In addition to the issued patents listed above, GBSGB's intellectual property portfolio includes a total of 14 USPTO and 41 international patents pending:
The Company runs a lean drug development program and minimizes expenses, including personnel, overhead, and fixed capital expenses (such as lab and diagnostic equipment), through strategic partnerships with universities, hospitals, suppliers, Contract Research Organizations (“CROs”), and Contract Manufacturing Organizations (“CMOs”). Through these research and development agreements, the Company has created a virtual pipeline for the further development of novel medicines based on ingredients originally derived from the cannabis plant and other plant-based traditional medicines. The partners bring both expertise and infrastructure at a reasonable cost to the life sciences program. In most instances, the Company has also negotiated with these partners to keep 100% of the ownership of the IP within GBSGB for original patent filings.
The Company currently has on-going research agreements with the following institutions covering the indicated areas of research:
Chaminade University: Broad-based research program to support the drug discovery platform that has yielded many of the Company’s original patents to date in the areas of neurodegenerative diseases, heart disease, inflammatory diseases, neuropathic and chronic pain. They have also performed the bioassay portion of the Cannabis Metabolomics study performed with the University of Athens, Greece and the Company. Our collaborations with Chaminade also led to the development of our PhAROS™ drug discovery platform.
University of Athens: Broad-based metabolomics analysis of over 100 cannabis genotypes including both hemp and THC-producing cannabis varieties, in combination with the Company’s bioassay data linking genotypes and potential disease-remediations. This project has the potential to define active ingredients from plant-derived mixtures beyond the standard cannabinoids and terpenoids. The discovery potential is huge, and novel agents have recently been discovered. Novel ligands have been identified and are being validated. This project will ultimately yield novel patent-protected therapies.
Michigan State University: Preclinical work using a cutting-edge, multi-cellular model of the human immune system and a multi-cell model of the brain to validate our MEMs for use in the treatment of COVID-19-related cytokine release syndromes (COVID-CRS). MSU has performed experiments using their novel model of the human-immune system that have allowed GBSGB to prepare cannabis-based formulas for the potential treatment of virally-induced hyperinflammation/cytokine storm syndrome that has led to the majority of COVID-19 deaths. Positive proof-of-concept results have guided the development of these selectively anti-inflammatory MEM.
The University of Seville: Bringing their novel expertise to the development and functional testing of time-released and disease-targeted nanoparticles of cannabis-based minimum essential mixtures for oral administration. These specialized nanoparticles are being used for the precise and time-released delivery of several of our therapies, including the Company’s chronic pain MEMs used in the preclinical animal testing performed at the NRC Canada. The University of Seville has completed functional testing on nanoparticles containing myrcene, nerolidol, and beta-caryophyllene for our chronic pain MEMs. In cell-based assays, the effectiveness and kinetics of the nanoparticle-forms of these terpenes were compared with the “naked” terpenes both individually and in mixtures. In all cases, the effectiveness of the nanoparticles was superior to the naked terpenes, however, the mixtures were dramatically more effective than the individuals. Recently, our partners at the University of Seville have completed the formulation of new cannabis-based ingredients for inclusion into the oral, time-released nanoparticle format for the completion of our maximally effective MEMs for chronic pain. The results from Seville are very promising, and these nanoparticles have entered the animal testing phase at the NRC in Halifax.
The National Research Center (NRC) of Canada, Halifax, Nova Scotia: Four animal-phase studies are being performed by Dr. Lee Ellis’ group at the NRC. 1) Parkinson’s Disease: In Q1 of 2020, an animal safety and efficacy study was completed for the Company’s equimolar MEMs for the treatment of the motor symptoms of Parkinson’s disease, and the NRC has demonstrated that the company’s PD formulations were able to reduce behavioral changes associated with the loss of dopamine-producing neurons, which underlies the pathology of Parkinson’s disease in the animal model. Based on achieving the statistically significant reduction in Parkinson’s disease symptomology in these equimolar MEMs, the Company completed a final phase of testing in August of 2021, which identified five defined cannabinoid ratio (DCR)-MEMs that were more effective than the root equimolar MEM. 2) Chronic Pain: In Q1 of 2019, the Company started a safety and efficacy study in animals for our Chronic Pain (CP) formulas. The midterm results for these preclinical pain studies were promising, but the study was significantly delayed by the COVID pandemic. These preclinical studies have resumed and are progressing well. 3 & 4) Depression and Anxiety: Minimum essential mixtures of plant-based ingredients from kava and the related Piper plant family are being evaluated now.
The University of Cadiz: Testing the safety and efficacy of the above-mentioned time-released nanoparticles in rodent models of chronic pain. Proof of concept complete for one formulation.
University of Hawaii: Validating the efficacy of a complex cannabis-based mixture for the treatment of cardiac hypertrophy and cardiac disease in a rodent model. Proof of concept work is complete in rodents, and we are seeking commercialization partners.
The Company has plant-based therapeutic products in the following stages of drug development: Discovery, Pre-Clinical, and entering the Clinical Phase. It has also licensed therapeutic products that the Company intends to develop through partners, labeled Partner Programs.
The completion of discovery, preclinical studies, clinical trials, and the required regulatory submissions required for obtaining US FDA pre-market approvals for pharmaceutical products (and equivalent approvals from other corresponding agencies worldwide) is traditionally a long and expensive process. However, the Company asserts that its proprietary, PhAROS™, AI-enabled, drug discovery engine; plant-inspired formulations; lean development program; novel regulatory strategy; experienced development partners; and aggressive licensing of these products at early clinical stages can mitigate some of the risks. The Company uses a combination of in silico discovery methods and automated screening of cellular and animal models of disease to decrease the time in Discovery prior to filing novel patent applications for disease-specific therapeutics. Through GBSGB, the Company’s original patent applications cover new chemical entities (“NCE”) based on discovery and validation of minimum essential mixtures derived from complex, plant-based therapeutics. The Company plans to use an Exploratory IND/Phase 0 Program that gets the Company to First-in-Human sooner than traditional programs, which reduces translational risks, and includes preliminary efficacy measures for responsible development decisions. In contrast, a traditional phased-development path would not provide any efficacy measures until Phase II. After the completion of our Phase 0 study for PD, which compares the efficacies of multiple related cannabinoid-based formulations, the Company plans to advance the lead PD drug candidate using an adaptive trial design that is more efficient than the traditional phased-development pathway. Through GBSGB, the Company has entered into research contracts, partnerships, and/or joint ventures with several respected, independent contract research organizations, medical schools, universities, and with other scientific consultants to increase developmental efficiencies. If and when one or more of the Company’s drugs, therapies or treatments are approved by the US FDA, the Company will seek to market them under licensing arrangements with major biotechnology or pharmaceutical companies.
There can be no assurance that we will ever be able to enter into any joint ventures or other arrangements with third parties to finance our drug development program or that if we are able to do so, that any of our projected therapies will ever be approved by the US FDA. Even if we obtain US FDA approval to market one of our therapies, there can be no assurance that it could be successfully marketed or would not be superseded by another plant-based therapy produced by one or more of our competitors. It also may be anticipated that even if we enter into a joint venture development with a financially stable pharmaceutical or institutional partner, we will still be required to raise significant additional capital in the future to achieve the strategic goals of the Company. There can be no assurance that we will be able to obtain such additional capital on reasonable terms, if at all. If the Company fails to achieve its goal of producing one or more plant-inspired pharmaceuticals or therapies, it would have a material adverse effect on our future financial condition and business prospects.
On March 24, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC. Pursuant to the Teco MIPA, the Company agreed to sell 100% of its membership interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC (the "Teco Subsidiaries") for approximately $8 million, which amount includes a cash payment at closing, the extinguishment of certain liabilities owed to the purchaser and affiliates of the purchaser, and an 8% promissory note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with 483 Management, LLC. Pursuant to the Nopah MIPA, the Company agreed to sell its 100% membership interest in GB Sciences Nopah, LLC ("Nopah"), which holds a Nevada medical marijuana cultivation certificate. As consideration, the Company would receive $300,000 as a reduction to the balance of the 0% Note payable dated October 23, 2017 and accounts payable of $74,647, which were owed to an affiliate of the purchaser.
The closing of the Teco and Nopah sales was contingent upon the successful transfer of the Nevada cultivation and production licenses. On December 14, 2021, the Company received approval from the Nevada Cannabis Compliance Board for the transfer of cannabis cultivation and extraction licenses held by its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC (the "Nevada Subsidiaries"). Consequently, all conditions to closing the sales of the 100% membership interests in the Nevada Subsidiaries were satisfied, and the transactions formally closed on December 31, 2021. After the closing date, the Company retains no ownership interest in the Nevada Subsidiaries.
The decrease in both periods is attributable to less debt outstanding as the result of the repayment of $500,000 debt principal during the March 31, 2022 quarter and a decrease in amortization expense from debt discounts during the current year period as the result of prior amortization of discounts on outstanding debt.
SIGNATURES
In accordance with the requirements of the Securities Exchange Act of 1934, the Registrant has caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
GB SCIENCES, INC.
|
|
|
|
|
Date: November 8, 2022
|
By:
|
/s/ John Poss
|
|
|
John Poss, Chief Executive Officer
(Principal Executive Officer)
|
|
|
|
|
|
GB SCIENCES, INC.
|
|
|
|
| Date: November 8, 2022 |
By:
|
/s/ Zach Swarts
|
|
|
Zach Swarts, Chief Financial Officer
(Principal Financial Officer)
|
Exhibit 10.1
$300,000 NOTE EXTENSION AGREEMENT
This $300,000 Note Extension Agreement (the “Agreement”) is made by and between Dickinson Hughes LLC (the “Note Holder”), and GB Sciences, Inc. (the “Company”). The Note Holder and the Company are sometimes referred to hereinafter as the “Parties”).
WHEREAS on February 23, 2021, the Company issued to the Note Holder a note in the face amount of $300,000 (the “$300,000 Note”);
WHEREAS, pursuant to the $300,000 Note, all principal and interest became due and payable to the Note Holder on February 23, 2022 (the “Original Due Date);
WHEREAS on August 18, 2017, the Company issued to the Note Holder a note in the face amount of $250,000 (“Associated Note 1”);
WHEREAS on November 6, 2017, the Company issued to the Note Holder a second note in the face amount of $250,000 (“Associated Note 2” and together with Associated Note 1, the “Associated Notes”);
WHEREAS the Company has repaid to the Note Holder all principal due under Associated Note 1 and Associated Note 2 but there is interest that remains to be paid on both Associated Notes (the “Remaining Interest Due on the Associated Notes”);
NOW, THEREFORE, in consideration of the mutual covenants and promises and other good and valuable consideration, it is mutually agreed as follows:
| |
1.
|
Extension of Original Due Date: In exchange for the payment by the Company to the Note Holder of the of Remaining Interest Due on the Associated Notes and for increasing the interest rate in accordance with paragraph 2 below, the Original Due Date of the $300,000 Note shall be extended to October 1, 2023. Thereafter, the legal obligations of the Company to the Note Holder under the $300,000 Note shall be the same as though the Original Due Date had been October 1, 2023.
|
| |
2.
|
Increase in Interest Rate: Beginning October 1, 2022, the rate of interest on the $300,000 Note will be increased from 6.0% to 8.0% per annum.
|
3. General:
(a) Binding of Agreement. The provisions of this Agreement shall inure to the benefit of and be binding upon the Parties and each and all of their respective heirs, legal representatives, successors and assigns. No other person or entity will have or acquire any right by virtue of this Agreement.
(b) Amendments; Waivers: No provision of this Agreement shall be modified, waived or discharged unless the modification, waiver or discharge is agreed to in writing and signed by both Parties. No waiver by any Party of any breach of, or of compliance with, any condition or provision of this Agreement by the other Party shall be considered a waiver of any other condition or provision or of the same condition or provision at another time.
(c) Entire Agreement: This Agreement constitutes the entire agreement between the Parties regarding the terms and conditions of the Agreement. This Agreement supersedes all prior negotiations, representations or agreements between the Parties, whether written or oral.
(d) Counterparts: This Agreement may be executed by the Parties in counterparts, each of which shall be deemed an original and which together shall constitute one instrument.
(e) Headings: Each and all of the headings contained in this Agreement are for reference purposes only and shall not in any manner affect the construction or interpretation of this Agreement or be deemed a part of this Agreement for any purpose.
(f) Savings Provision: To the extent that any provision of this Agreement or any paragraph, term, provision, sentence, phrase, clause or word of this Agreement shall be found to be illegal or unenforceable for any reason, such paragraph, term, provision, sentence, phrase, clause or word shall be modified or deleted in such a manner as to make this Agreement, as so modified, legal and enforceable under applicable laws. The remainder of this Agreement shall continue in full force and effect.
(g) Construction: The language of this Agreement and of each and every paragraph, term and provision of this Agreement shall, in all cases, for any and all purposes, and in any and all circumstances whatsoever be construed according to its fair meaning, not strictly for or against either Party, and with no regard whatsoever to the identity or status of any person or persons who drafted all or any portion of this Agreement.
IN WITNESS WHEREOF, the Parties and the Company have executed this Agreement as of the dates written below.
GB Sciences, Inc.
Dated: 9/28/2022
/s/ John Poss
By: John Poss, CEO
Dickinson Hughes LLC
Dated: 10/3/2022
/s/ Robert Moody Jr.
By: Robert Moody Jr.
authorized member and/or manager
Exhibit 10.2
$560,000 NOTE EXTENSION AGREEMENT
This $560,000 Note Extension Agreement (the “Agreement”) is made by and between Robert Moody Jr. (the “Note Holder”), and GB Sciences, Inc. (the “Company”). The Note Holder and the Company are sometimes referred to hereinafter as the “Parties”).
WHEREAS on August 3, 2017, the Company issued to the Note Holder a note in the face amount of $560,000 (the “$560,000 Note”);
WHEREAS, pursuant to the $560,000 Note, all principal and interest became due and payable to the Note Holder on August 2, 2020 (the “Original Due Date);
WHEREAS on August 18, 2017, the Company issued to the Note Holder a note in the face amount of $250,000 (“Associated Note 1”);
WHEREAS on November 6, 2017, the Company issued to the Note Holder a second note in the face amount of $250,000 (“Associated Note 2” and together with Associated Note 1, the “Associated Notes”);
WHEREAS the Company has repaid to the Note Holder all principal due under Associated Note 1 and Associated Note 2 but there is interest that remains to be paid on both Associated Notes (the “Remaining Interest Due on the Associated Notes”);
NOW, THEREFORE, in consideration of the mutual covenants and promises and other good and valuable consideration, it is mutually agreed as follows:
| |
1.
|
Extension of Original Due Date: In exchange for the payment by the Company to the Note Holder of the of Remaining Interest Due on the Associated Notes and for increasing the interest rate in accordance with paragraph 2 below, the Original Due Date of the $560,000 Note shall be extended to October 1, 2023. Thereafter, the legal obligations of the Company to the Note Holder under the $560,000 Note shall be the same as though the Original Due Date had been October 1, 2023.
|
| |
2.
|
Increase in Interest Rate: Beginning October 1, 2022, the rate of interest on the $560,000 Note will be increased from 6.0% to 8.0% per annum.
|
3. General:
(a) Binding of Agreement. The provisions of this Agreement shall inure to the benefit of and be binding upon the Parties and each and all of their respective heirs, legal representatives, successors and assigns. No other person or entity will have or acquire any right by virtue of this Agreement.
(b) Amendments; Waivers: No provision of this Agreement shall be modified, waived or discharged unless the modification, waiver or discharge is agreed to in writing and signed by both Parties. No waiver by any Party of any breach of, or of compliance with, any condition or provision of this Agreement by the other Party shall be considered a waiver of any other condition or provision or of the same condition or provision at another time.
(c) Entire Agreement: This Agreement constitutes the entire agreement between the Parties regarding the terms and conditions of the Agreement. This Agreement supersedes all prior negotiations, representations or agreements between the Parties, whether written or oral.
(d) Counterparts: This Agreement may be executed by the Parties in counterparts, each of which shall be deemed an original and which together shall constitute one instrument.
(e) Headings: Each and all of the headings contained in this Agreement are for reference purposes only and shall not in any manner affect the construction or interpretation of this Agreement or be deemed a part of this Agreement for any purpose.
(f) Savings Provision: To the extent that any provision of this Agreement or any paragraph, term, provision, sentence, phrase, clause or word of this Agreement shall be found to be illegal or unenforceable for any reason, such paragraph, term, provision, sentence, phrase, clause or word shall be modified or deleted in such a manner as to make this Agreement, as so modified, legal and enforceable under applicable laws. The remainder of this Agreement shall continue in full force and effect.
