate involved. Changes in the regulatory approval process during the development period, changes in or
the enactment of additional statutes or regulations, or changes in the regulatory review process may cause delays in the approval or rejection
of an application. There is no guarantee that our collaborators and customers will ever be successful in obtaining regulatory approval
for any product that incorporates our products or technology. Even if regulatory approval is received, the manufacturing processes, post
approval clinical data, labeling, advertising and promotional activities for any such product will be subject to continual requirements
of and review by the FDA and other regulatory bodies. Our business may be materially harmed by our collaborators’ and customers’
inability to obtain or maintain regulatory approvals for their products of their failure to comply with applicable regulations.
In addition, we will be
dependent on, and have no control over, consumer demand for the products into which our products are incorporated. Consumer demand for
our collaborators’ and customers’ products could be adversely affected by, among other things, delays in health regulatory
approval, the loss of patent and other intellectual property rights protection, the emergence of competing products, including generic
drugs or biosimilars, the degree to which private and government drug plans subsidize payment for a particular product and changes in
the marketing strategies for such products. The healthcare industry has changed significantly over time, and we expect the industry to
continue to evolve. Some of these changes may have a material adverse effect on our collaborators and customers and thus may have a material
adverse effect on our business. If the products into which our products are incorporated do not gain market acceptance, our revenues and
profitability may be adversely affected.
The regulatory approval processes of
the FDA and comparable foreign regulatory authorities are lengthy, time consuming, and inherently unpredictable. If we are ultimately
unable to obtain regulatory approval for our product candidates, we will be unable to generate product revenue and our business will be
substantially harmed.
The time required to
obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable, typically takes many years following the
commencement of clinical trials, and depends upon numerous factors, including the type, complexity and novelty of the product
candidates involved. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval
may change during the course of a product candidate’s clinical development and may vary among jurisdictions, which may cause
delays in the approval or the decision not to approve an application. Regulatory authorities have substantial discretion in the
approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require
additional preclinical, clinical or other studies. We have not submitted for, or obtained regulatory approval for any product
candidate, and it is possible that none of our existing product candidates or any product candidates we may seek to develop in the
future will ever obtain regulatory approval. Applications for our product candidates could fail to receive regulatory approval for a
variety of reasons. This lengthy approval process, as well as the unpredictability of the results of clinical trials, may result in
our failing to obtain regulatory approval to market any of our product candidates, which would significantly harm our business,
results of operations, and prospects.
Our or our customers’ product
candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory
approval, limit their commercial potential, or result in significant negative consequences.
Adverse events or other
undesirable side effects caused by our or our customers’ product candidates could cause us or regulatory authorities to interrupt,
delay, or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by regulatory
authorities. Side effects related to a drug or biologic could affect patient recruitment, the ability of enrolled patients to complete
the study, and/or result in potential product liability claims.
Additionally, if one or
more of our or our customers’ product candidates receives marketing approval, and we or others later identify undesirable side effects
or adverse events caused by such products, a number of potentially significant negative consequences could result. Regulatory authorities
may withdraw approvals of such product or impose restrictions on distribution. They may require additional warnings or contraindications
on the product label that could diminish the usage or otherwise limit the commercial success of the product. We or our customers may be
required to change the way the product is manufactured, be forced to suspend manufacturing the product or required to create a risk evaluation
and mitigation strategy (“REMS”). In addition, our reputation may suffer. Any of these events could prevent us from achieving
or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results
of operations, and prospects.
Even if we or our customers obtain regulatory
approval for a product candidate, our products will remain subject to extensive regulatory scrutiny.
If any of our or our customers’
product candidates are approved, they will be subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage,
advertising, promotion, sampling, record-keeping, conduct of post-marketing studies, and submission of safety, efficacy, and other post-market
information, including both federal and state requirements in the United States and requirements of comparable foreign regulatory authorities.
Ongoing regulatory requirements include ensuring that quality control and manufacturing and production procedures conform to cGMP regulations,
and we will be subject to continual review and inspections to assess compliance with cGMP regulations and adherence to commitments made
in any regulatory filings. Accordingly, we and others with whom we work must continue to expend time, money, and effort in all areas of
regulatory compliance.
Any regulatory approvals
that we or our customers receive for our products will be subject to limitations on the approved indicated uses for which the product
may be marketed and promoted or to the conditions of approval (including the requirement to implement a REMS), or contain requirements
for potentially costly post-marketing testing. Any new legislation addressing drug or biologic safety issues could result in delays in
product development or commercialization, or increased costs to assure manufacturing compliance. The FDA and other agencies, including
the Department of Justice, closely regulate and monitor the post-approval marketing and promotion of products to ensure that they are
manufactured, marketed and distributed only for the approved indications and in accordance with the provisions of the approved labeling.
Promotional communications with respect to prescription drugs and biologics are subject to a variety of legal and regulatory restrictions
and must be consistent with the information in the product’s approved label. The holder of an approved NDA must submit new or supplemental
applications and obtain approval for certain changes to the approved product, product labeling, or manufacturing process. We could also
be asked to conduct post-marketing manufacturing changes to verify the safety and efficacy of our products in general. An unsuccessful
post-marketing study or failure to complete such a study could result in the withdrawal of marketing approval and thereby affect the need
for our manufacturing services.
If a regulatory agency
discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with
the facility where the product is manufactured, or disagrees with the promotion, marketing or labeling of a product, such regulatory agency
may impose restrictions on that product or us, including, but not limited to, requiring withdrawal or recall of the product from the market,
imposing civil or criminal penalties, and imposing restrictions on ability to continue to manufacture the product(s). Any government investigation
of alleged violations of law could require us to expend significant time and resources in response, and could generate negative publicity.
Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our and our customers’ ability
to commercialize and generate revenue from our products. If regulatory sanctions are applied or if regulatory approval is withdrawn, the
value of our Company and our operating results will be adversely affected.
In addition, the FDA’s
regulations, policies or guidance may change and new or additional statutes or government regulations in the United States and other jurisdictions
may be enacted that could further restrict or regulate our post-approval manufacturing activities. We cannot predict the likelihood, nature
or extent of adverse government regulation that may arise from pending or future legislation or administrative action. If we are not able
to achieve and maintain regulatory compliance, we may not be permitted to continue manufacturing products for our customers’ products
and/or product candidates, which would adversely affect our ability to generate revenue and achieve or maintain profitability.
If the FDA were to begin to enforce
regulation of LDTs, we could incur substantial costs and delays associated with trying to obtain pre-market clearance or approval and
costs associated with complying with post-market requirements.
As an LDT, our MDx
Testing Services are currently subject to enforcement discretion by the FDA. In October 2014, the FDA issued two draft
guidance documents: “Framework for Regulatory Oversight of Laboratory Developed Tests,” which provides an overview of
how the FDA would regulate LDTs through a risk-based approach, and “FDA Notification and Medical Device Reporting for
Laboratory Developed Tests”, which provides guidance on how the FDA intends to collect information on existing LDTs, including
adverse event reports. Pursuant to the Framework for Regulatory Oversight draft guidance, LDT manufacturers would be subject to
medical device registration, listing, and adverse event reporting requirements. Many LDT manufacturers would be required to either
submit a pre-market application and receive the FDA’s approval before an LDT may be marketed or submit a pre-market
notification in advance of marketing. The Framework for Regulatory Oversight draft guidance states that within six months after the
guidance documents are finalized, all laboratories will be required to give notice to the FDA. On November 18, 2016, however,
the FDA announced that it would not release final versions of these guidance documents and would instead continue to work with
stakeholders, the new administration and Congress to determine the right approach. On January 13, 2017, the FDA released a
discussion paper on LDTs outlining a possible risk-based approach for FDA and CMS oversight of LDTs. According to the 2017
discussion paper, previously marketed LDTs would not be expected to comply with most or all FDA oversight requirements
(grandfathering), except for adverse event and malfunction reporting. In addition, certain new and significantly modified LDTs would
not be expected to comply with pre-market review unless the agency determines such tests could lead to patient harm. Since LDTs
currently on the market would be grandfathered in, pre-market review of new and significantly modified LDTs could be phased-in over
a four-year period, as opposed to the nine years proposed in the Framework for Regulatory Oversight draft guidance. In addition,
tests introduced after the effective date, but before their phase-in date, could continue to be offered during pre-market
review.
The discussion paper notes
that the FDA would focus on analytical and clinical validity as the basis for marketing authorization. The FDA anticipates laboratories
that already conduct proper validation should not be expected to experience new costs for validating their tests to support marketing
authorization and laboratories that conduct appropriate evaluations would not have to collect additional data to demonstrate analytical
validity for FDA clearance or approval. The evidence of the analytical and clinical validity of all LDTs would be made publicly available.
LDT manufacturers would be encouraged to submit prospective change protocols in their pre-market submission that outline specific types
of anticipated changes, the procedures that will be followed to implement them, and the criteria that will be met prior to implementation.
