If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Exchange Act. ☐
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall hereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
We are also offering to those purchasers, if any, whose purchase of our common stock in this offering would otherwise result in such purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity, in lieu of purchasing common stock, to purchase pre-funded warrants to purchase shares of our common stock. The purchase price of each pre-funded warrant will equal the price per share at which shares of our common stock are being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will equal $0.0001 per share of common stock. For each pre-funded warrant purchased in this offering in lieu of common stock, we will reduce the number of shares of common stock being sold in the offering on a one-for-one basis.
Each pre-funded warrant is exercisable for one share of our common stock (subject to adjustment as provided for therein) at any time at the option of the holder until such pre-funded warrant is exercised in full, provided that the holder will be prohibited from exercising pre-funded warrants for shares of our common stock if, as a result of such exercise, the holder, together with its affiliates, would own more than 4.99% of the total number of shares of our common stock then issued and outstanding. However, any holder may increase such percentage to any other percentage not in excess of 9.99%, provided that any increase in such percentage shall not be effective until 61 days after such notice to us.
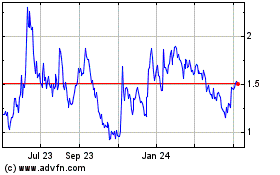
Our common stock is listed on The Nasdaq Capital Market under the symbol “CYTH.” The closing price of our common stock on November 16, 2022, as reported by The Nasdaq Capital Market, was $1.27 per share.
The public offering price per share of common stock and/or any pre-funded warrant, together with the common warrant that accompanies common stock or a pre-funded warrant will be determined between us and the underwriter in this offering at the time of pricing, and may be at a discount to the current market price. Therefore, the price of $1.27 per share of common stock used throughout this prospectus may not be indicative of the actual public offering price for our common stock, our pre-funded warrants and the common warrants. There is no established public trading market for the pre-funded warrants or common warrants, and we do not expect a market to develop. In addition, we do not intend to apply for the listing of the pre-funded warrants or common warrants on any national securities exchange. Without an active trading market, the liquidity of the common warrants and the pre-funded warrants will be limited.
The underwriter expects to deliver the shares of common stock, common warrants and pre-funded warrants, if any, to purchasers in the offering on or about , 2022.
Prospectus dated , 2022.
|
Prospectus Summary
|
1
|
| |
|
|
Risk Factors
|
6 |
| |
|
|
Disclosure Regarding Forward-looking Statements
|
17 |
| |
|
|
Use Of Proceeds
|
18 |
| |
|
|
Dividend Policy
|
18 |
| |
|
|
Capitalization
|
19 |
| |
|
|
Dilution
|
20 |
| |
|
|
Description Of Securities
|
21 |
| |
|
|
Underwriting
|
25 |
| |
|
|
Legal Matters
|
28 |
| |
|
|
Experts
|
28 |
| |
|
|
Where You Can Find More Information
|
28 |
| |
|
|
Incorporation by Reference
|
28 |
________________________________________
ABOUT THIS PROSPECTUS
The registration statement of which this prospectus forms a part that we have filed with the Securities and Exchange Commission (the “SEC”) includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus and the related exhibits filed with the SEC, together with the additional information described under the headings “Where You Can Find More Information” and “Incorporation by Reference” before making your investment decision.
You should also read and consider the information in the documents to which we have referred you under the caption “Where You Can Find More Information” in this prospectus. In addition, this prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find More Information.”
The market data and certain other statistical information used throughout this prospectus are based on independent industry publications, governmental publications, reports by market research firms or other independent sources. Some data are also based on our good faith estimates.
References herein to the "Company," "Registrant," "we," "us," "our" and "our company" refer to Cyclo Therapeutics, Inc., a Nevada corporation and its subsidiaries.
Certain monetary amounts, percentages and other figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables or charts and figures expressed as percentages in the text may not total 100% or, as applicable, when aggregated may not be the arithmetic aggregation of the percentages that precede them.
________________________________________
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS PROSPECTUS, INCORPORATED BY REFERENCE IN THIS PROSPECTUS OR IN ANY FREE WRITING PROSPECTUS WE MAY AUTHORIZE TO BE DELIVERED OR MADE AVAILABLE TO YOU. WE HAVE NOT, AND THE UNDERWRITER HAS NOT, AUTHORIZED ANYONE TO PROVIDE YOU WITH DIFFERENT INFORMATION. WE ARE NOT MAKING AN OFFER OF THESE SECURITIES IN ANY STATE WHERE THE OFFER IS NOT PERMITTED. YOU SHOULD NOT ASSUME THAT THE INFORMATION PROVIDED IN THIS PROSPECTUS IS ACCURATE AS OF ANY DATE OTHER THAN THE DATE ON THE FRONT OF THIS PROSPECTUS.
No person is authorized in connection with this prospectus to give any information or to make any representations about us, the securities offered hereby or any matter discussed in this prospectus, other than the information and representations contained in this prospectus. If any other information or representation is given or made, such information or representation may not be relied upon as having been authorized by us.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before investing in our securities. You should carefully read the entire prospectus, including “Risk Factors” as well as the documents that we incorporate by reference into this prospectus, including our financial statements and notes thereto, before making an investment decision.
Corporate Overview
We are a clinical stage biotechnology company that develops cyclodextrin-based products for the treatment of disease. We filed a Type II Drug Master File with the U.S. Food and Drug Administration (“FDA”) in 2014 for our lead drug candidate, Trappsol® Cyclo™ (hydroxypropyl beta cyclodextrin) as a treatment for Niemann-Pick Type C disease (“NPC”). NPC is a rare and fatal cholesterol metabolism disease that impacts the brain, lungs, liver, spleen, and other organs. In 2015, we launched an International Clinical Program for Trappsol® Cyclo™ as a treatment for NPC. In 2016, we filed an Investigational New Drug application (“IND”) with the FDA, which described our Phase I clinical plans for a randomized, double blind, parallel group study at a single clinical site in the U.S. The Phase I study evaluated the safety of Trappsol® Cyclo™ along with markers of cholesterol metabolism and markers of NPC during a 14-week treatment period of intravenous administration of Trappsol® Cyclo™ every two weeks to participants 18 years of age and older. The IND was approved by the FDA in September 2016, and in January 2017 the FDA granted Fast Track designation to Trappsol® Cyclo™ for the treatment of NPC. Initial patient enrollment in the U.S. Phase I study commenced in September 2017, and in May 2020 we announced Top Line data showing a favorable safety and tolerability profile for Trappsol® Cyclo™ in this study.
We have also completed a Phase I/II clinical study approved by several European regulatory bodies, including those in the United Kingdom, Sweden and Italy, and in Israel. The Phase I/II study evaluated the safety, tolerability and efficacy of Trappsol® Cyclo™ through a range of clinical outcomes, including neurologic, respiratory, and measurements of cholesterol metabolism and markers of NPC. Consistent with the U.S. study, the European/Israel study administered Trappsol® Cyclo™ intravenously to NPC patients every two weeks in a double-blind, randomized trial, but differs in that the study period was for 48 weeks (24 doses). The first patient was dosed in this study in July 2017, and in March of 2021 we announced that 100% of patients who completed the trial improved or remained stable, and 89% met the efficacy outcome measure of improvement in at least two domains of the 17-domain NPC severity scale.
Additionally, in February 2020 we had a face-to-face “Type C” meeting with the FDA with respect to the initiation of our pivotal Phase III clinical trial of Trappsol® Cyclo™ based on the clinical data obtained to date. At that meeting, we also discussed with the FDA submitting a New Drug Application (NDA) under Section 505(b)(1) of the Federal Food, Drug, and Cosmetic Act for the treatment of NPC in pediatric and adult patients with Trappsol® Cyclo™. A similar request was submitted to the European Medicines Agency (“EMA”) in February 2020, seeking scientific advice and protocol assistance from the EMA for proceeding with a Phase III clinical trial in Europe. In October 2020 we received a “Study May Proceed” notification from the FDA with respect to the proposed Phase III clinical trial, and in June of 2021 we commenced enrollment in TransportNPC, a pivotal Phase II study of Trappsol® Cyclo™ for the treatment of NPC.
Preliminary data from our clinical studies suggest that Trappsol® Cyclo™ releases cholesterol from cells, crosses the blood-brain-barrier in individuals suffering from NPC, and results in neurological and neurocognitive benefits and other clinical improvements in NPC patients. The full significance of these findings will be determined as part of the final analysis of these clinical trials.
On May 17, 2010, the FDA designated Trappsol® Cyclo™ as an orphan drug for the treatment of NPC, which would provide us with the exclusive right to sell Trappsol® Cyclo™ for the treatment of NPC for seven years following FDA drug approval. In April 2015, we also obtained Orphan Drug Designation for Trappsol® Cyclo™ in Europe, which will provide us with 10 years of market exclusivity following regulatory approval, which period will be extended to 12 years upon acceptance by the EMA’s Pediatric Committee of our pediatric investigation plan (PIP) demonstrating that Trappsol® Cyclo™ addresses the pediatric population. On January 12, 2017, we received Fast Track Designation from the FDA, and on December 1, 2017, the FDA designated NPC a Rare Pediatric Disease.
We are also exploring the use of cyclodextrins in the treatment of Alzheimer’s disease. In January 2018, the FDA authorized a single patient IND expanded access program using Trappsol® Cyclo™ for the treatment of Alzheimer’s disease. After 18 months of treatment in this geriatric patient with late-onset disease, the disease was stabilized and the drug was well tolerated. The patient also exhibited signs of improvement with less volatility and shorter latency in word-finding. We prepared a synopsis for an early stage protocol using Trappsol® Cyclo™ intravenously to treat Alzheimer’s disease that was presented to the FDA in January of 2021. We received feedback from the FDA on this synopsis in April 2021 and incorporated the feedback into an IND for a Phase II study for the treatment of Alzheimer’s disease with of Trappsol® Cyclo™ that we submitted to the FDA in November 2021. In December of 2021, we received IND clearance from the FDA, allowing us to proceed with our Phase II study of Trappsol® Cyclo™ for the treatment of Alzheimer’s disease. We expect to begin enrollment in this study during 2022.
We filed an international patent application in October 2019 under the Patent Cooperation Treaty directed to the treatment of Alzheimer’s disease with cyclodextrins, and we are pursuing national and regional stage applications based on this international application. The terms of any patents resulting from these national or regional stage applications would be expected to expire in 2039 if all the requisite maintenance fees are paid.
We also continue to operate our legacy fine chemical business, consisting of the sale of cyclodextrins and related products to the pharmaceutical, nutritional, and other industries, primarily for use in diagnostics and specialty drugs. However, our core business has transitioned to a biotechnology company primarily focused on the development of cyclodextrin-based biopharmaceuticals for the treatment of disease from a business that had been primarily reselling basic cyclodextrin products.
