Additional Proxy Soliciting Materials (definitive) (defa14a)
April 28 2021 - 7:01AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND
EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
SCHEDULE 14A
Proxy Statement Pursuant to Section 14(a) of the Securities Exchange Act of 1934
(Amendment No.__)
Filed by the Registrant
|
☑
|
Filed by a Party other than the Registrant
|
☐
|
Filed by the Registrant
Filed by a Party other than the Registrant
Check the appropriate box:
|
☐
|
Preliminary Proxy Statement
|
|
☐
|
Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2))
|
|
☐
|
Definitive Proxy Statement
|
|
☑
|
Definitive Additional Materials
|
|
☐
|
Soliciting Material Pursuant to § 240.14a-12
|
|
Adverum Biotechnologies, Inc.
|
|
(Name of Registrant as Specified In Its Charter)
|
|
|
|
|
|
(Name of Person(s) Filing Proxy Statement if Other Than the Registrant)
|
Payment of Filing Fee (Check the appropriate box)
|
☑
|
No fee required.
|
|
☐
|
Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11.
|
|
1.
|
Title of each class of securities to which transaction applies:
|
|
|
|
|
|
|
|
2.
|
Aggregate number of securities to which transaction applies:
|
|
|
|
|
|
|
|
3.
|
Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined):
|
|
|
|
|
|
|
|
4.
|
Proposed maximum aggregate value of transaction:
|
|
|
|
|
|
|
|
5.
|
Total fee paid:
|
|
|
|
|
|
|
|
☐
|
Fee paid previously with preliminary materials.
|
|
☐
|
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration
statement number, or the Form or Schedule and the date of its filing.
|
|
6.
|
Amount Previously Paid:
|
|
|
|
|
|
|
|
7.
|
Form, Schedule or Registration Statement No.:
|
|
|
|
|
|
|
|
8.
|
Filing Party:
|
|
|
|
|
|
|
|
9.
|
Date Filed:
|
|
|
|
|
|
|
On April 28, 2021, Adverum Biotechnologies, Inc. mailed the following letter to stockholders:
Your Board is guiding Adverum to successfully execute on its mission to establish ocular gene therapy as
a one-time treatment that preserves patient sight for life. The current standard of care for treating two of the leading causes of blindness is burdensome, often requiring an eye injection every four to eight weeks and a full day for treatment
and recovery in order to avoid vision loss. For millions of patients living with these debilitating ocular diseases, this regimen often persists for the rest of their lives.Adverum is working to change the treatment paradigm.ADVM-022 is an
advanced gene therapy product designed to treat two of the leading causes of blindness (wet age-related macular degeneration and diabetic macular edema) with a single in-office intravitreal injection. We believe it has the potential to be a
“one-and-done” approach for millions of patients around the
world. A
Conversation with Your Director Nominees Vote “FOR” ALL directors on the WHITE proxy card to support Adverum’s successful commercial launch of a transformative gene therapy to treat two of the leading causes of blindness.
Dawn Svoronos Reed Tuckson, M.D. Tom Woiwode, Ph.D. A Conversation with Your Director Nominees
at Adverum’s Upcoming Annual Meeting: Adverum invited our nominees to share with you in their own words how they view the tremendous opportunities ahead, and why they are the leaders we need at this critical time in our journey.