(g) Construction: The language of this Agreement and of each and every paragraph, term and provision of this Agreement shall, in all cases, for any and all purposes, and in any and all circumstances whatsoever be construed according to its fair meaning, not strictly for or against either Party, and with no regard whatsoever to the identity or status of any person or persons who drafted all or any portion of this Agreement.
IN WITNESS WHEREOF, the Parties and the Company have executed this Agreement as of the dates written below.
GB Sciences, Inc.
Dated: 9/28/2022
/s/ John Poss
By: John Poss, CEO
Robert Moody Jr.
Dated: 10/3/2022
/s/ Robert Moody Jr.
By: Robert Moody Jr.
Exhibit 31.1
CERTIFICATION OF PRINCIPAL EXECUTIVE AND FINANCIAL OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, John Poss, certify that:
1.I have reviewed this quarterly report on Form 10-Q of GB Sciences, Inc.;
2.Based on my knowledge, the quarterly report does not contain any untrue statement of material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this quarterly report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of and for the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures; and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent function):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls.
|
Date: November 8, 2022
|
/s/ John Poss
|
|
|
John Poss, Chief Executive Officer
(Principal Executive Officer)
|
Exhibit 31.2
CERTIFICATION OF PRINCIPAL EXECUTIVE AND FINANCIAL OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Zach Swarts, certify that:
1.I have reviewed this quarterly report on Form 10-Q of GB Sciences, Inc.;
2.Based on my knowledge, the quarterly report does not contain any untrue statement of material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this quarterly report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of and for the periods presented in this report;
4.The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures; and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent function):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal controls.
| Date: November 8, 2022 |
/s/ Zach Swarts
|
|
|
Zach Swarts, Chief Financial Officer
(Principal Financial Officer)
|
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of GB Sciences, Inc. (the “Company”) on Form 10-Q for the quarter ended September 30, 2022, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, John Poss, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
| Date: November 8, 2022 |
/s/ John Poss
|
|
|
John Poss, Chief Executive Officer
(Principal Executive Officer)
|
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Quarterly Report of GB Sciences, Inc. (the “Company”) on Form 10-Q for the quarter ended September 30, 2022, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Zach Swarts, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission or its staff upon request.
| Date: November 8, 2022 |
/s/ Zach Swarts
|
|
|
Zach Swarts, Chief Financial Officer
(Principal Financial Officer)
|
FORM 10-K
Table of Contents
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________________
FORM 10-K
__________________________
(Mark One)
|
|
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the Fiscal Year Ended March 31, 2022
|
|
☐
|
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE EXCHANGE ACT
|
For the transition period from __________ to ___________
Commission file number: 000-55462
GB SCIENCES, INC.
(Exact name of registrant as specified in its charter)
____________________
|
Nevada
|
59-3733133
|
|
(State or other Jurisdiction of
|
(IRS Employer I.D. No.)
|
|
Incorporation or Organization)
|
|
___________________________
3550 W. Teco Avenue
Las Vegas, Nevada 89118
Phone: (866) 721-0297
(Address and telephone number of
principal executive offices)
___________________________
Securities registered under Section 12 (b) of the Exchange Act:
|
Title of each class
|
|
Name of each exchange on which registered
|
|
None
|
|
None
|
Securities registered under Section 12(g) of the Exchange Act:
|
Common Stock $.0001 Par Value
|
|
Title of Class
|
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer", "accelerated filer", "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.).
| |
Large accelerated filer ☐
|
Accelerated filer ☐
|
| |
Non-accelerated filer ☐
|
Smaller reporting company ☑
|
| |
|
Emerging growth company ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting stock held by non-affiliates of the registrant computed by reference to the price at which the common equity was last sold as of the last business day of the registrant’s most recently completed second fiscal quarter, that being September 2021, was approximately $11.3 million.
Total shares outstanding on June 30, 2022, were 329,204,224.
Documents Incorporated by Reference
None
GB SCIENCES, INC.
FORM 10-K
TABLE OF CONTENTS
PART I
DISCLOSURE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K of GB Sciences, Inc., a Nevada corporation and its subsidiaries (the “Company”), contains “forward-looking statements,” as defined in the United States Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “could”, “expects”, “plans”, “intends”, “anticipates”, believes”, “estimates”, “predicts” or “continue”, which list is not meant to be all-inclusive, and other such negative terms and comparable technology. These forward-looking statements, include, without limitation, statements about market opportunity, strategies, competition, expected activities and expenditures as we pursue business our plan, and the adequacy of available cash reserves. Although we believe the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Actual results may differ materially from the predictions discussed in these forward-looking statements. The economic environment within which we operate could materially affect actual results. Additional factors that could materially affect these forward-looking statements and/or predictions include among other things:
(i) product demand, market and customer acceptance of any or all of the Company’s products, equipment and other goods,
(ii) ability to obtain financing to expand its operations,
(iii) ability to attract and retain qualified personnel,
(iv) the results, cost and timing of our preclinical studies and clinical trials, including any delays to such clinical trials relating to enrollment or site initiation, as well as the number of required trials for regulatory approval and the criteria for success in such trials,
(v) our dependence on third parties in the conduct of our preclinical studies and clinical trials,
(vi) legal and regulatory developments in the United States and foreign countries, including any actions or advice that may affect the design, initiation, timing, continuation, progress or outcome of clinical trials or result in the need for additional clinical trials,
(vii) the results of our preclinical studies and earlier clinical trials of our product candidates may not be predictive of future results and we may not have favorable results in our ongoing or planned clinical trials,
(viii) the difficulties and expenses associated with obtaining and maintaining regulatory approval of our product candidates, and the indication and labeling under any such approval,
(ix) our plans and ability to develop and commercialize our product candidates,
(x) successful development of our commercialization capabilities, including sales and marketing capabilities, whether alone or with potential future collaborators,
(xi) the size and growth of the potential markets for our product candidates, the rate and degree of market acceptance of our product candidates and our ability to serve those markets,
(xii) the success of competing therapies and products that are or become available,
(xiii) our ability to limit our exposure under product liability lawsuits, shareholder class action lawsuits or other litigation,
(xiv) our ability to obtain and maintain intellectual property protection for our product candidates,
(xv) our ability to obtain and maintain third-party manufacturing for our product candidates on commercially reasonable terms,
(xvi) delays, interruptions or failures in the manufacture and supply of our product candidates,
(xvii) the performance of third parties upon which we depend, including third-party contract research organizations, or CROs, contract manufacturing organizations, or CMOs, contractor laboratories and independent contractors,
(xviii) the timing and outcome of current and future legal proceedings,
(xix) our ability to maintain proper functionality and security of our internal computer and information systems and prevent or avoid cyberattacks, malicious intrusion, breakdown, destruction, loss of data privacy or other significant disruption,
(xx) the adequacy of capital reserves and liquidity including, but not limited to, access to additional borrowing capacity,
(xxi) the extent to which health epidemics and other outbreaks of communicable diseases, including the ongoing COVID-19 pandemic, could disrupt our operations or materially and adversely affect our business and financial conditions, and
(xxii) general industry and market conditions and growth rates, unexpected natural disasters, and other factors, which we have little or no control: and any other factors discussed in the Company’s filings with the Securities and Exchange Commission (“SEC”).
You should refer to Part I Item 1A. “Risk Factors” of this Annual Report on Form 10-K for a discussion of material factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report on Form 10-K will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
You should read this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K and have filed as exhibits to this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
ITEM 1. DESCRIPTION OF BUSINESS
Unless the context indicates otherwise, all references to “GB” and “GB Sciences” refers solely to GB Sciences, Inc., a Nevada corporation, and all references to “the Company,” “we”, “us” or “our” in this Annual Report refers to GB Sciences and its consolidated subsidiaries.
Overview
GB Sciences, Inc. (“the Company”, “GB Sciences”, “we”, “us”, or “our”) is a plant-inspired, biopharmaceutical research and development company creating patented, disease-targeted formulations of cannabis- and other plant-inspired therapeutic mixtures for the prescription drug market through its wholly owned Canadian subsidiary, GbS Global Biopharma, Inc. (“GBSGB”).
Through GBSGB, the Company is engaged in the research and development of plant-inspired medicines, with virtual operations in North America and Europe. GBSGB’s assets include a portfolio of intellectual property containing both proprietary plant-inspired formulations and our AI-enabled drug discovery platform, as well as critical research contracts and key supplier arrangements. The Company’s intellectual property portfolio, which is held by GBSGB, contains six issued U.S. and three issued foreign patents, as well as 18 U.S. and 49 foreign patent-pending applications. On October 14th, 2021, we filed the nonprovisional USPTO patent application entitled “METHOD AND SYSTEMS FOR PHYTOMEDICINE ANALYTICS FOR RESEARCH OPTIMIZATION AT SCALE" to further protect aspects of our proprietary drug discovery engine, PhAROS™, which stands for Phytomedical Analytics for Research Optimization at Scale. On March 1st, 2022, the Company’s newest patent was issued by the U.S. Patent and Trademark Office (USPTO) for a cannabinoid-containing mixture designed to treat cardiac hypertrophy, often present in advanced heart disease. The Company’s newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor associated hearing loss and urinary cystitis.
GBSGB’s intellectual property covers a range of over 65 medical conditions, from which five drug development programs are in the preclinical stage of drug development including our formulations for Parkinson’s disease ("PD"), chronic pain, COVID-related cytokine release syndrome, depression/anxiety, and cardiovascular therapeutic programs. The Company’s primary focus is on preparing its lead program for the treatment of the motor symptoms of Parkinson's disease for a first-in-human clinical trial. Depending on the results of ongoing preclinical studies, the Company intends to move forward with clinical trials for its chronic pain and COVID-related cytokine release syndrome therapies after PD. The Company’s formulations for chronic pain, anxiety, and depression are currently in preclinical animal studies with researchers at the National Research Council Canada. The Company also recently received positive preclinical proof-of-concept data supporting its complex mixtures for the treatment of Cytokine Release Syndrome related to COVID-19, and its lead candidates will be optimized based on late-stage preclinical studies at Michigan State University. Proof-of-concept studies in animals that support our heart disease formulations have been successfully completed at the University of Hawaii. The Company runs a lean drug development program through GBSGB and takes effort to minimize expenses, including personnel, overhead, and fixed capital expenses through strategic partnerships with Universities and Contract Research Organizations (“CROs”). Our productive research and development network includes distinguished universities, hospitals, and Contract Research Organizations.
We were incorporated in the State of Delaware on April 4, 2001, under the name “Flagstick Venture, Inc.” On March 28, 2008, stockholders owning a majority of our outstanding common stock approved changing our then name “Signature Exploration and Production Corp.” as our business model had changed.
On April 4, 2014, we changed our name from Signature Exploration and Production Corporation to Growblox Sciences, Inc. Effective December 12, 2016, the Company amended its Certificate of Corporation pursuant to shareholder approval, and the Company’s name was changed from Growblox Sciences, Inc. to GB Sciences, Inc.
Effective April 8, 2018, Shareholders of the Company approved the change in corporate domicile from the State of Delaware to the State of Nevada and increase in the number of authorized capital shares from 250,000,000 to 400,000,000. Effective August 15, 2019, Shareholders of the Company approved an increase in authorized capital shares from 400,000,000 to 600,000,000.
Business Strategy
Drug Discovery and Development of Novel Cannabis- and Other Plant-Inspired Therapies
Through its wholly owned Canadian subsidiary, GBS Global Biopharma, Inc. ("GBSGB"), the Company has conducted ground-breaking research embracing the rational design of plant-inspired medicines led by Dr. Andrea Small-Howard, the Company’s President, Chief Science Officer, and Director. In the early days, Small-Howard and Dr. Helen Turner, Vice President of Innovation and Dean of the Natural Sciences and Mathematics Department at Chaminade University, posited that minimum essential mixtures of plant-based ingredients would provide more targeted and effective treatments for specific disease conditions than either single ingredient or whole plant formulations. They started with cannabis-based drug discovery and developed a rapid screening and assaying system that tested thousands of combinations of cannabinoids and terpenes in vitro against cell-based models of disease. This process identified precise mixtures of cannabinoids and terpenes, many of which contained no THC, to treat categories of disease conditions, including neurological disorders, inflammation, heart disease, metabolic syndrome, and chronic neuropathic pain. More recently, a similar approach has been applied to the discovery and validation of therapies informed by plants described in a variety of Traditional Medical Systems. These rich discovery efforts have yielded new preclinical programs; for example, our anxiety and depression formulations that contain minimum essential mixtures of compounds derived from plants in the Piper plant family, such as kava.
Currently, the Company’s drug discovery engine involves both a data analytics/machine learning tool to expedite drug discovery and high throughput screening of cell and animal models of disease. As previously mentioned, the Company initially explored the potential medical uses of specific mixtures derived from cannabis-based raw materials, but our early in silico tools have now been improved, and they are becoming increasingly effective for investigating the medical applications of potential therapeutic mixtures from any plant-derived starting material. In 2014, the Company developed its first rapid screening and assaying system which tested thousands of combinations of cannabinoids and terpenes against cell-based models of diseases. This process has been refined over the years and now has identified precise mixtures of cannabinoids and terpenes, many of which contained no THC, to treat categories of disease conditions, including neurological disorders, inflammation, heart disease, metabolic syndrome, chronic and neuropathic pain. Through GBSGB, the Company has filed for patent protection on these plant-inspired, minimum essential mixtures, and they are validating them in disease-specific animal models in preparation for human trials.
The Company’s drug discovery process combines: 1) PhAROS™: Phytomedical Analytics for Research Optimization at Scale for the prediction of minimum essential mixtures from plant-based materials, and 2) HTS: high throughput screening to refine and validate plant-inspired, minimum essential mixtures in well-established cell and animal models of diseases. This combined approach to drug discovery increases research efficiency and accuracy reducing the time from ideation to patenting from 7 years to 1.5 years. The Company now uses its PhAROS™ Drug Discovery Platform to ‘pre-validate’ therapeutic mixtures. PhAROS can both prioritize and eliminate some potential combinations, which reduces time and resources used in the discovery period. PhAROS™ can also be used to identify and predict the efficacy of plant-inspired, minimum essential mixtures for specific diseases in silico, which are then tested by screening in cell and animal models. Screening of plant-inspired mixtures for drug discovery involves the testing of specific combinations of plant chemicals from many naturally occurring plants and the use of live models for these diseases that have been well established by other researchers. The Company refines the potential therapeutic mixtures pre-validated by PhAROS™ to optimize their effectiveness using cell and animal models. Based on data from disease-specific assays, therapeutic formulations are refined during the HTS screening process by removing compounds that do not act synergistically with the others in the mixtures. The goal is to identify minimum essential mixtures (MEM) that retain the efficacy of the whole plant extracts, but with the manufacturing and quality control advantages of single ingredient pharmaceutical products.
In October of 2021, GBSGB began its first preclinical animal trial of non-cannabis-based formulations that were discovered and pre-validated using our PhAROS™ drug discovery platform. The National Research Council of Canada (“NRC”) are testing the Company’s proprietary, psychotropic plant-based formulas for the treatment of depression and anxiety. For these novel psychotropic drug candidates, the Company used the PhAROS™ platform to identify new ingredients to improve upon an initial formulation for anxiety based on traditional medicine. The original plant mixture was derived from the kava plant, but some elements of kava are thought to cause liver toxicity. PhAROS™ identified ingredients from the Piper plant family as a substitute for the functionality of the ingredients in question without the potentially adverse safety profiles of those original ingredients. The Piper plant family includes pepper plants that are used worldwide in traditional medicines. The Company’s new psychotropic formulations are currently in preclinical trials at the Zebrafish Toxicology, Genomics and Neurobiology Lab at the NRC, led by Dr. Lee Ellis, Research Officer and Team Lead. The ongoing work between the NRC and the Company has produced strong and applicable data for the evaluation of its therapies, and this trial could provide novel treatment options for patients with depression and anxiety.