Risks Associated With our Business
Our ability to execute our business strategy is subject to numerous risks, as more fully described in the section captioned “Risk Factors” immediately following this prospectus summary. You should read these risks before you invest in our securities. In particular, risks associated with our business include, but are not limited to, the following:
| |
●
|
We have suffered recent losses and our future profitability is uncertain.
|
| |
●
|
Even with the proceeds from this offering, we will need additional capital to fund our operations as planned.
|
| |
●
|
We have not received approval for any drug candidate for commercial sale and, as a result, we have never generated any revenue from the sale of biopharmaceutical products, and expect to continue to incur significant financial losses in the future, which makes it difficult to assess our future viability.
|
| |
●
|
We are largely dependent upon the success of our Trappsol® Cyclo™ product, which may never receive regulatory approval.
|
| |
●
|
Even if Trappsol® Cyclo™ receives regulatory approval, we may not be successful in our commercialization efforts and Trappsol® Cyclo™ may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
|
| |
●
|
The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects.
|
| |
●
|
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
|
| |
●
|
Later discovery of previously unknown problems could limit our ability to market or sell Trappsol® Cyclo™, even if it is initially approved, and can expose us to product liability claims.
|
| |
●
|
We rely in part on third parties for research and clinical trials for products using Trappsol® Cyclo™.
|
| |
●
|
We currently have no marketing and sales organization for our pharmaceutical candidates and may have to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to generate product revenue.
|
| |
●
|
We rely upon third parties for the manufacture of Trappsol® Cyclo™ and are dependent on their quality and effectiveness.
|
| |
●
|
We face competition from well-funded companies to treat NPC.
|
| |
●
|
The rights we rely upon to protect our unpatented trade secrets may be inadequate.
|
| |
|
|
| |
● |
We cannot ensure that patent rights relating to inventions described and claimed in our pending patent applications will issue, that patents based on our patent applications will not be challenged and rendered invalid and/or unenforceable, or that third parties will not find ways to circumvent our patent rights or claim co-ownership thereof.
|
| |
●
|
The pharmaceutical business is subject to increasing government price controls and other restrictions on pricing, reimbursement and access to drugs, which could adversely affect our future revenues and profitability.
|
| |
●
|
We are dependent on our executive officers, and we may not be able to pursue our current business strategy effectively if we lose them.
|
Corporate and other Information
We were organized as a Florida corporation on August 9, 1990, with operations beginning in July 1992. In conjunction with a restructuring in 2000, we changed our name from Cyclodextrin Technologies Development, Inc. to CTD Holdings, Inc. We changed our name to Cyclo Therapeutics, Inc. in September 2019 to better reflect our current business, and on November 6, 2020, we reincorporated from the State of Florida to the State of Nevada. Our principal offices are located at 6714 NW 16th Street, Suite B, Gainesville, FL 32653, and our telephone number is (386) 418-8060. We maintain a website at www.cyclotherapeutics.com. Information contained on our website does not constitute part of this prospectus.
We are a “smaller reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller reporting company until the last day of any fiscal year for so long as either (1) the market value of our shares of common stock held by non-affiliates does not equal or exceed $250 million as of the prior June 30th, or (2) our annual revenues did not equal or exceed $100 million during such completed fiscal year and the market value of our shares of common stock held by non-affiliates did not equal or exceed $700.0 million as of the prior June 30th. To the extent we take advantage of any reduced disclosure obligations, it may also make it difficult to compare our financial statements with other public companies.
Available Information
Because we are subject to the information and reporting requirements of the Exchange Act, we file or furnish, as applicable, annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that website is www.sec.gov. We make available on our website at www.cyclotherapeutics.com, free of charge, copies of these reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
The Offering
|
Common Stock Offered by Us
|
|
7,870,000 shares. |
| |
|
|
|
Pre-funded Warrants Offered by Us
|
|
We are also offering to certain purchasers whose purchase of our common stock in this offering would otherwise result in the purchaser, together with its affiliates, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase pre-funded warrants in lieu of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. Each pre-funded warrant will be exercisable for one share of common stock. The purchase price of each pre-funded warrant and the accompanying common warrant will equal the price at which the common stock and the accompanying common warrant are being sold to the public in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until exercised in full. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because we will issue one common warrant for each share of common stock and for each pre-funded warrant to purchase one share of common stock sold in this offering, the number of common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold.
|
| |
|
|
|
Common Warrants Offered by Us
|
|
We are issuing to purchasers of shares of our common stock and/or pre-funded warrants in this offering a common warrant to purchase one share of our common stock for each share and/or pre-funded warrant purchased in this offering. Because a common warrant to purchase share(s) of our common stock is being sold together in this offering with each share of common stock and, in the alternative, each pre-funded warrant to purchase one share of common stock, the number of common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold. The common warrants will be exercisable at an exercise price of $ per share (representing % of the price at which a share of common stock and accompanying common warrant are sold to the public in this offering) for a five-year period beginning on the effective date of an amendment to our articles of incorporation increasing the number of authorized shares of common stock to at least 30,000,000. No fractional shares of common stock will be issued in connection with the exercise of a common warrant. In lieu of fractional shares, we will round up to the next whole share. |
| |
|
|
|
Common stock outstanding prior to this offering (1)
|
|
8,481,848 shares
|
| |
|
|
|
Public offering price:
|
|
Assumed combined public offering price of $1.27 per share of common stock and accompanying common warrant, or pre-funded warrant and accompanying common warrant, as applicable, which is equal to the last reported sale price per share of our common stock on The Nasdaq Capital Market on November 16, 2022. |
| |
|
|
|
Common stock outstanding after this offering (1)
|
|
16,351,848 shares (assuming we sell only shares of common stock and no pre-funded warrants, and none of the common warrants issued in this offering are exercised).
|
| |
|
|
|
Use of proceeds
|
|
We estimate that we will receive net proceeds from this offering of approximately $9,100,000 based upon an assumed offering price of $1.27 per share of common stock and accompanying common warrant, or pre-funded warrant and accompanying common warrant, as applicable, after deducting the underwriting discount and commissions and estimated offering expenses payable by us. We currently intend to use the net proceeds we receive from this offering to (i) continue with our pivotal Phase III trial for the treatment of NPC with Trappsol® Cyclo™, (ii) fund further development of our preclinical programs towards IND filings and clinical trials for the treatment of Alzheimer’s disease with Trappsol® Cyclo™ and (iii) fund working capital and general corporate purposes using any remaining amounts. See “Use of Proceeds” on page 18. |
|
Lock-Up
|
|
Our directors and executive officers have agreed not to offer for sale, issue, sell, contract to sell, pledge or otherwise dispose of any of our common stock or securities convertible into common stock for a period of days commencing on the date of this prospectus.
|
| |
|
|
|
Risk Factors
|
|
You should carefully read the “Risk Factors” section of this prospectus beginning on page 6 for a discussion of factors that you should consider before deciding to invest in our securities.
|
| |
|
|
|
Trading Symbol and Listing
|
|
Our common stock is listed on The Nasdaq Capital Market under the symbol “CYTH”. We do not intend to apply for listing of the common warrants or pre-funded warrants on any national securities exchange or trading system.
|
(1) Unless we indicate otherwise, the number of shares of our common stock outstanding after this offering is based on 8,481,848 shares of common stock outstanding on November 21, 2022, and excludes the following:
| |
●
|
425,646 shares of our common stock issuable upon the exercise of stock options, with a weighted-average exercise price of $5.17 per share;
|
| |
●
|
2,480,042 shares of our common stock reserved for issuance under our 2021 Equity Incentive Plan; and |
| |
●
|
2,045,846 shares of our common stock issuable upon the exercise of warrants, with a weighted-average exercise price of $11.22 per share. |
Unless otherwise noted, the information in this prospectus assumes:
| |
●
|
no exercise of the outstanding options and warrants described above; and
|
| |
●
|
no exercise of common warrants.
|
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the following risk factors in addition to other information in this prospectus before purchasing our securities. The risks and uncertainties described below are those that we currently deem to be material and that we believe are specific to our company, our industry and our securities. In addition to these risks, our business may be subject to risks currently unknown to us. We also update risk factors from time to time in our periodic reports on Forms 10-K, 10-Q and 8-K which will be incorporated by reference in this prospectus. If any of these or other risks actually occurs, our business may be adversely affected, the trading price of our securities may decline and you may lose all or part of your investment.
We have suffered recent losses and our future profitability is uncertain.
We have incurred net losses of approximately $14.3 million and $8.9 million for the years ended December 31, 2021 and December 31, 2020, respectively, and approximately $4.3 million and $10.6 million for the three and nine months ended September 30, 2022, respectively. Our recent losses have predominantly resulted from research and development expenses for our Trappsol® Cyclo™ product and other general operating expenses, including personnel costs. We believe our expenses will continue to increase as we conduct clinical trials and continue to seek regulatory approval for the use of Trappsol® Cyclo™ in the treatment of NPC and Alzheimer’s disease. As a result, we expect our operating losses to continue until such time, if ever, that product sales, licensing fees, royalties and other sources generate sufficient revenue to fund our operations. We cannot predict when, if ever, we might achieve profitability and cannot be certain that we will be able to sustain profitability, if achieved.
Even with the proceeds from our recent public offerings, we will need additional capital to fund our operations as planned.
For the year ended December 31, 2021 and nine months ended September 30, 2022, our operations used approximately $15.0 million and $12.4 million in cash, respectively. Cash used in operations consisted of cash on hand and cash raised through public offerings and private placements of our securities. At December 31, 2021, the Company had a cash balance of approximately $16.6 million and current liabilities of approximately $3.8 million, and at September 30, 2022, the Company had a cash balance of approximately $4.3 million and current liabilities of approximately $2.9 million. Although we raised approximately $10.8 million in our November 2021 public offering and approximately $8.0 million from the exercise of warrants originally issued in our December 2020 offering, we will need additional capital to continue our research and development programs, conduct clinical trials, seek regulatory approvals and manufacture and market our products. We will seek such additional funds through public or private equity or debt financings and other sources. We cannot be certain that adequate additional funding will be available to us on acceptable terms, if at all. In addition, in order to issue additional equity securities, we will need to obtain stockholder approval to increase the number of our authorized shares of common stock. If we cannot raise the additional funds required for our anticipated operations, we may be required to reduce the scope of or eliminate our research and development programs, delay our clinical trials and the ability to seek regulatory approvals, downsize our general and administrative infrastructure, or seek alternative measures to avoid insolvency. If we raise additional funds through future offerings of shares of our common stock or other securities, such offerings would cause dilution of current stockholders’ percentage ownership in the Company, which could be substantial. Future offerings also could have a material and adverse effect on the price of our common stock.
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern.
Our auditors, WithumSmith+Brown, PC, have indicated in their report on our consolidated financial statements for the fiscal year ended December 31, 2021, that conditions exist that raise substantial doubt about our ability to continue as a going concern due to our recurring losses from operations and significant accumulated deficit. In addition, we continue to experience negative cash flows from operations. A “going concern” opinion could impair our ability to finance our operations through the sale of equity. Our ability to continue as a going concern will depend upon the availability of equity financing which represents the primary source of cash flows that will permit us to meet our financial obligations as they come due and continue our research and development efforts.
We have not received approval for any drug candidate for commercial sale and, as a result, we have never generated any revenue from the sale of biopharmaceutical products, and expect to continue to incur significant financial losses in the future, which makes it difficult to assess our future viability.
While we sell cyclodextrins for use and research in numerous industries, we have not yet received the necessary regulatory approvals to commercially sell any biopharmaceutical products. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk, including risks related to the regulatory approval process. Because the focus of our business has transitioned to the development of cyclodextrin-based products for the treatment of disease, we anticipate that our expenses will increase substantially as we:
| |
●
|
continue our ongoing and planned development of Trappsol® Cyclo™ for multiple indications;
|
| |
|
|
| |
●
|
initiate, conduct and complete ongoing, anticipated or future preclinical studies and clinical trials for our current and future product candidates;
|
| |
|
|
| |
●
|
seek marketing approvals for product candidates that successfully complete clinical trials; and
|
| |
|
|
| |
●
|
establish a sales, marketing and distribution infrastructure to commercialize products for which we may obtain marketing approval.
|
We will continue to incur significant losses until such time, if ever, as we are able to commercialize our drug candidates. If we are not able to do so we may not sustain a viable business.
Risks Related to Product Development, Regulatory Approval and Commercialization
We are largely dependent upon the success of our Trappsol® Cyclo™ product, which may never receive regulatory approval for the treatment of disease.
