Orgenesis Announces Collaboration Agreement with SCTbio to Expand POCare Sites in the Czech Republic and Enhance Capabilities for the Production of Lentivirus Vectors
May 18 2023 - 7:30AM

(NASDAQ: ORGS) (“Orgenesis”), a global biotech
company working to unlock the full potential of cell and gene
therapies (CGT) through its US-based point-of-care (POCare)
services subsidiary, Morgenesis LLC (“Morgenesis”), has signed a
collaboration agreement with SCTbio, a full-service contract
development and manufacturing organization (CDMO) specializing in
cell-based therapy and viral vectors. The goal of the collaboration
is to open new point-of-care (POCare) sites within the Czech
Republic and leverage SCTbio’s GMP facility for the production of
lentivirus vectors to support Morgenesis’ POCare worldwide
customers, including use in CAR-T cell and gene therapies (CGTs).
Orgenesis’ POCare Platform, including its
Orgenesis Mobile Processing Units and Labs (OMPULs), overcomes
conventional processing challenges by enabling high-quality
standards and sterile, scalable onsite processing of CGTs, near
local hospitals and treatment facilities. The POCare centers and
OMPULs are designed to shorten the implementation time of new
production, while offering a more cost-effective environment and
enabling local scalability.
Lentiviral vectors are part of the family of
retroviral vectors and are a fundamental component of the cell
therapy manufacturing process, ensuring the delivery of the
therapeutic genes into the patient’s cells. SCTbio brings expertise
in retroviral vector GMP production with a deep knowledge of
state-of-the-art manufacturing technologies and quality control
(QC) methods.
Ludek Sojka, Chief Executive Officer at SCTbio,
stated, “We are looking forward to collaborating with Orgenesis.
Its POCare Platform is a unique technology supporting the
development of potentially breakthrough CGTs, and we recognize the
great potential for utilizing the long-term experience of our team
and our GMP lentiviral vector manufacturing capabilities to support
Orgenesis-powered breakthroughs in Central Europe and beyond.”
Vered Caplan, CEO of Orgenesis, commented, “This
partnership reflects our commitment to helping bring potential
breakthrough cell therapies to market in a cost-effective,
high-quality, and scalable manner. We look forward to teaming with
SCTbio to expand our POCare CGT production services to the region,
as well as leveraging their lentiviral vector production as a key
element supporting our CAR-T customers in the EU.”
About SCTbioSCTbio is a cell-based therapy and
retroviruses vector CDMO spun out from SOTIO Group in 2021, led by
a tight-knit team that has been through every stage of process and
product development over the last decade, bringing SOTIO’s
autologous cell therapies into phase III. From its 2,000 sqm
facility in Central Europe, SCTbio offers personal attention
through long-term manufacturing partnerships, offering strategic
development and regulatory guidance alongside full services
covering GMP production, testing and logistics of advanced therapy
medicinal products, including genetically modified and viral
vectors.
The breadth of its experience means that SCTbio
delivers the highest quality collaboration through a range of
services ensuring GMP compliance for the full life cycle of drug
development, that include technology transfers, process
development, clinical manufacturing services, fill & finish,
quality control, QA/QP release, storage and logistics.
SCTbio is member of PPF Group.To learn more, visit
SCTbio.com.
About OrgenesisOrgenesis is a
global biotech company working to unlock the full potential of cell
and gene therapies (CGTs) in an affordable and accessible format at
the point of care. The Orgenesis POCare Platform is comprised of
three enabling components: a pipeline of licensed POCare
Therapeutics that are processed and produced in closed, automated
POCare Technology systems across a collaborative POCare Network.
Orgenesis identifies promising new therapies and leverages its
POCare Platform to provide a rapid, globally harmonized pathway for
these therapies to reach and treat large numbers of patients at
lowered costs through efficient, scalable, and decentralized
production. The POCare Network brings together patients, doctors,
industry partners, research institutes and hospitals worldwide to
achieve harmonized, regulated clinical development and production
of the therapies. www.orgenesis.com.
Notice Regarding Forward-Looking
Statements This press release contains forward-looking
statements which are made pursuant to the safe harbor provisions of
Section 27A of the Securities Act of 1933, as amended, and Section
21E of the Securities and Exchange Act of 1934, as amended. These
forward-looking statements involve substantial uncertainties and
risks and are based upon our current expectations, estimates and
projections and reflect our beliefs and assumptions based upon
information available to us at the date of this release. We caution
readers that forward-looking statements are predictions based on
our current expectations about future events. These forward-looking
statements are not guarantees of future performance and are subject
to risks, uncertainties and assumptions that are difficult to
predict. Our actual results, performance or achievements could
differ materially from those expressed or implied by the
forward-looking statements as a result of a number of factors,
including, but not limited to, our reliance on, and our ability to
grow, our point-of-care cell therapy platform and OMPUL business,
our ability to achieve and maintain overall profitability, our
ability to manage our research and development programs that are
based on novel technologies, our ability to control key elements
relating to the development and commercialization of therapeutic
product candidates with third parties, the timing of completion of
clinical trials and studies, the availability of additional data,
outcomes of clinical trials of our product candidates, the
potential uses and benefits of our product candidates, our ability
to manage potential disruptions as a result of the COVID-19
pandemic, the sufficiency of working capital to realize our
business plans and our ability to raise additional capital, the
development of our POCare strategy, our trans differentiation
technology as therapeutic treatment for diabetes, the technology
behind our in-licensed ATMPs not functioning as expected, our
ability to further our CGT development projects, either directly or
through our JV partner agreements, and to fulfill our obligations
under such agreements, our license agreements with other
institutions, our ability to retain key employees, our competitors
developing better or cheaper alternatives to our products, risks
relating to legal proceedings against us and the risks and
uncertainties discussed under the heading "RISK FACTORS" in Item 1A
of our Annual Report on Form 10-K for the fiscal year ended
December 31, 2021, and in our other filings with the Securities and
Exchange Commission. We undertake no obligation to revise or update
any forward-looking statement for any reason.
IR contact for Orgenesis:Crescendo
Communications, LLCTel: 212-671-1021Orgs@crescendo-ir.com
Communications contact for
Orgenesis/SCTbioIB CommunicationsNeil Hunter / Michelle
BoxallTel +44 (0)20 8943
4685neil@ibcomms.agency / michelle@ibcomms.agency
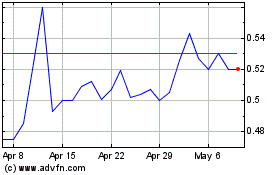
Orgenesis (NASDAQ:ORGS)
Historical Stock Chart
From Mar 2024 to Apr 2024
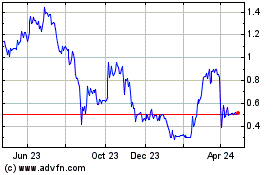
Orgenesis (NASDAQ:ORGS)
Historical Stock Chart
From Apr 2023 to Apr 2024