The U.S. Patent and Trademark Office allows complex mixtures to be claimed as Active Pharmaceutical Ingredients ("APIs"). Through GBSGB, the Company has six issued patents, plus a series of pending patents containing plant-derived complex mixtures and minimum essential mixtures that act as therapeutic agents for specific disease categories, as described below. The Company’s pending patents are protected whether the individual compounds are derived from the cannabis plant, another plant, synthetically produced, or derived from a combination of sources for the individual chemical compounds in these mixtures. On March 1st, 2022, the Company’s newest patent was issued by the U.S. Patent and Trademark Office (USPTO) for a cannabinoid-containing mixture designed to treat cardiac hypertrophy, often present in advanced heart disease. The Company’s newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor associated hearing loss and urinary cystitis. This year, our growing intellectual property portfolio was augmented with additional patent-protections for our PhAROS™ drug discovery platform, new PhAROS™ discovered, non-cannabis formulations, and improved formulations for our PD therapeutics.
Drug Development Progress
The Company has made significant strides in the past year with respect to both its drug discovery research and product development programs. The Company, through GBSGB, now has five preclinical phase product development programs and is aggressively preparing its lead formulations for the treatment of Parkinson’s disease for a first-in-human clinical trial. Our lead program in Parkinson’s disease is being prepared for a first-in-human trial through the following essential steps: a) creating clinical prototypes by combining our proprietary Parkinson’s formulas with a convenient oral delivery system; b) performing a dose response study in rodents to establish the correct range of active ingredients for our first-in-human trial; c) performing necessary ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicology) tests on the clinical prototypes; and d) selecting a Contract Research Organization (CRO) to prepare an Investigational New Drug (IND) application to the US FDA to begin our first-in-human trial. In addition to our work in preparing the Parkinson’s formulation for a First-in-Human trial, the Company’s chronic pain, anxiety, and depression formulations are currently in preclinical animal studies with Dr. Lee Ellis of the National Research Council ("NRC") Canada in Halifax, Nova Scotia. We received positive preclinical, proof-of-concept data supporting our minimum essential mixtures for the treatment of Cytokine Release Syndrome in COVID-19 (COVID-CRS) and other severe hyperinflammatory conditions. GBSGB’s lead COVID-CRS candidates will be optimized based on late-stage preclinical studies with Dr. Norbert Kaminski at Michigan State University.
For the Company’s lead program in PD therapeutics, the efficacy of our original formulations has been improved and the Company has filed a new patent application family to protect our defined cannabinoid ratio-minimum essential mixtures (DCR-MEMs) for the treatment of Parkinsonian motor symptoms. The Company had announced previously that it has obtained the statistically significant reduction of Parkinson’s-disease like symptoms using proprietary cannabinoid-containing MEMs in an animal model of Parkinson’s disease ("PD"). Three of the Company’s PD formulations significantly reduced the PD-like motor symptoms. In addition, the toxicity studies for these original PD formulas came back without any significant negative findings. These initial efficacious PD formulations were equimolar minimum essential mixtures (E-MEMs), wherein, each contained three cannabinoids combined at an equimolar ratio (1:1:1). In the past year 2020-2021, the Company has screened an additional sixty-three variations of the original three equimolar MEMs and identified a total of twenty-two DCR-MEMs with optimized ratios of cannabinoids that produced a statistically significant reduction in OHDA induced motor symptoms. Five of these twenty-two efficacious MEMs outperformed the original equimolar cannabinoid MEMs. A new patent application has been filed to protect these DCR-MEMs. These important preclinical results will be included in GBS’ Investigational New Drug ("IND") application with the US FDA to enter human clinical trials as soon as possible. New therapies to address Parkinson’s disease symptoms are needed to help those afflicted with this debilitating disease. The combined direct and indirect costs associated with Parkinson’s disease are estimated at $52 billion in the U.S. alone.
This year, we are working with Catalent Pharma on the preparation of clinical prototypes of our proprietary cannabinoid-based formulations for Parkinson’s disease in Catalent Pharma’s proprietary Zydis® delivery system. Catalent Pharma’s Zydis® delivery system is an Orally Disintegrating Tablet format (“ODT”) that should be ideal for delivering our cannabinoid-ratio controlled formulations to Parkinson’s patients. More than 50% of Parkinson’s patients have trouble swallowing, but the Zydis® format delivers the active ingredients into the mouth by dispersion without needing water or the ability to swallow. To ready the Company’s Parkinson’s disease therapies for a First-in-Human trial, the initial clinical prototypes of our Defined Cannabinoid Ratio (DCR)-MEM have been formulated by Catalent Pharma using Catalent’s Zydis® Orally Disintegrating Tablet technology and they are being evaluated in stability and functional testing. As mentioned above, the ODT format was selected for the PD formulas because it dissolves on the tongues of patients without the need to swallow for ease of use in patients with PD, who often have difficulties with swallowing. Previously, the Company has completed two proof-of-concept studies for its MEM. Now, the Company is performing a Feasibility Study that will produce and validate the clinical prototypes for its DCR-MEM. The Company selected Catalent for the delivery of their PD therapies due to Catalent’s prior experience in working on US FDA-approved, cannabinoid-containing drugs, their Schedule I drug manufacturing facilities, their familiarity with US FDA and international regulatory and manufacturing requirements, their expertise in tackling formulation challenges, and their ability to achieve the stability and dosing necessary for these novel therapeutic mixtures. In addition to its Zydis® technology, Catalent has early drug development services and additional oral drug delivery solutions available for the efficient delivery of the Company's proprietary APIs.
Additionally, the Company has selected the University of Lethbridge to start our required dose response study in a rodent model of Parkinson’s disease, which will help us to establish the correct dosing for our first-in-human trial. Prior to filing our IND application, we must conduct ADMET testing on the clinical prototypes of our Parkinson’s medication being formulated for us by Catalent Pharma. The Company has identified a Contract Research Organization that will perform the ADMET testing. In the IND application for our novel Parkinson’s disease therapy, the ADMET testing data will be combined with the Chemistry Manufacturing and Controls (CMC) data prepared by Catalent Pharma and our proof-of-concept data (National Research Council Canada). In the near future, we expect to announce the selection of the Contract Research Organization that will write the IND-application and run the first-in-human trials for our novel treatment for the motor symptoms of Parkinson’s disease.
For its lead chronic pain program, the Company is testing its MEM for chronic pain both as encapsulated, time-released nanoparticles, as well as in non-encapsulated forms of these therapeutic mixtures in an animal model at the NRC in Halifax, Nova Scotia. In preparation for human clinical trials, our standard MEM and the time-released MEM are currently being compared in an animal model that demonstrates their potential effectiveness at treating chronic pain. The early results from this preclinical research project look very promising. However, the COVID pandemic adversely affected the progress on this study, but we are happy to report that we are back on track to continue with the testing of these promising chronic pain formulations.
In late summer of 2021, the Company received positive proof-of-concept data from a human immune cell model supporting the efficacy of their proprietary MEM designed for the suppression of COVID-related, cytokine release syndromes (CRS) while preserving key anti-viral immune responses. Based on this new positive proof-of-concept data, GBSGB converted their provisional patent application entitled, “CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF CYTOKINE RELEASE SYNDROME WHILE PRESERVING KEY ANTI-VIRAL IMMUNE REACTIONS” to a nonprovisional patent application on August 18, 2021. The best performing MEM will be further developed in preparation for clinical studies to evaluate their anti-inflammatory potential in the treatment of severely ill COVID-19 patients contending with Cytokine Release Syndrome (CRS) and associated hyperinflammatory conditions, such as macrophage activation syndrome (MAS) and acute respiratory distress syndrome (ARDS). CRS, MAS, and ARDS are the leading causes of deaths in COVID-19 patients. The Company’s proof-of-concept study was performed at Michigan State University using a state-of-the-science human immune model. In the Company’s proof-of-concept study, immune cells from human donors were co-cultured together in one of four treatment groups: untreated (no inflammatory stimulus), inflammatory stimulus, control (inflammatory stimulus + vehicle from cannabinoid mixtures), or pre-treatment with the cannabinoid mixture + inflammatory stimulus. Then a panel of cytokines and inflammatory markers was measured from each of these treatment groups from different immune cell types within the co-cultured cells at four time points to determine whether the Company’s MEMs were able to alter the levels of pro-inflammatory cytokines or other inflammatory agents. The Company’s COVID-CRS formulations showed potential for the selective inhibition of pro-inflammatory processes in response to viral- and bacterial-triggered hyperinflammation in a human immune cell model. These positive proof-of-concept results support the potential for some of these mixtures to accomplish our therapeutic goals, but, ultimately, clinical trial results will determine whether they are efficacious. The Company’s plant-based drug discovery platform is advancing biopharmaceutical research at a time when thousands are dying from COVID-19. The next step is to further develop our plant-inspired drugs and eventually bring them to human trials so that the use of well-defined cannabinoid mixtures in clinical practice can become a reality.
As mentioned above, the Company announced that the NRC Canada is testing our proprietary, psychotropic plant-based formulas for the treatment of depression and anxiety in preclinical animal studies. The Company has leveraged its patent-pending PhAROS™ (Phytomedical Analytics for Research Optimization at Scale) platform to identify these combinations of plant compounds for novel drug candidates to treat depression and anxiety. These are the company’s first non-cannabis formulations to enter preclinical studies. For these novel psychotropic drug candidates, the Company used the PhAROS™ platform to identify new ingredients to improve upon an initial formulation for anxiety based on traditional medicine. The original plant mixture was derived from the kava plant, but some elements of kava are thought to cause liver toxicity. PhAROS™ identified ingredients from the Piper plant family as a substitute for the functionality of the ingredients in question without the potentially adverse safety profiles of those original ingredients. The Piper plant family includes pepper plants that are used worldwide in traditional medicines. The Global Anxiety Disorder and Depression Treatment Market size is forecast to reach USD 19.81 Billion by 2028 according to Reports & Data.
Favorable Research Updates from our university collaborators reveal the promise in our discovery programs including: 1) Multiple MEM discovery projects using and advancing our proprietary PhAROS™ drug discovery platform in conjunction with Chaminade University, 2) the Company’s Cannabis Metabolomics Project with both Chaminade University of Honolulu, Hawai’i and the University of Athens, Greece, and 3) the Company’s Time-Released Nanoparticles for Delivery of Cannabis-based Ingredients with the University of Seville, Spain and the University of Cadiz, Spain.
This year, our growing intellectual property portfolio was augmented with additional patent-protections for our PhAROS™ drug discovery platform that were filed in July of 2021 and in October of 2021. The Company, through GBSGB, also filed for protection of new PhAROS™ discovered, non-cannabis formulations in July of 2021. In September of 2021, the Company filed a patent application for the Company’s improved DCR-MEM formulations for our PD therapeutic program. These new patent applications expanded upon the solid foundation of intellectual property developed over the past six years. In 2020, the three patents which protect formulations for the Company’s lead therapeutic programs were issued by the USPTO. The issuance of U.S. Patent No. 10,653,640 entitled "Cannabinoid-Containing Complex Mixtures for the Treatment of Neurodegenerative Diseases" on May 19, 2020 protects methods of using GBSGB’s proprietary cannabinoid-containing complex mixtures (CCCM™) for treating Parkinson’s Disease. This was an important milestone in the development of these vitally important therapies and validates GBSGB’s drug discovery platform. In the US alone, the combined direct and indirect costs associated with Parkinson’s disease are estimated at $52 billion, and new therapies to address Parkinson’s disease symptoms are greatly needed. This was also the first time that a US patent has been awarded for a cannabis-based complex mixture defined using this type of drug discovery method. The first US patent for PD therapies validated our drug discovery platform and strengthened our intellectual property portfolio of unique CCCM’s™, each targeting one of up to 60 specific clinical applications.
The issuance of the Company’s second and third US patents for active pharmaceutical ingredients that are complex mixtures identified by our biotech platform further confirmed that the Company’s pharmaceutical compositions can be patent protected for use as biopharmaceutical and nutraceutical products. The US Patent entitled “Myrcene-Containing Complex Mixtures Targeting TRPV1” protects methods of using our proprietary MEMs for the treatment of pain disorders related to arthritis, shingles, irritable bowel syndrome, sickle cell disease, and endometriosis. In the US alone, chronic pain represents an estimated health burden of between $560 and $650 billion dollars, and an estimated 20.4% of U.S. adults suffer from chronic pain that significantly decreases their quality of life. Despite the widespread rates of addiction and death, opioids remain the standard of care treatment for most people with chronic pain. The Company believes that it is important to create safer, less addictive alternatives to opioids for the treatment of chronic pain disorders, like GBSGB’s myrcene-containing MEMs.
The Company's third issued US Patent entitled "Cannabinoid-Containing Complex Mixtures for the Treatment of Mast-Cell-Associated or Basophil-Mediated Inflammatory Disorders" protects methods of using the Company’s proprietary MEMs for treating Mast Cell Activation Syndrome (MCAS). MCAS is a severe immunological condition in which mast cells inappropriately and excessively release inflammatory mediators, resulting in a range of severe chronic hyperinflammatory symptoms and life-threatening anaphylaxis attacks. Receiving this patent for the treatment of MCAS using our MEMs is an important milestone in the development of this urgently needed medicine. There is no single recommended treatment for MCAS patients. Instead, they attempt to manage MCAS symptoms primarily by avoiding ‘triggers’ and using rescue medicines for their severe hyperinflammatory attacks. Therefore, MCAS patients need new therapeutic options to control their mast cell related symptoms, and our MEMs were designed to simultaneously control multiple inflammatory pathways within mast cells as a comprehensive treatment option. The Company is strategically targeting MCAS for two additional reasons. By focusing on a rare disease with no known cure, our company can apply for the U.S. Food and Drug Administration’s expedited approval process, which allows clinically successful treatments to get to market both quicker and more cost effectively. Gaining approval from the US FDA for the entire anti-inflammatory market would be extremely time consuming and cost prohibitive. Demonstrating that our MEMs are safe for the treatment of MCAS would favorably position our Company for clinical testing of these MEMs as potential treatments for other related inflammatory disorders, such as inflammatory bowel disease, thereby widening the target market and drastically shortening the development cycle and costs.
The Company’s fourth US Patent was issued on March 1, 2022 for a cannabinoid-containing mixture designed to treat cardiac hypertrophy, often present in advanced heart disease. Gb Sciences’ newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor associated hearing loss and urinary cystitis. Despite multiple categories of prescription heart medications on the market, heart disease remains the leading cause of death in the United States for people of most racial and ethnic groups. Alternative therapeutic approaches are still needed, especially for the treatment of advanced heart disease. The market for prescription heart disease medications is predicted to rise to $64 billion dollars in the US by 2026, with future market growth fueled by innovative new therapeutic approaches.
Intellectual Property
GBSGB retained Fenwick & West, a Silicon Valley based law firm focusing on life sciences and high technology companies with a nationally top-ranked intellectual property practice, to develop strategies for the protection of the Company's intellectual property. The status of the intellectual property portfolio is as follows. Unless otherwise indicated, all patents listed below are assigned to the Company's wholly-owned subsidiary, GBS Global Biopharma, Inc.