Our lead drug candidate, Trappsol® Cyclo™ is the focus of much of our management team’s development efforts. The product is currently designated as an orphan drug for the treatment of NPC in the United States and Europe. We plan to continue to make substantial investment in continued research and development of our Trappsol® Cyclo™ product in connection with obtaining approval for marketing the product for the treatment of NPC, as well as Alzheimer’s disease. The potential population of NPC patients is small, and our ability to market the drug for use other than research is severely constrained by regulatory restrictions. In the course of its development, our Trappsol® Cyclo™ drug product will be subject to extensive and rigorous government regulation through the European Medicines Agency in the E.U. and through the Food and Drug Administration (FDA) in the United States. Regulatory approval in any jurisdiction cannot be guaranteed. There can be no guarantees that our product will be effective and safe in the treatment of NPC, Alzheimer’s disease or any other disease nor is there any guarantee that it will be deemed by the regulatory agencies of any jurisdiction to be effective and safe. Despite the time and expense involved in developing a drug candidate, failure of a drug candidate can occur at any stage of development and for many reasons, including without limitation negative or inconclusive results from pre-clinical data or clinical trials. Failure to comply with applicable regulatory requirements in any jurisdiction, either before or after product approval, may subject us to administrative or judicially imposed sanctions.
Even if Trappsol® Cyclo™ receives regulatory approval, we may not be successful in our commercialization efforts and Trappsol® Cyclo™ may fail to achieve the degree of market acceptance by physicians, patients, healthcare payors and others in the medical community necessary for commercial success.
Even if Trappsol® Cyclo™ receives regulatory approval, we may not be successful in our commercialization efforts and market acceptance by physicians, patients, third-party payors and others in the medical community may be less than estimated. Market acceptance will require us to build and maintain strong relationships with healthcare professionals involved in the treatment of NPC. The number of healthcare professionals associated with treatment centers that address NPC is limited. A failure to build or maintain these important relationships with these healthcare professionals and treatment centers could result in lower market acceptance. Our efforts to educate physicians, patients, third-party payors and others in the medical community on the benefits of Trappsol® Cyclo™ may require significant resources and may never be successful. The degree of market acceptance of Trappsol® Cyclo™, if approved for commercial sale, will depend on a number of factors, including:
| |
●
|
its efficacy;
|
| |
|
|
| |
●
|
limitations or warnings or any restrictions on the use of Trappsol® Cyclo™, together with other medications, and the prevalence and severity of any side effects;
|
| |
|
|
| |
●
|
the availability and efficacy of alternative treatments;
|
| |
|
|
| |
●
|
the effectiveness of sales and marketing efforts and the strength of marketing and distribution support;
|
| |
|
|
| |
●
|
the cost-effectiveness of Trappsol® Cyclo™ compared to alternative therapies and the ability to offer such drug for sale at competitive prices; and
|
| |
|
|
| |
●
|
availability and amount of coverage and reimbursement from government payors, managed care plans and other third-party payors.
|
The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our product claims or that any regulatory authority whose approval we will require in order to market and sell our products in any territory will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that clinical trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our regulatory submissions and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including FDA approval. Clinical trials are expensive and complex, can take many years and have uncertain outcomes. We cannot predict whether we will encounter problems with any of our completed, ongoing or planned clinical trials that will cause us or regulatory authorities to delay or suspend clinical trials, or delay the analysis of data from completed or ongoing clinical trials. We estimate that clinical trials of Trappsol® Cyclo™ for the treatment of NPC will continue for several years, but they may take significantly longer to complete. Failure can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future therapeutic candidates, including but not limited to:
| |
●
|
delays in securing clinical investigators or trial sites for the clinical trials;
|
| |
●
|
delays in obtaining institutional review board and other regulatory approvals to commence a clinical trial;
|
| |
●
|
slower than anticipated patient recruitment and enrollment;
|
| |
●
|
negative or inconclusive results from clinical trials;
|
| |
●
|
unforeseen safety issues;
|
| |
●
|
uncertain dosing issues;
|
| |
●
|
an inability to monitor patients adequately during or after treatment; and
|
| |
●
|
problems with investigator or patient compliance with the trial protocols.
|
A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials, even after seeing promising results in earlier clinical trials. Despite the results reported in earlier clinical trials for Trappsol® Cyclo™, we do not know whether any Phase III or other clinical trials we may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market Trappsol® Cyclo™. If later-stage clinical trials do not produce favorable results, our ability to obtain regulatory approval for Trappsol® Cyclo™ may be adversely impacted.
Later discovery of previously unknown problems could limit our ability to market or sell Trappsol® Cyclo™, even if it is initially approved, and can expose us to product liability claims.
Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with any third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| |
●
|
refusals or delays in the approval of applications or supplements to approved applications;
|
| |
|
|
| |
●
|
refusal of a regulatory authority to review pending market approval applications or supplements to approved applications;
|
| |
|
|
| |
●
|
restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market or voluntary or mandatory product recalls or seizures;
|
| |
|
|
| |
●
|
fines, warning letters, or holds on clinical trials;
|
| |
|
|
| |
●
|
import or export restrictions;
|
| |
|
|
| |
●
|
injunctions or the imposition of civil or criminal penalties;
|
| |
|
|
| |
●
|
restrictions on product administration, requirements for additional clinical trials, or changes to product labeling requirements; or
|
| |
|
|
| |
●
|
recommendations by regulatory authorities against entering into governmental contracts with us.
|
Discovery of previously unknown problems or risks relating to our product could also subject us to potential liabilities through product liability claims.
If we do not obtain required approvals in other countries in which we aim to market our products, we will be limited in our ability to export or sell the products in those markets.
Our lack of experience in conducting clinical trials in any jurisdiction may negatively impact the approval process in those jurisdictions where we intend to seek approval of Trappsol® Cyclo™. If we are unable to obtain and maintain required approval from one or more foreign jurisdictions where we would like to sell Trappsol® Cyclo™, we will be unable to market products as intended, our international market opportunity will be limited and our results of operations will be harmed.
We rely in part on third parties for research and clinical trials for products using Trappsol® Cyclo™.
We rely on contract research organizations (“CROs”), academic institutions, corporate partners, and other third parties to assist us in managing, monitoring, and otherwise carrying out clinical trials and research activities. We rely or will rely heavily on these parties for the execution of our clinical studies and control only certain aspects of their activities. Accordingly, we may have less control over the timing and other aspects of these clinical trials than if we conducted them entirely on our own. Although we rely on these third parties to manage the data from clinical trials, we will be responsible for confirming that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. Our failure, or the failure of third parties on which we rely, to comply with the strict requirements relating to conducting, recording, and reporting the results of clinical trials, or to follow good clinical practices, may delay the regulatory approval process or cause us to fail to obtain regulatory approval for Trappsol® Cyclo™.
We currently have no marketing and sales organization for our pharmaceutical candidates and may have to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to generate product revenue.
We have no internal sales, marketing or distribution capabilities for the sale of biopharmaceutical products. If any of our drug candidates ultimately receives regulatory approval, we may not be able to effectively market and distribute it. We may have to seek collaborators, especially for marketing and sales outside of the United States, or invest significant amounts of financial and management resources to develop internal sales, distribution and marketing capabilities. We may not be able to enter into collaborations or hire consultants or external service providers to assist us in sales, marketing and distribution functions on acceptable financial terms, or at all. In addition, our product revenues and our profitability, if any, may be lower if we rely on third parties for these functions than if we were to market, sell and distribute products that we develop ourselves. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. Even if we determine to perform sales, marketing and distribution functions ourselves, we could face a number of additional related risks, including:
| |
●
|
we may not be able to attract and build an effective marketing department or sales force;
|
| |
|
|
| |
●
|
the cost of establishing a marketing department or sales force may exceed our available financial resources and the revenue generated by our product candidates that we may develop, in-license or acquire; and
|
| |
|
|
| |
●
|
our direct sales and marketing efforts may not be successful.
|
We rely upon third parties for the manufacture of Trappsol® Cyclo™ and are dependent on their quality and effectiveness.
Trappsol® Cyclo™ requires precise, high-quality manufacturing. The failure to achieve and maintain high manufacturing standards, including the failure to conform to c-GMP (current Good Manufacturing Practice), or to detect or control anticipated or unanticipated manufacturing errors or the frequent occurrence of such errors, could result in discontinuance or delay of ongoing or planned clinical trials, delays or failures in product testing or delivery, cost overruns, product recalls or withdrawals, patient injury or death, and other problems that could seriously hurt our business. Contract drug manufacturers often encounter difficulties involving production yields, quality control and quality assurance and shortages of qualified personnel. These manufacturers are subject to stringent regulatory requirements, including the FDA’s c-GMP regulations and similar foreign laws and standards. If our contract manufacturers fail to maintain ongoing compliance at any time, the production of our product candidates could be interrupted, resulting in delays or discontinuance of our clinical trials, additional costs and loss of potential revenues.
We face competition from well-funded companies to treat NPC.
We face competition from other entities, including pharmaceutical and biotechnology companies and governmental institutions that are working on supporting orphan drug designations and clinical trials for the neurological manifestations of NPC. Some of these entities are well-funded, with more financial, technical and personnel resources than we have, and have more experience than we do in designing and implementing clinical trials. If we are unable to compete effectively against our current or future competitors, sales of our Trappsol® Cyclo™ product may not grow and our financial condition may suffer.
Our business and operations would suffer in the event of computer system failures or security breaches.
In the ordinary course of our business, we collect, store and transmit confidential information, including intellectual property, proprietary business information and personal information. Despite the implementation of security measures, our internal computer systems, and those of our contract research organizations, or CROs, and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyberattacks, natural disasters, fire, terrorism, war and telecommunication and electrical failures. Cyberattacks are increasing in their frequency, sophistication and intensity. Cyberattacks could include the deployment of harmful malware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. Significant disruptions of our information technology systems or security breaches could adversely affect our business operations and/or result in the loss, misappropriation, and/or unauthorized access, use or disclosure of, or the prevention of access to, confidential information (including trade secrets or other intellectual property, proprietary business information and personal information), and could result in financial, legal, business and reputational harm to us. If such disruptions were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Further, the COVID-19 pandemic has resulted in a significant number of our employees and partners working remotely, which increases the risk of a data breach or issues with data and cybersecurity. To the extent that any disruption or security breach results in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development of our future product candidates could be delayed.
We are subject to risks arising from COVID-19.
The COVID-19 coronavirus has spread across the globe and is impacting worldwide economic activity. A pandemic, including COVID-19 or other public health epidemic, poses the risk that we or our employees, CROs, suppliers, manufacturers and other partners may be prevented from conducting business activities for an indefinite period of time, including due to the spread of the disease or shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the full impact that COVID-19 could have on our business, the continued spread of COVID-19 could disrupt our clinical trials, supply chain and the manufacture or shipment of our cyclodextrin products, and other related activities, which could have a material adverse effect on our business, financial condition and results of operations. COVID-19 has also had an adverse impact on global economic conditions which could impair our ability to raise capital when needed. While we have not yet experienced any disruptions in our business or other negative consequences relating to COVID-19, the extent to which the COVID-19 pandemic impacts our results will depend on future developments that are highly uncertain and cannot be predicted.
Risks Related to Our Intellectual Property
The rights we rely upon to protect our unpatented trade secrets may be inadequate.
To manufacture and produce Trappsol® Cyclo™, we rely primarily on unpatented trade secrets, know-how and technology which are difficult to protect, especially in the pharmaceutical industry, where much of the information about a product must be made public during the regulatory approval process. We seek to protect trade secrets, in part, by entering into confidentiality agreements with third-party manufacturers, employees, consultants and others. These parties may breach or terminate these agreements or may refuse to enter into such agreements with us, and we may not have adequate remedies for such breaches. Furthermore, these agreements may not provide meaningful protection for our trade secrets or other proprietary information and may not provide an adequate remedy in the event of unauthorized use or disclosure of confidential information or other breaches of the agreements. Despite our efforts to protect our trade secrets, we or others may unintentionally or willfully disclose our proprietary information to competitors.
