Verve Therapeutics Shares Tumble on FDA Study Hold Update
December 05 2022 - 10:51AM
Dow Jones News
By Colin Kellaher
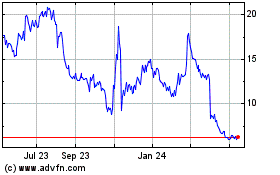
Shares of Verve Therapeutics Inc. slid more than 15% on Monday
after the genetic-medicines company reported details of the U.S.
Food and Drug Administration's clinical hold on planned studies of
its lead drug candidate.
The Cambridge, Mass., company, which is seeking FDA approval for
a U.S. study of Verve-101, the cardiovascular disease heterozygous
familial hypercholesterolemia, said the agency has requested
additional preclinical data relating to potency differences between
human and non-human cells, risks of germline editing, and
off-target analyses in non-hepatocyte cell types.
Verve said the FDA also requested available data from an ongoing
Phase 1 study in New Zealand and the U.K., and that the agency
wants the company to modify the U.S. trial protocol to incorporate
additional contraceptive measures and to increase the length of the
staggering interval between dosing of participants.
Verve, which filed in October for FDA approval of the planned
study, in early November said it received notification of the hold,
and that it expected an official letter with the FDA's questions
within 30 days.
Verve on Monday said it plans to submit a response to the FDA as
expeditiously as possible, adding that it expects to report initial
safety and pharmacodynamic data from the dose-escalation portion of
the New Zealand/U.K. study in the second half of 2023.
Verve shares were recently changing hands at $20.05, down more
than 16%.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
December 05, 2022 10:36 ET (15:36 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Verve Therapeutics (NASDAQ:VERV)
Historical Stock Chart
From Mar 2024 to Apr 2024
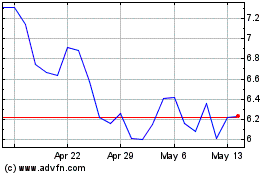
Verve Therapeutics (NASDAQ:VERV)
Historical Stock Chart
From Apr 2023 to Apr 2024