Issued Patents
|
Title: CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF NEURODEGENERATIVE DISEASES
|
| |
|
|
|
|
|
|
|
|
|
U.S. Patent Number:
|
|
10,653,640
|
|
Expiration date:
|
October 23, 2038
|
|
Issued:
|
|
May 19, 2020
|
|
Inventors:
|
|
Andrea Small-Howard et al.
|
| |
|
|
|
|
|
|
U.S. Patent protection was granted for GBSGB’s Cannabinoid-Containing Complex Mixtures for the treatment of Parkinson’s disease.
|
|
Title: MYRCENE-CONTAINING COMPLEX MIXTURES TARGETING TRPV1
|
| |
|
|
|
|
|
|
|
|
|
U.S. Patent Number:
|
|
10,709,670
|
|
Expiration date:
|
May 22, 2038
|
|
Issued:
|
|
July 14, 2020
|
|
Inventors:
|
|
Andrea Small-Howard, et al.
|
| |
|
|
|
|
|
|
GBSGB’s MCCMs are protected in the U.S. for use in the treatment of pain related to arthritis, shingles, irritable bowel syndrome, sickle cell disease, and endometriosis.
|
|
Title: CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF MAST CELL-ASSOCIATED OR BASOPHIL-MEDIATED INFLAMMATORY DISORDERS
|
| |
|
|
|
|
|
|
|
|
|
U.S. Patent Number:
|
|
10,857,107
|
|
Expiration date:
|
January 31, 2038
|
|
Issued:
|
|
December 8, 2020
|
|
Inventors:
|
|
Andrea Small-Howard et al.
|
| |
|
|
|
|
|
|
U.S. Patent protection was granted for GBSGB’s Cannabinoid-Containing Complex Mixtures for the treatment of Mast Cell Activation Syndrome (MCAS).
|
Title: TRPV1 ACTIVATION-MODULATING COMPLEX MIXTURES OF CANNABINOIDS AND/OR TERPENES
|
| |
|
|
|
|
|
|
|
|
|
|
|
11,260,044 |
|
|
|
|
|
|
March 1, 2022 |
|
Inventors: |
Andrea Small-Howard et al.
|
| |
|
|
|
|
|
| U.S. Patent protection was granted for GBSGB’s Cannabinoid-Containing Complex Mixtures designed to treat cardiac hypertrophy, often present in advanced heart disease. The Company’s newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor associated hearing loss and urinary cystitis. |
|
Title: METHODS AND COMPOSITIONS FOR PREVENTION AND TREATMENT OF CARDIAC HYPERTROPHY
|
| |
|
|
|
|
|
|
|
|
|
Inventor:
|
|
Alexander Stokes
|
|
Assignee:
|
University of Hawai'i
|
|
U.S. Patent Number:
|
|
9,084,786
|
|
Issued:
|
July 21, 2015
|
|
U.S. Patent Number:
|
|
10,137,123
|
|
Issued:
|
|
November 27, 2018
|
|
E.U. Patent Number:
|
|
2,635,281
|
|
Issued:
|
|
March 14, 2018
|
|
Hong Kong Patent Number:
|
|
14102182.8
|
|
Issued:
|
March 14, 2018
|
| |
|
GBSGB has sublicensed from Makai Biotechnology, LLC these two issued USPTO patents and two issued international patents for the prevention and treatment of heart failure due to cardiac hypertrophy through therapeutic regulation of TRPV1.
|
|
Title: METHOD FOR PRODUCING A PHARMACEUTICAL COMPOSITION OF POLYMERIC NANOPARTICLES FOR TREATING NEUROPATHIC PAIN CAUSED BY PERIPHERAL NERVE COMPRESSION
|
|
Spain Patent Number:
|
|
ES2582287
|
|
Inventors:
|
Lucia Martin Banderas, Mercedes Fernandez Arevalo, Esther Berrocoso Dominguez, Juan Antonio Mico Segura
|
|
Issued:
|
|
September 29, 2017
|
|
Assignees:
|
Universidad de Sevilla, Universidad de Cadiz, Centro de Investigacion Biomedica En Red
|
| |
|
Exclusive worldwide license held by GBS Global Biopharma, Inc. Claims benefit of Spanish Patent Application No. P201500129 (Pub. No. ES 2582287). GBSGB holds the exclusive rights to commercialize these cannabinoid-containing, time-released, oral nanoparticles for the treatment of neuropathic pain.
|
In addition to the issued patents listed above, GBSGB's intellectual property portfolio includes a total of ten USPTO and thirty-five international patents pending:
|
Title
|
Jurisdiction
|
Application Number
|
Other International Applications Filed
|
Continuation of
|
|
CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF NEURODEGENERATIVE DISEASES
|
US
|
USPTO 16/844,713
PCT/US2017/055989
|
AU, CA, CN, EP, HK, IL, JP
|
15/729,565
|
|
MYRCENE-CONTAINING COMPLEX MIXTURES TARGETING TRPV1
|
US
|
USPTO 16/878,295
PCT/US2018/033956
|
AU, CA, CN, EP, HK, IL, JP
|
15/986,316
|
|
CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF MAST CELL-ASSOCIATED OR BASOPHIL-MEDIATED INFLAMMATORY DISORDERS
|
US
|
USPTO 17/065,400
PCT/US2018/016296
|
AU, CA, CN, EP, HK, IL, JP
|
15/885,620
|
| TRPV1 ACTIVATION-MODULATING COMPLEX MIXTURES OF CANNABINOIDS AND/OR TERPENES |
US |
USPTO 16/420,004 PCT/US2019/033618 |
AU, CA, CN, EP, HK, IL, JP |
17/576,485 |
|
THERAPEUTIC NANOPARTICLES ENCAPSULATING TERPENOIDS AND/OR CANNABINOIDS
|
US
|
USPTO 16/686,069
PCT/ES2019/070765
|
|
|
|
TREATMENT OF PAIN USING ALLOSTERIC MODULATOR OF TRPV1
|
US
|
USPTO 16/914,205
PCT/US2020/039989
|
|
|
|
CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF CHRONIC INFLAMMATORY DISORDERS
|
US
|
USPTO 63/067,269 (provisional)
|
|
|
|
CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF CYTOKINE RELEASE SYNDROME WHILE PRESERVING KEY ANTI-VIRAL IMMUNE REACTIONS
|
US
|
USPTO 17/406,035
PCT/US2021/046584
|
|
|
|
IN SILICO META-PHARMACOPEIA ASSEMBLY FROM NON-WESTERN MEDICAL SYSTEMS USING ADVANCED DATA ANALYTIC TECHNIQUES TO IDENTIFY AND DESIGN PHYTOTHERAPEUTIC STRATEGIES
|
US
|
USPTO 17/501,498
PCT/US2021/055056
|
|
|
|
METHODS AND COMPOSITIONS FOR PREVENTION AND TREATMENT OF CARDIAC HYPERTROPHY
|
EU
|
EPO 3,348,267
|
IN, CN
|
|
|
METHOD FOR PRODUCING A PHARMACEUTICAL COMPOSITION OF POLYMERIC NANOPARTICLES FOR TREATING NEUROPATHIC PAIN CAUSED BY PERIPHERAL NERVE COMPRESSION
|
WIPO/PCT
|
WIPO 2016/128591
PCT/ES2016/000016
|
EU, CA
|
|
|
CANNABINOID-CONTAINING FORMULATIONS FOR PARKINSONIAN MOVEMENT DISORDERS
|
US
|
USPTO 63/249,482 (provisional)
|
|
|
|
METHODS AND COMPOSITIONS FOR THE IDENTIFICATION OF NOVEL THERAPEUTIC APPROACHES TO MIGRAINE USING THE PHAROS IN SILICO DRUG DISCOVERY PLATFORM
|
US
|
USPTO 63/221,334
(provisional)
|
|
|
|
METHOD AND COMPOSITIONS FOR THE PHYTOMEDICAL COMPONENT SUPPLY CHAIN DECISION SUPPORT USING THE PHAROS IN SILICO DRUG DISCOVERY PLATFORM
|
US
|
USPTO 63/221,358
(provisional)
|
|
|
|
METHODS AND COMPOSITIONS FOR NOVEL PAIN THERAPIES INCLUDING OPIOID-ALTERNATIVE STRATEGIES IDENTIFIED USING THE PHAROS IN SILICO DRUG DISCOVERY PLATFORM
|
US
|
USPTO 63/221,364
(provisional)
|
|
|
|
METHODS AND COMPOSITIONS FOR NOVEL PAIN THERAPIES TARGETED TO SPECIFIC PAIN SUBTYPES IDENTIFIED USING THE PHAROS IN SILICO DRUG DISCOVERY PLATFORM
|
US
|
USPTO 63/221,366
(provisional)
|
|
|
|
METHODS AND COMPOSITIONS DEVELOPMENT OF NOVEL THERAPEUTICS BASED ON PIPER SPECIE-CONTAINING PHYTOMEDICINES FOR ANXIETY AND ASSOCIATED DISORDERS USING THE PHAROS IN SILICO DRUG DISCOVERY PLATFORM
|
US
|
USPTO 63/221,367
(provisional)
|
|
|
|
METHODS AND COMPOSITIONS FOR DECONVOLUTION OF COMPLEX PHYTOMEDICAL FORMULAE FOR CANCER TO IDENTIFY TARGETED STRATEGIES FOR CANCER PAIN AND CYTOTOXIC THERAPEUTIC CANDIDATES USING THE PHAROS IN SILICO DRUG DISCOVERY PLATFORM
|
US
|
USPTO 63/221,371
(provisional)
|
|
|
Partnering Strategy
The Company runs a lean drug development program and minimizes expenses, including personnel, overhead, and fixed capital expenses (such as lab and diagnostic equipment), through strategic partnerships with universities, hospitals, suppliers, Contract Research Organizations (“CROs”), and Contract Manufacturing Organizations (“CMOs”). Through these research and development agreements, the Company has created a virtual pipeline for the further development of novel medicines based on ingredients originally derived from the cannabis plant and other plant-based traditional medicines. The partners bring both expertise and infrastructure at a reasonable cost to the life sciences program. In most instances, the Company has also negotiated with these partners to keep 100% of the ownership of the IP within GBSGB for original patent filings.
The Company currently has on-going research agreements with the following institutions covering the indicated areas of research:
Chaminade University: Broad-based research program to support the drug discovery platform that has yielded many of the Company’s original patents to date in the areas of neurodegenerative diseases, heart disease, inflammatory diseases, neuropathic and chronic pain. They have also performed the bioassay portion of the Cannabis Metabolomics study performed with the University of Athens, Greece and the Company. Our collaborations with Chaminade also led to the development of our PhAROS™ drug discovery platform.
University of Athens: Broad-based metabolomics analysis of over 100 cannabis genotypes including both hemp and THC-producing cannabis varieties, in combination with the Company’s bioassay data linking genotypes and potential disease-remediations. This project has the potential to define active ingredients from plant-derived mixtures beyond the standard cannabinoids and terpenoids. The discovery potential is huge, and novel agents have recently been discovered. Novel ligands have been identified and are being validated. This project will ultimately yield novel patent-protected therapies.
Michigan State University: Preclinical work using a cutting-edge, multi-cellular model of the human immune system and a multi-cell model of the brain to validate our MEMs for use in the treatment of COVID-19-related cytokine release syndromes (COVID-CRS). MSU has performed experiments using their novel model of the human-immune system that have allowed GBSGB to prepare cannabis-based formulas for the potential treatment of virally-induced hyperinflammation/cytokine storm syndrome that has led to the majority of COVID-19 deaths. Positive proof-of-concept results have guided the development of these selectively anti-inflammatory MEM.
The University of Seville: Bringing their novel expertise to the development and functional testing of time-released and disease-targeted nanoparticles of cannabis-based minimum essential mixtures for oral administration. These specialized nanoparticles are being used for the precise and time-released delivery of several of our therapies, including the Company’s chronic pain MEMs used in the preclinical animal testing performed at the NRC Canada. The University of Seville has completed functional testing on nanoparticles containing myrcene, nerolidol, and beta-caryophyllene for our chronic pain MEMs. In cell-based assays, the effectiveness and kinetics of the nanoparticle-forms of these terpenes were compared with the “naked” terpenes both individually and in mixtures. In all cases, the effectiveness of the nanoparticles was superior to the naked terpenes, however, the mixtures were dramatically more effective than the individuals. Recently, our partners at the University of Seville have completed the formulation of new cannabis-based ingredients for inclusion into the oral, time-released nanoparticle format for the completion of our maximally effective MEMs for chronic pain. The results from Seville are very promising, and these nanoparticles have entered the animal testing phase at the NRC in Halifax.
The National Research Center (NRC) of Canada, Halifax, Nova Scotia: Four animal-phase studies are being performed by Dr. Lee Ellis’ group at the NRC. 1) Parkinson’s Disease: In Q1 of 2020, an animal safety and efficacy study was completed for the Company’s equimolar MEMs for the treatment of the motor symptoms of Parkinson’s disease, and the NRC has demonstrated that the company’s PD formulations were able to reduce behavioral changes associated with the loss of dopamine-producing neurons, which underlies the pathology of Parkinson’s disease in the animal model. Based on achieving the statistically significant reduction in Parkinson’s disease symptomology in these equimolar MEMs, the Company completed a final phase of testing in August of 2021, which identified five defined cannabinoid ratio (DCR)-MEMs that were more effective than the root equimolar MEM. 2) Chronic Pain: In Q1 of 2019, the Company started a safety and efficacy study in animals for our Chronic Pain (CP) formulas. The midterm results for these preclinical pain studies were promising, but the study was significantly delayed by the COVID pandemic. These preclinical studies have resumed and are progressing well. 3 & 4) Depression and Anxiety: Minimum essential mixtures of plant-based ingredients from kava and the related Piper plant family are being evaluated now.
The University of Cadiz: Testing the safety and efficacy of the above-mentioned time-released nanoparticles in rodent models of chronic pain. Proof of concept complete for one formulation.
University of Hawaii: Validating the efficacy of a complex cannabis-based mixture for the treatment of cardiac hypertrophy and cardiac disease in a rodent model. Proof of concept work is complete in rodents, and we are seeking commercialization partners.
Path to Market: Drug Development Stages and Proposed Clinical Trials
The Company has plant-based therapeutic products in the following stages of drug development: Discovery, Pre-Clinical, and entering the Clinical Phase. It has also licensed therapeutic products that the Company intends to develop through partners, labeled Partner Programs.
The completion of discovery, preclinical studies, clinical trials, and the required regulatory submissions required for obtaining US FDA pre-market approvals for pharmaceutical products (and equivalent approvals from other corresponding agencies worldwide) is traditionally a long and expensive process. However, the Company asserts that its proprietary, PhAROS™, AI-enabled, drug discovery engine; plant-inspired formulations; lean development program; novel regulatory strategy; experienced development partners; and aggressive licensing of these products at early clinical stages can mitigate some of the risks. The Company uses a combination of in silico discovery methods and automated screening of cellular and animal models of disease to decrease the time in Discovery prior to filing novel patent applications for disease-specific therapeutics. Through GBSGB, the Company’s original patent applications cover new chemical entities (“NCE”) based on discovery and validation of minimum essential mixtures derived from complex, plant-based therapeutics. The Company plans to use an Exploratory IND/Phase 0 Program that gets the Company to First-in-Human sooner than traditional programs, which reduces translational risks, and includes preliminary efficacy measures for responsible development decisions. In contrast, a traditional phased-development path would not provide any efficacy measures until Phase II. After the completion of our Phase 0 study for PD, which compares the efficacies of multiple related cannabinoid-based formulations, the Company plans to advance the lead PD drug candidate using an adaptive trial design that is more efficient than the traditional phased-development pathway. Through GBSGB, the Company has entered into research contracts, partnerships, and/or joint ventures with several respected, independent contract research organizations, medical schools, universities, and with other scientific consultants to increase developmental efficiencies. If and when one or more of the Company’s drugs, therapies or treatments are approved by the US FDA, the Company will seek to market them under licensing arrangements with major biotechnology or pharmaceutical companies.
There can be no assurance that we will ever be able to enter into any joint ventures or other arrangements with third parties to finance our drug development program or that if we are able to do so, that any of our projected therapies will ever be approved by the US FDA. Even if we obtain US FDA approval to market one of our therapies, there can be no assurance that it could be successfully marketed or would not be superseded by another plant-based therapy produced by one or more of our competitors. It also may be anticipated that even if we enter into a joint venture development with a financially stable pharmaceutical or institutional partner, we will still be required to raise significant additional capital in the future to achieve the strategic goals of the Company. There can be no assurance that we will be able to obtain such additional capital on reasonable terms, if at all. If the Company fails to achieve its goal of producing one or more plant-inspired pharmaceuticals or therapies, it would have a material adverse effect on our future financial condition and business prospects.
Other Operations
On March 24, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC. Pursuant to the Teco MIPA, the Company agreed to sell 100% of its membership interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC (the "Teco Subsidiaries") for approximately $8 million, which amount includes a cash payment at closing, the extinguishment of certain liabilities owed to the purchaser and affiliates of the purchaser, and an 8% promissory note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with 483 Management, LLC. Pursuant to the Nopah MIPA, the Company agreed to sell its 100% membership interest in GB Sciences Nopah, LLC ("Nopah"), which holds a Nevada medical marijuana cultivation certificate. As consideration, the Company would receive $300,000 as a reduction to the balance of the 0% Note payable dated October 23, 2017 (Note 4) and accounts payable of $74,647, which were owed to an affiliate of the purchaser.
The closing of the Teco and Nopah sales was contingent upon the successful transfer of the Nevada cultivation and production licenses. On December 14, 2021, the Company received approval from the Nevada Cannabis Compliance Board for the transfer of cannabis cultivation and extraction licenses held by its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC (the "Nevada Subsidiaries"). Consequently, all conditions to closing the sales of the 100% membership interests in the Nevada Subsidiaries were satisfied, and the transactions formally closed on December 31, 2021. After the closing date, the Company retains no ownership interest in the Nevada Subsidiaries.