If we fail to maintain trade secret protection, our competitive position may be adversely affected. Competitors may also independently discover our trade secrets. Enforcement of claims that a third party has illegally obtained and is using trade secrets is expensive, time consuming and uncertain. If our competitors independently develop equivalent knowledge, methods and know-how, we would not be able to assert our trade secrets against them and our business could be harmed.
We cannot ensure that patent rights relating to inventions described and claimed in our pending patent applications will issue, that patents based on our patent applications will not be challenged and rendered invalid and/or unenforceable, or that third parties will not find ways to circumvent our patent rights or claim co-ownership thereof.
We have patent applications pending with respect to the treatment of Alzheimer’s disease with Trappsol® Cyclo™. However, we cannot predict:
| |
●
|
if and when patents may issue based on our patent applications;
|
| |
|
|
| |
●
|
the scope of protection of any patent issuing based on our patent applications;
|
| |
|
|
| |
●
|
whether the claims of any patent issuing based on our patent applications will provide protection against competitors;
|
| |
|
|
| |
●
|
whether or not third parties will find ways to invalidate or circumvent our patent rights, or claim co-ownership rights in our patent rights, which may impact our ability to enforce our patent rights against third parties;
|
| |
|
|
| |
●
|
whether or not others will obtain patents claiming aspects similar to those covered by our patents and patent applications; or
|
| |
|
|
| |
●
|
whether we will need to initiate litigation or administrative proceedings to enforce and/or defend our patent rights which will be costly whether we win or lose.
|
We cannot be certain that the claims in our pending patent applications will be considered patentable by the U.S. Patent and Trademark Office or by patent offices in foreign countries. Even if the patents do issue based on our patent applications, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. Furthermore, even if they are unchallenged, our patents may not adequately exclude third parties from practicing relevant technology or prevent others from designing around our claims. If the breadth or strength of our intellectual property position with respect to our product candidates is threatened, it could dissuade companies from collaborating with us and threaten our ability to commercialize our product candidates. It is possible that third parties with whom we have collaborated may contend that they co-own patent rights we have filed, which, if correct and in the absence of an agreement to the contrary, could prevent us from asserting the patent rights against our competitors. Furthermore, in the event of litigation or administrative proceedings, we cannot be certain that the claims in any of our issued patents will be considered valid by courts in the United States or foreign countries.
We are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our product candidates.
There is a substantial amount of litigation over patent and other intellectual property rights in the biotechnology industry. Whether or not a product infringes a patent involves complex legal and factual considerations, the determination of which is often uncertain. Searches typically performed to identify potentially infringed patents of third parties are often not conclusive and, because patent applications can take many years to issue, there may be applications now pending, which may later result in issued patents which our current or future products may infringe or be alleged to infringe. In addition, our competitors or other parties may assert that our product candidates and the methods employed may be covered by patents held by them. If any of our products infringes a valid patent, we could be prevented from manufacturing or selling such product unless we are able to obtain a license or able to redesign the product in such a manner as to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid infringement, nor does a later redesign protect the Company from prior infringement. We are aware of third party U.S. patents and patent applications, which may be relevant to our lead product candidate Trappsol® Cyclo™ for treating Niemann-Pick Type C disease, and may be relevant to the use of Trappsol® Cyclo™ for treating Alzheimer’s disease. Although we believe that we would not infringe a valid claim of those patents or pending patent applications, if issued, the owner of the patent rights may disagree with our assessment and bring an infringement action against us. There is no assurance that a court would find in our favor on questions of infringement or validity. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and can divert our management’s attention from operating our business.
We may need to initiate lawsuits to protect or enforce our intellectual property rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual property rights, which would harm our ability to compete in the market.
In order to protect or enforce our intellectual property rights, we may initiate patent, trademark and related litigation against third parties, such as infringement suits or requests for injunctive relief. Our ability to establish and maintain a competitive position may be achieved in part by prosecuting claims against others who we believe to be infringing its rights. Any lawsuits that we initiate could be expensive, take significant time and divert our management’s attention from other business concerns and the outcome of litigation to enforce our intellectual property rights in patents, trade secrets or trademarks is highly unpredictable. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, or adversely affect our ability to distribute any products that are subject to such litigation. In addition, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, including attorney fees, if any, may not be commercially valuable.
Risks Related to Legal and Regulatory Compliance Matters
The pharmaceutical business is subject to increasing government regulation and reform, including with respect to price controls, reimbursement and access to drugs, which could adversely affect our future revenues and profitability.
To the extent our products are developed, commercialized, and successfully introduced to market, they may not be considered cost-effective, and third-party or government reimbursement might not be available or sufficient. Globally, governmental and other third-party payors are becoming increasingly aggressive in attempting to contain health care costs by strictly controlling, directly or indirectly, pricing and reimbursement and, in some cases, limiting or denying coverage altogether on the basis of a variety of justifications, and we expect pressures on pricing and reimbursement from both governments and private payors inside and outside the U.S. to continue.
If we obtain the required regulatory approval to sell our drug candidates, we will be subject to substantial pricing, reimbursement, and access pressures from state Medicaid programs, private insurance programs and pharmacy benefit managers, and the implementation of U.S. health care reform legislation that is increasing these pricing pressures. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, instituted comprehensive health care reform, and includes provisions that, among other things, reduce and/or limit Medicare reimbursement, and impose new and/or increased taxes. The future of the Affordable Care Act and its constituent parts are uncertain at this time.
In almost all markets, pricing and choice of prescription pharmaceuticals are subject to governmental control. Therefore, the price of our products and their reimbursement in Europe and in other countries is and will be determined by national regulatory authorities. Reimbursement decisions from one or more of the European markets may impact reimbursement decisions in other European markets. A variety of factors are considered in making reimbursement decisions, including whether there is sufficient evidence to show that treatment with the product is more effective than current treatments, that the product represents good value for money for the health service it provides, and that treatment with the product works at least as well as currently available treatments.
The continuing efforts of government and insurance companies, health maintenance organizations, and other payors of health care costs to contain or reduce costs of health care may affect our future revenues and profitability or those of our potential customers, suppliers, and collaborative partners, as well as the availability of capital.
United States federal and state privacy laws, and equivalent laws of other nations, may increase our costs of operation and expose us to civil and criminal sanctions.
Regulation of data processing is evolving, as federal, state, and foreign governments continue to adopt new, or modify existing, laws and regulations addressing data privacy and security, and the collection, processing, storage, transfer, and use of data. These new or proposed laws and regulations are subject to differing interpretations and may be inconsistent among jurisdictions, and guidance on implementation and compliance practices are often updated or otherwise revised, which adds to the complexity of processing personal data. These and other requirements could require us or our collaborators to incur additional costs to achieve compliance, limit our competitiveness, necessitate the acceptance of more onerous obligations in our contracts, restrict our ability to use, store, transfer, and process data, impact our or our collaborators’ ability to process or use data in order to support the provision of our products, affect our or our collaborators’ ability to offer our products in certain locations, or cause regulators to reject, limit or disrupt our clinical trial activities.
We and our collaborators may be subject to federal, state and foreign data protection laws and regulations (i.e., laws and regulations that address privacy and data security). In the United States, numerous federal and state laws and regulations, including federal health information privacy laws, state personal information laws, state data breach notification laws, state health information privacy laws and federal and state consumer protection laws and regulations that govern the collection, use, disclosure and protection of health-related and other personal information could apply to our operations or the operations of our collaborators. In addition, we may obtain health information from third parties (including research institutions from which we obtain clinical trial data) that are subject to privacy and security requirements under the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH. Depending on the facts and circumstances, we could be subject to civil or criminal penalties if we knowingly use or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
Risks Related to Employee Matters
We are dependent on our executive officers, and we may not be able to pursue our current business strategy effectively if we lose them.
Our success to date has largely depended on the efforts and abilities of our executive officers, namely N. Scott Fine, our Chief Executive Officer, and Lise Kjems, MD, PhD, our Chief Medical Officer. Our ability to manage our operations and meet our business objectives could be adversely affected if, for any reason, such officers do not remain with us.
Our employees, clinical trial investigators, CROs, consultants, vendors and any potential commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards.
We are exposed to the risk of fraud or other misconduct by our employees, clinical trial investigators, CROs, consultants, vendors and any potential commercial partners. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (i) U.S. laws and regulations or those of foreign jurisdictions, including those laws that require the reporting of true, complete and accurate information, (ii) manufacturing standards, (iii) federal and state health and data privacy, security, fraud and abuse, government price reporting, transparency reporting requirements, and other healthcare laws and regulations in the United States and abroad or (iv) laws that require the true, complete and accurate reporting of financial information or data. Such misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct applicable to all of our employees, as well as a disclosure program and other applicable policies and procedures, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant civil, criminal and administrative penalties, damages, fines, disgorgement, individual imprisonment, exclusion from government funded healthcare programs, such as Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, additional integrity reporting and oversight obligations, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
If we fail to comply with the U.S. federal Anti-Kickback Statute and similar state and foreign country laws, we could be subject to criminal and civil penalties and exclusion from federally funded healthcare programs including the Medicare and Medicaid programs and equivalent third country programs, which would have a material adverse effect on our business and results of operations.
A provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration, directly or indirectly, in cash or in kind, to induce or reward the referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable, in whole or in part, by Medicare, Medicaid or any other federal healthcare program. The federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, many states have adopted laws similar to the federal Anti-Kickback Statute that apply to activity in those states, and some of these laws are even broader than the federal Anti-Kickback Statute in that their prohibitions may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
While we believe our operations will be in compliance with the federal Anti-Kickback Statute and similar state laws, we cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
Risks Related To Our Fine Chemical Business
A small number of our customers account for a substantial portion of our revenue and receivables, and the loss of any of these customers would materially decrease our revenues.
In 2021, four major customers accounted for 73% of total revenues. Accounts receivable balances for these major customers represented 94% of total accounts receivable at December 31, 2021. For the three months ended September 30, 2022, two customers accounted for 86% of total revenues, and for the nine months ended September 30, 2022, three major customers accounted for 67% of total revenues. We have a supply contract with only one of our major customers. The loss of one of these customers would materially decrease our revenues if we were unable to replace such customers.
We are dependent on certain third-party suppliers.
We purchase the Trappsol® cyclodextrin products we sell from third-party suppliers and depend on those suppliers for the cyclodextrins we use in our Aquaplex® products. We are also dependent on outside manufacturers that use lyophilization techniques for our Aquaplex® products. We purchase substantially all of our Trappsol® products from bulk manufacturers and distributors in the U.S., Japan, China, and Europe. Although products are available from multiple sources, an unexpected interruption of supply, or material increases in the price of products, for any reason, such as regulatory requirements, import restrictions, loss of certifications, power interruptions, fires, hurricanes, war or other events could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We may be negatively affected by currency exchange rate fluctuations.
Our earnings and cash flows are influenced by currency fluctuations due to the geographic diversity of our suppliers, which may have a significant impact on our financial results. As we buy inventory from foreign suppliers, the change in the value of the U.S. dollar in relation to the Euro, Yen and Yuan has an effect on our cost of inventory, and will continue to do so. We buy most of our products from outside the U.S. using U.S. dollars. Our main supplier of specialty cyclodextrins and complexes, Cyclodextrin Research & Development Laboratory, is located in Hungary and its prices are set in Euros. The cost of our bulk inventory often changes due to fluctuations in the U.S. dollar. These products currently represent a significant portion of our revenues. When we experience short-term increases in currency fluctuation or supplier price increases, we are often not able to raise our prices sufficiently to maintain our historical margins and therefore, our margins on these sales may decline. If the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions may adversely affect our results of operations and financial condition.