DAWNI joined our Board in December 2020 with great excitement for Adverum’s life-changing therapy
and to help Adverum accelerate its commercialization efforts. Today, I am more confident than ever in our advanced treatment, our team and the path ahead for Adverum to transform patient treatment and reduce the burden of care.My career spans
over 30 years in the biopharmaceutical industry. During my more than 20 years at Merck, I spearheaded and supported dozens of global pharmaceutical product launches, many of which were mass-market opportunities.Simply put – I know what it takes
to bring a product to market. I know the challenges and the pitfalls.And I know the opportunities for all stakeholders – including patients, providers, and stockholders – when you get it right. I intend to support Adverum in doing just
that. REEDI’ve always made decisions first and foremost with patients top of mind – and I joined Adverum because it was clear the team shares this commitment. Patients are the drivers behind Adverum’s mission and are at the center of our
commercialization strategy. As a director, I bring deep experience in the policy and regulatory sides of thehealthcare industry – from provider, to payer to regulator.I have a strong understanding of the complex reimbursement environment,
particularly when it comes to disruptive therapies like our gene therapy product ADVM-022 and I have a proven track record of enhancing patient access, which is critical to our business at this time.TOMI have made a career creating and growing
biotech companies and bring perspective and insights as a significant stockholder of Adverum.As a Managing Director at Versant Ventures, I have been involved in and supported the founding and financing of multiple successful gene therapy and
gene editing companies, including Audentes Therapeutics, Coda Biotherapeutics, CRISPR Therapeutics, Graphite Bio and Passage Bio, among others. Our firm has been an early mover in the space, and is a strong supporter of the innovative approach
being pursued at Adverum. Adverum is approaching an important milestone– commercialization of ADVM-022. What unique skills and experiences do you bring to help Adverum prepare for and navigate this stage of growth?
TOMAs a significant investor of Adverum, my interests are strongly aligned with yours. As you have
seen over the past several years, our Board is committed to enhancing value for stockholders.Our efforts have already allowed our stock to significantly outperform our gene therapy peers. We are focusing on continuing to generate data on
efficacy and inflammation management and advancing ADVM-022 to late stage trials and an eventual submission to the U.S. Food and Drug Administration (FDA) in 2024. REEDAnd we’re not stopping there. We expect our upcoming milestones, including
reporting clinical data and achievingpre-commercialization, clinical and development milestones, to drive significant value for our stockholders throughout the rest of 2021 and beyond. DAWNWe are laser-focused on bringing ADVM-022 to market
and delivering this transformative gene therapy to patients globally. As we announced previously, having aligned with the FDA in the first quarter of 2021, we are planning to accelerate our clinical development program by conducting two global
Phase 3 trials in wet AMD, which we expect to initiate in parallel in the fourth quarter of this year.Following Phase 3 trials, we are targeting a Biologics License Application submission for wet AMD in 2024. We have also completed patient
enrollment in the Phase 2 trial of ADVM-022 for DME. Of course, patient safety is our top priority. While data from our OPTIC study in wet AMD shows that ADVM-022 continues to be well tolerated with ocular cell grades and steroid eye drop use
decreasing over time, we are relentlessly committed to ensuring a seamless patient experience with the most predictable outcome and providing the highest quality product. As such, we are pursuing multiple paths to further improve results with
respect to inflammation management and prophylaxis. This includes weekly meetings with experts from our scientific advisory board and Key Opinion Leaders in our field to review data generated on areal-time basis, solicit input, implement their
recommendations in our studies and apply the learnings from data we are collecting.Importantly, all instances of inflammation that have occurred in our OPTIC study to date are mild to moderate, limited to the front of the eye and are not vision
threatening. What are your plans to drive value creation for Adverum’s stockholders? What is the Board most focused on with respect to ADVM-022?
Looking ahead, we have carefully designed our two upcoming Phase 3 trials to enable us to
demonstrate favorable efficacy and safety profiles for patients.The entire Adverum Board is focused on this important objective to successfully mitigate inflammation, and we look forward to sharing further updates as the program
progresses. REEDAdverum has an incredible opportunity to disrupt and transform the current standard of care for millions of patients around the world facing vision loss. In 2020 alone, $11.5 billion was spent globally on approved anti-VEGF
therapies1 targeting the treatment of ocular diseases. We believe ADVM-022 is uniquely positioned to potentially disrupt this sizable market, and we are encouraged not only by the unmet needs, but also by our constructive discussions with the
FDA, which have allowed us to accelerate our clinical development program for ADVM-022. Adverum’s director nominees directly reflect the critical needs of a company working to develop and launch the first mass market gene therapy. We have the
right technology, team and strategy in place to meet patient needs and deliver value for our stockholders. DAWNAdverum has frequently refreshed its Board to ensure the skillsets of its directors match its current needs. We are focused on
having the right leadership, at the right time. In fact, we are currently in process to recruit a high-quality independent director with commercial gene therapy experience to be named in 2021. We have been actively engaging with many of our
stockholders about top candidates given the scarcity of gene therapy products that have been approved and have already interviewed candidates to fill this slot. TOMWe have a very strong Board with a passion for Adverum’s mission and a track
record of delivering value. Our directors bring highly relevant experience and capabilities to help us execute on our strategy as we move towards commercialization of ADVM-022.This team knows how to build successful biotech companies. What
makes you confident you are the best directors to oversee Adverum’s strategy and create value at this point in Adverum’s history? What about the rest of the Board? Can you talk about the Board’s governance and recent refreshment efforts? We
appreciate your continued support as we work to drive value for ALL stockholders.