Competition
The biotech industry is subject to intense and increasing competition. We face potential competition from many different sources, including large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, and medical technology companies. Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future. Some of our competitors may have substantially greater capital resources, facilities and infrastructure then we have, which may enable them to compete more effectively in this market. These competitors include Cara Therapeutics Inc., Corbus Pharmaceuticals Holdings Inc., Zynerba Pharmaceuticals Inc., Tetra Bio-Pharma, Inc., Revive Therapeutics, Inc., Axim Biotechnologies, Inc., and Emerald BioScience, Inc., among others.
There are several organizations that may be developing or marketing therapies for the indications that we are pursuing. Many of our competitors, including many of the organizations named above, have substantially greater financial, technical and human resources than we do and significantly greater experience in the development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of competitors.
We believe the key competitive factors that will affect the development and commercial success of our product candidates, if approved for marketing, are likely to be their safety, efficacy and tolerability profile, reliability, convenience of dosing, price and reimbursement from government and third-party payers. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payers seeking to encourage the use of generic products. Generic products that broadly address these indications are currently on the market for the indications that we are pursuing, and additional products are expected to become available on a generic basis over the coming years. If our product candidates achieve marketing approval, we expect that they will be priced at a significant premium over competitive generic products.
Government Regulation and Federal Policy
Government authorities in the U.S. (including federal, state and local authorities) and in other countries extensively regulate, among other things, the manufacturing, research and clinical development, marketing, labeling and packaging, storage, distribution, post-approval monitoring and reporting, advertising and promotion, export and import of pharmaceutical products, such as those we are developing. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Moreover, failure to comply with applicable regulatory requirements may result in, among other things, warning letters, clinical holds, civil or criminal penalties, recall or seizure of products, injunction, disbarment, partial or total suspension of production or withdrawal of the product from the market. Any agency or judicial enforcement action could have a material adverse effect on us.
FDA Regulation
In the U.S., the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”) and its implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The process required by the FDA before product candidates may be marketed in the U.S. generally involves the following:
●completion of extensive preclinical laboratory tests and preclinical animal studies, all performed in accordance with the FDA’s Good Laboratory Practice (“GLP”) regulations. Preclinical testing generally includes evaluation of our product candidates in the laboratory or in animals to characterize the product and determine safety and efficacy;
●submission to the FDA of an Investigational New Drug application ("IND"), which must become effective before human clinical trials may begin and must be updated annually;
●performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication;
●submission to the FDA of a New Drug Application ("NDA") after completion of all pivotal clinical trials;
●a determination by the FDA within 60 days of its receipt of an NDA to file the NDA for review;
●satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities at which the active pharmaceutical ingredient (“API”) and finished drug product are produced and tested to assess compliance with cGMP regulations;
●satisfactory completion of an FDA pre-approval inspection of one or more of the clinical sites at which the clinical trials were conducted;
●at the discretion of the FDA, a public Advisory Committee Meeting where the data is reviewed by experts who discuss the data and give their opinion (which the FDA is not obliged to follow) of the adequacy of the data to support an approval; and
●FDA review and approval of an NDA prior to any commercial marketing or sale of the drug in the U.S.
We rely, and expect to continue to rely on third parties for the production, distribution, shipping and storage of clinical and commercial quantities of our product candidates. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution or require substantial resources to correct. In addition, discovery of previously unknown problems with a product or the failure to comply with applicable requirements may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal or recall of the product from the market or other voluntary, FDA-initiated or judicial action that could delay or prohibit further marketing. Newly discovered or developed safety or effectiveness data may require changes to a product’s approved labeling, including the addition of new warnings and contraindications, and also may require the implementation of other risk management measures. Also, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development.
In addition to regulations in the U.S., we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the U.S. have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application (“CTA”) must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of an investigational drug under European Union regulatory systems, we must submit a marketing authorization application. The application used to file the NDA in the U.S. is similar to that required in Europe, with the exception of, among other things, country-specific document requirements. For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Cannabis Regulation
Although the Company has completely divested of its cannabis cultivation and production facilities effective December 31, 2021, the Company has owned and operated subsidiaries that were involved in the manufacturing and distribution of cannabis products under State law. These operations were subject to prohibition under United States federal law.
Under the Controlled Substances Act (“CSA”), the policies and regulations of the Federal government and its agencies are that cannabis (marijuana) is a Schedule 1 narcotic that is addictive and has no medical benefit. Accordingly, and a range of activities including cultivation and the personal use of cannabis is prohibited and subject to prosecution and criminal penalties. Unless and until Congress amends the CSA with respect to medical cannabis, there is a risk that the federal authorities may enforce current federal law, and we may be deemed to have engaged in producing, cultivating, or dispensing cannabis in violation of federal law, or we may be deemed to have facilitated the sale or distribution of drug paraphernalia in violation of federal law with respect to our Company’s divested business operations. Active enforcement of the current federal regulatory position on cannabis may thus indirectly and adversely affect our strategic goals, revenues and profits. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated federal policy remains uncertain. See “Risk Factors” below. The U.S. Supreme Court declined to hear a case brought by San Diego County, California that sought to establish federal preemption over state medical cannabis laws. The preemption claim was rejected by every court that reviewed the case. The California 4th District Court of Appeals wrote in its unanimous ruling, “Congress does not have the authority to compel the states to direct their law enforcement personnel to enforce federal laws.” However, in another case, the U.S. Supreme Court held that, as long as the CSA contains prohibitions against cannabis, under the Commerce Clause of the United States Constitution, the United States may criminalize the production and use of cannabis even where states approve its use for medical purposes.
In an effort to provide guidance to federal law enforcement, the Department of Justice (“DOJ”) issued Guidance Regarding Cannabis Enforcement to all United States attorneys in a memorandum from Deputy Attorney General David Ogden on October 19, 2009, in a memorandum from Deputy Attorney General James Cole on June 29, 2011 and in a memorandum from Deputy Attorney General James Cole on August 29, 2013. Each memorandum provides that the DOJ is committed to the enforcement of the CSA, but, the DOJ is also committed to using its limited investigative and prosecutorial resources to address the most significant threats in the most effective, consistent and rational way.
The August 29, 2013 memorandum provides updated guidance to federal prosecutors concerning cannabis enforcement in light of state laws legalizing medical and recreational cannabis possession in small amounts.
The memorandum sets forth certain enforcement priorities that are important to the federal government:
| |
•
|
Distribution of cannabis to children;
|
| |
•
|
Revenue from the sale of cannabis going to criminals;
|
| |
•
|
Diversion of medical cannabis from states where it is legal to states where it is not;
|
| |
•
|
Using state authorized cannabis activity as a pretext of another illegal drug activity;
|
| |
•
|
Preventing violence in the cultivation and distribution of cannabis;
|
| |
•
|
Preventing drugged driving;
|
| |
•
|
Growing cannabis on federal property; and
|
| |
•
|
Preventing possession or use of cannabis on federal property.
|
On January 4, 2018, Attorney General Jeff Sessions revoked the Ogden Memo and the Cole Memos.
The DOJ has not historically devoted resources to prosecuting individuals whose conduct is limited to possession of small amounts of cannabis for use on private property but has relied on state and local law enforcement to address cannabis activity. In the event the DOJ reverses its stated policy and begins strict enforcement of the CSA in states that have laws legalizing medical cannabis and recreational cannabis in small amounts, there may be a direct and adverse impact to our business and our revenue and profits. Furthermore, H.R. 83, enacted by Congress on December 16, 2014, provides that none of the funds made available to the DOJ pursuant to the 2015 Consolidated and Further Continuing Appropriations Act may be used to prevent certain states, including Nevada and California, from implementing their own laws that authorized the use, distribution, possession, or cultivation of medical cannabis.
In contrast to federal policy, the majority of U.S. states, four U.S. territories, and the District of Columbia have laws and/or regulations that recognize, in one form or another, legitimate medical uses for cannabis and adult recreational use of cannabis. Many other states are considering similar legislation.
Employees
As of March 31, 2022, we had five employees, including three full-time employees.
ITEM 1A. RISK FACTORS
You should carefully consider the risks, uncertainties and other factors described below, in addition to the other information set forth in this Annual Report on Form 10-K, including our financial statements and the related notes thereto. Any of these risks, uncertainties and other factors could materially and adversely affect our business, financial condition, results of operation and cash flows. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment. An investment in our securities is speculative and involves a high degree of risk. You should not invest in our securities if you cannot bear the economic risk of your investment for an indefinite period of time and cannot afford to lose your entire investment. There may be additional risks that we do not presently know of or that we currently believe are immaterial which could also impair our business and financial position. See also “Cautionary Note Regarding Forward-Looking Statements.”
RISKS RELATING TO OUR BUSINESS AND INDUSTRY
We have a limited operating history, which may make it difficult for investors to predict future performance based on current operations.
We have a limited operating history upon which investors may base an evaluation of our potential future performance. In particular, we have not proven that we can supply growing equipment in a manner that enables us to be profitable and meet customer requirements, develop intellectual property to enhance our product lines, obtain the necessary permits to develop medical grade cannabis, develop and maintain relationships with key manufacturers and strategic partners to extract value from our intellectual property, raise sufficient capital in the public and/or private markets, or respond effectively to competitive pressures. As a result, there can be no assurance that we will be able to develop or maintain consistent revenue sources, or that our operations will be profitable and/or generate positive cash flows.
Any forecasts we make about our operations may prove to be inaccurate. We must, among other things, determine appropriate risks, rewards, and level of investment in our product lines, respond to economic and market variables outside of our control, respond to competitive developments and continue to attract, retain and motivate qualified employees. There can be no assurance that we will be successful in meeting these challenges and addressing such risks and the failure to do so could have a materially adverse effect on our business, results of operations and financial condition. Our prospects must be considered in light of the risks, expenses, and difficulties frequently encountered by companies in the early stage of development. As a result of these risks, challenges and uncertainties, the value of your investment could be significantly reduced or completely lost.
Our independent auditors’ report for the fiscal years ended March 31, 2022 and 2021 have expressed doubts about our ability to continue as a going concern;
Due to the uncertainty of our ability to meet our current operating and capital expenses, in our audited annual financial statements as of and for the years ended March 31, 2022 and 2021 our independent auditors included a note to our financial statements regarding concerns about our ability to continue as a going concern. The Company has incurred recurring losses and has generated limited revenue since inception. These factors and the need for additional financing in order for the Company to meet its business plan, raise substantial doubt about the ability to continue as a going concern. The presence of the going concern note to our financial statements may have an adverse impact on the relationships we are developing and plan to develop with third parties as we continue the commercialization of our products and could make it challenging and difficult for us to raise additional financing, all of which could have a material adverse impact on our business and prospects and result in a significant or complete loss of your investment.
We have incurred significant losses in prior periods, and losses in the future could cause the quoted price of our Common Stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due and on our cash flows.
We have incurred significant losses in prior periods. For the years ended March 31, 2022 and 2021, we had net losses of $530,873 and $3,725,027, respectively, and we had an accumulated deficit of $104,580,122 and $103,886,232 respectively. Any losses in the future could cause the quoted price of our common stock to decline or have a material adverse effect on our financial condition, our ability to pay our debts as they become due, and on our cash flows.
We will need additional capital to sustain our operations and will need to seek further financing, which we may not be able to obtain on acceptable terms or at all. If we fail to raise additional capital, as needed, our ability to implement our business plan could be compromised.
We have limited capital resources and operations. To date, our operations have been funded primarily from the proceeds of debt and equity financings. We expect to require substantial additional capital in the near future to implement our strategies, develop our intellectual property base, and establish our targeted levels of commercial production. There is no assurance that we will be able to raise the amount of capital needed for future growth plans.
Even if financing is available, it may not be on terms that are acceptable. If unable to raise the necessary capital at the times required, the Company may have to materially change the business plan, including delaying implementation of aspects of the business plan or curtailing or abandoning the business plan. Even if we obtain financing for our near-term operations, we expect that we will require additional capital thereafter, especially if we are to develop our Science division and start to conduct, individually or with joint venture partners, pre-clinical and clinical trials for potential pharmaceutical, or nutraceutical products derived from cannabis. Our capital needs will depend on numerous factors including: (i) our profitability; (ii) the release of competitive products by our competition; (iii) the level of our investment requirements for research and development; and (iv) the amount of our capital expenditures, including acquisitions. We cannot assure you that we will be able to obtain capital in the future to meet our needs.
If we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership held by our existing stockholders will be reduced and our stockholders may experience significant dilution. In addition, new securities may contain rights, preferences or privileges that are senior to those of our common stock. If we raise additional capital by incurring debt, this will result in increased interest expense. If we raise additional funds through the issuance of securities, market fluctuations in the price of our shares of common stock could limit our ability to obtain equity financing.
We cannot give you any assurance that any additional financing will be available to us, or if available, will be on terms favorable to us. If we are unable to raise capital when needed, our business, financial condition, and results of operations would be materially adversely affected, and we could be forced to reduce or discontinue our operations.
Drug research and development programs typically involves huge expenditures, long periods to obtain FDA approvals and the potential that such prospective pharmaceutical products will not prove to be safe and effective.
The production of FDA-approved pharmaceutical products and related drug is typically a highly expensive a long and drawn out process, typically involving hundreds of millions of dollars and a decade or more to achieve. Although we believe that some, if not all, of our planned cannabinoid based pharmaceutical protocols can qualify for “orphan drug” status and be accelerated through the FDA approval process, there can be no assurance that this will be the case.
In addition, we do not now have, and do not expect in the foreseeable future to have, the capital resources to fund our drug discovery programs, nor do we have the infrastructure to conduct such program alone. For that reason, we intend to engage in joint ventures with third parties, including hospitals, clinics, foundations and other qualified sources. Although we are in preliminary discussions with various potential partners, to date, we have not entered into any definitive drug development joint venture or partnership agreement. Our failure or inability to enter into one or more drug development agreements will materially and adversely affect our ability to develop our Science division. Even if we are able to obtain such joint drug development agreements there can be no assurance that it will be on terms and conditions that will be favorable to us.
There is the further risk that the anticipated costs of producing an FDA approved drug will not escalate to the point that will cause us and any of our prospective development partners to abandon such efforts.
Even if we do develop an FDA-approved pharmaceutical product, there is the risk that it will not be saleable to a major pharmaceutical company (either before or after completion of the FDA approval process), or that other competing drugs will not be produced providing the same medical benefits.
Accordingly, there is a significant risk that we will never be able to generate a return on our investment, and we could lose our entire investment in GBS Global Biopharma, Inc.. Either of such events, would have a material adverse effect on our business prospects and equity value.
There has been limited study on the effects of cannabinoids and future clinical research studies may lead to conclusions that dispute or conflict with our understanding and belief regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabinoid-based active ingredients.
Many of the Company's products involve the use of complex mixtures of cannabinoids. Research regarding the medical benefits, viability, safety, efficacy and dosing of cannabinoids remains in relatively early stages. There have been few clinical trials on the benefits of cannabinoids conducted by us or by others, but the number of trials is growing.
Future research and clinical trials may draw opposing conclusions to statements contained in the articles, reports and studies we have relied on or could reach different or negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing or other facts and perceptions related to cannabinoid-containing prescription medicines. However, our proprietary formulations will have been through the rigorous premarket approval process of the US FDA prior to marketing.
Federal law prohibits the use of cannabis for the purposes in which the Company has previously engaged.
Under the federal Controlled Substances Act (“CSA”), cannabis is deemed to be a Schedule One narcotic that has no medical benefit. Therefore, a range of activities including cultivation and the personal use of cannabis is prohibited and is a criminal offense. Unless and until Congress amends the CSA with respect to medical cannabis, as to the timing or scope of any which amendments there can be no assurance, there is a risk that federal authorities may enforce current federal law. The risk of strict enforcement of the CSA in light of Congressional activity, judicial holdings, and stated federal policy remains uncertain.
The current policy and regulations of the Federal government and its agencies, including the U.S. Drug Enforcement Agency and the FDA, are that cannabis has no medical benefit and a range of activities including cultivation and use of cannabis for personal use is prohibited on the basis of Federal law. Although the majority of states and the District of Columbia have passed legislation permitting the cultivation and dispensing of medical cannabis, these laws are, in many jurisdictions, subject to strict regulation and limitations and are still being developed. Active enforcement of the current federal regulatory position on cannabis on a regional or national basis may directly and adversely affect the Company even though it was allowed by state regulation in the various states in which the Company operated. Accordingly, although the Company was successful in obtaining state cultivation and production licenses in Nevada and other states and operated pursuant to such licenses, the operations were in violation of federal law. If existing federal laws are enforced by the United States Department of Justice or the FDA, it is possible that the Company could be prosecuted for its former operations in cannabis cultivation and production under state licensing.