Risks Related To Our Common Stock and This Offering
Our management has broad discretion as to the use of the net proceeds from this offering.
We currently intend to use the net proceeds that we receive from this offering to (i) continue with our pivotal Phase III trial for the treatment of NPC with Trappsol® Cyclo™, (ii) fund further development of our preclinical programs towards IND filings and clinical trials for the treatment of Alzheimer’s disease with Trappsol® Cyclo™ and (iii) fund working capital and general corporate purposes using any remaining amounts. Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in “Use of Proceeds.” Accordingly, you will have to rely upon the judgment of our management with respect to the use of the proceeds. Our management may spend a portion or all of the net proceeds from this offering in ways that holders of our common stock may not desire or that may not yield a significant return or any return at all. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may also invest the net proceeds from this offering in a manner that does not produce income or that loses value.
There is no market for our common warrants or pre-funded warrants.
The public offering price for the securities will be determined by negotiations between us and the underwriter, and may not be indicative of prices that will prevail in the trading market. We do not intend to apply to list the common warrants or pre-funded warrants on The Nasdaq Capital Market or any nationally recognized trading system, and accordingly, there will be no trading market for such warrants. In the absence of an active public trading market:
| |
●
|
you may not be able to resell your securities at or above the public offering price;
|
| |
|
|
| |
●
|
the market price of our common stock may experience more price volatility; and
|
| |
|
|
| |
●
|
there may be less efficiency in carrying out your purchase and sale orders.
|
The ability to exercise our common warrants is contingent upon stockholder approval.
We do not have a sufficient number of authorized shares of common stock to cover the exercise in full of all of the common warrants to be issued in this offering. Accordingly, pursuant to the terms thereof, the common warrants will not be exercisable until the effective date of an amendment to our articles of incorporation increasing the number of authorized shares of common stock to at least 30,000,000. We intend to seek approval from our stockholders at our next annual meeting to increase the number of our authorized shares of common stock to at least 30,000,000. However, our stockholders may reject such proposal, which could delay or prevent the ability of holders of our common warrants to exercise them.
The market price of our common stock may be highly volatile, and you could lose all or part of your investment.
The trading price of our common stock and warrants is likely to be volatile. This volatility may prevent you from being able to sell your securities at or above the price you paid for your securities. Our stock price and warrant price could be subject to wide fluctuations in response to a variety of factors, which include:
| |
●
|
whether we achieve our anticipated corporate objectives;
|
| |
●
|
changes in financial or operational estimates or projections;
|
| |
●
|
termination of the lock-up agreement or other restrictions on the ability of our stockholders and other security holders to sell shares after this offering; and
|
| |
●
|
general economic or political conditions in the United States or elsewhere.
|
In addition, the stock market in general, and the stock of clinical stage biotechnology companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future.
You will incur immediate and substantial dilution as a result of this offering. Assuming the sale by us of 7,870,000 shares of common stock and accompanying common warrants in this offering at an assumed combined public offering price of $1.27 per share of common stock and accompanying common warrants, which is equal to the last reported sale price per share of our common stock on The Nasdaq Capital Market on November 16, 2022, after deducting the underwriting discount and commissions and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $0.54 per share. For a further description of the dilution that investors in this offering may experience, see “Dilution.”
In the past, we have issued shares of common stock and warrants in private placements of our securities, and we have issued shares of common stock as compensation to our officers and directors. Our issuance of shares of common stock in the future, and the exercise of outstanding warrants or warrants that we may issue in the future, may result in additional dilution to investors in this offering.
If we are delisted from The Nasdaq Capital Market, and our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not maintain a listing on Nasdaq and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
Our failure to meet the continued listing requirements of The Nasdaq Capital Market could result in a de-listing of our securities.
If we fail to satisfy the continued listing requirements of Nasdaq, such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to de-list our securities. Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we would take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our securities, prevent our common stock from dropping below the Nasdaq minimum bid price requirement or prevent future non-compliance with Nasdaq’s listing requirements.
We will indemnify and hold harmless our officers and directors to the maximum extent permitted by Nevada law.
Our bylaws provide that we will indemnify and hold harmless our officers and directors against claims arising from our activities, to the maximum extent permitted by Nevada law. If we were called upon to perform under our indemnification agreement, then the portion of our assets expended for such purpose would reduce the amount otherwise available for our business.
Because we do not expect to pay dividends for the foreseeable future, investors seeking cash dividends should not purchase shares of common stock.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings, if any, to finance the expansion of our business. As a result, we do not anticipate paying any cash dividends in the foreseeable future. Our payment of any future dividends will be at the discretion of our Board of Directors after taking into account various factors, including but not limited to our financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. Accordingly, investors seeking cash dividends should not purchase our shares.
Common warrants are speculative in nature.
The common warrants offered pursuant to this prospectus do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the common warrants may exercise their right to acquire the common stock and pay an exercise price of $ , prior to five years from the date of issuance, after which date any unexercised common warrants will expire and have no further value. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the common warrants, and, consequently, whether it will ever be profitable for holders of the common warrants to exercise those warrants.
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
The information contained in this prospectus contains certain forward-looking statements. All statements other than statements of historical facts contained or incorporated by reference in this prospectus, including statements regarding our future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “will,” “may,” “future,” “plan,” “intend” and “expect” and similar expressions generally identify forward-looking statements.
These forward-looking statements are not guarantees and are subject to known and unknown risks, uncertainties and assumptions that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by such forward-looking statements. Although we believe that our plans, intentions and expectations reflected in the forward-looking statements are reasonable, we cannot be sure that they will be achieved. Particular uncertainties that could cause our actual results to be materially different than those expressed in our forward-looking statements include: our history of losses; our inability to receive regulatory approval for our products; later discovery of previously unknown problems; reliance on third parties; competition between us and other companies in the industry; delays in the development of products; our ability to raise additional capital; continued services of our executive management team; and statements of assumption underlying any of the foregoing, as well as other factors set forth under the caption “Risk Factors” on page 6 of this prospectus.
All subsequent written and oral forward-looking statements attributable to us, or persons acting on our behalf, are expressly qualified in their entirety by the foregoing. Except as required by law, we undertake no obligation to update any forward-looking statement, whether as a result of new information, future events or otherwise.
USE OF PROCEEDS
We estimate that the net proceeds from this offering will be approximately $9,100,000, assuming a public offering price of $1.27 per share of common stock and common warrant, after deducting the underwriting discount and commissions and estimated offering expenses payable by us and assuming no exercise of the common warrants. We will only receive additional proceeds from the exercise of the common warrants issuable in connection with this offering if such warrants are exercised at their exercise price of $ and the holders of such warrants pay the exercise price in cash upon such exercise. Such proceeds with respect to the common warrants could not exceed $ .
The foregoing discussion assumes no sale of pre-funded warrants.
We intend to use the net proceeds from this offering to (i) continue with our pivotal Phase III trial for the treatment of NPC with Trappsol® Cyclo™, (ii) fund further development of our preclinical programs towards IND filings and clinical trials for the treatment of Alzheimer’s disease with Trappsol® Cyclo™ and (iii) fund working capital and general corporate purposes using any remaining amounts. Pending these uses, we intend to invest the funds in short-term, investment grade, interest-bearing securities. It is possible that, pending their use, we may invest the net proceeds in a way that does not yield a favorable, or any, return for us.
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot currently allocate specific percentages of the net proceeds that we may use for the purposes specified above, and we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds will vary depending on numerous factors, including our ability to obtain additional financing. We may find it necessary or advisable to use the net proceeds for other purposes, and our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds from this offering. See “Risk Factors” for a discussion of certain risks that may affect our intended use of the net proceeds from this offering.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends on our common stock at any time in the foreseeable future. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our Board and will depend on, among other factors, our financial condition, operating results, capital requirements, general business conditions, the terms of any future credit agreements and other factors that our Board may deem relevant.
CAPITALIZATION
The following table sets forth our cash and cash equivalents, debt obligations, and capitalization as of September 30, 2022 (i) on an actual basis, and (ii) on a pro forma basis to give effect to the issuance and sale of shares of our common stock and common warrants in this offering at an assumed combined public offering price of $1.27 per share and accompanying common warrant, equal to the last reported sale price per share of our common stock on The Nasdaq Capital Market on November 16, 2022, for total net proceeds of approximately $9,100,000 (assuming no sale of pre-funded warrants).
| |
|
As of September 30, 2022
|
|
| |
|
Actual
|
|
|
Pro Forma (1)
|
|
| |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$ |
4,290,198 |
|
|
$ |
13,393,251 |
|
|
Stockholders’ (deficit) equity:
|
|
|
|
|
|
|
|
|
|
Common stock, $0.001 par value, 20,000,000 shares authorized; 8,455,442 issued and outstanding actual; 16,351,848 shares issued and outstanding pro forma
|
|
|
846 |
|
|
|
1,635 |
|
|
Preferred stock, par value $.0001 per share, 5,000,000 shares authorized, 0 issued and outstanding
|
|
|
- |
|
|
|
- |
|
|
Additional paid-in capital
|
|
|
64,430,566 |
|
|
|
73,533,619 |
|
|
Accumulated deficit
|
|
$ |
(58,818,952 |
) |
|
$ |
(58,818,952 |
) |
|
Total stockholders’ equity (deficit)
|
|
|
5,612,460 |
|
|
|
14,716,302 |
|
|
Total capitalization
|
|
$ |
8,487,523 |
|
|
|
17,591,365 |
|
| (1) |
Each $0.50 increase (decrease) in the assumed public offering price of $1.27 per share and accompanying common warrant would increase (decrease) cash and cash equivalents, working capital, total assets, total liabilities, additional paid-in capital and total stockholders’ (deficit) equity by $3,660,000, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting the underwriting discount and commissions. Similarly, each increase (decrease) of 500,000 shares of common stock and accompanying common warrant offered by us would increase (decrease) each of cash and cash equivalents, working capital, total assets, additional paid-in capital and total stockholders’ (deficit) equity by $635,000 assuming the public offering price of $1.27 per share and accompanying common warrant remains the same, and after deducting the underwriting discount and commissions. |
The foregoing pro forma information is illustrative only, and our capitalization following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table together with our financial statements and the related notes incorporated into in this prospectus.
The above discussion and table is based on 8,455,452 shares of common stock outstanding on September 30, 2022, and excludes the following:
| |
●
|
425,646 shares of our common stock issuable upon the exercise of stock options, with a weighted-average exercise price of $5.17 per share;
|
| |
●
|
2,505,718 shares of our common stock reserved for issuance under our 2021 Equity Incentive Plan;
|
| |
●
|
2,048,188 shares of our common stock issuable upon the exercise of warrants, with a weighted-average exercise price of $11.26 per share; and
|
| |
●
|
shares of common stock issuable upon exercise of the common warrants issued in this offering.
|
DILUTION
If you invest in our securities in this offering, your interest will be diluted to the extent of the difference between the public offering price per share of common stock and accompanying common warrant and the net tangible book value per share of common stock immediately after this offering.
Our net tangible book value is the amount of our total tangible assets less our total liabilities. We had a net tangible book value as of September 30, 2022 of $2,822,832, or $0.33 share of common stock.
Pro forma as adjusted net tangible book value is our pro forma net tangible book value, plus the effect of the sale of our securities in this offering at the assumed public offering price of $1.27 per share of common stock and accompanying common warrant and after deducting the underwriting discount and commissions and other estimated offering expenses payable by us. Our net tangible book value as of September 30, 2022 would have been approximately $11,925,885, or $0.73 per share. This amount represents an immediate increase in net tangible book value of approximately $0.40 per share to our existing stockholders, and an immediate dilution of $0.54 per share to new investors participating in this offering. Dilution per share to new investors is determined by subtracting net tangible book value per share after this offering from the public offering price per share paid by new investors.
