iBio Selected to Produce ATB Therapeutics’ Bioengineered Antibody-Toxin Fusion Proteins
December 01 2020 - 4:30PM

iBio, Inc. (NYSEA:IBIO) (“iBio” or the “Company”), a biotech
innovator and biologics contract development and manufacturing
organization (“CDMO”), today announced that it has entered into its
first Statement of Work (“SoW”) under a Master Services Agreement
with Belgium-based ATB Therapeutics
(“
atbtherapeutics”) to produce its bioengineered
antibody-toxin fusion proteins using iBio’s
FastPharming® System.
The fusion proteins, called
atbodies™, are being designed for the treatment of
cancers. iBio will develop a manufacturing process and assays for
select atbodies per the SoW.
“We are pleased to be chosen as the process
development and manufacturing partner for
atbtherapeutics, whose platform technology offers
a unique approach for hard-to-treat hematological malignancies and
solid tumors,” said Tom Isett, Chairman & CEO of iBio. “We are
looking forward to helping atbtherapeutics rapidly
build a scalable manufacturing process so that its
atbody drug candidates may quickly reach the
clinic and begin to realize their potential in oncology.”
Bertrand Magy, CEO of
atbtherapeutics, commented: “We are thrilled to be
working with iBio, a high-quality, customer-focused CDMO whose
proven expertise should be highly valuable as we prepare for our
first safety trials. With iBio’s support, we believe we will be
able to rapidly progress toward the clinic with our lead product
candidates for the treatment of cancer.”
About
atbtherapeutics
atbtherapeutics is a Belgian
pioneering biopharmaceutical company building an oncology pipeline
of antibody-toxin-bioengineered ‘atbodies.’
atbody novel biologic is differentiated by a
unique composition and novel mechanism of action, together aiming
to extend the therapeutic window of current targeted therapies. The
atbody is a novel therapeutic that can only be
achieved using atbtherapeutics’ proprietary
plant-based atbiofarm technology.
atbtherapeutics is advancing its first program in
hematological malignancies and is developing a second program for
solid tumors in its discovery portfolio. For more information,
visit www.atbtherapeutics.com.
About iBio, Inc.
iBio is a global leader in plant-based biologics
manufacturing. Its FastPharming® System combines
vertical farming, automated hydroponics, and glycan engineering
technologies to rapidly deliver high-quality monoclonal antibodies,
vaccines, bioinks and other proteins. The Company’s subsidiary,
iBio CDMO LLC, provides FastPharming Contract
Development and Manufacturing Services. iBio’s
Glycaneering Development Service™ includes an
array of new glycosylation technologies for engineering
high-performance recombinant proteins. Additionally, iBio is
developing proprietary products, which include IBIO-100 for the
treatment of fibrotic diseases, and vaccines for infectious
diseases. For more information, visit www.ibioinc.com.
FORWARD-LOOKING
STATEMENTSCertain statements in this press release
constitute "forward-looking statements" within the meaning of the
federal securities laws. Words such as "may," "might," "will,"
"should," "believe," "expect," "anticipate," "estimate,"
"continue," "predict," "forecast," "project," "plan," "intend" or
similar expressions, or statements regarding intent, belief, or
current expectations, are forward-looking statements. These
forward-looking statements are based upon current estimates and
assumptions and include statements regarding helping ATB
Therapeutics rapidly build a scalable manufacturing process so that
its atbody drug candidates may quickly reach the
clinic and begin to realize their potential in oncology and the
Company’s proven expertise being highly valuable as ATB
Therapeutics prepares for its first safety trials. While the
Company believes these forward-looking statements are reasonable,
undue reliance should not be placed on any such forward-looking
statements, which are based on information available to us on the
date of this release. These forward-looking statements are subject
to various risks and uncertainties, many of which are difficult to
predict that could cause actual results to differ materially from
current expectations and assumptions from those set forth or
implied by any forward-looking statements. Important factors that
could cause actual results to differ materially from current
expectations include, among others, the Company’s ability to help
ATB Therapeutics rapidly build a scalable manufacturing process
using iBio’s FastPharming® System, the Company’s
ability to obtain regulatory approvals for commercialization of its
product candidates, including its COVID-19 vaccines, or to comply
with ongoing regulatory requirements, regulatory limitations
relating to its ability to promote or commercialize its product
candidates for specific indications, acceptance of its product
candidates in the marketplace and the successful development,
marketing or sale of products, its ability to maintain its license
agreements, the continued maintenance and growth of its
intellectual property portfolio, its ability to establish and
maintain collaborations, its ability to obtain or maintain the
capital or grants necessary to fund its research and development
activities, competition, its ability to retain its key employees or
maintain its NYSE American listing, and the other risk factors
discussed in the Company’s most recent Annual Report on Form 10-K
and the Company’s subsequent filings with the SEC, including
subsequent periodic reports on Forms 10-Q and 8-K. The information
in this release is provided only as of the date of this release,
and we undertake no obligation to update any forward-looking
statements contained in this release on account of new information,
future events, or otherwise, except as required by law.
Contacts:
Stephen KilmeriBio, Inc.Investor Relations(646)
274-3580 skilmer@ibioinc.com
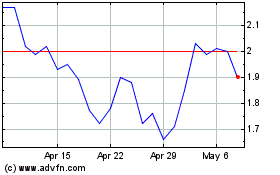
iBio (AMEX:IBIO)
Historical Stock Chart
From Mar 2024 to Apr 2024
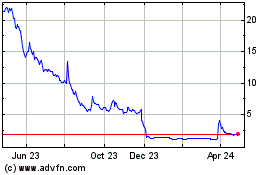
iBio (AMEX:IBIO)
Historical Stock Chart
From Apr 2023 to Apr 2024