Because the Company's sales were subject to IRC 280E, we owe federal income taxes even though we incurred losses.
Under the federal Controlled Substances Act (“CSA”), cannabis is deemed to be a Schedule One narcotic that has no medical benefit. The production and distribution of Schedule One narcotics is subject to Internal Revenue Code Section 280E, which prohibits the Company from deducting any ordinary and necessary business expenses from taxable gross profit related to the sale of cannabis products. Without the deduction of business expenses, the Company owes income taxes in the amount of $896,495, including accrued penalties and interest, despite having generated net losses and substantial net operating loss carryforwards. The Company does not currently have sufficient resources to pay those taxes, and if we are unable to pay those taxes we may be subject to penalties and IRS enforcement action.
Because the business activities of some of our former customers were illegal under Federal law, we may be deemed to have aided and abetted illegal activities through the products that we provided to those customers. As a result, we may be subject to actions by law enforcement authorities which would materially and adversely affect our business.
Until the December 31, 2021 sale of the Nevada Subsidiaries, we provided products to customers that were engaged in businesses involving the possession, use, cultivation, and transfer of cannabis. As a result, law enforcement authorities may seek to bring an action or actions against us, including, but not limited, to a claim of aiding and abetting another’s criminal activities. Such an action would have a material effect on our business and operations.
If we incur substantial liability from litigation, complaints, or enforcement actions, our financial condition could suffer.
Our previous participation in the cannabis industry may lead to litigation, formal or informal complaints, enforcement actions, and inquiries by various federal, state, or local governmental authorities against the Company. Litigation, complaints, and enforcement actions involving these subsidiaries could consume considerable amounts of financial and other corporate resources, which could have a negative impact on our sales, revenue, profitability, and growth prospects.
We could have difficulty accessing the service of banks, which may make it difficult for us to operate.
Since the use of cannabis is illegal under Federal law, there is an argument that banks should not accept for deposit funds from businesses involved with the cannabis industry. Consequently, such businesses often have difficulty finding a bank willing to accept their business.
On February 14, 2014, the U.S. government issued rules allowing banks to legally provide financial services to state licensed marijuana businesses. A memorandum issued by the Justice Department to federal prosecutors re-iterated guidance previously given, this time to the financial industry that banks can do business with legal marijuana businesses and “may not” be prosecuted. The Treasury Department's Financial Crimes Enforcement Network (FinCEN) issued guidelines to banks that “it is possible to provide financial services" to state-licensed marijuana businesses and still be in compliance with federal anti-money laundering laws.
Notwithstanding the above federal guidelines and in addition to potential federal sanctions, regulators in the states in which we are able to conduct business may make it difficult for local banks to do business with companies considered to have engaged in cultivating and distributing cannabis. Furthermore, banks may be reluctant to do business with us because of past participation in the cannabis industry. Failure to maintain a permanent banking relationship could have a material and adverse effect on our future business operations.
We face intense competition and many of our competitors have greater resources that may enable them to compete more effectively.
The industry in which we operate is subject to intense and increasing competition. Some of our competitors have greater capital resources, facilities and diversity of product lines, which may enable them to compete more effectively in this market. Our competitors may devote their resources to developing and marketing products that will directly compete with our product lines. Due to this competition, there is no assurance that we will not encounter difficulties in obtaining revenues and market share or in the positioning of our products. There are no assurances that competition in our respective industries will not lead to reduced prices for our products. If we are unable to successfully compete with existing companies and new entrants to the market this will have a negative impact on our business and financial condition.
If we fail to protect or develop our intellectual property, our business could be adversely affected.
Our viability will depend, in part, on our ability to develop and maintain the proprietary aspects of our technology to distinguish our products from our competitors’ products. We will rely on patents, copyrights, trademarks, trade secrets, and confidentiality provisions to establish and protect our intellectual property.
Any infringement or misappropriation of our intellectual property could damage its value and limit our ability to compete. We may have to engage in litigation to protect the rights to our intellectual property, which could result in significant litigation costs and require a significant amount of our time. In addition, our ability to enforce and protect our intellectual property rights may be limited in certain countries outside the United States, which could make it easier for competitors to capture market position in such countries by utilizing technologies that are similar to those developed or licensed by us.
Competitors may also harm our sales by designing products that mirror the capabilities of our products or technology without infringing on our intellectual property rights. If we do not obtain sufficient protection for our intellectual property, or if we are unable to effectively enforce our intellectual property rights, our competitiveness could be impaired, which would limit our growth and future revenue.
We may also find it necessary to bring infringement or other actions against third parties to seek to protect our intellectual property rights. Litigation of this nature, even if successful, is often expensive and time-consuming to prosecute and there can be no assurance that we will have the financial or other resources to enforce our rights or be able to enforce our rights or prevent other parties from developing similar technology or designing around our intellectual property.
Although we believe that our intellectual property does not and will not infringe upon the patents or violate the proprietary rights of others, it is possible such infringement or violation has occurred or may occur, which could have a material adverse effect on our business.
We are not aware of any infringement by us of any person’s or entity’s intellectual property rights. In the event that products we sell are deemed to infringe upon the patents or proprietary rights of others, we could be required to modify our products or obtain a license for the manufacture and/or sale of such products or cease selling such products. In such event, there can be no assurance that we would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon our business.
There can be no assurance that we will have the financial or other resources necessary to enforce or defend a patent infringement or proprietary rights violation action. If our products or proposed products are deemed to infringe or likely to infringe upon the patents or proprietary rights of others, we could be subject to injunctive relief and, under certain circumstances, become liable for damages, which could also have a material adverse effect on our business and our financial condition.
Our trade secrets may be difficult to protect.
Our success depends upon the skills, knowledge, and experience of our scientific and technical personnel, our consultants and advisors, as well as our licensors and contractors. Because we operate in a highly competitive industry, we rely in part on trade secrets to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We enter into confidentiality or non-disclosure agreements with our corporate partners, employees, consultants, outside scientific collaborators, developers, and other advisors. These agreements generally require that the receiving party keep confidential and not disclose confidential information developed by the receiving party or made known to the receiving party by us during the course of the receiving party’s relationship with us. These agreements also generally provide that inventions conceived by the receiving party in the course of rendering services to us will be our exclusive property, and we enter into assignment agreements to perfect our rights.
These confidentiality, inventions and assignment agreements may be breached and may not effectively assign intellectual property rights to us. Our trade secrets also could be independently discovered by competitors, in which case we would not be able to prevent the use of such trade secrets by our competitors. The enforcement of a claim alleging that a party illegally obtained and was using our trade secrets could be difficult, expensive and time consuming and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. The failure to obtain or maintain meaningful trade secret protection could adversely affect our competitive position.
Our future success depends on our key executive officers and our ability to attract, retain, and motivate qualified personnel.
Our future success largely depends upon the continued services of our executive officers and management team. If one or more of our executive officers are unable or unwilling to continue in their present positions, we may not be able to replace them readily, if at all. Additionally, we may incur additional expenses to recruit and retain new executive officers. If any of our executive officers joins a competitor or forms a competing company, we may lose some of our potential customers. Finally, we do not maintain “key person” life insurance on any of our executive officers. Because of these factors, the loss of the services of any of these key persons could adversely affect our business, financial condition, and results of operations, and thereby an investment in our stock.
Our continuing ability to attract and retain highly qualified personnel will also be critical to our success because we will need to hire and retain additional personnel as our business grows. There can be no assurance that we will be able to attract or retain highly qualified personnel. We face significant competition for skilled personnel in our industry. This competition may make it more difficult and expensive to attract, hire, and retain qualified managers and employees. Because of these factors, we may not be able to effectively manage or grow our business, which could adversely affect our financial condition or business. As a result, the value of your investment could be significantly reduced or completely lost.
We may not be able to effectively manage our growth or improve our operational, financial, and management information systems, which would impair our results of operations.
In the near term, we intend to expand the scope of our operations activities significantly. If we are successful in executing our business plan, we will experience growth in our business that could place a significant strain on our business operations, finances, management and other resources. The factors that may place strain on our resources include, but are not limited to, the following:
| |
●
|
The need for continued development of our financial and information management systems;
|
| |
●
|
The need to manage strategic relationships and agreements with manufacturers, customers and partners; and
|
| |
●
|
Difficulties in hiring and retaining skilled management, technical, and other personnel necessary to support and manage our business.
|
Additionally, our strategy could produce a period of rapid growth that may impose a significant burden on our administrative and operational resources. Our ability to effectively manage growth will require us to substantially expand the capabilities of our administrative and operational resources and to attract, train, manage, and retain qualified management and other personnel. There can be no assurance that we will be successful in recruiting and retaining new employees or retaining existing employees.
We cannot provide assurances that our management will be able to manage this growth effectively. Our failure to successfully manage growth could result in our sales not increasing commensurately with capital investments or otherwise materially adversely affecting our business, financial condition, or results of operations.
If we are unable to continually innovate and increase efficiencies, our ability to attract new customers may be adversely affected.
In the area of innovation, we must be able to develop new technologies and products that appeal to our customers. This depends, in part, on the technological and creative skills of our personnel and on our ability to protect our intellectual property rights. We may not be successful in the development, introduction, marketing, and sourcing of new technologies or innovations, that satisfy customer needs, achieve market acceptance, or generate satisfactory financial returns.
Litigation may adversely affect our business, financial condition, and results of operations.
From time to time in the normal course of our business operations, we may become subject to litigation that may result in liability material to our financial statements as a whole or may negatively affect our operating results if changes to our business operations are required. The cost to defend such litigation may be significant and may require a diversion of our resources. There also may be adverse publicity associated with litigation that could negatively affect customer perception of our business, regardless of whether the allegations are valid or whether we are ultimately found liable. Insurance may not be available at all or in sufficient amounts to cover any liabilities with respect to these or other matters. A judgment or other liability in excess of our insurance coverage for any claims could adversely affect our business and the results of our operations.
If we fail to implement and maintain proper and effective internal controls and disclosure controls and procedures pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, our ability to produce accurate and timely financial statements and public reports could be impaired, which could adversely affect our operating results, our ability to operate our business, and investors’ views of us.
As of March 31, 2022, management assessed the effectiveness of our internal controls over financial reporting. Management concluded, as of the fiscal year ended March 31, 2022, that our internal controls and procedures were not effective to detect the inappropriate application of U.S. GAAP rules. Management concluded that our internal controls were adversely affected by deficiencies in the design or operation of our internal controls, which management considered to be material weakness; specifically, no member of our board of directors qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act.
The failure to implement and maintain proper and effective internal controls and disclosure controls could result in material weaknesses in our financial reporting such as errors in our financial statements and in the accompanying footnote disclosures that could require restatements. Investors may lose confidence in our reported financial information and disclosure, which could negatively impact our stock price.
We do not expect that our internal controls over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. Over time, controls may become inadequate because changes in conditions or deterioration in the degree of compliance with policies or procedures may occur. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Our insurance coverage may be inadequate to cover all significant risk exposures; because we have operated in the cannabis industry, we have a difficult time obtaining the various insurances that are desired to operate our business, which may expose us to additional risk and financial liabilities.
We will be exposed to liabilities that are unique to the products we provide. While we intend to maintain insurance for certain risks, the amount of our insurance coverage may not be adequate to cover all claims or liabilities, and we may be forced to bear substantial costs resulting from risks and uncertainties of our business. It is also not possible to obtain insurance to protect against all operational risks and liabilities. The failure to obtain adequate insurance coverage on terms favorable to us, or at all, could have a material adverse effect on our business, financial condition and results of operations. We do not have any business interruption insurance. Any business disruption or natural disaster could result in substantial costs and diversion of resources. We do not have directors' and officers' liability insurance in place and could incur substantial costs to indemnify our directors and officers against any claims that may arise. We currently have insurance coverage in place for workers' compensation.
Insurance that is otherwise readily available is more difficult for us to find, and more expensive, because we have engaged in the cannabis industry. There are no guarantees that we will be able to find such insurances in the future, or that the cost will be affordable to us. If we are forced to go without such insurances, it may prevent us from entering into certain business sectors, may inhibit our growth, and may expose us to additional risk and financial liabilities.
RISKS RELATED TO AN INVESTMENT IN OUR SECURITIES
We expect to experience volatility in the price of our common stock, which could negatively affect stockholders’ investments.
The trading price of our common stock may be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. The stock market in general has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of companies with securities traded in those markets. Broad market and industry factors may seriously affect the market price of companies’ stock, including ours, regardless of actual operating performance. All of these factors could adversely affect your ability to sell your shares of common stock or, if you are able to sell your shares, to sell your shares at a price that you determine to be fair or favorable.
Our common stock is categorized as “penny stock,” which may make it more difficult for investors to sell their shares of common stock due to suitability requirements.
Our common stock is categorized as “penny stock”. The Securities and Exchange Commission (the “SEC”) has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exceptions. The price of our common stock is significantly less than $5.00 per share and is therefore considered “penny stock.” This designation imposes additional sales practice requirements on broker-dealers who sell to persons other than established customers and accredited investors. The penny stock rules require a broker-dealer buying our securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase the securities given the increased risks generally inherent in penny stocks. These rules may restrict the ability and/or willingness of brokers or dealers to buy or sell our common stock, either directly or on behalf of their clients, may discourage potential stockholders from purchasing our common stock, or may adversely affect the ability of stockholders to sell their shares.
Financial Industry Regulatory Authority (“FINRA”) sales practice requirements may also limit a stockholder’s ability to buy and sell our common stock, which could depress the price of our common stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require a broker-dealer to have reasonable grounds for believing that the investment is suitable for that customer before recommending an investment to a customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative, low-priced securities will not be suitable for at least some customers. Thus, the FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our shares of common stock, have an adverse effect on the market for our shares of common stock, and thereby depress our price per share of common stock.
The elimination of monetary liability against our directors, officers, and employees under Nevada law and the existence of indemnification rights for or obligations to our directors, officers, and employees may result in substantial expenditures by us and may discourage lawsuits against our directors, officers, and employees.
Our Articles of Incorporation contain a provision permitting us to eliminate the personal liability of our directors to us and our stockholders for damages for the breach of a fiduciary duty as a director or officer to the extent provided by Nevada law. We may also have contractual indemnification obligations under any future employment agreements with our officers. The foregoing indemnification obligations could result in us incurring substantial expenditures to cover the cost of settlement or damage awards against directors and officers, which we may be unable to recoup. These provisions and the resulting costs may also discourage us from bringing a lawsuit against directors and officers for breaches of their fiduciary duties and may similarly discourage the filing of derivative litigation by our stockholders against our directors and officers even though such actions, if successful, might otherwise benefit us and our stockholders. We do not have directors' and officers' liability insurance in place and could incur substantial costs to indemnify our directors and officers against any claims that may arise.
We may issue additional shares of common stock in the future, which could cause significant dilution to all stockholders.
Our Articles of Incorporation authorize the issuance of up to 600,000,000 shares with a par value of $0.0001 per share. As of June 30, 2022, we had 329,204,224 shares of common stock outstanding. However, we require additional capital and will likely issue additional shares of Common Stock in the future in connection with one or more financings or an acquisition. Such issuances may not require the approval of our stockholders. In addition, certain of our outstanding rights to purchase additional shares of common stock or securities convertible into our common stock are subject to full-ratchet anti-dilution protection, which could result in the right to purchase significantly more shares of common stock being issued or a reduction in the purchase price for any such shares or both. Any issuance of additional shares of our common stock, or equity securities convertible into our common stock, including but not limited to, warrants, and options, will dilute the percentage ownership interest of all stockholders, may dilute the book value per share of our common stock, and may negatively impact the market price of our common stock.
Because we do not intend to pay any cash dividends on our common stock, our stockholders will not be able to receive a return on their shares unless they sell them.
We intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. Declaring and paying future dividends, if any, will be determined by our Board, based upon earnings, financial condition, capital resources, capital requirements, restrictions in our Articles of Incorporation, contractual restrictions, and such other factors as our Board deems relevant. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell them. There is no assurance that stockholders will be able to sell shares when desired.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
ITEM 2. PROPERTY
The company does not own or lease any physical premises. Our executive officers and employees work remotely in a "virtual office" setting, and our mailing address is 3550 W. Teco Avenue, Las Vegas, NV 89118.