The following table illustrates this per share dilution:
|
Assumed public offering price per share of common stock (attributing no value to the warrants)
|
|
$ |
1.27 |
|
|
Net tangible book value per share as of September 30, 2022
|
|
$ |
0.33 |
|
|
Increase in net tangible book value per share after this offering
|
|
$ |
0.40 |
|
|
Pro forma as adjusted net tangible book value per share after giving effect to this offering
|
|
$ |
0.73 |
|
|
Dilution in net tangible book value per share to new investors
|
|
$ |
0.54 |
|
Each $0.50 increase (decrease) in the assumed public offering price of $1.27 per share and accompanying common warrant would increase (decrease) the net tangible book value per share by $0.62, and the dilution per share to new investors in this offering by $0.82, assuming the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discount and commissions and estimated offering expenses payable by us. Each increase of 500,000 in the number of shares of common stock and accompanying common warrants sold in this offering would increase (decrease) our net tangible book value per share by approximately $0.43 and the dilution per share to new investors in this offering by $0.50, assuming that the public offering price per share and accompanying common warrants remains the same and after deducting the underwriting discount and commissions and estimated offering expenses payable by us.
The foregoing discussion and table do not take into account further dilution to new investors that could occur upon the exercise of outstanding warrants having a per share exercise or conversion price less than the per share offering price to the public in this offering.
The above discussion and table is based on 8,455,452 shares of common stock outstanding on September 30, 2022, and excludes the following:
| |
●
|
425,646 shares of our common stock issuable upon the exercise of stock options, with a weighted-average exercise price of $5.17 per share;
|
| |
●
|
2,505,718 shares of our common stock reserved for issuance under our 2021 Equity Incentive Plan;
|
| |
●
|
2,048,188 shares of our common stock issuable upon the exercise of warrants, with a weighted-average exercise price of $11.26 per share; and
|
| |
●
|
shares of common stock issuable upon exercise of the common warrants issued in this offering.
|
DESCRIPTION OF SECURITIES
The following description of our capital stock and provisions of our articles of incorporation and bylaws are summaries and are qualified by reference to the articles of incorporation and bylaws that have been filed with the SEC as exhibits to the registration statement of which this prospectus forms a part.
Description of Existing Securities
Common Stock
We are authorized to issue 20,000,000 shares of common stock, $0.001 par value per share, of which 8,481,848 are outstanding on the date of this prospectus. Holders of shares of our common stock are entitled to one vote per share on all matters submitted to a vote of the stockholders and are not entitled to cumulative voting rights. Our shares of our common stock do not carry any preemptive, conversion or subscription rights, and there are no sinking fund or redemption provisions applicable to the shares of our common stock. Holders of our common stock are entitled to receive dividends and other distributions in cash, stock or property as may be declared by our Board of Directors from time to time out of our assets or funds legally available for dividends or other distributions, subject to dividend or distribution preferences that may be applicable to any then outstanding shares of preferred stock. In the event of our voluntary or involuntary liquidation, dissolution or winding up, holders of shares of our common stock are entitled to share ratably in the assets legally available for distribution to stockholders after payment of all debts and other liabilities and satisfaction of the liquidation preference, if any, granted to the holders of any preferred stock then outstanding. All outstanding shares of our common stock are fully paid and nonassessable.
Preferred Stock
We are authorized to issue 5,000,000 shares of preferred stock, $0.001 par value per share, none of which are issued or outstanding on the date of this prospectus. Our certificate of incorporation authorizes our Board of Directors to establish one or more series of preferred stock (including convertible preferred stock). Unless required by law, the authorized shares of preferred stock will be available for issuance without further action by you. Our Board of Directors is able to determine, with respect to any series of preferred stock, the powers (including voting powers), preferences and relative, participating, optional or other special rights, and the qualifications, limitations or restrictions thereof, including, without limitation:
| |
●
|
the designation of the series;
|
| |
●
|
the number of shares of the series, which our Board of Directors may, except where otherwise provided in the preferred stock designation, increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares then outstanding);
|
| |
●
|
whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series;
|
| |
●
|
the dates at which dividends, if any, will be payable;
|
| |
●
|
the redemption rights and price or prices, if any, for shares of the series;
|
| |
●
|
the terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series;
|
| |
●
|
the amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of our affairs;
|
| |
●
|
whether the shares of the series will be convertible into shares of any other class or series, or any other security, of the Company or any other corporation, and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate adjustments, the date or dates as of which the shares will be convertible and all other terms and conditions upon which the conversion may be made;
|
| |
●
|
restrictions on the issuance of shares of the same series or of any other class or series; and
|
| |
●
|
the voting rights, if any, of the holders of the series.
|
We could issue a series of preferred stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of the holders of our common stock might believe to be in their best interests or in which the holders of our common stock might receive a premium for your common stock over the market price of the common stock. Additionally, the issuance of preferred stock may adversely affect the holders of our common stock by restricting dividends on the common stock, diluting the voting power of the common stock or subordinating the liquidation rights of the common stock. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our common stock.
Description of Securities in this Offering
The following summary of certain terms and provisions of the common warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the common warrant and pre-funded warrant, the forms of which have been filed as exhibits to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the forms of common warrant and pre-funded warrant for a complete description of the terms and conditions thereof.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Existing Securities”
in this prospectus.
Common Warrants
We are selling to investors in this offering shares of our common stock with accompanying common warrants to purchase 7,870,000 shares of our common stock and/or pre-funded warrants with accompanying common warrants to purchase 7,870,000 shares of our common stock at an assumed combined purchase price of $1.27 for shares and accompanying common warrants and $1.27 for pre-funded warrants and accompanying common warrants.
Each common warrant will be exercisable at an exercise price of $ per share, subject to adjustment.
We do not have a sufficient number of authorized shares of common stock to cover the exercise in full of all of the common warrants to be issued in this offering. Accordingly, pursuant to the terms thereof, the common warrants will not be exercisable until the effective date of an amendment to our articles of incorporation increasing the number of authorized shares of common stock to at least 30,000,000. We intend to seek approval from our stockholders at our next annual meeting to increase the number of our authorized shares of common stock to at least 30,000,000. However, our stockholders may reject such proposal, which could delay or prevent the ability of holders of our common warrants to exercise them. Once the number of authorized shares of our common stock has been increased to at least 30,000,000, the common warrants will be exercisable until the five-year anniversary of the date of such increase, but not thereafter.
Subject to limited exceptions, a holder of common warrants will not have the right to exercise any portion of its common warrants if the holder, together with its affiliates, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of our common stock outstanding immediately after giving effect to such exercise (the “Beneficial Ownership Limitation”); provided, however, that upon 61 days’ prior notice to the Company, the holder may increase or decrease the Beneficial Ownership Limitation, provided that in no event shall the Beneficial Ownership Limitation exceed 9.99%.
The common warrants contain a “cashless exercise” feature that allows holders to exercise the common warrants without a cash payment to the Company upon the terms set forth in the common warrants, if, at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available for the issuance of the shares to the exercising common warrant holder.
In the case of certain fundamental transactions affecting the Company, a holder of common warrants, upon exercise of such common warrants after such fundamental transaction, will have the right to receive, in lieu of shares of the Company’s common stock, the same amount and kind of securities, cash or property that such holder would have been entitled to receive upon the occurrence of the fundamental transaction, had the common warrants been exercised immediately prior to such fundamental transaction. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the common warrants have the right to require us or a successor entity to redeem the common warrants for cash in the amount of the Black-Scholes Value (as defined in the common warrants) of the unexercised portion of the common warrants concurrently with or within 30 days following the consummation of a fundamental transaction. However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our board of directors, the holders of the common warrants will only be entitled to receive from us or our successor entity, as of the date of consummation of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the common warrant that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction.
The exercise price and number of the shares of our common stock issuable upon the exercise of the common warrants will be subject to adjustment in the event of any stock dividends and splits, recapitalization, reorganization or similar transaction, as described in the common warrants.
We do not intend to list the common warrants on any securities exchange or nationally recognized trading system. Except as otherwise provided in the common warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the common warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their common warrants.
Pre-Funded Warrants
Each pre-funded warrant offered hereby will have an initial exercise price per share equal to $0.0001. The pre-funded warrants will be immediately exercisable and may be exercised at any time until the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The pre-funded warrants will be issued separately from the accompanying common warrants and may be transferred separately immediately thereafter.
The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99 % of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s pre-funded warrants up to 9.99 % of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock. No fractional shares of common stock will be issued in connection with the exercise of a pre-funded warrant. In lieu of fractional shares, we will round up to the next whole share.
At any time, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrant to us together with the appropriate instruments of transfer.
We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading system. Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their pre-funded warrants.
Nevada Anti-Takeover Statutes
The following provisions of the Nevada Revised Statutes (“NRS”) could, if applicable, have the effect of discouraging takeovers of our company.
Transactions with Interested Stockholders. The NRS prohibits a publicly-traded Nevada company from engaging in any business combination with an interested stockholder for a period of three years following the date that the stockholder became an interested stockholder unless, prior to that date, the board of directors of the corporation approved either the business combination itself or the transaction that resulted in the stockholder becoming an interested stockholder.
An “interested stockholder” is defined as any entity or person beneficially owning, directly or indirectly, 10% or more of the outstanding voting stock of the corporation and any entity or person affiliated with, controlling, or controlled by any of these entities or persons. The definition of “business combination” is sufficiently broad to cover virtually any type of transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition or otherwise benefit its own interests rather than the interests of the corporation and its stockholders.
In addition, business combinations that are not approved and therefore take place after the three year waiting period may also be prohibited unless approved by the board of directors and stockholders or the price to be paid by the interested stockholder is equal to the highest of (i) the highest price per share paid by the interested stockholder within the 3 years immediately preceding the date of the announcement of the business combination or in the transaction in which he or she became an interested stockholder, whichever is higher; (ii) the market value per common share on the date of announcement of the business combination or the date the interested stockholder acquired the shares, whichever is higher; or (iii) if higher for the holders of preferred stock, the highest liquidation value of the preferred stock.
Acquisition of a Controlling Interest. The NRS contains provisions governing the acquisition of a “controlling interest” and provides generally that any person that acquires 20% or more of the outstanding voting shares of an “issuing corporation,” defined as Nevada corporation that has 200 or more stockholders at least 100 of whom are Nevada residents (as set forth in the corporation’s stock ledger); and does business in Nevada directly or through an affiliated corporation, may be denied voting rights with respect to the acquired shares, unless a majority of the disinterested stockholder of the corporation elects to restore such voting rights in whole or in part.
The statute focuses on the acquisition of a “controlling interest” defined as the ownership of outstanding shares sufficient, but for the control share law, to enable the acquiring person, directly or indirectly and individually or in association with others, to exercise (i) one-fifth or more, but less than one-third; (ii) one-third or more, but less than a majority; or (iii) a majority or more of the voting power of the corporation in the election of directors.
The question of whether or not to confer voting rights may only be considered once by the stockholders and once a decision is made, it cannot be revisited. In addition, unless a corporation’s articles of incorporation or bylaws provide otherwise (i) acquired voting securities are redeemable in whole or in part by the issuing corporation at the average price paid for the securities within 30 days if the acquiring person has not given a timely information statement to the issuing corporation or if the stockholders vote not to grant voting rights to the acquiring person’s securities; and (ii) if voting rights are granted to the acquiring person, then any stockholder who voted against the grant of voting rights may demand purchase from the issuing corporation, at fair value, of all or any portion of their securities.
The provisions of this section do not apply to acquisitions made pursuant to the laws of descent and distribution, the enforcement of a judgment, or the satisfaction of a security interest, or acquisitions made in connection with certain mergers or reorganizations.