In addition,
legislative proposals addressing the FDA’s oversight of LDTs have been introduced in Congress. For example, in March 2020, the
“Verifying Accurate Leading-edge IVCT Development Act of 2020,” or VALID Act, was officially introduced in Congress. The
bill proposes a risk-based approach that would subject many LDTs to FDA regulation by creating a new in vitro clinical test, or
IVCT, category of regulated products. As proposed, the bill grandfathers many existing LDTs from the proposed premarket approval,
quality systems, and labeling requirements, respectively, but would require such tests to comply with other regulatory requirements
(e.g., registration and listing, adverse event reporting). The VALID Act was re-introduced in a slightly modified form in
June 2021, and the bill continues to be the subject of active discussions. However, we cannot predict if this (or any other
bill) will be enacted in its current (or any other) form and cannot quantify the effect of such proposals on our business.
If we fail to comply with laboratory
licensing requirements, we could lose the ability to offer our clinical testing services or experience disruptions to our business.
CLIA is a federal law
regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the
diagnosis, prevention, or treatment of disease. CLIA is intended to ensure the quality and reliability of clinical laboratories in the
United States by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency
testing, patient test management, quality control, quality assurance and inspections. Clinical laboratories must be certified under CLIA
in order to perform testing on human specimens, unless they fall within an exception to CLIA certification, such as research laboratories
that test human specimens but do not report patient-specific results for the diagnosis, prevention, or treatment of any disease or impairment
of, or the assessment of the health of individual patients. CLIA certification is also required to be eligible to bill Federal and State
healthcare programs, as well as many private third-party payers, for diagnostic testing and services. Currently, we are supplying our
iCTC capture assay and associated testing services under the research exception to CLIA. If we expand our laboratory testing services
so that the research exception no longer applies to our iCTC capture, we will no longer be able to offer these services. Further, if we
fail to comply with the CLIA research exception with respect to our iCTC capture assay, we could be found to have violated FDA or CLIA
regulations or guidances and could have to stop offering these services and potentially be assessed substantial penalties.
Healthcare legislative measures aimed
at reducing healthcare costs may have a material adverse effect on our business and results of operations.
Third party payors are
developing increasingly sophisticated methods of controlling healthcare costs. In both the United States and certain foreign jurisdictions,
there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our products
profitably. In particular, in the United States in 2010, the ACA was enacted. In addition, other legislative changes have been proposed
and adopted in the United States since the ACA was enacted. The repeal of or changes in some or all of the ACA and complying with any
new legislation or reversing changes implemented under the ACA could be time-intensive and expensive, resulting in a material adverse
effect on our business.
There have been, and likely
will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at containing or lowering
the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government,
insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or
impose price controls may adversely affect the demand for our product candidates, if we obtain regulatory approval, including: our ability
to receive or set a price that we believe is fair for our products; our ability to generate revenue and achieve or maintain profitability;
the level of taxes that we are required to pay; and the availability of capital. We expect that the ACA, as well as other healthcare reform
measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous
coverage criteria, lower reimbursement, and new payment methodologies. This could lower the price that we receive for any approved product.
Any denial in coverage or reduction in reimbursement from Medicare or other government-funded programs may result in a similar denial
or reduction in payments from private payors, which may prevent us from being able to generate sufficient revenue, attain profitability
or commercialize our product candidates, if approved.
Our employees, independent contractors,
consultants, commercial partners and vendors may engage in misconduct or other improper activities, including non-compliance with regulatory
standards and requirements.
We are exposed to the
risk of fraud, misconduct or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors.
Misconduct by these parties could include intentional, reckless and negligent conduct that fails to: comply with applicable laws and regulations
of the FDA and other comparable foreign regulatory authorities; provide true, complete and accurate information to the FDA and other comparable
foreign regulatory authorities; comply with manufacturing standards we have established; comply with healthcare fraud and abuse laws in
the United States and similar foreign fraudulent misconduct laws; or report financial information or data accurately or to disclose unauthorized
activities to us.
If we or our customers
obtain FDA approval of any of our products and begin commercializing those products in the United States, our potential exposure under
such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. We have adopted
a code of business conduct and ethics, but it is not always possible to identify and deter misconduct by employees and third parties,
and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses
or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such
laws. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions
could have a significant impact on our business, including the imposition of significant fines or other sanctions.
If we fail to comply with healthcare
laws, we could face substantial penalties and our business, operations and financial conditions could be adversely affected.
Healthcare providers,
physicians and payors play a primary role in the recommendation and prescription of any product candidates for which our customers may
obtain marketing approval. Restrictions under applicable federal, state and foreign healthcare laws and regulations may affect our ability
to operate and expose us to areas of risk, including activities that potentially harm consumers and analogous state and foreign laws and
regulations.
Because of the breadth
of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities
could, despite our efforts to comply, be subject to challenge under one or more of such laws. Efforts to ensure that our business arrangements
will comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities
will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable
healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting
our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative
penalties, damages, disgorgement, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, and
curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
In addition, the approval and commercialization of any of our customers’ product candidates outside the United States will also
likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
Risks Related to Personnel:
Our failure to manage our growth in
operations and acquisitions of new product lines and new businesses could harm our business.
The recent growth in our
operations could place a significant strain on our current management resources. We have a limited number of personnel and expect to continue
to have a limited number of personnel for the foreseeable future.
To manage such growth,
we may need to improve our:
| • | operations and financial systems; |
| • | procedures and controls; and |
| • | training and management of our employees. |
If we are unable to continue to retain
the services of Dr. Hayward, we may not be able to continue our operations.
Our success depends
to a significant extent upon the continued service of Dr. James A. Hayward, our CEO. On July 28, 2016, we entered into an
employment agreement with Dr. Hayward. The initial term was from July 1, 2016 through June 30, 2017, with
automatic one-year renewal periods. As of June 30, 2022, the employment contract automatically renewed for an
additional year. Loss of the services of Dr. Hayward could significantly harm our business, results of operations and
financial condition. We do not maintain key-person insurance on the life of Dr. Hayward.
We may have conflicts of interest with
our affiliates and related parties, and in the past we have engaged in transactions and entered into agreements with affiliates that were
not negotiated at arms’ length.
We have engaged, and may
in the future engage, in transactions with affiliates and other related parties. These transactions may not have been, and may not be,
on terms as favorable to us as they could have been if obtained from non-affiliated persons. While an effort has been made, and will continue
to be made, to enter into transactions with affiliated persons and other related parties at rates and on terms as favorable as would be
charged by others, there will always be an inherent conflict of interest between our interests and those of our affiliates and related
parties. The Company may be adversely impacted if any related party agreement or transaction is made on unfavorable terms.
Risks Relating to Our Common Stock and
Other Securities:
There are a large number of shares of
common stock underlying our outstanding options and warrants and the sale of these shares may depress the market price of our common stock
and cause immediate and substantial dilution to our existing stockholders.
As of July 27, 2022, we
had 8,982,520 shares of common stock issued and outstanding, outstanding options to purchase 1,063,143 shares of common stock, outstanding
warrants to purchase 2,239,963 shares of common stock, and 2,766,248 shares available for grant under our 2005 and 2020 Equity Incentive
Plans. The issuance of shares upon exercise of our outstanding options and warrants will cause immediate and substantial dilution to our
stockholders and any sale thereof may depress the market price of our common stock.
We may be required to repurchase certain
of our warrants.
Under our warrants sold
privately that have registration rights, in the event of a “Fundamental Transaction” (as defined in the related warrant agreement,
which generally includes any merger with another entity, the sale, transfer or other disposition of all or substantially all of our assets
to another entity, or the acquisition by a person of more than 50% of our common stock), each warrant holder will have the right at any
time prior to the consummation of the Fundamental Transaction to require us to repurchase the warrant for a purchase price in cash equal
to the Black Scholes value (as calculated under the warrant agreement) of the then remaining unexercised portion of such warrant on the
date of such Fundamental Transaction, which may materially adversely affect our financial condition and/or results of operations and may
prevent or deter a third party from acquiring us.
If we fail to comply with the continuing
listing standards of Nasdaq, our securities could be delisted, which could limit investors’ ability to make transactions in our
common stock and subject us to additional trading restrictions.
Our common stock is listed
on Nasdaq under the symbol “APDN”. For our common stock to continue to be listed on Nasdaq, we must meet the current continued
listing requirements, which provide, among other things, that a company may be delisted if the bid price of its stock drops below $1.00
for a period of 30 consecutive business days.
We may in the future decide
to enact a reverse stock split to comply with Nasdaq’s minimum bid price requirement. However, even if we enact such a reverse stock
split, there can be no assurance that we would be able to maintain compliance with Nasdaq’s minimum bid price or other listing requirements.
If we were unable to meet these requirements, our common stock could be delisted from Nasdaq. The effect of a reverse stock split on the
market price of our common stock cannot be predicted with any certainty, and the history of similar reverse stock split combinations for
companies in like circumstances is varied. It is possible that the per share price of the common stock after the reverse stock split will
not rise in proportion to the reduction in the number of shares of the common stock outstanding resulting from the reverse stock split,
effectively reducing our market capitalization, and there can be no assurance that the market price per post-reverse split share will
either exceed or remain in excess of the Nasdaq prescribed minimum bid price for a sustained period of time. The market price of our common
stock may vary based on other factors that are unrelated to the number of shares outstanding, including our future performance.