ITEM 3. LEGAL PROCEEDINGS
On April 11, 2022, the Company was served notice of a lawsuit filed in the Eighth Judicial District Court in Clark County, Nevada by an individual who alleges he was shot by a security guard at the Teco Facility in May of 2020. The alleged incident occurred after the claimant broke into the Teco Facility during closing hours. GB Sciences, Inc. and its former subsidiaries GB Sciences Nevada, LLC and GB Sciences Las Vegas, LLC, along with the security provider, Protective Force International, Inc., were named as defendants in the lawsuit. The Company holds a certificate of insurance with the insurer for Protective Force International and believes it may have coverage under that policy in the event the Company is found liable for damages, however, the Company denies any liability and intends to vigorously defend the lawsuit. We are unable to make any determination at this time as to the likelihood or amount of damages.
On April 22, 2020, the Company failed to repay any of the outstanding balance of the Convertible Promissory Note Payable to Iliad Research and Trading, L.P., resulting in a default. On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594. The Company's obligation to Iliad was satisfied in full on December 16, 2020 upon payment of $3,006,015 pursuant to the Judgment Settlement Agreement.
On April 22, 2020, the Company was served notice of a lawsuit filed in the Eighth Judicial District Court in Clark County, Nevada by a contractor who had been hired to perform architectural and design services. The lawsuit demanded payment of $73,050 for the services provided. On September 17, 2020, the Company entered into a Mutual Compromise, Settlement, and Release Agreement with the contractor and made payment of $25,000 in full satisfaction of the alleged debt and reduced the cost of the related fixed asset by $48,050.
We are currently not involved in any other material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not Applicable
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
GB Sciences, Inc.’s common stock is quoted on the OTCQB under the symbol "GBLX".
For the periods indicated, the following table sets forth the high and low per share intra-day sales prices per share of common stock. These prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions.
|
Fiscal Year 2022
|
|
High ($)
|
|
|
Low ($)
|
|
|
Fourth Quarter
|
|
$ |
0.04 |
|
|
$ |
0.02 |
|
|
Third Quarter
|
|
|
0.04 |
|
|
|
0.02 |
|
|
Second Quarter
|
|
|
0.06 |
|
|
|
0.03 |
|
|
First Quarter
|
|
|
0.07 |
|
|
|
0.04 |
|
| |
|
|
|
|
|
|
|
|
|
Fiscal Year 2021
|
|
|
|
|
|
|
|
|
|
Fourth Quarter
|
|
$ |
0.04 |
|
|
$ |
0.13 |
|
|
Third Quarter
|
|
|
0.06 |
|
|
|
0.03 |
|
|
Second Quarter
|
|
|
0.03 |
|
|
|
0.03 |
|
|
First Quarter
|
|
|
0.04 |
|
|
|
0.03 |
|
As of June 30, 2022, there were 183 holders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of shareholders, we are unable to estimate the total number of beneficial holders.
Dividend Policy
Cash dividends have never been declared or paid on common stock and dividends are not anticipated on common stock in the foreseeable future. Future earnings, if any, will be retained to finance the expansion business and for general corporate purposes. There is no assurance we will pay dividends in the future. Future dividend policy is within the discretion of the board of directors and will depend upon various factors, including results of operations, financial condition, capital requirements and investment opportunities.
Recent Sales of Unregistered Securities
On May 9, 2022 the Company entered into a Placement Agent's Agreement with its brokers for the private placement of up to $565,000 in units at a price of $0.03 per unit. For each unit purchased, the investor will receive one share of the Company's common stock and one warrant to purchase one share of the Company's common stock at a price of $0.10 for a period of five years. As of the date of this report, the Company has received $125,000 under the private placement and issued 4,166,667 shares of its common stock and 4,166,667 warrants to purchase one share of the Company's common stock at $0.10 for five years.
ITEM 6. SELECTED FINANCIAL DATA
As a "smaller reporting company" as defined by Item 10 of Regulation S-K, the Company is not required to provide information required by this Item.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion of the plan of operation, financial condition and results of operations should be read in conjunction with the Company’s financial statements, and notes thereto, included elsewhere herein. This discussion contains forward-looking statements that involve risks and uncertainties. Actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors including, but not limited to, those discussed in this Annual Report.
Executive Overview
GB Sciences, Inc. (“the Company”, “GB Sciences”, “we”, “us”, or “our”) is a plant-inspired, biopharmaceutical research and development company creating patented, disease-targeted formulations of cannabis- and other plant-inspired therapeutic mixtures for the prescription drug market through its wholly owned Canadian subsidiary, GbS Global Biopharma, Inc. (“GBSGB”).
Through GBSGB, the Company is engaged in the research and development of plant-inspired medicines, with virtual operations in North America and Europe. GBSGB’s assets include a portfolio of intellectual property containing both proprietary plant-inspired formulations and our AI-enabled drug discovery platform, as well as critical research contracts and key supplier arrangements. The Company’s intellectual property portfolio, which is held by GBSGB, contains six issued U.S. and three issued foreign patents, as well as 18 U.S. and 49 foreign patent-pending applications. On October 14th, 2021, we filed the nonprovisional USPTO patent application entitled “METHOD AND SYSTEMS FOR PHYTOMEDICINE ANALYTICS FOR RESEARCH OPTIMIZATION AT SCALE" to further protect aspects of our proprietary drug discovery engine, PhAROS™, which stands for Phytomedical Analytics for Research Optimization at Scale. On March 1st, 2022, the Company’s newest patent was issued by the U.S. Patent and Trademark Office (USPTO) for a cannabinoid-containing mixture designed to treat cardiac hypertrophy, often present in advanced heart disease. The Company’s newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor associated hearing loss and urinary cystitis.
GBSGB’s intellectual property covers a range of over 65 medical conditions, from which five drug development programs are in the preclinical stage of drug development including our formulations for Parkinson’s disease ("PD"), chronic pain, COVID-related cytokine release syndrome, depression/anxiety, and cardiovascular therapeutic programs. The Company’s primary focus is on preparing its lead program for the treatment of the motor symptoms of Parkinson's disease for a first-in-human clinical trial. Depending on the results of ongoing preclinical studies, the Company intends to move forward with clinical trials for its chronic pain and COVID-related cytokine release syndrome therapies after PD. The Company’s formulations for chronic pain, anxiety, and depression are currently in preclinical animal studies with researchers at the National Research Council Canada. The Company also recently received positive preclinical proof-of-concept data supporting its complex mixtures for the treatment of Cytokine Release Syndrome related to COVID-19, and its lead candidates will be optimized based on late-stage preclinical studies at Michigan State University. Proof-of-concept studies in animals that support our heart disease formulations have been successfully completed at the University of Hawaii. The Company runs a lean drug development program through GBSGB and takes effort to minimize expenses, including personnel, overhead, and fixed capital expenses through strategic partnerships with Universities and Contract Research Organizations (“CROs”). Our productive research and development network includes distinguished universities, hospitals, and Contract Research Organizations.
Recent Developments
Divestiture of Nevada Cannabis Operations
On March 24, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC. Pursuant to the Teco MIPA, the Company agreed to sell 100% of its membership interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC (the "Teco Subsidiaries") for approximately $8 million, which amount includes a cash payment at closing, the extinguishment and/or repayments of certain liabilities owed to the purchaser and affiliates of the purchaser, and an 8% promissory note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with 483 Management, LLC. Pursuant to the Nopah MIPA, the Company agreed to sell its 100% membership interest in GB Sciences Nopah, LLC ("Nopah"), which holds a Nevada medical marijuana cultivation certificate. As consideration, the Company would receive $312,315 in consideration in the form of a $237,668 reduction to the outstanding principal and accrued interest balances of the 0% Note payable dated October 23, 2017, and extinguishment of accounts payable of $74,647, which were owed to an affiliate of the purchaser.
The closing of the Teco and Nopah sales was contingent upon the successful transfer of the Nevada cultivation and production licenses. On December 14, 2021, the Company received approval from the Nevada Cannabis Compliance Board for the transfer of cannabis cultivation and extraction licenses held by its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC (the "Nevada Subsidiaries"). Consequently, all conditions to closing the sales of the 100% membership interests in the Nevada Subsidiaries were satisfied, and the transactions formally closed on December 31, 2021. After the closing date, the Company retains no ownership interest in the Nevada Subsidiaries.
As consideration for the membership interests, the Company received cash payments of $1,648,772 (including $400,000 in advance payments received during the nine months ended December 31, 2021), the extinguishment of debt and current liabilities owed to affiliates of the purchaser of $3,462,854, and a $3,025,000 8% note receivable.
Intellectual Property Portfolio
On March 1, 2022, the USPTO issued U.S. Patent No. 11,260,044 entitled TRPV1 ACTIVATION-MODULATING COMPLEX MIXTURES OF CANNABINOIDS AND/OR TERPENES. The patent covers intellectual property for a cannabinoid-containing mixture designed to treat cardiac hypertrophy, often present in advanced heart disease. The newly issued patent also covers the use of these receptor-targeted formulations for the treatment of TRPV1-receptor-associated hearing loss and urinary cystitis.
In October of 2021, GBSGB began its first preclinical animal trial of non-cannabis-based formulations that were discovered and pre-validated using our PhAROS™ drug discovery platform. The National Research Council of Canada (“NRC”) are testing the Company’s proprietary, psychotropic plant-based formulas for the treatment of depression and anxiety. For these novel psychotropic drug candidates, the Company used the PhAROS™ platform to identify new ingredients to improve upon an initial formulation for anxiety based on traditional medicine. The original plant mixture was derived from the kava plant, but some elements of kava are thought to cause liver toxicity. PhAROS™ identified ingredients from the Piper plant family as a substitute for the functionality of the ingredients in question without the potentially adverse safety profiles of those original ingredients. The Piper plant family includes pepper plants that are used worldwide in traditional medicines.
In late summer of 2021, the Company received positive proof-of-concept data from a human immune cell model supporting the efficacy of their proprietary MEM designed for the suppression of COVID-related, cytokine release syndromes (CRS) while preserving key anti-viral immune responses. Based on this new positive proof-of-concept data, GBSGB converted their provisional patent application entitled, “CANNABINOID-CONTAINING COMPLEX MIXTURES FOR THE TREATMENT OF CYTOKINE RELEASE SYNDROME WHILE PRESERVING KEY ANTI-VIRAL IMMUNE REACTIONS” to a nonprovisional patent application on August 18, 2021.
On October 14th, 2020, GB Sciences filed a provisional patent application to protect its machine learning algorithm for the prediction of novel active ingredients from traditional, plant-based medical preparations. The new provisional patent application is entitled “In Silico Meta-Pharmacopeia Assembly from Non-Western Medical Systems Using Advanced Data Analytic Techniques to Identify and Design Phytotherapeutic Strategies”. GBSGB’s proprietary data analytics tool uses in silico convergence analysis to deconvolve modes of action and predict desirable components of plant-based formulations established in traditional medical practice based on computational consensus analysis across cultures and medical systems.
On September 23rd, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting their Cannabinoid Containing Complex Mixtures (CCCMs) for the Treatment of Mast Cell Activation Syndrome (MCAS). The patent is owned by the Company’s Canadian entity, GBS Global Biopharma, Inc. MCAS is a severe immunological condition in which mast cells inappropriately and excessively release inflammatory mediators, resulting in a range of severe chronic hyperinflammatory symptoms and life-threatening anaphylaxis attacks. There is no single recommended treatment for MCAS patients. Instead, patients, with their doctor’s guidance, attempt to manage MCAS symptoms primarily by avoiding ‘triggers’ and using rescue medicines for their severe hyperinflammatory attacks. Therefore, MCAS patients need new therapeutic options to control their mast cell related symptoms, and the Company’s CCCM™ were designed to simultaneously control multiple inflammatory pathways within mast cells as a comprehensive treatment option. The application, entitled “Cannabinoid-Containing Complex Mixtures for the Treatment of Mast Cell-Associated or Basophil-Mediated Inflammatory Disorders” was originally filed on January 31, 2018 and describes CCCMs that can be used for the treatment of Crohn's disease, Inflammatory Bowel Disease (IBD), Irritable Bowel Syndrome (IBS), rheumatoid arthritis, osteoarthritis, allergic asthma, Chronic Obstructive Pulmonary Disease (COPD), psoriasis, eczema, urticarias, dermatitis, mastocytosis, or anaphylactic sting. Claims for these additional indications will be examined by the USPTO in the future. On December 8, 2020, the patent was issued as United States Patent 10,857,107.
On April 7th, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting Cannabinoid Containing Complex Mixtures ("CCCMs") for the Treatment of Parkinson’s disease (PD), which is owned by the Company’s Canadian entity, GBS Global Biopharma, Inc. On May 19, 2020, the patent was issued as United States Patent 10,653,640.
On May 12th, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting Myrcene Containing Complex Mixtures ("MCCMs") for the Treatment of Neuropathic Pain. Intellectual property rights to this application and the MCCM contained within it are owned by the Company’s Canadian entity, GBS Global Biopharma, Inc. The Company's MCCMs are protected for use in the treatment of pain related to arthritis, shingles, irritable bowel syndrome, sickle cell disease, and endometriosis. The patent was issued on July 14, 2020 as United States Patent 10,709,670.
Results of Operations
The following table sets forth certain of our Statement of Operations data from continuing operations:
| |
|
For the Years Ended
|
|
| |
|
March 31,
|
|
| |
|
2022
|
|
|
2021
|
|
| |
|
|
|
|
|
|
|
|
|
SALES REVENUE
|
|
$ |
- |
|
|
$ |
- |
|
|
COST OF GOODS SOLD
|
|
|
- |
|
|
|
- |
|
|
GROSS PROFIT (LOSS)
|
|
|
- |
|
|
|
- |
|
|
GENERAL AND ADMINISTRATIVE EXPENSES
|
|
|
1,868,734 |
|
|
|
2,001,617 |
|
|
LOSS FROM OPERATIONS
|
|
|
(1,868,734 |
) |
|
|
(2,001,617 |
) |
|
OTHER INCOME (EXPENSE)
|
|
|
|
|
|
|
|
|
|
Gain on extinguishment
|
|
|
22,405 |
|
|
|
467,872 |
|
|
Gain on settlement of accounts payable
|
|
|
- |
|
|
|
422,414 |
|
|
Gain on deconsolidation
|
|
|
5,206,208 |
|
|
|
- |
|
|
Interest expense
|
|
|
(474,768 |
) |
|
|
(1,285,460 |
) |
|
Loss on modification of line of credit
|
|
|
- |
|
|
|
(650,000 |
) |
|
Loss on impairment of note receivable
|
|
|
(3,025,000 |
) |
|
|
- |
|
|
Debt default penalty
|
|
|
- |
|
|
|
(286,059 |
) |
|
Loss on disposal
|
|
|
(15,639 |
) |
|
|
- |
|
|
Other income
|
|
|
9,000 |
|
|
|
- |
|
|
Total other income/(expense)
|
|
|
1,722,206 |
|
|
|
(1,331,233 |
) |
|
LOSS BEFORE INCOME TAXES
|
|
|
(146,528 |
) |
|
|
(3,332,850 |
) |
|
Income tax expense
|
|
|
- |
|
|
|
- |
|
|
LOSS FROM CONTINUING OPERATIONS
|
|
|
(146,528 |
) |
|
|
(3,332,850 |
) |
|
Net loss from discontinued operations
|
|
|
(384,345 |
) |
|
|
(392,177 |
) |
|
NET LOSS
|
|
$ |
(530,873 |
) |
|
$ |
(3,725,027 |
) |
General and Administrative Expenses. General and administrative expense decreased $132,883 to $1,868,734 for the year ended March 31, 2022 as compared to $2,001,617 for the same period last year. The decrease is attributable primarily to reduced compensation paid to executives and board members. The Company is continuing its efforts to maintain administrative costs at a minimum and to make the best use of its limited resources in advancing research & development of the Company's intellectual property portfolio.
Gain on extinguishment. The Company recorded a gain on extinguishment of $22,405 In connection with the Second Promissory Note Modification agreement with 483 Management, LLC. The gain on extinguishment of $467,872 for the year ended March 31, 2021 relates to the Judgment Settlement Agreement with Iliad Research & Trading, L.P. In order to settle the lawsuit brought by Iliad, the Company paid $3,006,014 in full satisfaction of the principal and accrued interest balance of $3,473,886.
Gain on settlement of accounts payable. During the year ended March 31, 2021, the Company settled accounts payable at a discount in exchange for immediate lump sum payments and recorded income from cancellation of accounts payable totaling $422,414.
Gain on deconsolidation. The Company recorded a gain on deconsolidation of $5,206,208 related to the sale of its membership interests in the Nevada Subsidiaries during the year ended March 31, 2022.
