UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
SCHEDULE
14A
Proxy
Statement Pursuant to Section 14(a) of the
Securities
Exchange Act of 1934
(Amendment
No. )
Filed
by the Registrant ☒
Filed
by a Party other than the Registrant ☐
Check
the appropriate box:
| ☐ |
Preliminary
Proxy Statement |
| |
|
| ☐ |
Confidential,
for Use of the Commission Only (as Permitted by Rule 14a-6(e)(2)) |
| |
|
| ☐ |
Definitive
Proxy Statement |
| |
|
| ☒ |
Definitive
Additional Materials |
| |
|
| ☐ |
Soliciting
Material under § 240.14a-12 |
AIM
ImmunoTech Inc.
(Name
of Registrant as Specified in its Charter)
(Name
of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment
of Filing Fee (Check all boxes that apply):
| ☒ |
No
fee required |
| |
|
| ☐ |
Fee
paid previously with preliminary materials |
| |
|
| ☐ |
Fee
computed on table in exhibit required by Item 25(b) per Exchange Act Rules 14a-6(i)(1) and 0-11 |

AIM
ImmunoTech Board Files Definitive Proxy Statement and Sends Letter to Shareholders
Highlights
Significant Progress Made in Recent Years – and Especially Over Past 18 Months – in Repurposing Lead Drug Ampligen in Oncology
to Benefit Patients and All Shareholders
Calls
on Shareholders to Support Continued Positive Momentum by Re-Electing the AIM Board and Discarding Any Materials Received from Jorgl
Activist Group
OCALA,
Fla., September 19, 2022 — The Board of Directors (“Board”) of AIM ImmunoTech Inc. (NYSE: American AIM) (“AIM”
or the “Company”), today announced that the Company has filed its Definitive Proxy Statement in connection with AIM’s
upcoming 2022 Annual Meeting of Stockholders (the “Annual Meeting”), to be held on November 3, 2022, and has mailed this
statement, along with a letter, to shareholders.
The
full text of the letter follows.
September
19, 2022
Dear
fellow shareholders:
Thank
you for your investment and your trust in AIM ImmunoTech (“AIM” or the “Company”). Now is an exciting time for
us as we continue to strive to bring new life-saving oncology therapies to market to benefit patients and create value for you, our shareholders.
As we will describe in more detail below, we have made significant progress in recent years – and especially over the past 18 months
– in repurposing our lead drug, Ampligen, into oncology. We also have strong momentum building upon the positive interim results
in published data from preeminent cancer centers that we are now seeing from our clinical trials.
Our
2022 Annual Meeting of Stockholders (the “Annual Meeting”) will be held on November 3, 2022 and we are writing to ask for
your support of our mission to bring life-saving therapies to market.
This
year your vote is especially important because Jonathan Jorgl, an AIM shareholder who first purchased 1,000 AIM shares on June 27, 2022,
has nominated two director candidates (together with Mr. Jorgl, the “Jorgl Activist Group”) for election to the Board at
the upcoming Annual Meeting. If these two individuals were elected, they would control the Board.
Alarmingly,
as they disclosed for the first time only last week, the Jorgl Activist Group’s proxy campaign is being funded by an individual
whose role and identity the Jorgl Activist Group previously concealed, most likely because he recently pled guilty to criminal charges
of wire fraud relating to fraudulent securities trading, material misrepresentations to investors and misuse of funds. As a result, he
was recently sentenced by a federal court to three years of probation and ordered to pay hundreds of thousands of dollars in restitution.
Additionally,
the Board believes that the Jorgl Activist Group has an undisclosed arrangement with another individual who pled guilty to insider trading
criminal charges brought by the Department of Justice, and settled insider trading civil charges brought by the SEC. More details about
the Jorgl Activist Group are contained in the enclosed proxy materials.
The
Board has unanimously determined that Mr. Jorgl’s director nominations did not comply with AIM’s bylaws and are invalid.
Mr. Jorgl is attempting to fight this determination in a case currently pending before a court, but the critical point is this: we strongly
urge you to ignore any proxy materials you receive from the Jorgl Activist Group and vote on the Company’s WHITE proxy card
for your current highly qualified directors to protect your investment.
