Adaptive Biotechnologies Gets FDA EUA for T-Detect to Confirm Recent or Prior Covid-19 Infection
March 05 2021 - 7:28PM
Dow Jones News
By Josh Beckerman
Adaptive Biotechnologies Corp. said the U.S. Food and Drug
Administration issued an Emergency Use Authorization for T-Detect
COVID to confirm recent or prior Covid-19 infection.
Shares were recently up 18% to $50 after hours.
The company said the T-cell-based based test is the first
indication resulting from Adaptive's TCR-Antigen Map collaboration
with Microsoft Corp.
"People who have been unsure about a prior infection will now
have another way to know if they had the virus," the company said.
"We have proven that it is possible to read how T cells detect
disease in the blood, and this is just the beginning of a pipeline
of tests for many other indications," the company said.
The EUA was based on a clinical validation study showing that
T-Detect COVID demonstrated sensitivity of 97.1% from date of
diagnosis using RT-PCR, Adaptive said.
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
March 05, 2021 19:13 ET (00:13 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
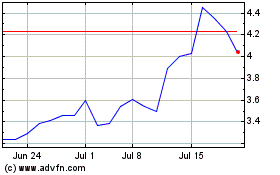
Adaptive Biotechnologies (NASDAQ:ADPT)
Historical Stock Chart
From Mar 2024 to Apr 2024
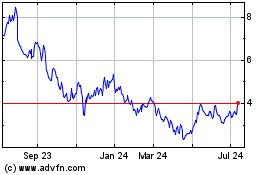
Adaptive Biotechnologies (NASDAQ:ADPT)
Historical Stock Chart
From Apr 2023 to Apr 2024