UNDERWRITING
Pursuant to the underwriting agreement with EF Hutton, division of Benchmark Investments, LLC, as the sole book-running manager of this offering. Subject to the terms and conditions set forth in an underwriting agreement by and between us and the underwriter, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase from us, the respective number of shares of our common stock, pre-funded warrants and common warrants set forth opposite their name below.
|
Underwriter
|
|
Number of
Shares
|
|
|
Number of
Pre-
Funded
Warrants
|
|
|
Number of
Shares
Underlying
Common
Warrants
|
|
|
EF Hutton, division of Benchmark Investments, LLC
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total:
|
|
|
|
|
|
|
|
|
|
|
|
|
We have agreed to indemnify underwriter against certain liabilities, including liabilities under the Securities Act relating to losses or claims resulting from material misstatements in or omissions from this prospectus, the registration statement of which this prospectus is a part, certain free writing prospectuses that may be used in the offering and in certain marketing materials used in connection with this offering and to contribute to payments the underwriter may be required to make in respect of those liabilities.
The underwriter is offering the shares, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the shares, and other conditions contained in the underwriting agreement, such as the receipt by the underwriter of officers’ certificates and legal opinions. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
Discount, Commissions and Expenses
The following table shows the public offering price, underwriting discount and proceeds before expenses to us.
| |
|
Per
Share
and
Related
Common
Warrant
|
|
|
Per Pre-
Funded
Warrant
and
Related
Common
Warrant
|
|
|
Total
|
|
|
Offering price
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Underwriting discount and commissions(1)
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
Proceeds to us, before offering expenses
|
|
$ |
|
|
|
$ |
|
|
|
$ |
|
|
|
(1)
|
The underwriting discount and commissions is 7.0% of the gross proceeds of this offering, or $ per share of common stock, or pre-funded warrant, and accompanying common warrant.
|
The underwriter initially proposes to offer part of the shares of common stock directly to the public at the offering price listed on the cover page of this prospectus and part to certain dealers. After the initial offering of the shares of common stock, the offering price and other selling terms may from time to time be varied by the underwriter.
The estimated offering expenses payable by us, exclusive of the underwriting discount, are approximately $ million, which includes legal, accounting and printing costs, expenses incurred and various other fees associated with the registration and listing of our common stock. We have also agreed to pay the expenses of the underwriters in connection with the offering, including filing fees and investor presentation expenses, as well as underwriter’s counsel legal fees, up to an aggregate $75,000.
Right of First Refusal
We have granted the underwriter a 12-month right of first refusal to act as sole bookrunning underwriter or sole placement agent, as the case may be, for any financing by us so long as this offering raises gross proceeds in excess of $6.5 million.
Lock-Up Agreements
Other than with respect to certain issuances, without the prior consent of the underwriter we will not for a period of 90 days after the date of offering, other than with respect to the offering (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company; (ii) file or caused to be filed any registration statement with the Securities and Exchange Commission relating to the offering of any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company other than with respect to a registration statement on Form S-8 or S-4; (iii) complete any offering of debt securities of the Company, other than entering into a line of credit with a traditional bank; or (iv) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of capital stock of the Company, whether any such transaction described in clause (i), (ii), (iii) or (iv) above is to be settled by delivery of shares.
Listing
Our common stock is listed on The Nasdaq Capital Market under the symbol “CYTH.”
Electronic Distribution
In connection with this offering, the underwriter or securities dealers may distribute prospectuses by electronic means, such as e-mail. In addition, the underwriter may facilitate Internet distribution for this offering to certain of its Internet subscription customers. The underwriter may allocate a limited number of shares for sale to its online brokerage customers. An electronic prospectus is available on the Internet websites maintained by the underwriter. Other than the prospectus in electronic format, the information on the websites of the underwriter is not part of this prospectus.
Other
The underwriter and its affiliates are full-service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriter and certain of its affiliates have provided from time to time, and may provide in the future, investment and commercial banking and financial advisory services to us and our affiliates in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions. In the ordinary course of its various business activities, the underwriter and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments of ours. The underwriter and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
This prospectus does not constitute an offer to sell to, or a solicitation of an offer to buy from, anyone in any country or jurisdiction (a) in which such an offer or solicitation is not authorized; (b) in which any person making such offer or solicitation is not qualified to do so; or (c) in which any such offer or solicitation would otherwise be unlawful. No action has been taken that would, or is intended to, permit a public offer of the shares of common stock or possession or distribution of this prospectus or any other offering or publicity material relating to the shares in any country or jurisdiction (other than the United States) where any such action for that purpose is required. Accordingly, the underwriter has undertaken that it will not, directly or indirectly, offer or sell any shares or have in its possession, distribute or publish any prospectus, form of application, advertisement or other document or information in any country or jurisdiction except under circumstances that will, to the best of its knowledge and belief, result in compliance with any applicable laws and regulations and all offers and sales of shares by it will be made on the same terms.
Canada. The common stock may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the common stock must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
United Kingdom. This prospectus and any other material in relation to the shares of common stock described herein is only being distributed to, and is only directed at, persons in the United Kingdom who are “qualified investors” or otherwise in circumstances which do not require publication by the Company of a prospectus pursuant to section 85(1) of the UK Financial Services and Markets Act 2000. Any investment or investment activity to which this prospectus relates is available only to, and will be engaged in only with, investment professionals falling within Article 19(5), or high net worth entities falling within Article 49(2), of the UK Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 or other persons to whom such investment or investment activity may lawfully be made available (together, “relevant persons”). Persons who are not relevant persons should not take any action on the basis of this prospectus and should not act or rely on it.
Switzerland. The securities will not be offered, directly or indirectly, to the public in Switzerland and this prospectus does not constitute a public offering prospectus as that term is understood pursuant to article 652a or 1156 of the Swiss Federal Code of Obligations.
European Economic Area. In relation to each Member State of the European Economic Area that has implemented the European Prospectus Directive (each, a “Relevant Member State”), an offer of our shares may not be made to the public in a Relevant Member State other than:
| |
●
|
to any legal entity which is a qualified investor, as defined in the European Prospectus Directive;
|
| |
●
|
to fewer than 150 natural or legal persons (other than qualified investors as defined in the European Prospectus Directive), subject to obtaining the prior consent of the representatives for any such offer; or
|
| |
●
|
in any other circumstances falling within Article 3(2) of the European Prospectus Directive;
|
provided that no such offer of our shares shall require us or the underwriter to publish a prospectus pursuant to Article 3 of the European Prospectus Directive or supplement prospectus pursuant to Article 16 of the European Prospectus Directive.
For the purposes of this description, the expression an “offer to the public” in relation to the securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the expression may be varied in that Relevant Member State by any measure implementing the European Prospectus Directive in that member state, and the expression “European Prospectus Directive” means Directive 2003/71/EC (and amendments hereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State. The expression 2010 PD Amending Directive means Directive 2010/73/EU.
We have not authorized and do not authorize the making of any offer of securities through any financial intermediary on our behalf, other than offers made by the underwriter and its affiliates, with a view to the final placement of the securities as contemplated in this document. Accordingly, no purchaser of the shares, other than the underwriter, is authorized to make any further offer of shares on our behalf or on behalf of the underwriter.
LEGAL MATTERS
Selected legal matters with respect to the validity of the securities offered by this prospectus will be passed upon for us by Fox Rothschild LLP, 101 Park Avenue, New York, NY 10178. Certain legal matters in connection with this offering will be passed upon for the underwriter by Lowenstein Sandler LLP, New York, New York.
EXPERTS
WithumSmith+Brown, PC, our independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended December 31, 2021, as set forth in their report, which is incorporated by reference in this prospectus and elsewhere in this registration statement. WithumSmith+Brown, PC’s report includes an explanatory paragraph relating to our ability to continue as a going concern. Our consolidated financial statements are incorporated by reference in reliance on WithumSmith+Brown, PC’s report, given on their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is a part of the registration statement on Form S-1 we filed with the SEC under the Securities Act and does not contain all the information set forth in the registration statement. Whenever a reference is made in this prospectus to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated herein by reference for a copy of such contract, agreement or other document. Because we are subject to the information and reporting requirements of the Exchange Act, we file or furnish, as applicable, annual, quarterly and current reports, proxy statements and other information with the SEC. The SEC also maintains a web site that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The website address is www.sec.gov. The information on the SEC’s website is not part of this prospectus, and any references to this website or any other website are inactive textual references only. Our Internet address is www.adnas.com. The information found on our website is not part of this prospectus and investors should not rely on any such information in deciding whether to invest.
INCORPORATION BY REFERENCE
We have elected to incorporate certain information by reference into this prospectus. By incorporating by reference, we can disclose important information to you by referring you to other documents we have filed or will file with the SEC. The information incorporated by reference is deemed to be part of this prospectus, except for information incorporated by reference that is superseded by information contained in this prospectus. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below that we have previously filed with the SEC, except in each case the information contained in such document to the extent “furnished” and not “filed”:
All reports and other documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act prior to the termination of this offering, including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement but excluding any information furnished and not filed with the SEC, shall be deemed to be incorporated by reference in this prospectus and to be a part hereof from the date of filing such reports and other documents.
Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
You may obtain copies of these documents on the website maintained by the SEC at http://www.sec.gov. We will furnish to you, upon written or oral request, a copy of any or all of the documents that have been incorporated by reference, including exhibits to these documents.
You may obtain from us copies of the documents incorporated by reference in this prospectus, at no cost, by requesting them in writing or by telephone at:
Cyclo Therapeutics, Inc.
6714 NW 16th Street, Suite B
Gainesville, FL 32653
(386) 418-8060
7,870,000 SHARES OF COMMON STOCK
PRE-FUNDED WARRANTS TO PURCHASE UP TO 7,870,000 SHARES OF COMMON STOCK
COMMON WARRANTS TO PURCHASE UP TO 7,870,000 SHARES OF COMMON STOCK
SHARES OF COMMON STOCK UNDERLYING THE PRE-FUNDED WARRANTS AND COMMON WARRANTS
PROSPECTUS
EF Hutton
division of Benchmark Investments, LLC
, 2022
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the costs and expenses incurred by us in connection with the sale of the common stock being registered by this registration statement. All amounts shown are estimates, except for the Securities and Exchange Commission (“SEC”) registration fee and FINRA fee.
|
SEC registration fee
|
|
$ |
2,204.00 |
|
|
FINRA filing fee
|
|
$ |
3,500.00 |
|
|
Accounting fees and expenses
|
|
$ |
15,000.00 |
|
|
Legal fees and expenses
|
|
$ |
125,000.00 |
|
|
Miscellaneous expenses
|
|
$ |
46,500.00 |
|
| |
|
|
|
|
|
Total
|
|
$ |
192,204 |
|
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Nevada law provides that a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation (i.e., a “non-derivative proceeding”), by reason of the fact that he or she is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys' fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with the action, suit or proceeding if he or she:
| |
●
|
Is not liable under Section 78.138 of the Nevada Revised Statutes for breach of his or her fiduciary duties to the corporation; or
|
| |
●
|
Acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
|
In addition, a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor (i.e., a “derivative proceeding”), by reason of the fact that he or she is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys' fees actually and reasonably incurred by him or her in connection with the defense or settlement of the action or suit if he:
| |
●
|
Is not liable under Section 78.138 of the Nevada Revised Statutes for breach of his or her fiduciary duties to the corporation; or
|
| |
●
|
Acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation.
|
Under Nevada law, indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
To the extent that a director, officer, employee or agent of a corporation has been successful on the merits or otherwise in defense of any non-derivative proceeding or any derivative proceeding, or in defense of any claim, issue or matter therein, the corporation is obligated to indemnify him or her against expenses, including attorneys' fees, actually and reasonably incurred in connection with the defense.