In
contemplating your vote, we ask that you consider the following:
The
Current Management Team and Board is Successfully Executing on the Company’s Business and Capital Allocation Strategy
| |
● |
The
Company and its management team installed in 2016 has gone from having little cash, a sustained period of poor share price performance
resulting in the threat of delisting from the New York Stock Exchange American, inadequate reserves of Ampligen to support major
oncology clinical trials, and no such trials underway, to now having financial stability after manufacturing an adequate supply of
Ampligen to support clinical trials. This was a vital first step that the Company was unable to achieve previously and has allowed
us to move forward with our development process. |
| |
|
|
| |
● |
AIM
has sufficient liquid assets (~$41.7 million per the Company’s 10-Q for the period ended June 30, 2022) to advance our priority
development pipeline and fund operations through the end of 2023. |
| |
|
|
| |
● |
Further,
we have also initiated and are helping to fund several ongoing oncology clinical trials and early access programs in large potential
markets with lethal unmet medical needs such as advanced pancreatic cancer and advanced recurrent ovarian cancer. |
| |
|
|
| |
● |
Our
clinical trials have been yielding consistently encouraging, statistically significant interim results with positive safety profiles,
which also have been published in peer reviewed journals. We have achieved multiple potentially game-changing clinical and regulatory
milestones this year and expect this positive momentum to continue throughout the rest of 2022 and into next year. |
| |
|
|
| |
● |
We
received notification from the U.S. Food and Drug Administration (“FDA”) that the FDA’s Clinical Hold on AIM’s
investigational new drug (“IND”) application for a Phase 2 study of Ampligen as a therapy for locally advanced pancreatic
cancer (AMP-270) has been lifted and the Company may proceed with the study. |
| |
|
|
| |
● |
In
April of this year, we announced several positive developments related to Ampligen, including the following: |
| |
○ |
The
Independent Investigators from preeminent oncology research centers presented strongly positive data in three different cancer indications
at the prestigious American Association for Cancer Research Annual Meeting. |
| |
|
|
| |
○ |
The
Erasmus Medical Center presented published data (Cancers) demonstrating Ampligen’s potential to offer beneficial anti-tumor
effects in late-stage pancreatic cancer patients, including Ampligen treatment’s association with higher progression-free and
overall survival in late-stage pancreatic cancer patients when compared to well-matched historical controls. |
| |
|
|
| |
○ |
We
reported positive data from a Phase 2a study evaluating Ampligen as a component of a chemokine modulatory (CKM) regimen for the treatment
of colorectal cancer metastatic to the liver. |
|
○ |
We
reported positive data from a Phase 1 study evaluating Ampligen for the treatment of stage 4 metastatic triple negative breast cancer. |
|
● |
Further,
on July 28, 2022, we reported positive preliminary pilot study data from our ongoing Expanded Access Program (AMP-511) evaluating
Ampligen in patients with chronic fatigue symptoms following COVID infections (a form of “Long COVID”). |
|
○ |
The
preliminary data from this uncontrolled clinical protocol found that patients reported significant improvements in fatigue symptoms
after treatment with Ampligen compared to baseline, which the investigators considered a clinically significant decrease in fatigue-related
measures. Based on these early results, we are working to move forward with a Phase 2 controlled trial in post COVID Conditions (also
known as “Long COVID”). |
An
Experienced, Well-Qualified Board
Your
Board has the right experience, skill sets and deep knowledge of the Company and its drug candidates to continue overseeing the successful
execution of our strategy to deliver therapies for patients and value for our shareholders. More detailed biographies of our directors
are contained in the enclosed proxy materials.
| |
● |
Stewart
L. Appelrouth possesses key financial and regulatory expertise as a certified public accountant with over 40 years of accounting
and audit experience. |
| |
|
|
| |
● |
Thomas
K. Equels, M.S. J.D., Executive Vice Chairman, Chief Executive Officer and President, has over 25 years of experience as a practicing
attorney specializing in complex business litigation. He also has extensive experience in clinical trial design and development,
creating intellectual property concepts, and in financing drug development. |
| |
|
|
| |
● |
Dr.
William M. Mitchell, Chairman, has extensive medical industry experience, including as a Professor of Pathology at Vanderbilt
University School of Medicine, a board-certified physician and a former member of the Board of Directors of Chronix Biomedical, a
company involved in next generation DNA sequencing for medical diagnostics. |
We
encourage you, as an AIM shareholder, to support the Board – and ensure the Company has the leadership to protect investors –
by voting online, by phone or by mail, using the WHITE proxy card.
***
WE
URGE YOU TO COMPLETE, DATE, AND SIGN THE ENCLOSED WHITE PROXY CARD AND MAIL IT PROMPTLY IN THE POSTAGE-PAID ENVELOPE PROVIDED,
OR VOTE BY TELEPHONE OR THE INTERNET AS INSTRUCTED ON THE WHITE PROXY CARD, WHETHER OR NOT YOU PLAN TO ATTEND THE ANNUAL MEETING.
THE
BOARD RECOMMENDS A VOTE “FOR ALL” OF OUR BOARD’S NOMINEES
(STEWART
L. APPELROUTH, THOMAS K. EQUELS AND WILLIAM M. MITCHELL)
ON
PROPOSAL 1 USING THE ENCLOSED WHITE PROXY CARD.
Sincerely,
The
AIM ImmunoTech Board of Directors
If
you have any questions or need assistance voting, please contact the Company’s proxy solicitor Morrow Sodali LLC (“Morrow
Sodali”) using the below information.