If our common stock were
to be delisted from Nasdaq, our common stock could begin to trade on one of the markets operated by OTC Markets Group, including OTCQX,
OTCQB or OTC Pink (formerly known as the “pink sheets”), as the case may be. In such event, our common stock could be subject
to the “penny stock” rules which among other things require brokers or dealers to approve investors’ accounts,
receive written agreements and determine investor suitability for transactions and disclose risks relating to investing in the penny stock
market. Any such delisting of our common stock could have an adverse effect on the market price of, and the efficiency of the trading
market for our common stock, not only in terms of the number of shares that can be bought and sold at a given price, but also through
delays in the timing of transactions and less coverage of us by securities analysts, if any. Also, if in the future we were to determine
that we need to seek additional equity capital, it could have an adverse effect on our ability to raise capital in the public or private
equity markets.
Any material weaknesses in our internal
control over financial reporting in the future could adversely affect investor confidence, impair the value of our common stock and increase
our cost of raising capital.
Any failure to remedy
deficiencies in our internal control over financial reporting that may be discovered or our failure to implement new or improved controls,
or difficulties encountered in the implementation of such controls, could harm our operating results, cause us to fail to meet our reporting
obligations or result in material misstatements in our financial statements. Any such failure could, in turn, affect the future ability
of our management to certify that internal control over our financial reporting is effective. Inferior internal control over financial
reporting could also subject us to the scrutiny of the SEC and other regulatory bodies which could cause investors to lose confidence
in our reported financial information and could subject us to civil or criminal penalties or stockholder litigation, which could have
an adverse effect on our results of operations and the market price of our common stock.
In addition, if we
or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting, the
disclosure of that fact, even if quickly remedied, could reduce the market’s confidence in our financial statements and harm
our share price. Furthermore, deficiencies could result in future non-compliance with Section 404 of the Sarbanes-Oxley Act of 2002.
Such non-compliance could subject us to a variety of administrative sanctions, including review by the SEC or other regulatory
authorities.
If we are unable to obtain additional financing our business
operations may be harmed or discontinued.
Our continuation as a going concern is dependent
upon our future revenues and our ability to commercialize more products, obtain additional capital and attain profitable operations. We
will require additional funds to complete the continued development and commercialization of our products, product manufacturing, and
to fund expected additional losses from operations, until revenues are sufficient to cover our operating expenses. If we are unsuccessful
in obtaining any necessary additional financing, we will most likely be forced to reduce or terminate our operations.
We may require additional financing which may in turn require
the issuance of additional shares of common stock, preferred stock or other debt or equity securities (including convertible securities)
and which would dilute the ownership held by our stockholders.
We may need to raise funds through either
debt or the sale of our shares of our common stock in order to achieve our business goals. Any additional shares issued would
further dilute the percentage ownership held by the stockholders. Furthermore, if we raise funds in equity transactions through the
issuance of convertible securities which are convertible at the time of conversion at a discount to the prevailing market price,
substantial dilution is likely to occur resulting in a material decline in the price of your shares. Our public offerings completed
in November 2014, April 2015, December 2018, and November 2019, our registered direct offerings during
January 2021 and February 2022, our registered direct public offering and concurrent private placement during
November 2015, our private placements completed in November 2016, June 2017, and August 2019, and our
registered direct offering in December 2017 resulted in dilution to investors and future offerings of securities could result
in further dilution to investors.
We may require additional financing in the future, which may
not be available or, if available, may be on terms that cause a decline in the value of the shares of our common stock held by stockholders.
If we raise capital in the future by issuing additional
securities, our stockholders may experience a decline in the value of the shares of our common stock they currently hold or may acquire
prior to any such financing. In addition, such securities may have rights senior to the rights of holders of our shares of common stock.
Risks Related to this Offering:
Management will have broad discretion as to the use of proceeds
from this offering and we may use the net proceeds in ways with which you may disagree.
We
intend to use the net proceeds of this offering for the further development of our Therapeutic DNA Production and MDx Testing Services,
as well as general corporate purposes, which may include research and development expenses, capital expenditures, working capital and
general and administrative expenses, and potential acquisitions of or investments in businesses, products and technologies that complement
our business, although we have no present commitments or agreements to make any such acquisitions or investments as of the date of this
prospectus. Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds
in ways that do not improve our results of operations or enhance the value of our common stock. Accordingly, you will be relying on the
judgment of our management on the use of net proceeds, and you will not have the opportunity, as part of your investment decision, to
assess whether the proceeds are being used appropriately. Our failure to apply these funds effectively could have a material adverse effect
on our business and cause the price of our common stock to decline.
Pending these uses, we intend to invest the funds
in short-term, investment grade, interest-bearing securities. It is possible that, pending their use, we may invest the net proceeds in
a way that does not yield a favorable, or any, return for us.
The public offering price will be set by our board of directors
and does not necessarily indicate the actual or market value of our common stock.
Our board of directors will approve the public
offering price and other terms of this offering after considering, among other things: the number of shares authorized in our Certificate
of Incorporation; the current market price of our common stock; trading prices of our common stock over time; the volatility of our common
stock; our current financial condition and the prospects for our future cash flows; the availability of and likely cost of capital of
other potential sources of capital; the characteristics of interested investors and market and economic conditions at the time of the
offering. The offering price is not intended to bear any relationship to the book value of our assets or our past operations, cash flows,
losses, financial condition, net worth or any other established criteria used to value securities. The public offering price may not be
indicative of the fair value of the common stock.
If you purchase the common stock or pre-funded warrants sold
in this offering, you will experience immediate dilution as a result of this offering and future equity issuances.
Because the price per share of our common stock
and pre-funded warrants being offered is higher than the book value per share of our common stock, you will suffer immediate substantial
dilution in the net tangible book value of the common stock you purchase in this offering. See the section entitled “Dilution”
of this prospectus for a more detailed discussion of the dilution you will incur if you purchase common stock and pre-funded warrants
in this offering. The issuance of additional shares of our common stock in future offerings could be dilutive to stockholders if they
do not invest in future offerings. Moreover, to the extent that we issue options or warrants to purchase, or securities convertible into
or exchangeable for, shares of our common stock in the future and those options, warrants or other securities are exercised, converted
or exchanged, stockholders may experience further dilution.
There is no public market for the pre-funded warrants or
Series Warrants being offered in this offering.
There is no established public trading market
for the pre-funded warrants or Series Warrants being offered in this offering, and we do not expect a market to develop. In addition,
we do not intend to apply to list the pre-funded warrants or Series Warrants on any securities exchange or nationally recognized trading
system, including The Nasdaq Stock Market. Without an active market, the liquidity of the pre-funded warrants or Series Warrants will
be limited.
Holders of pre-funded warrants or Series Warrants purchased
in this offering will have no rights as common stockholders until such holders exercise their pre-funded warrants or Series Warrants
and acquire our common stock.
Until holders of pre-funded warrants or Series
Warrants acquire shares of our common stock upon exercise of the pre-funded warrants or Series Warrants, as applicable, holders of pre-funded
warrants or Series Warrants will have no rights with respect to the shares of our common stock underlying such pre-funded warrants or
Series Warrants. Upon exercise of the pre-funded warrants or Series Warrants, the holders will be entitled to exercise the rights of
a common stockholder only as to matters for which the record date occurs after the exercise date.
Provisions of the Series Warrants and pre-funded warrants
offered by this prospectus could discourage an acquisition of us by a third party.
In addition to the discussion of the provisions
of our Certificate of Incorporation, certain provisions of the Series Warrants and pre-funded warrants offered by this prospectus could
make it more difficult or expensive for a third party to acquire us. Such Series Warrants and pre-funded warrants prohibit us from engaging
in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes
our obligations under the Series Warrants and pre-funded warrants. Further, the Series Warrants and pre-funded warrants provide that,
in the event of certain transactions constituting “fundamental transactions,” with some exception, holders of such the Series
Warrants and pre-funded warrants will have the right, at their option, to require us to repurchase such the Series Warrants and pre-funded
warrants at a price described in the Series Warrants and pre-funded warrants. These and other provisions of the Series Warrants and pre-funded
warrants offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial
to you.
The sale of our common stock and the Series Warrants in this
offering could result in the reset of the exercise price of certain outstanding warrants.
We have outstanding warrants to purchase 717,813
shares of our common stock with an exercise price of $2.80 per share that may be subject to further adjustment. Subject to certain exceptions,
the terms of these warrants provide that (i) if we sell common stock at a price per share less than the then-current exercise price,
or securities which are convertible or exercisable into shares of common stock at an effective per share price less than the then current
exercise price, then we are required to reduce the exercise price of the warrants to be the lower price of such subsequent sale, or (ii) if
we sell securities which are convertible or exercisable into shares of common stock at a price which varies or may vary with the market
price of the shares of our common stock, including by way of one or more reset(s) to a fixed price, the holders of such securities
have the right to substitute the variable price for the exercise price. The exercise price of such warrants cannot adjust below a specified
minimum exercise price, which is $1.47 for 458,813 of such warrants, $1.58 for 159,000 of such warrants, $1.38 for 50,000 of such warrants
and $1.36 for 50,000 of such warrants.
