AbCellera's Covid-19 Treatment Gets Recommendation From European Regulators
March 05 2021 - 5:00PM
Dow Jones News
By Kimberly Chin
AbCellera Biologics Inc. on Friday said European regulators
recommended the use of its Covid-19 treatment in confirmed Covid-19
patients, ages 12 years and older, that don't require supplemental
oxygen or are at risk of progressing to more severe issues.
A committee of the European Medicines Agency that evaluates the
use of therapies in humans issued a positive opinion for the use of
AbCellera's bamlanivimab treatment alone as well as together with
etesevimab.
The regulator reviewed the Phase 2 and Phase 3 trial results
from AbCellera and Eli Lilly & Co., its collaborator in
developing antibody therapies to prevent and treat Covid-19. The
trial's results showed that bamlanivimab could reduce viral load
and symptoms, as well as hospitalizations by about 70%. The use of
bamlanivimab and etesevimab together indicated it could reduce the
risk of Covid-19 hospitalizations and death by 70% in patients with
mild to moderate symptoms.
The EMA committee's recommendation can be used by the European
Union to make decisions on whether to authorize therapies for use.
Bamlanivimab received emergency use authorization by the Food and
Drug Administration last month.
AbCellera is a Canada-based biotechnology company.
Write to Kimberly Chin at kimberly.chin@wsj.com
(END) Dow Jones Newswires
March 05, 2021 16:45 ET (21:45 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
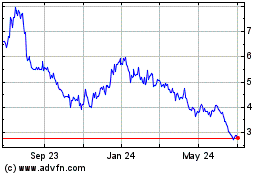
AbCellera Biologics (NASDAQ:ABCL)
Historical Stock Chart
From Mar 2024 to Apr 2024
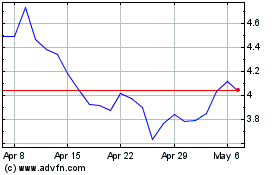
AbCellera Biologics (NASDAQ:ABCL)
Historical Stock Chart
From Apr 2023 to Apr 2024