Footnotes1
Based on 2020 public filings from Regeneron, Roche and NovartisForward-looking StatementsStatements contained in this letter regarding the events or results that may occur in the future are “forward-looking statements” within the meaning of the
Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding Adverum’s expectations that its two global Phase 3 trials in wet AMD will initiate in parallel in the fourth quarter of
2021, that it will report clinical data and achieve pre-commercialization, clinical and development milestones throughout the rest of 2021 and beyond, and that it will name a high-quality independent director with commercial gene therapy
experience in 2021 and submit a BLA to the FDA in 2024. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include risks inherent to, without
limitation: Adverum’s novel technology, which makes it difficult to predict the time and cost of product candidate development and obtaining regulatory approval; the results of early clinical trials not always being predictive of future
results; and the potential for future complications or side effects in connection with use of ADVM-022. Risks and uncertainties facing Adverum are described more fully in Adverum’s Annual Report on Form 10-K for the year ended December 31, 2020
and any subsequent filings with the SEC under the heading “Risk Factors.” All forward-looking statements contained in this letter speak only as of the date on which they were made. Adverum undertakes no obligation to update such statements to
reflect events that occur or circumstances that exist after the date on which they were made.Important InformationAdverum Biotechnologies, Inc. (“Adverum”) has filed a definitive proxy statement and form of associated WHITE proxy card with the
U.S. Securities and Exchange Commission (the “SEC”) in connection with the solicitation of proxies for Adverum’s 2021 Annual Meeting (the “Proxy Statement”). Adverum, its directors and certain of its executive officers and employees will be
participants in the solicitation of proxies from stockholders in respect of the 2021 Annual Meeting. Information regarding the names of Adverum’s directors, executive officers and employees and their respective interests in Adverum by security
holdings or otherwise is set forth in the Proxy Statement. Details concerning the nominees of Adverum’s Board of Directors for election at the 2021 Annual Meeting are included in the Proxy Statement.BEFORE MAKING ANY VOTING DECISION, INVESTORS
AND STOCKHOLDERS OF ADVERUM ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH OR FURNISHED TO THE SEC, INCLUDING THE ADVERUM’S DEFINITIVE PROXY STATEMENT AND ANY SUPPLEMENTS THERETO AND ACCOMPANYING WHITE PROXY CARD, BECAUSE THEY CONTAIN
IMPORTANT INFORMATION. Investors andstockholders can obtain a copy of the Proxy Statement and other relevant documents filed by Adverum free of charge from the SEC’s website, www.sec.gov. Stockholders may also contact Innisfree M&A
Incorporated with questions or requests for additional copies of the proxy materials by calling toll free at (877) 750-9496. Your Vote Is Important, No Matter How Many or How Few Shares You Own If you have any questions, or need assistance in
voting your shares on the WHITE proxy card, please call the firm assisting Adverum with the solicitation of proxies: INNISFREE M&A INCORPORATEDStockholders Call Toll-Free at (877) 750-9496Banks and Brokers Call Collect (212)
750-5833
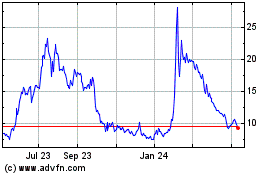
Adverum Biotechnologies (NASDAQ:ADVM)
Historical Stock Chart
From Mar 2024 to Apr 2024
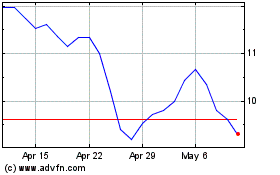
Adverum Biotechnologies (NASDAQ:ADVM)
Historical Stock Chart
From Apr 2023 to Apr 2024