Use
of Proceeds
We estimate that the net proceeds from this offering
will be approximately $10,900,696, assuming the sale of all the securities offered under this prospectus, after deducting the Placement
Agent fees and estimated offering expenses payable by us and assuming no exercise of the Series Warrants. However, this is a best efforts
offering with no minimum number of securities or amount of proceeds as a condition to closing, and we may not sell all or any of these
securities offered pursuant to this prospectus; as a result, we may receive significantly less in net proceeds. We will only receive additional
proceeds from the exercise of the Series A Warrants issuable in connection with this offering if such Series A Warrants are exercised
at their exercise price of $4.00 and the holders of such Series A Warrants pay the exercise price in cash upon such exercise. We
will only receive additional proceeds from the exercise of the Series B Warrants issuable in connection with this offering if such Series
B Warrants are exercised at their exercise price of $4.00 and the holders of such Series B Warrants pay the exercise price in cash upon
such exercise. Such proceeds with respect to the Series A Warrants and Series B Warrants could not exceed, in the aggregate, $24,000,000.
The foregoing discussion assumes no sale of pre-funded
warrants.
We
intend to use the net proceeds from this offering for the further development of our Therapeutic DNA Production Services and
MDx Testing Services, as well as general corporate purposes, which may include research and development expenses, capital
expenditures, working capital and general and administrative expenses, and potential acquisitions of or investments in businesses,
products and technologies that complement our business, although we have no present commitments or agreements to make any such
acquisitions or investments as of the date of this prospectus. Pending these uses, we intend to invest the funds in short-term,
investment grade, interest-bearing securities. It is possible that, pending their use, we may invest the net proceeds in a way that
does not yield a favorable, or any, return for us.
Our expected use of net proceeds from this offering
represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot currently
allocate specific percentages of the net proceeds that we may use for the purposes specified above, and we cannot predict with certainty
all of the particular uses for the net proceeds to be received upon the completion of this offering, or the amounts that we will actually
spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds will vary depending on numerous factors,
including our ability to obtain additional financing. We may find it necessary or advisable to use the net proceeds for other purposes,
and our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding
the application of the net proceeds from this offering. See “Risk Factors” for a discussion of certain risks that may affect
our intended use of the net proceeds from this offering.
Market
Price of our Common Stock and Related Stockholder Matters
Market Information
Our common stock is listed on The Nasdaq Stock
Market under the symbol “APDN.” A description of the common stock that we are issuing in this offering is set forth under
the heading “Description of Securities” beginning on page 34 of this prospectus. We do not intend to apply for
the listing of the pre-funded warrants or Series Warrants that are part of this offering on any national securities exchange.
The last reported sale price for our common stock
on August 3, 2022 was $4.10 per share.
Holders
As of July 27, 2022, we had 409 record holders
of our common stock, and no preferred stock issued and outstanding. The number of record holders was determined from the records of our
transfer agent and does not include beneficial owners of common stock whose shares are held in the names of various security brokers,
dealers, and registered clearing agencies. The transfer agent of our common stock and publicly traded warrants is American Stock Transfer &
Trust Company, 6201 15th Avenue, Brooklyn, New York 11219.
Dividend Policy
We have never declared or paid any cash dividends
on our common stock. We do not anticipate paying any cash dividends to stockholders in the foreseeable future. In addition, any future
determination to pay cash dividends will be at the discretion of the board of directors and will be dependent upon our financial condition,
results of operations, capital requirements, and such other factors as the board of directors deem relevant.
Capitalization
The following table sets forth our capitalization
as of March 31, 2022:
| • | on a pro forma, as adjusted basis, after giving effect to the application of the net proceeds of this
offering and after deducting the Placement Agent fees and estimated offering expenses payable by us. |
The information set forth in the following table
should be read in conjunction with and is qualified in its entirety by “Use of Proceeds” above, as well as our “Management’s
Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the notes to those financial
statements incorporated by reference in this prospectus. See “The Offering” in this prospectus for information relating to
the expected number of shares of our common stock to be outstanding after this offering.
| |
|
As of March 31, 2022 |
|
| |
|
Actual |
|
|
Pro Forma, As
Adjusted for
this Offering* |
|
| Cash and cash equivalents |
|
$ |
6,512,784 |
|
|
$ |
17,413,480 |
|
| Stockholders’ Equity (Deficit): |
|
|
|
|
|
|
|
|
| Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; -0- shares issued and outstanding as of March 31, 2022 |
|
|
- |
|
|
|
|
|
| Series A Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; -0- shares outstanding as of March 31, 2022 |
|
|
- |
|
|
|
|
|
| Series B Preferred stock, par value $0.001 per share; 10,000,000 shares authorized; -0- shares outstanding as of March 31, 2022 |
|
|
- |
|
|
|
|
|
| Common stock, $0.001 par value per share; 200,000,000 shares authorized; 8,234,320 shares issued and outstanding as of March 31, 2022 |
|
|
8,236 |
|
|
|
11,236 |
|
| Additional paid-in capital |
|
|
298,351,897 |
|
|
|
287,339,141 |
|
| Accumulated deficit |
|
|
(290,712,648 |
) |
|
|
(290,712,648) |
|
| Total Stockholders’ Equity (Deficit) |
|
$ |
7,647,485 |
|
|
$ |
(3,362,271) |
|
*Assumes a $12,000,000 capital raise with net cash proceeds of $10,900,696
and sale of 3,000,000 shares of our common stock in this offering.
The discussion and table above are based on
8,234,320 shares of our common stock outstanding as of March 31, 2022, which excludes 6,000,000 shares of our common stock
that may be issued upon exercise of pre-funded warrants and Series Warrants issued in this offering, 1,067,614 shares of common stock
issuable upon exercise of outstanding options, 2,988,163 shares of common stock issuable upon exercise of outstanding warrants, 2,761,777
shares available for grant under our 2005 and 2020 Equity Incentive Plans as of such date. The discussion and table above assume no sale
of pre-funded warrants, which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis.
Dilution
If you invest in our common stock and/or pre-funded
warrants in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering
price per share of our common stock and the as adjusted net tangible book value per share of our common stock after this offering. Our
net tangible book value as of March 31, 2022 was approximately $10.2 million, or $1.24 per share of our common stock (based upon
8,234,320 shares of our common stock outstanding). Net tangible book value per share is equal to our total tangible assets less our total
liabilities, divided by the number of shares of our outstanding common stock.
After giving effect to the sale of shares of our
common stock and accompanying Series Warrants in this offering at the public offering price of $4.00 per share, and after deducting the
Placement Agent fee and estimated offering expenses payable by us, and excluding the proceeds, if any, from the exercise of the Series
Warrants and pre-funded warrants, if any, issued in this offering, our as adjusted net tangible book value as of March 31, 2022 would
have been approximately ($795,350), or ($0.07) per share of common stock. This represents an immediate decrease in as adjusted net tangible
book value of $1.31 per share to our existing stockholders, and an immediate dilution of $3.93 per share to new investors purchasing
securities in this offering at the public offering price.
The following table illustrates this dilution on
a per share basis:
| Public offering price per share and accompanying Series Warrant |
|
|
|
|
|
$ |
4.00 |
|
| Historical net tangible book value per share as of March 31, 2022 |
|
$ |
1.24 |
|
|
|
|
|
| Pro forma decrease in net tangible book value per share attributable to investors in this offering |
|
$ |
(1.31) |
|
|
|
|
|
| As adjusted net tangible book value per share after giving effect to this offering |
|
|
|
|
|
$ |
(0.07) |
|
| Dilution per share to investors participating in this offering |
|
|
|
|
|
$ |
3.93 |
|
The foregoing discussion and table do not take
into account further dilution to investors in this offering that could occur upon the exercise of outstanding options and warrants, including
the pre-funded warrants and Series Warrants offered in this offering, having a per share exercise price less than the public offering
price per share in this offering.
The discussion and table above are based on
8,234,320 shares of our common stock outstanding as of March 31, 2022, which excludes 6,000,000 shares of our common stock that
may be issued upon exercise of pre-funded warrants and Series Warrants issued in this offering, 1,067,614 shares of common stock issuable
upon exercise of outstanding options, 2,988,163 shares of common stock issuable upon exercise of outstanding warrants, 2,784,085 shares
available for grant under our 2005 and 2020 Equity Incentive Plans as of such date.
The discussion and table above assume no sale of pre-funded warrants,
which, if sold, would reduce the number of shares of common stock that we are offering on a one-for-one basis.
To the extent that our outstanding options or warrants
are exercised, new options are issued under our equity incentive plan, or additional shares of our common stock are issued in the future,
there may be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because
of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating
plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could
result in further dilution to our stockholders.