MORROW
SODALI
509
Madison Avenue
Suite
1206
New
York, NY 10022
Banks
and Brokers Call: (203) 658-9400
Stockholders
Call Toll Free: (800) 662-5200
E-mail:
AIM@investor.morrowsodali.com
About
AIM ImmunoTech Inc.
AIM
ImmunoTech Inc. is an immuno-pharma company focused on the research and development of therapeutics to treat multiple types of cancers,
immune disorders, and viral diseases, including COVID-19. The Company’s lead product, Ampligen® (rintatolimod) is an immuno-modulator
with broad spectrum activity being developed for globally important cancers, viral diseases and disorders of the immune system.
Ampligen
is currently being used as a monotherapy to treat pancreatic cancer patients in an Early Access Program (EAP) approved by the Inspectorate
of Healthcare in the Netherlands at Erasmus Medical Center and AIM plans to initiate a Phase 2 clinical study in 2022. The Company also
has multiple ongoing clinical trials to evaluate Ampligen as a combinational therapy for the treatment of a variety of solid tumor types
both underway and planned at major cancer research centers. Additionally, Ampligen is approved in Argentina for the treatment of severe
chronic fatigue syndrome (CFS) and is currently being evaluated in many aspects of SARS-CoV-2/COVID-19 myalgic encephalomyelitis/chronic
fatigue syndrome (ME/CFS) and Post COVID Conditions.
For
more information, please visit aimimmuno.com and connect with the Company on Twitter, LinkedIn, and Facebook.
Forward-Looking
Statements
This
press release contains certain forward-looking statements that involve risks, uncertainties and assumptions that are difficult to predict.
Words and expressions reflecting optimism, satisfaction or disappointment with current prospects, as well as words such as “believes,”
“hopes,” “intends,” “estimates,” “expects,” “projects,” “plans,”
“anticipates” and variations thereof, or the use of future tense, identify forward-looking statements, but their absence
does not mean that a statement is not forward-looking. The Company’s forward-looking statements are not guarantees of performance,
and actual results could vary materially from those contained in or expressed by such statements due to risks, uncertainties and other
factors. The Company urges investors to consider specifically the various risk factors identified in its most recent Form 10-K, and any
risk factors or cautionary statements included in any subsequent Form 10-Q or Form 8-K, filed with the Securities and Exchange Commission.
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release.
Except as required by law, the Company does not undertake any responsibility to update any forward-looking statements to take into account
events or circumstances that occur after the date of this press release.
Important
Information
The
Company has filed a definitive proxy statement and associated WHITE proxy card with the SEC in connection with the solicitation
of proxies for the Company’s Annual Meeting. Details concerning the nominees of the Company’s Board of Directors for election
at the Annual Meeting are included in the proxy statement. BEFORE MAKING ANY VOTING DECISION, INVESTORS AND STOCKHOLDERS OF THE COMPANY
ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH OR FURNISHED TO THE SEC, INCLUDING THE COMPANY’S PROXY STATEMENT AND ANY AMENDMENTS
OR SUPPLEMENTS THERETO, WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION.
Investors
and stockholders will be able to obtain a copy of the definitive proxy statement, any amendments or supplements thereto and other documents
filed by the Company free of charge from the SEC’s website, www.sec.gov. Copies of these materials will also be available free
of charge on AIM’s Investor Relations website at https://aimimmuno.com/sec-filings/.
Participants
in the Solicitation
The
Company, its directors and certain of its executive officers are participants in the solicitation of proxies from stockholders in respect
of the Annual Meeting. Information regarding the names of the Company’s directors and executive officers and their respective interests
in the Company by security holdings or otherwise is set forth in the Company’s Definitive Proxy Statement, filed with the SEC on
September 19, 2022. To the extent holdings of such participants in the Company’s securities have changed since the amounts described
in the Definitive Proxy Statement, such changes have been or will be reflected on Initial Statements of Beneficial Ownership on Form
3 or Statements of Change in Ownership on Form 4 filed with the SEC. These documents can be obtained free of charge from the sources
indicated above.
Investor
Contacts:
JTC
Team, LLC
Jenene
Thomas
833-475-8247
AIM@jtcir.com
OR
Morrow
Sodali
AIM@investor.MorrowSodali.com
Media
Contact:
Longacre
Square Partners
Dan
Zacchei / Joe Germani
dzacchei@longacresquare.com
/ jgermani@longacresquare.com
AIM ImmunoTech (AMEX:AIM)
Historical Stock Chart
From Mar 2024 to Apr 2024
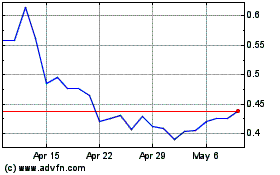
AIM ImmunoTech (AMEX:AIM)
Historical Stock Chart
From Apr 2023 to Apr 2024