Beam Therapeutics Says FDA Lifts Clinical Hold on IND Application for BEAM-201
December 02 2022 - 7:10AM
Dow Jones News
By Chris Wack
Beam Therapeutics Inc. said Friday that the U.S. Food and Drug
Administration has lifted the clinical hold and cleared the
Investigational New Drug application for BEAM-201 for the treatment
of relapsed/refractory T-cell acute lymphoblastic leukemia/T-cell
lymphoblastic lymphoma.
The biotechnology company said its BEAM-201 is a specific
anti-CD7, multiplex-edited, allogeneic chimeric antigen receptor
T-cell development candidate.
"The FDA's clearance of our IND for BEAM-201 is an exciting
moment for Beam and for the field of gene editing, as it represents
the first IND clearance for a multiplex-base edited investigational
drug," said John Evans, chief executive of Beam.
Beam shares were up 11% to $51 in premarket trading.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
December 02, 2022 06:55 ET (11:55 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
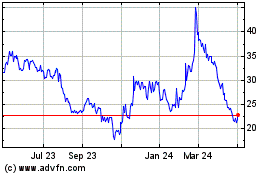
Beam Therapeutics (NASDAQ:BEAM)
Historical Stock Chart
From Mar 2024 to Apr 2024
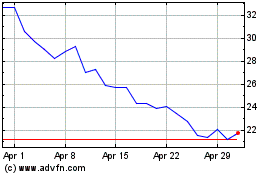
Beam Therapeutics (NASDAQ:BEAM)
Historical Stock Chart
From Apr 2023 to Apr 2024