SECURITY
OWNERSHIP OF CERTAIN BENEFICIAL OWNERS
AND MANAGEMENT
The following table sets forth certain information
regarding the shares of our common stock beneficially owned as of July 27, 2022, (i) by each person who is known to us to beneficially
own 5% or more of the outstanding common stock, (ii) by each of our principal executive officer, our principal financial officer
and our other executive officers and by each of our directors and (iii) by all executive officers and directors as a group.
Unless otherwise indicated below, each person or
entity has an address in care of our principal executive offices at 50 Health Sciences Drive, Stony Brook, New York 11790.
| Name and Address of Beneficial Owner | |
Title of Class | |
Number of
Shares
Owned(1)(2) | | |
Percentage
of Class(3) | |
| Executive Officers and Directors: | |
| |
| | | |
| | |
| James A. Hayward | |
Common Stock | |
| 506,481 | (4) | |
| 5.41 | % |
| Yacov A. Shamash | |
Common Stock | |
| 26,225 | (5) | |
| * | |
| John Bitzer, III | |
Common Stock | |
| 57,155 | (6)(7) | |
| * | |
| Robert B. Catell | |
Common Stock | |
| 24,340 | (11) | |
| * | |
| Joseph D. Ceccoli | |
Common Stock | |
| 23,847 | (8) | |
| * | |
| Beth M. Jantzen | |
Common Stock | |
| 72,518 | (12) | |
| * | |
| Judith Murrah | |
Common Stock | |
| 83,990 | (13) | |
| * | |
| Clay Shorrock | |
Common Stock | |
| 46,546 | (16) | |
| | |
| Scott L. Anchin | |
Common Stock | |
| 24,288 | (15) | |
| * | |
| Sanford R. Simon | |
Common Stock | |
| 23,488 | (9) | |
| * | |
| Elizabeth Schmalz | |
Common Stock | |
| 22,150 | (10) | |
| * | |
| All directors and officers as a group (11 persons) | |
Common Stock | |
| 911,028 | (14) | |
| 9.38 | % |
| 5% Stockholders: | |
| |
| | | |
| | |
| Dillon Hill | |
Common Stock | |
| 658,739 | (17) | |
| 6.83 | % |
* indicates less than one percent
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power
with respect to the shares shown. Except as indicated by footnote and subject to community property laws where applicable, to our knowledge,
the stockholders named in the table have sole voting and investment power with respect to all shares of common stock shown as beneficially
owned by them. A person is deemed to be the beneficial owner of securities that can be acquired by such person within 60 days upon the
exercise of options, warrants or convertible securities (in any case, the “Currently Exercisable Options”). |
| (2) | Does not include the remaining unvested shares subject to options granted on November 1, 2021 pursuant to the 2020 Equity Incentive
Plan, which vest 100% of the underlying shares on the one-year anniversary of grant, including 29,845 for each of Mr. Anchin, Ms. Schmalz
and Mr. Catell, 30,094 for Mr. Simon, 30,840 for Mr. Ceccoli, 31,586 for Mr. Bitzer and 31,834 for Mr. Shamash. |
| (3) | Based upon 8,982,520
shares of common stock outstanding as of July 27, 2022. Each beneficial owner’s
percentage ownership is determined by assuming that the Currently Exercisable Options that
are beneficially held by such person (but not those held by any other person) have been exercised
and converted. |
| (4) | Includes 372,295 shares underlying Currently Exercisable Options. |
| (5) | Includes 24,640 shares underlying Currently Exercisable Options. |
| (6) | Includes 21,920 shares underlying Currently Exercisable Options
for Mr. Bitzer. |
| (7) | Includes 34,563 shares of common stock owned by Delabarta, Inc. (“Delabarta”), a wholly-owned subsidiary of ABARTA, Inc.
(“ABARTA”). Mr. Bitzer is former President and a member of the board of directors of each of Delabarta and ABARTA. Mr. Bitzer
disclaims beneficial ownership of the shares held by Delabarta except to the extent of his pecuniary interest therein. |
| (8) | Includes 23,278 shares underlying Currently Exercisable Options. |
| (9) | Includes 23,416 shares underlying Currently Exercisable Options. |
| (10) | Includes 21,374 shares underlying Currently Exercisable Options. |
| (11) | Includes 22,400 shares underlying Currently Exercisable Options. |
| (12) | Includes 72,446 shares underlying Currently Exercisable Options. |
| (13) | Includes 81,443 shares underlying Currently Exercisable Options. |
| (14) | Includes 733,796 shares underlying Currently Exercisable Options. |
| (15) | Includes 24,038 shares underlying Currently Exercisable Options. |
| (16) | Includes 46,546 shares underlying Currently Exercisable Options. |
| (17) | This information is based on a Form 13G/A filed with the SEC on January 21, 2021 by
Bruce Grossman. Bruce Grossman reported sole and shared voting and sole and shared dispositive power of 658,739 shares of common stock
underlying currently exercisable warrants. The address of Bruce Grossman is c/o Dillon Hill Capital LLC, 200 Business Park Drive, Suite 306,
Armonk, NY 10504. |
CERTAIN
RELATIONSHIPS AND RELATED TRANSACTIONS
Other Relationships
On
July 17, 2019, we issued $1.5 million of secured convertible notes (the “July 2019 Notes”), bearing interest at
a rate of 6% per annum, in a non-brokered private placement with an accredited investor, Dillon Hill Capital, LLC. Dillion Hill Capital,
LLC and Dillion Hill Investment Company (together the “Warrant Investors”) are both controlled by Bruce Grossman, who is a
beneficial holder of more than five percent of our common stock. See “Security Ownership of Certain Beneficial Owners
and Management.”
On
November 15, 2019, we closed an underwritten public offering where we issued and sold 2,285,000 shares of our common stock and
2,285,000 accompanying common warrants (the “2019 Warrants”) each with the right to purchase one share of our common
stock at an exercise price of $5.25 per share. In such offering, the Warrant Investors purchased certain of the 2019 Warrants. On
October 7, 2020, we entered into Warrant Exercise Agreements (each, a “Warrant Exercise Agreement”) with each of
the Warrant Investors, whereby 318,000 of our 2019 Warrants were exercised. The gross proceeds to the Company from this partial
exercise of the 2019 Warrants were $1,669,500.
On
October 9, 2020, the Company entered into a letter agreement with Dillon Hill Capital, LLC as sole holder of the July 2019 Notes
for the repayment in full of such notes, in an aggregate amount of $1,665,581, representing their outstanding principal amount plus accrued
but unpaid interest through their scheduled maturity date. The Company paid such payoff amount on October 9, 2020.
In
consideration of this partial exercise of the 2019 Warrants and of the consent to repayment of the July 2019 Notes, the Company
agreed to issue pursuant to the Warrant Exercise Agreement (the “Private Placement”), in addition to the 318,000 shares of
common stock issued upon exercise of the 2019 Warrants, 159,000 replacement warrants (the “Replacement Warrants”) to the
Warrant Investors, which is an amount equal to one-half the amount of the 2019 Warrants exercised pursuant to the Warrant Exercise Agreements.
The Replacement Warrants have an average exercise price of $2.80, the closing price on The Nasdaq Capital Market of the Company’s
common stock on the date of the applicable warrant exercise.
The
quantity, issue date, exercise price and expiration date of the Replacement Warrants are listed in the table below:
| Quantity |
|
|
Issuance Date |
|
Exercise Price |
|
|
Expiration Date |
| 159,000 |
|
|
October 7,
2020 |
|
$ |
2.80 |
|
|
October 7,
2025 |
| 50,000 |
|
|
December 9, 2020 |
|
$ |
2.80 |
|
|
December 9, 2025 |
| 50,000 |
|
|
December 10, 2020 |
|
$ |
2.80 |
|
|
December 10, 2025 |
Each
Replacement Warrant is exercisable for one share of common stock beginning on the date of issuance thereof and ending on the five-year
anniversary of such date. The exercise price and number of shares of common stock issuable upon exercise of the Replacement Warrants are
subject to adjustment in the event of any stock dividend, split, recapitalization, reorganization or similar transaction, as described
in the Replacement Warrants. Subject to limited exceptions, a holder of a Replacement Warrant will not have the right to exercise any
portion of its Replacement Warrant if the holder, together with its affiliates, would beneficially own in excess of 9.99% of the number
of shares of common stock outstanding immediately after giving effect to such exercise (the “Beneficial Ownership Limitation”);
provided that upon 61 days’ prior notice to the Company, the holder may elect to increase or decrease the Beneficial Ownership Limitation,
although in no event may the Beneficial Ownership Limitation exceed 9.99%. Each Replacement Warrant includes an adjustment provision that,
subject to certain exceptions, reduces its exercise price if the Company issues common stock or common stock equivalents at a price lower
than the then-current exercise price of such Replacement Warrant, subject to a minimum exercise price of 21% of such Replacement Warrant’s
initial exercise price per share. Under certain limited circumstances, including that the daily volume weighted average price of the common
stock for each of 20 consecutive trading days has exceeded three times the exercise price of such Replacement Warrant, the Company may
call for cancellation of all or any portion of such Replacement Warrant for which a notice of exercise has not yet been delivered for
consideration equal to $0.001 per share of common stock for which such Replacement Warrant is excisable.