Further, Nevada law permits a Nevada corporation to purchase and maintain insurance or to make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise for any liability asserted against him or her and liability and expenses incurred by him or her in his or her capacity as a director, officer, employee or agent, or arising out of his or her status as such, whether or not the corporation has the authority to indemnify him or her against such liability and expenses.
Our bylaws provide that, the Company shall, to the fullest extent permitted by the laws of the State of Nevada, indemnify any person who is or was a director or officer of the Company or any predecessor of the Company or is or was serving at the Company’s request as a director, officer, employee or agent of another corporation, partnership, joint venture, trust, or other entity (each such person, an “Indemnitee”) against expenses, including attorneys’ fees, judgments, fines, and amounts paid in settlement, actually and reasonably incurred by the Indemnitee in connection with any threatened, pending, or completed action, suit, or proceeding, whether civil, criminal, administrative, or investigative, other than a proceeding by or in the right of the Company, to which the Indemnitee is, was, or is threatened to be made a party by reason of being an Indemnitee, if the Indemnitee either: (a) did not breach, through intentional misconduct, fraud, or a knowing violation of law, the Indemnitee’s fiduciary duties as a director or officer to act in good faith and in the interests of the Company; or (b) acted in good faith and in a manner the Indemnitee reasonably believed to be in or not opposed to the best interests of the Company and, with respect to any criminal action or proceeding, had no reasonable cause to believe the Indemnitee’s conduct was unlawful.
Additionally, our bylaws provide that the Company shall, to the fullest extent permitted by the laws of the State of Nevada, indemnify any Indemnitee against expenses, including attorneys’ fees and amounts paid in settlement, actually and reasonably incurred by the Indemnitee in connection with any threatened, pending, or completed suit or action by or in the right of the Corporation to which the Indemnitee is, was, or is threatened to be made a party by reason of being an Indemnitee, if the Indemnitee either: (a) did not breach, through intentional misconduct, fraud, or a knowing violation of law, the Indemnitee’s fiduciary duties as a director or officer to act in good faith and in the interests of the Company; or (b) acted in good faith and in a manner the Indemnitee reasonably believed to be in or not opposed to the best interests of the Company.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
Over the past three years, we have issued and sold the following securities without registration under the Securities Act:
On April 24, 2020, the Company completed a private placement of common stock to a group of accredited investors that included several directors of the Company and members of management (the “April 2020 Private Placement”). Investors in the April 2020 Private Placement purchased a total of 200,000 shares of common stock at a price of $10.00 per share, resulting in gross proceeds to the Company of $2,000,000. The Company did not utilize a financial adviser or placement agent in connection with the April 2020 Private Placement. However, pursuant to terms of the Placement Agency Agreement with ThinkEquity, the Company paid ThinkEquity (i) a cash fee in the amount of $29,637, representing 8% of the gross proceeds in the April 2020 Private Placement received from investors that were first introduced to the Company by ThinkEquity in connection with the May 2019 Private Placement, and (ii) a warrant to purchase 2,223 shares of common stock, representing 6% of the shares of common stock purchased by such investors in the April 2020 Private Placement, at an exercise price of $11.00 per share (110% of the price per share paid by investors in the April 2020 Private Placement).
On August 27, 2020, the Company completed a private placement of common stock to a group of accredited investors that included several directors of the Company and members of management (the “August 2020 Private Placement”). Investors in the August 2020 Private Placement purchased a total of 283,111 “Units” at a price of $10.00 per Unit, resulting in gross proceeds to the Company of $2,831,114. Each Unit consisted of one share of common stock and a seven-year warrant (“Warrant”) to purchase one share of common stock at an exercise price of $15.00 per share.
In January 2021, the Company issued 10,000 shares of common stock with a value of $50,300 to a consultant for services.
ITEM 16. EXHIBITS
The following exhibits are filed as part of this registration statement:
|
Exhibits
|
|
|
|
1.1*
|
|
Form of Underwriting Agreement
|
|
2.1
|
|
Agreement and Plan of Merger, dated November 4, 2020, by and between Cyclo Therapeutics, Inc., a Florida corporation, and Cyclo Therapeutics, Inc., a Nevada corporation (incorporated by reference to Exhibit 2.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2020).
|
|
3.1
|
|
Articles of Incorporation of Cyclo Therapeutics, Inc., a Nevada corporation (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2020).
|
|
3.2
|
|
Certificate of Amendment to Articles of Incorporation of Cyclo Therapeutics, Inc., filed June 24, 2021 (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 24, 2021).
|
|
3.3
|
|
Bylaws of Cyclo Therapeutics, Inc., a Nevada corporation (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 10, 2020).
|
|
4.1*
|
|
Form of common warrant
|
|
4.2*
|
|
Form of pre-funded warrant
|
|
4.3
|
|
Form of Warrant issued to investors in private placements conducted in 2016, 2017 and 2018 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed June 8, 2016).
|
|
4.4
|
|
Form of Warrant, dated May 31, 2019, issued by CTD Holdings, Inc. to investors and ThinkEquity, a division of Fordham Financial Management, Inc., and its designees (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed June 4, 2019).
|
|
4.5
|
|
Form of Warrant, dated August 27, 2020, issued by Cyclo Therapeutics, Inc. to investors in a private placement conducted in August 2020 (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed September 2, 2020).
|
|
4.6
|
|
Form of Public Warrant (incorporated by reference to Exhibit 4.5 to Company’s Registration Statement on S-1 filed November 16, 2020)
|
|
4.7
|
|
Form of Warrant Agency Agreement between the Company and vStock Transfer LLC (incorporated by reference to Exhibit 4.6 to Company’s Registration Statement on S-1 filed November 16, 2020)
|
|
4.8
|
|
Form of Representative’s Warrant. (incorporated by reference to Exhibit 4.7 to Company’s Registration Statement on S-1 filed November 16, 2020)
|
|
5.1*
|
|
Opinion of Fox Rothschild LLP
|
|
10.1
|
|
Securities Purchase and Collaboration Agreement dated as of April 9, 2014 between CTD Holdings, Inc. and Novit, L.P. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 15, 2014).
|
|
10.2†
|
|
Employment Agreement between the Company and N. Scott Fine, dated as of September 14, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed October 16, 2015).
|
|
10.3†
|
|
Amendment to Employment Agreement between the Company and N. Scott Fine, dated as of November 7, 2017 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 8, 2017).
|
|
10.4
|
|
Promissory Note in the original principal amount of $265,000, dated January 21, 2016, by Crit, Inc. DBA Commercial Gates & Electric, in favor of CTD Holdings, Inc. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed January 27, 2016).
|
|
10.5
|
|
Mortgage, dated January 21, 2016, by Crit, Inc. DBA Commercial Gates & Electric, in favor of CTD Holdings, Inc. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed January 27, 2016).
|
|
10.6
|
|
Commercial Contract between Alchem Laboratories Corporation and Nanosonic Products Inc., entered into September 6, 2016 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed December 20, 2016).
|
|
10.7†
|
|
Employment Agreement between the Company and Dr. Sharon H. Hrynkow, dated as of September 14, 2015 (incorporated by reference to Exhibit 10.10 to the Company’s Annual Report on Form 10-K filed March 15, 2019).
|
|
10.8†
|
|
Amendment to Employment Agreement between the Company and Dr. Sharon H. Hrynkow, dated as of November 8, 2017 (incorporated by reference to Exhibit 10.11 to the Company’s Annual Report on Form 10-K filed March 15, 2019).
|
|
10.9†
|
|
2019 Omnibus Equity Incentive Plan (incorporated by reference to Appendix B to the Company’s Proxy Statement on Schedule 14A filed July 19, 2019).
|
|
10.10†
|
|
2021 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed June 24, 2021).
|
|
10.11
|
|
Promissory Note, dated May 4, 2020, by Cyclodextrin Technologies Development, Inc., a wholly-owned subsidiary of the Company, in favor of BBVA USA (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed May 6, 2020).
|
|
10.12†
|
|
Employment Agreement between the Company and N. Scott Fine, dated as of February 28, 2022 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed March 2, 2022).
|
|
10.13†
|
|
Employment Agreement between the Company and Lise Kjems, dated as of September 27, 2021 (incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-K filed March 11, 2022).
|
|
10.14†
|
|
Employment Agreement between the Company and Michael Lisjak, dated as of February 28, 2022 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed March 2, 2022).
|
|
10.15†
|
|
Employment Agreement between the Company and Joshua Fine, dated as of February 28, 2022 (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed March 2, 2022).
|
|
10.16†
|
|
Employment Agreement between the Company and Jeffrey Tate, dated as of February 28, 2022 (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed March 2, 2022).
|
|
21.1
|
|
Subsidiaries (incorporated by reference to Exhibit 21.1 to the Company’s Annual Report on Form 10-K filed April 16, 2018).
|
|
23.1*
|
|
Consent of WithumSmith+Brown, PC
|
|
23.2*
|
|
Consent of Fox Rothschild LLP (included in Exhibit 5.1)
|
|
107**
|
|
Filing Fee Table
|
* Filed herewith.
** Previously filed.
† Management contract or compensatory plan or arrangement.
ITEM 17. UNDERTAKINGS
(a) The undersigned registrant hereby undertakes:
(1) to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) that, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) that, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(6) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(7) The undersigned registrant hereby undertakes that:
(i) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(ii) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, on November 21, 2022.
| |
CYCLO THERAPEUTICS, INC.
|
|
| |
|
|
|
| |
By:
|
/s/ N. Scott Fine
|
|
| |
|
N. SCOTT FINE
Chief Executive Officer
(principal executive officer)
Date: November 21, 2022
|
|
KNOW ALL PERSONS BY THESE PRESENTS, that each individual whose signature appears below constitutes and appoints N. Scott Fine, with full authority to act without the others, his true and lawful attorney-in-fact and agent with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any and all amendments to this registration statement, and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| By: |
/s/ N. Scott Fine |
|
By: |
/s/ Joshua M. Fine |
|
| |
N. SCOTT FINE |
|
|
JOSHUA M. FINE |
|
| |
Chief Executive Officer; Director |
|
|
Chief Financial Officer |
|
| |
(principal executive officer) |
|
|
(principal financial and accounting officer) |
|
| Date: |
November 21, 2022 |
|
|
Date: November 21, 2022 |
|
| By: |
/s/ C.E. RICK STRATTAN |
|
|
By: |
/s/ William S. Shanahan |
|
| |
C.E. RICK STRATTAN |
|
|
|
WILLIAM S. SHANAHAN |
|
| |
Director |
|
|
|
Director |
|
| Date: |
November 21, 2022 |
|
|
Date: |
November 21, 2022 |
|
| |
|
|
|
|
|
|
| By: |
/s/ Jeffrey L. Tate |
|
|
By: |
/s/ F. Patrick Ostronic |
|
| |
JEFFREY L. TATE |
|
|
|
F. PATRICK OSTRONIC |
|
| |
Chief Operating Officer; Director |
|
|
|
Director |
|
| Date: |
November 21, 2022 |
|
|
Date: |
November 21, 2022 |
|
| |
|
|
|
|
|
|
| By: |
/s/ Markus W. Sieger |
|
|
By: |
/s/ Randall M. Toig |
|
| |
MARKUS W. SIEGER |
|
|
|
RANDALL M. TOIG |
|
| |
Chairman of the Board, Director |
|
|
|
Director |
|
| Date: |
November 21, 2022 |
|
|
Date: |
November 21, 2022 |
|
Cyclo Therapeutics (NASDAQ:CYTH)
Historical Stock Chart
From Mar 2024 to Apr 2024
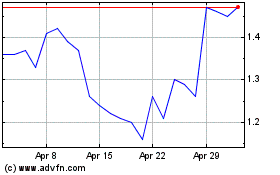
Cyclo Therapeutics (NASDAQ:CYTH)
Historical Stock Chart
From Apr 2023 to Apr 2024