As
of October 9, 2020, all of the obligations and liabilities of the Company and its affiliates under the July 2019 Notes, the
related purchase agreement, and related Security Agreements, and any other related documents and instruments, was automatically satisfied
in full, and all related liens, mortgages or other security interests were automatically released.
Director Independence
Our
board of directors has determined that currently and at all times during the fiscal year ended September 30, 2021, each of our
directors other than Dr. Hayward and Mr. Anchin—consisting of John Bitzer, III, Robert B. Catell, Joseph D.
Ceccoli, Yacov A. Shamash, Sanford R. Simon, and Elizabeth M. Schmalz—are and were “independent” as
defined by the listing standards of Nasdaq, constituting a majority of independent directors on our board of directors as required
by the rules of Nasdaq. Our board of directors considers in its evaluation of independence whether any director has a
relationship with us that would interfere with the exercise of independent judgment in carrying out his or her responsibilities of a
director.
Description
of Securities
The following description of our common stock,
pre-funded warrants and accompanying Series Warrants summarizes the material terms and provisions of the securities that we may issue
in connection with this offering. It may not contain all the information that is important to you. For the complete terms of our common
stock, please refer to our Certificate of Incorporation and our by-laws (“By-Laws”), which are filed as exhibits to the registration
statement which includes this prospectus. See “Where You Can Find More Information” and “Incorporation by Reference.”
The Delaware General Corporation Law (“DGCL”) may also affect the terms of these securities. The summary below is qualified
in its entirety by reference to our Certificate of Incorporation and By-Laws, each as in effect at the time of any offering of securities
under this prospectus.
As of July 27, 2022, our authorized capital
stock consists of 200,000,000 shares of common stock, par value $0.001 per share, of which 8,982,520 shares were issued and outstanding,
and 10,000,000 shares of preferred stock, par value $0.001 per share, of which no shares were issued and outstanding. In addition, as
of July 27, 2022, there were issued and outstanding options to purchase 1,063,143 shares of common stock, warrants to purchase 2,239,963
shares of our common stock and 2,778,556 shares available for grant under our 2020 Equity Incentive Plan. The authorized and unissued
shares of common stock and preferred stock are available for issuance without further action by our stockholders.
Common Stock
Each stockholder of our common stock is entitled
to one vote for each share issued and outstanding held on all matters to be voted upon by the stockholders. Our shares of common stock
have no preemptive, conversion, or redemption rights. The rights, preferences, and privileges of the holders of common stock are subject
to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock. Upon the sale of substantially
all of our stock or assets or dissolution, liquidation or winding up, and after all liquidation preferences payable to any series of preferred
stock entitled thereto have been satisfied, our remaining assets shall be distributed to all holders of common stock and any similarly
situated stockholders who are not entitled to any liquidation preference or, if there be an insufficient amount to pay all such stockholders,
then ratably among such holders. All of our issued and outstanding shares of common stock are fully paid and non-assessable. The holders
of shares of our common stock will be entitled to such cash dividends as may be declared from time to time by our board of directors from
funds available therefor.
The shares of common stock offered by this prospectus,
when issued and paid for, will also be fully paid and non-assessable.
Our common stock is listed on The Nasdaq Capital
Market under the symbol “APDN.” American Stock Transfer & Trust Company, located in Brooklyn, New York, is the transfer
agent and registrar for our common stock.
Preferred Stock
Our Certificate of Incorporation provides that
our board of directors may, by resolution, designate classes of preferred stock in the future. The designated series of preferred stock
shall have such powers, designations, preferences and relative, participation or optional or other special rights and qualifications,
limitations or restrictions as shall be expressed in the resolution adopted by the board of directors. Once designated by our board of
directors, each series of preferred stock will have specific financial and other terms described in the documents that govern the preferred
stock, which include our Certificate of Incorporation and any certificates of designation that our board of directors may adopt. Prior
to the issuance of shares of each series of preferred stock, the board of directors is required by the DGCL and our Certificate of Incorporation
to adopt resolutions and file a certificate of designations with the Secretary of State of the State of Delaware. The certificate of designations
fixes for each class or series the designations, powers, preferences, rights, qualifications, limitations and restrictions, including,
but not limited to, some or all of the following:
| • | the number of shares constituting that series and the distinctive designation of that series, which number may be increased or decreased
(but not below the number of shares then outstanding) from time to time by action of the board of directors; |
| • | the dividend rate and the manner and frequency of payment of dividends on the shares of that series, whether dividends will be cumulative,
and, if so, from which date; |
| • | whether that series will have voting rights, in addition to any voting rights provided by law, and, if so, the terms of such voting
rights; |
| • | whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision
for adjustment of the conversion rate in such events as the board of directors may determine; |
| • | whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption; |
| • | whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount
of such sinking fund; |
| • | whether or not the shares of the series will have priority over or be on a parity with or be junior to the shares of any other series
or class in any respect; |
| • | the rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation,
and the relative rights or priority, if any, of payment of shares of that series; and |
| • | any other relative rights, preferences and limitations of that series. |
Although our board of directors has no intention
at the present time of doing so, it could authorize the issuance of a series of preferred stock that could, depending on the terms of
such series, impede the completion of a merger, tender offer or other takeover attempt.
Series Warrants
The following summary of certain terms and
provisions of the Series Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by,
the provisions of the Series Warrants, the forms of which are filed as exhibits to the registration statement of which this prospectus
forms a part. Prospective investors should carefully review the terms and provisions of the forms of Series Warrant for complete descriptions
of the terms and conditions of the Series Warrants.
We are selling to investors in this offering of
shares of our common stock and/or pre-funded warrants in this offering a Series A Warrant to purchase 1 share of our common stock
and a Series B Warrant to purchase 1 share of our common stock for each share and/or pre-funded warrant purchased in this offering
for a combined purchase price of 4.00. The Series A Warrants and the Series B Warrants are referred to herein together as the “Series
Warrants”.
Each Series A Warrant will be exercisable beginning
on the Initial Exercise Date, which is the date of closing, at an exercise price of $4.00 per share, subject to adjustment. The Series
A Warrants will be exercisable for five years from the Initial Exercise Date, but not thereafter. Each Series B Warrant will be exercisable
beginning on the Initial Exercise Date, at an exercise price of $4.00 per share, subject to adjustment. The Series B Warrants will be
exercisable for thirteen months from the Initial Exercise Date, but not thereafter. No fractional shares of common stock will be issued
in connection with the exercise of a Series Warrant. In lieu of fractional shares, we will round up to the next whole share.
Subject to limited exceptions, a holder of
Series Warrants will not have the right to exercise any portion of its Series Warrants if the holder, together with its affiliates, would
beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of our common stock outstanding
immediately after giving effect to such exercise (the “Beneficial Ownership Limitation”); provided, however, that upon 61
days’ prior notice to the Company, the holder may increase or decrease the Beneficial Ownership Limitation, provided that in no
event shall the Beneficial Ownership Limitation exceed 9.99%.
The Series Warrants contain a “cashless
exercise” feature that allows holders to exercise the Series Warrants without a cash payment to the Company upon the terms set
forth in the Series Warrants, if, at the time of exercise there is no effective registration statement registering, or the prospectus
contained therein is not available for the issuance of the shares to the exercising Series Warrant holder.
In the case of certain fundamental transactions
affecting the Company, a holder of Series Warrants, upon exercise of such Series Warrants after such fundamental transaction, will have
the right to receive, in lieu of shares of the Company’s common stock, the same amount and kind of securities, cash or property
that such holder would have been entitled to receive upon the occurrence of the fundamental transaction, had the Series Warrants been
exercised immediately prior to such fundamental transaction. In lieu of such consideration, a holder of Series Warrants may instead elect
to receive a cash payment based upon the Black-Scholes value of their Series Warrants.
The exercise price and number of the shares
of our common stock issuable upon the exercise of the Series Warrants will be subject to adjustment in the event of any stock dividends
and splits, recapitalization, reorganization or similar transaction, as described in the Series Warrants.
We do not intend to list the Series Warrants
on any securities exchange or nationally recognized trading system. Except as otherwise provided in the Series Warrants or by virtue
of such holder’s ownership of shares of our common stock, the holders of the Series Warrants do not have the rights or privileges
of holders of our common stock, including any voting rights, until they exercise their Series Warrants.
Pre-Funded Warrants
The following summary of certain terms and provisions
of pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions
of the pre-funded warrant, the form of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of
the terms and conditions of the pre-funded warrants.
Each pre-funded warrant offered hereby will
have an initial exercise price per share equal to $0.0001. The pre-funded warrants will be immediately exercisable and may be exercised
at any time until the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon
exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting
our common stock and the exercise price. The pre-funded warrants will be issued separately from the accompanying Series Warrants and
may be transferred separately immediately thereafter.
The pre-funded warrants will be exercisable, at
the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for
the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below).
A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own
more than 4.99 % of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from
the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s pre-funded
warrants up to 9.99 % of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such
percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this
offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding
common stock. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of
fractional shares, we will round up to the next whole share.
At any time, in lieu of making
the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may
elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according
to a formula set forth in the pre-funded warrants.
Subject to applicable laws, a pre-funded warrant
may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments
of transfer.
We do not intend to list the pre-funded warrants
on any securities exchange or nationally recognized trading system. Except as otherwise provided in the pre-funded warrants or by virtue
of such holder’s ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges
of holders of our common stock, including any voting rights, until they exercise their pre-funded warrants.
Possible Anti-Takeover Effects of Delaware Law and our Certificate
of Incorporation and By-Laws
Our Certificate of Incorporation contains provisions
that could make it more difficult to acquire control of our company by means of a tender offer, open market purchases, a proxy contest
or otherwise. A description of these provisions is set forth below.
Anti-Takeover Effects of Delaware Law
Companies incorporated in Delaware are subject
to the provisions of Section 203 of the DGCL, or Section 203, unless the corporation has “opted out” of these provisions
with an express provision in its original certificate of incorporation or an express provision in its certificate of incorporation or
by-laws resulting from a stockholders’ amendment approved by at least a majority of the outstanding voting shares. We have opted
out of Section 203 with an express provision in our Certificate of Incorporation. Therefore, the anti-takeover effects of Section 203
do not apply to us.
In general, Section 203 prohibits a publicly-held
Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year
period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed
manner. A “business combination” includes, among other things, a merger, asset or stock sale or other transaction resulting
in a financial benefit to the interested stockholder. An “interested stockholder” is a person who, together with affiliates
and associates, owns, or did own within three years prior to the determination of interested stockholder status, 15% or more of the corporation’s
voting stock.
Under Section 203, a business combination
between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions: before the stockholder
became interested, the board of directors approved either the business combination or the transaction which resulted in the stockholder
becoming an interested stockholder; upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder,
the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced,
excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee
stock plans, in some instances; or at or after the time the stockholder became interested, the business combination was approved by the
board of directors of the corporation and authorized at an annual or special meeting of the stockholders by the affirmative vote of at
least two-thirds of the outstanding voting stock which is not owned by the interested stockholder.
Election and Removal of Directors
Directors will be elected by a plurality of
the voting power of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of
directors. Our Certificate of Incorporation does not provide for a classified board of directors or for cumulative voting in the
election of directors. Under Article VIII of the Certificate of Incorporation and Section 3.13 of the By-Laws, directors
may be removed by the stockholders of the Company only for cause, and in such case only by the affirmative vote of the holders of at
least a majority of the voting power of the issued and outstanding shares of capital stock of the Company then entitled to vote in
the election of directors. On December 21, 2015, the Court of Chancery of the State of Delaware invalidated as a matter of law
provisions of the certificate of incorporation and bylaws of VAALCO Energy, Inc. (“VAALCO”), a Delaware
corporation, that permitted the removal of VAALCO’s directors by its stockholders only for cause. In In re VAALCO
Energy, Inc. Stockholder Litigation, Consol. C.A. No. 11775-VCL (Del. Ch. Dec. 21, 2015), the Court ruled from
the bench to hold that, in the absence of a classified board or cumulative voting, VAALCO’s “only for-cause”
director removal provisions conflict with Section 141(k) of the DGCL and are therefore invalid. Because the
Company’s Certificate of Incorporation and By-Laws contain similar “only for-cause” director removal provisions
and the Company does not have a classified board of directors or cumulative voting, the Company will not attempt to enforce the
foregoing “only for-cause” director removal provision in light of the recent VAALCO decision.
Size of Board and Vacancies
The authorized number of directors may be determined
by the board of directors, provided the board shall consist of at least one (1) member. No decrease in the number of directors constituting
the board shall shorten the term of any incumbent director.
Vacancies occurring on our board of directors for
any reason and newly created directorships resulting from an increase in the authorized number of directors may be filled only by a vote
of a majority of the remaining members of the board of directors, although less than a quorum, or by a sole remaining director, at any
meeting of the board of directors.
Amendment
The Certificate of Incorporation may be amended
in the manner prescribed by the DGCL. The board of directors is authorized to adopt, amend, alter or repeal the By-Laws by the affirmative
vote of at least a majority of the board of directors then in office. No amendment to the Certificate of Incorporation or the By-Laws
may adversely affect any indemnification right or protection of any director, officer, employee or other agent existing at the time of
such amendment, repeal or adoption of an inconsistent provision for or in respect of any act, omission or other matter occurring, or any
action or proceeding accruing or arising prior to such amendment, repeal or adoption of an inconsistent provision.
Authorized but Unissued Shares of Common Stock and of Preferred
Stock
We believe that the availability of the “Blank
Check” preferred stock under our Certificate of Incorporation provides us with flexibility in addressing corporate issues that may
arise. The board of directors has the power, subject to applicable law, to issue series of preferred stock that could, depending on the
terms of the series, impede the completion of a merger, tender offer or other takeover attempt that some, or a majority, of the stockholders
might believe to be in their best interests or in which stockholders might receive a premium for their stock over the then prevailing
market price of the stock. Our board of directors may issue preferred stock with voting rights or conversion rights that, if exercised,
could adversely affect the voting power of the holders of common stock.
The authorized shares of preferred stock, as well
as shares of common stock, will be available for issuance without further action by our stockholders, unless action is required by applicable
law or the rules of any stock exchange on which our securities may be listed. Having these authorized shares available for issuance
allows us to issue shares without the expense and delay of a special stockholders’ meeting. We may use additional shares for a variety
of purposes, including future public offerings to raise additional capital, to fund acquisitions and as employee compensation. The existence
of authorized but unissued shares of common stock and preferred stock could render more difficult or discourage an attempt to obtain control
of our company by means of a proxy contest, tender offer, merger or otherwise. The above provisions may deter a hostile takeover or delay
a change in control or management of our company.
Advance Notice Procedure
Our By-Laws provide an advance notice procedure
for stockholders to nominate director candidates for election or to bring business before an annual meeting of stockholders. Only persons
nominated by, or at the direction of, our board of directors or by a stockholder of record who has given proper and timely notice to our
secretary prior to the meeting at which such stockholder is entitled to vote and appears, will be eligible for election as a director.
In addition, any proposed business other than the nomination of persons for election to our board of directors must constitute a proper
matter for stockholder action pursuant to a proper notice of meeting delivered to us. For notice to be timely, it must generally be delivered
to our secretary not less than 90 nor more than 120 calendar days prior to the first anniversary of the previous year’s annual meeting
(or if the date of the annual meeting is more than 30 calendar days before or more than 60 calendar days after the anniversary date of
the previous year’s annual meeting, not earlier than the 120th calendar day prior to such meeting and not later than either the
90th calendar day prior to such meeting or the 10th calendar day after public disclosure of the date of such meeting is first made by
us). These advance notice provisions may have the effect of precluding the conduct of certain business at a meeting if the proper procedures
are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors
or otherwise attempt to obtain control of us.
Special Meetings of Stockholders
Our By-Laws provide that special meetings of stockholders
may be called only by the Chairman of the Board, the Chief Executive Officer, or the board of directors pursuant to a resolution adopted
by a majority of the board.
PLAN
OF DISTRIBUTION
Pursuant to an engagement
agreement, dated June 22, 2022 (the “Placement Agent Agreement”), we have engaged H.C. Wainwright & Co., LLC,
or the Placement Agent, to act as our exclusive placement agent to solicit offers to purchase the securities offered pursuant to this
prospectus on a reasonable best efforts basis. The engagement agreement does not give rise to any commitment by the Placement Agent to
purchase any of our securities, and the Placement Agent will have no authority to bind us by virtue of the engagement agreement. The Placement
Agent is not purchasing or selling any of the securities offered by us under this prospectus, nor is it required to arrange for the purchase
or sale of any specific number or dollar amount of securities. The Placement Agent has agreed to use reasonable best efforts to arrange
for the sale of the securities by us. The Placement Agent does not guarantee that it will be able to raise new capital in any prospective
offering. The Placement Agent may engage sub-agents or selected dealers to assist with the offering.
We will enter into a securities
purchase agreement directly with institutional investors, at such investor’s option, which purchase our securities in this offering.
Investors which do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase
of our securities in this offering.
We will deliver the securities being issued to
the investors upon receipt of investor funds for the purchase of the securities offered pursuant to this prospectus. We expect to deliver
the securities being offered pursuant to this prospectus on or about August 8, 2022. There is no minimum number of securities or amount
of proceeds that is a condition to closing of this offering.
Fees and Expenses
We
have agreed to pay the Placement Agent a cash fee equal to 7.0% of the aggregate gross proceeds raised in this offering and to reimburse
the Placement Agent for its legal fees and expenses and other out-of-pocket expenses in an amount up to $50,000 and for its closing costs
in an amount up to $15,950. We estimate the total offering expenses of this offering that will be payable by us, excluding the Placement
Agent fees and expenses, will be approximately $193,350.
Regulation M
The Placement Agent may be
deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act, and any commissions received by it and
any profit realized on the resale of the securities sold by it while acting as principal might be deemed to be underwriting discounts
or commissions under the Securities Act. As an underwriter, the Placement Agent would be required to comply with the requirements of the
Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and
regulations may limit the timing of purchases and sales of our securities by the Placement Agent acting as principal. Under these rules and
regulations, the Placement Agent (i) may not engage in any stabilization activity in connection with our securities and (ii) may
not bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted
under the Exchange Act, until it has completed its participation in the distribution.
Indemnification
We
have agreed to indemnify the Placement Agent against certain liabilities, including certain liabilities arising under the Securities Act,
or to contribute to payments that the Placement Agent may be required to make for these liabilities.
Determination of Offering Price
Our common stock is currently traded on The Nasdaq
Stock Market under the symbol “APDN.” On August 3, 2022 the closing price of our common stock was $4.10 per share.
There is a material disparity between the
offering price of the shares of our common stock being offered under this prospectus and pre-funded warrants and the market price of
the common stock at the date of this prospectus. We believe that the market price of our common stock at the date of this prospectus
is not the appropriate public offering price for the shares of our common stock because the market price is affected by a number of
factors. The final public offering price was determined by negotiation between us, the Placement Agent and the investors in this
offering. The principal factors considered by us and the Placement Agent in determining the final public offering price
included:
| • | the recent trading history of our common stock on The Nasdaq Capital Market, including market prices and trading volume of our common
stock; |
| • | the current market price of our common stock on The Nasdaq Capital Market; |
| • | the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; |
| • | the information set forth or incorporated by reference in this prospectus and otherwise available to the Placement Agent; |
| • | our past and present financial performance and an assessment of our management; |
| • | our prospects for future earnings and the present state of our products; |
| • | the current status of competitive products and product developments by our competitors; |
| • | our history and prospects, and the history and prospects of the industry in which we compete; |
| • | the general condition of the securities markets at the time of this offering; and |
| • | other factors deemed relevant by the Placement Agent and us. |
The final public offering price stated on the
cover page of this prospectus should not be considered an indication of the actual value of the shares of common stock and accompanying
Series Warrants and/or pre-funded warrants and accompanying Series Warrants sold in this offering. That price is subject to change as
a result of market conditions and other factors and we cannot assure you that the shares of common stock and accompanying Series Warrants
and/or pre-funded warrants and accompanying Series Warrants sold in this offering can be resold at or above the public offering price.
Lock-up Agreements
Our officers and directors, representing 15.13%
of our outstanding shares of common stock, have agreed with the Placement Agent to be subject to a lock-up period of 90 days following
the closing of this offering. This means that, during the applicable lock-up period, such persons may not offer for sale, contract to
sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly,
any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock. Certain
limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed
to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering, although
we will be permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. The lock-up
period is subject to an additional extension to accommodate for our reports of financial results or material news releases. The Placement
Agent may, in its sole discretion and without notice, waive the terms of any of these lock-up agreements.
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is American Stock Transfer & Trust Company, LLC.
Other Relationships
The Placement Agent and its affiliates have engaged,
and may in the future engage, in investment banking transactions and other commercial dealings in the ordinary course of business with
us or our affiliates. The Placement Agent has received, or may in the future receive, customary fees and commissions for these transactions.
In addition, in the ordinary course of their business
activities, the Placement Agent and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities
(or related derivative securities) for their own account and for the accounts of their customers. Such investments and securities activities
may involve securities and/or instruments of ours or our affiliates. The Placement Agent and its affiliates may also make investment recommendations
and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend
to clients that they acquire, long and/or short positions in such securities and instruments.
Indemnification
We have agreed to indemnify the Placement Agent
against liabilities relating to the offering arising under the Securities Act and the Exchange Act, liabilities arising from breaches
of some or all of the representations and warranties contained in the Placement Agent Agreement, and to contribute to payments that the
Placement Agent may be required to make for these liabilities.
Electronic Distribution
A prospectus in electronic format may be made available
on a website maintained by the Placement Agent and the Placement Agent may distribute prospectuses electronically. Other than the prospectus
in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus
forms a part, has not been approved and/or endorsed by us or the Placement Agent and should not be relied upon by investors.
Foreign Regulatory Restrictions on Purchase of Securities Offered
Hereby Generally
No action has been or will be taken in any jurisdiction
(except in the United States) that would permit a public offering of the securities offered by this prospectus, or the possession, circulation
or distribution of this prospectus or any other material relating to us or the securities offered hereby in any jurisdiction where action
for that purpose is required. Accordingly, the securities offered hereby may not be offered or sold, directly or indirectly, and neither
of this prospectus nor any other offering material or advertisements in connection with the securities offered hereby may be distributed
or published, in or from any country or jurisdiction except in compliance with any applicable rules and regulations of any such country
or jurisdiction.
The Placement Agent may arrange to sell securities
offered by this prospectus in certain jurisdictions outside the United States, either directly or through affiliates, where they are permitted
to do so. See “Where You Can Find More Information.”
Nasdaq Listing
Our common stock is listed on The Nasdaq Capital
Market under the symbol “APDN.”
Experts
Marcum
LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the years ended September 30, 2021 and 2020, as set forth in their report, which is incorporated
by reference in this prospectus and elsewhere in this registration statement. Marcum LLP’s report includes an explanatory paragraph
relating to our ability to continue as a going concern. Our consolidated financial statements are incorporated by reference in reliance
on Marcum LLP’s report, given on their authority as experts in accounting and auditing.
Legal
Matters
Certain legal matters relating to the issuance
of the securities offered by this prospectus will be passed upon for us by McDermott Will & Emery LLP, New York, New York. Certain
legal matters in connection with this offering will be passed upon for the Placement Agent by Ellenoff Grossman & Schole LLP,
New York, New York.
Where
you can find more information
This prospectus is a part of the registration statement
on Form S-1 we filed with the SEC under the Securities Act and does not contain all the information set forth in the registration
statement. Whenever a reference is made in this prospectus to any of our contracts, agreements or other documents, the reference may not
be complete and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other
documents incorporated herein by reference for a copy of such contract, agreement or other document. Because we are subject to the information
and reporting requirements of the Exchange Act, we file or furnish, as applicable, annual, quarterly and current reports, proxy statements
and other information with the SEC. The SEC also maintains a web site that contains reports, proxy and information statements and other
information regarding companies, such as ours, that file documents electronically with the SEC. The website address is www.sec.gov. The
information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive
textual references only. Our Internet address is www.adnas.com. The information found on our website is not part of this prospectus and
investors should not rely on any such information in deciding whether to invest.
Incorporation
by Reference
We have elected to incorporate certain information
by reference into this prospectus. By incorporating by reference, we can disclose important information to you by referring you to other
documents we have filed or will file with the SEC. The information incorporated by reference is deemed to be part of this prospectus,
except for information incorporated by reference that is superseded by information contained in this prospectus. This means that you must
look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously
incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below that
we have previously filed with the SEC, except in each case the information contained in such document to the extent “furnished”
and not “filed” (Commission File No. 001-36745):
All reports and other documents subsequently filed
by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all
such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration
statement but excluding any information furnished and not filed with the SEC, shall be deemed to be incorporated by reference in this
prospectus and to be a part hereof from the date of filing such reports and other documents.
Information in such future filings updates and
supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify
and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein
by reference to the extent that statements in the later filed document modify or replace such earlier statements. Any statement so modified
or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may obtain copies of these documents on the
website maintained by the SEC at http://www.sec.gov. We will furnish to you, upon written or oral request, a copy of any or all of the
documents that have been incorporated by reference, including exhibits to these documents. You may request a copy of those filings at
no cost by writing or telephoning us at Corporate Secretary, Applied DNA Sciences, Inc., 50 Health Sciences Drive, Stony Brook, New
York 11790, (631) 240-8800 or visiting our website at http://www.adnas.com. No information contained on our website is intended to be
included as part of, or incorporated by reference into, this prospectus.
$12,000,000
3,000,000 Shares of Common Stock
and Accompanying Series A Warrants to Purchase 3,000,000 Shares of Common Stock and Series B Warrants to Purchase 3,000,000
Shares of Common Stock
or
3,000,000 Pre-Funded Warrants to Purchase 3,000,000 Shares of Common Stock and Accompanying Series A Warrants to Purchase 3,000,000 Shares of Common
Stock and Series B Warrants to Purchase 3,000,000 Shares of Common Stock
Shares of Common Stock Underlying the Pre-Funded
Warrants and Series Warrants
PROSPECTUS

H.C. Wainwright &
Co.
August 4, 2022
Applied DNA Sciences (NASDAQ:APDN)
Historical Stock Chart
From Mar 2024 to Apr 2024
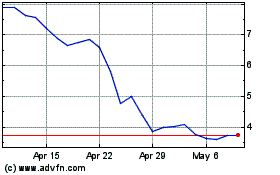
Applied DNA Sciences (NASDAQ:APDN)
Historical Stock Chart
From Apr 2023 to Apr 2024