Interest Expense. Interest for the year ended March 31, 2022 was $474,768, compared to $1,285,460 for the year ended March 31, 2021. The decrease is attributable to substantially less interest-bearing debt outstanding during the year ended March 31, 2022, as the result of the payoff of the note payable to Iliad Research and Trading, L.P. in December 2020. In addition, notes with balances totaling $2.1 million at December 31, 2021 stopped accruing interest beginning December 1, 2020, as the result of the Omnibus Amendment to the agreements surrounding the sale of the Company's Nevada Subsidiaries.
Loss on modification of line of credit. As a result of the Omnibus Amendment dated December 29, 2020, the Company accrued a modification expense of $650,000. The amount represents an increase to the note balance to a total of $1,025,000, which will reduce the note receivable issued to the Company at the closing of the sale of the Teco Facility.
Loss on impairment of note receivable. During the year ended March 31, 2022, the Company recorded an impairment charge of $3,025,000 related to the $3,025,000 note receivable from AJE Management LLC received from the sale of the Nevada Subsidiaries. The impairment charge was deemed necessary due to the note maker's failure to pay the interest payment due on April 1, 2022 and anticipated failure to make the payment due July 1, 2022, under a contractual provision that allows the deferral of payments to the next quarterly payment date in the event that cash flow from the Teco Facility is insufficient to make that quarter's payment.
Debt default penalty. The Company recorded a default penalty of $286,059 for the year ended March 31, 2021 related to the Company's failure to timely repay the principal and interest owed under the note payable to Iliad Research and Trading, L.P. on April 1, 2020. The penalty is 10% of the principal and accrued interest balances outstanding at the time of default.
Liquidity and Capital Resources
Current Liquidity
The Company will need additional capital to implement our strategies. There is no assurance that it will be able to raise the amount of capital needed for future growth plans. Even if financing is available, it may not be on terms that are acceptable. If unable to raise the necessary capital at the times required, the Company may have to materially change the business plan, including delaying implementation of aspects of the business plan or curtailing or abandoning the business plan. The Company represents a speculative investment and investors may lose all of their investment. In order to be able to achieve the strategic goals, the Company needs to further expand its business and financing activities. Based upon the cash position, it is necessary to raise additional capital by the end of the next quarter in order to continue to fund current operations. These factors raise substantial doubt about the ability to continue as a going concern. The Company is pursuing several alternatives to address this situation, including the raising of additional funding through equity or debt financings. In order to finance existing operations and pay current liabilities over the next twelve months, the Company will need to raise additional capital. No assurance can be given that the Company will be able to operate profitably on a consistent basis, or at all, in the future.
The principal sources of liquidity to date have been cash generated from sales of debt and equity securities.
At March 31, 2022, the Company had a cash balance of $233,893, other current assets excluding cash were $93,933, and our working capital deficit was $3,607,638. Current liabilities were $3,935,464, which consisted principally of $987,565 in notes and convertible notes payable, $1,657,008 in accounts payable, $394,396 in accrued liabilities, and $896,495 income taxes payable from discontinued operations. At March 31, 2021, the Company had a cash balance of $793,040, other current assets excluding cash were $256,251, current assets from discontinued operations were $2,494,564, and our working capital deficit was $5,054,593, net of working capital of $1,201,488 from discontinued operations. Current liabilities were $8,598,448, which consisted principally of $3,594,804 in notes and convertible notes payable, $1,412,459 in accounts payable, $1,451,687 in accrued liabilities, $84,913 of indebtedness to related parties, $761,509 of income taxes from discontinued operations, and $1,293,076 current liabilities from discontinued operations.
Sources and Uses of Cash
Operating Activities
Cash used in operations was $1,866,154 including $87,772 used in discontinued operations for the year ended March 31, 2022, compared to cash used of $2,185,220 including $118,644 used in discontinued operations for the year ended March 31, 2021. We anticipate that cash flows from operations will be insufficient to fund business operations for the next twelve-month period. Accordingly, we will have to generate additional liquidity or cash flow to fund our current and anticipated operations. This will likely require the sale of additional common stock or other securities. There is no assurance that we will be able to realize any significant proceeds from such sales, if at all.
Investing Activities
Cash flows provided by investing activities were $1,450,339, including $1,567 provided by discontinued operations for the year ended March 31, 2022, compared to cash provided by investing activities of $4,655,519, net of $103,729 used in discontinued operations for the year ended March 31, 2021. Cash provided by investing activities of continuing operations for the year ended March 31, 2022 consists of $1,648,772 proceeds from the sale of the Nevada Subsidiaries, offset by $200,000 paid to our attorneys to file patent applications. Cash provided by investing activities for the prior year relates to $5,051,923 proceeds received from the Wellcana note receivable, offset by $292,675 used to pay our attorneys and researchers to draft and file patent applications. Cash used in prior year investing activities was used to pay our attorneys and researchers to draft and file patent applications.
Financing Activities
During the year ended March 31, 2022 cash used in financing activities was $495,925 including $103,387 used in discontinued operations. For the year ended March 31, 2021, cash used in financing activities was $1,476,432, including $161,768 used in discontinued operations.
Cash used in financing activities of continuing operations for the year ended March 31, 2022 consisted of $575,000 used for principal payments on notes payable and $6,266 paid for brokerage fees from warrant exercises, offset by $138,728 proceeds from warrant exercises and $50,000 proceeds from a convertible note payable.
Cash used in financing activities of continuing operations for the year ended March 31, 2021 consisted of $3,156,014 used for principal payments of the note payable to Iliad Research and Trading, L.P., principal repayment of a related party note in the amount of $151,923, brokerage fees of $107,373 for warrant solicitations, and debt issuance fees of $74,750, offset by $1,075,396 proceeds from warrant exercises, $725,000 in proceeds from the issuance of convertible notes, and $375,000 in proceeds from a line of credit.
Notes and Convertible Notes Payable
0% Note Payable dated October 23, 2017
On October 23, 2017, the Company amended the existing Nevada Medical Marijuana Production License Agreement (“Amended Production License Agreement”). Per the terms of the Amended Production License Agreement, GB Sciences purchased the remaining percentage of the production license resulting in the 100% ownership of the license. GB Sciences also received 100% ownership of the cultivation license included in the original Nevada Medical Marijuana Production License Agreement. In exchange, GB Sciences made one-time payment of $500,000 and issued a 0% Promissory Note in the amount of $700,000 payable in equal monthly payments over a three-year period commencing on January 1, 2018. The present value of the note was $521,067 on the date of its issuance based on an imputed interest rate of 20.3% and the Company recorded a discount on notes payable of $178,933 related to the difference between the face value and present value of the note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") for the sale of its interest in GB Sciences Nopah, LLC. The Nopah sale was closed December 31, 2021 after successful transfer of the Nevada Medical Marijuana Cultivation Facility Registration Certificate on December 14, 2021. At close, the principal balance of the note was reduced from $369,445 to $190,272 and accounts payable totaling $74,647 to an affiliate of the purchaser were extinguished.
On March 4, 2022, the Company entered into the Second Promissory Note Modification Agreement, which reduced the total outstanding balance of principal and interest from $201,532 (at the time of the agreement) to $179,127 and modified the terms of the note to provide that the Company would make an immediate payment of $75,000, with $5,000 monthly payments thereafter until the note is repaid in full. The modification also provided that the note would bear interest at 8.0% per annum.
We evaluated the modification under the guidance in ASC 470-50 and determined that the modification represents an extinguishment because the change in the fair value of the note exceeded 10% of the carrying value of the note immediately prior to the modification. As a result, the Company recorded a gain on extinguishment of $22,405 equal to the change in the carrying value of the note resulting from the modification.
The Company made a $75,000 payment pursuant to the terms of the modification on March 4, 2022. At March 31, 2022, the outstanding balance of the note was $104,127 and accrued interest was $616.
8% Line of Credit dated July 24, 2020
On July 24, 2020, the Company entered into the Loan Agreement, 8% Secured Promissory Note, and Security Agreement (together, the "July 24 Note") with AJE Management, LLC, which established a revolving loan of up to $500,000 that the Company may draw on from time to time. The loan was collateralized by the Teco Facility, subject to the pre-existing lien held by CSW Ventures, L.P. in connection with the 8% Senior Secured Convertible Promissory Note dated February 28, 2019. Contemporaneously with the Loan Agreement, the Company and AJE Management entered into the Amendment to the Membership Interest Purchase Agreement with AJE Management. The amendment provides that any balances outstanding under the July 24 Note at the time of the close of the sale of the Teco Facility would be forgiven in exchange for a reduction to the $4,000,000 note receivable that the Company will receive as consideration for the sale of the Teco Facility. The reduction to the note receivable would be equal to 3 times the balance outstanding under the July 24 Note on the date of the close of the sale of the Teco Facility. The balance outstanding under the note plus accrued interest were permitted to be repaid at any time prior to the close of the sale of the Teco facility.
On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility. The Omnibus Amendment reduced the amount of the note receivable that the Company was to receive from the sale of the Teco Facility by $975,000 (three times $325,000 in advances made under the July 24 Note) to $3,025,000. Any advances made to the Company under the July 24 Note in excess of $325,000 were to reduce the amount of cash received upon close of the sale of Teco one-for-one, i.e., such advances would be considered advance payments of the $4,000,000 cash purchase price. No interest would accrue after November 30, 2020. The Company also agreed that it would not repay the balances outstanding under the July 24 Note prior to the closing of the Teco sale. As a result of the Omnibus Amendment, the Company accrued a modification expense of $650,000 during the year ended March 31, 2021. Prior to December 31, 2021, the Company received $50,000 in additional advances above $325,000 during the fiscal year ended March 31, 2021, bringing the total balance to $1,025,000, and accrued interest was $12,510. Upon close of the Teco sale on December 31, 2021, the note and accrued interest balances were forgiven and the Company has no further obligations related to the line of credit.
March 2017 and July 2017 Convertible Note Offerings
In March 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.60 per share for the period of three years. Between March 2017 and May 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $2,000,000. The Notes are payable within three years of issuance and are convertible into 8,000,000 shares of the Company’s common stock. The Company also issued 8,000,000 common stock warrants to the Noteholders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $1,933,693, which included $904,690 related to the relative fair value of beneficial conversion features and $1,029,003 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
In July 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.65 per share for the period of three years. Between July 2017 and December 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $7,201,000. The Notes are payable within three years of issuance and are convertible into 28,804,000 shares of the Company’s common stock. The Company also issued 28,804,000 common stock warrants to the Note holders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $7,092,796, which included $3,142,605 related to the relative fair value of beneficial conversion features and $3,950,191 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
All notes from the March and July 2017 offerings have passed their maturity dates. During the year ended March 31, 2022, the Company agreed to extensions with the holders of a total of $197,000 of the $1,257,000 that remains outstanding. For the $197,000 of extended notes, the Company agreed to reduce the conversion price to $0.10 per share and issued a total of 788,000 additional warrants to the holders of the notes with a term of three years and an exercise price of $0.10 per share. In exchange, the maturity date of the notes was extended to September 30, 2023. Using the Black-Scholes model, the Company valued the warrants at $13,396 and the change in the fair value of the conversion feature at $33,490. Because the change in the fair value of the conversion feature exceeded 10% of the carrying amount of the notes, the Company accounted for the modification of the notes as an extinguishment and recorded a discount on the new convertible notes of $46,886 related to the fair value of the new warrants issued and the change in the fair value of the conversion feature. The Company recorded interest expense of $26,127 on the new notes during the year ended March 31, 2022, of which $14,306 represented amortization of the note discounts. Accrued interest on the $197,000 extended notes is $56,152 at March 31, 2022, which includes $38,438 accrued prior to the extinguishments.
Three convertible notes totaling $1,060,000 held by the same investor are past maturity and are currently in default. On January 20, 2022, the Company repaid $500,000 of the principal balances owed to the investor, and one convertible note in the amount of $560,000 remains outstanding plus accrued interest on all three notes totaling $286,119. The Company intends to negotiate the terms of an extension of the remaining note and accrued interest with the note holder. The notes do not provide for a default penalty or penalty interest rate. Interest expense for the notes was $57,846 during the year ended March 31, 2022.
8% Senior Secured Convertible Promissory Note dated February 28, 2019
On February 28, 2019, the Company issued a $1,500,000 8% Senior Secured Convertible Promissory Note and entered into the Note Purchase Agreement and Security Agreement with CSW Ventures, L.P. (together, “CSW Note”). The note matured on August 28, 2020, and was convertible at any time until maturity into 8,823,529 shares of the Company’s common stock at $0.17 per share. Collateral pledged as security for the note includes all of the Company’s 100% membership interests in GB Sciences, Nevada, LLC and GB Sciences Las Vegas, LLC, which together represent substantially all of the Company’s cannabis cultivation and production operations and assets located at the Teco facility in Las Vegas, Nevada.
On December 29, 2020, the Company entered into the Omnibus Amendment, and the note holder agreed to cease interest accrual on the CSW Note after November 30, 2020. After conversions, the remaining principal balance and carrying amount of the note was $1,111,863 as of December 31, 2021. Accrued interest was $144,994.
Upon close of the Teco sale on December 31, 2021, the note and accrued interest balances were extinguished in exchange for a reduction of 110% of the balances of accrued interest and principal outstanding to the $4 million cash payment. The 10% increase to the balances owed under the note totaled $125,686, and the Company recorded that amount as a reduction of the gain on deconsolidation.
8% Convertible Promissory Note dated April 23, 2019
On April 23, 2019, the Company entered into the Note Purchase Agreement with Iliad Research and Trading, L.P. ("Iliad") and issued an 8% Convertible Promissory Note with a face value of $2,765,000. The Note was issued with original issue discount of $265,000 and is convertible into shares of the Company’s common stock at a price of $0.17 per share at the option of the note holder at any time until the Note is repaid. The Note matured on April 22, 2020. A total discount of $440,000 was recorded on the note, which includes $265,000 of original issue discount and $175,000 in fees paid to brokers.
During the year ended March 31, 2020, the Company honored the conversion of a total of a total of $125,000 of accrued interest on the Iliad Note at reduced conversion rates. On October 30, 2019, the Company received notice of the conversion of $75,000 at $0.06 per share and issued 1,250,000 shares of its common stock. The fair value of the shares issued exceeded the fair value of the shares issuable under the original terms of the Note by $64,706, and the Company recorded an induced conversion expense. On November 18, 2019, the Company received notice of the conversion of $50,000 of the note balance at $0.0375 per share and issued 1,333,333 shares of its common stock.
On April 22, 2020, the Company failed to make payment of the principal and accrued interest due under the Iliad Note, resulting in a default. Upon the occurrence of the default, the principal and accrued interest balances outstanding increased by 10%. As the result of the default, Company recorded an expense of $9,559 related to a 10% increase in the accrued interest balance and $276,500 related to the 10% increase in the principal balance, totaling $286,059 which is recorded as debt default penalty on the statement of operations for the year ended March 31, 2021.
On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. The lawsuit further sought to compel the Company to participate in arbitration pursuant to the arbitration provisions contained within the Note Purchase Agreement and to prohibit the Company to raise funds through the issuance of its common stock unless the note is paid in full simultaneously with such issuance. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594 plus reasonable attorney's fees and costs and accrued post-judgment interest at the default rate of 15% per annum.
On November 20, 2020, the Company, Iliad, and Wellcana Plus, LLC entered into the Judgment Settlement Agreement, whereby Iliad agreed to discharge all amounts owed to it by the Company upon receipt of payment totaling $3,006,015 directly from the proceeds of the Wellcana Note Receivable on or before December 8, 2020. On December 8, 2020, Wellcana failed to make payment to the Company. On December 9, 2020, the Company entered into a letter agreement with Iliad extending the Judgment Settlement agreement in exchange for payment of $25,000 plus $25,000 per week until the payment totaling $3,006,015 is received by Iliad, with such payments not reducing the amount owed under the Judgment Settlement Agreement. On December 16, 2020, Wellcana made payment of the full amount owed to the Company, of which $3,006,015 was paid directly to Iliad in full satisfaction of the Judgment Settlement Agreement. On December 18, 2020, Iliad filed a Satisfaction of Judgment in the Third Judicial District Court of Salt Lake County in the State of Utah, and the lawsuit was dismissed. The Company has no further obligations to Iliad.
December 2020 $625,000 6% Convertible Notes
On December 18, 2020, the Company began an offering of 6.0% convertible notes for the purpose of funding a pre-clinical study of the Company's patent-pending Cannabinoid-Containing Complex Mixtures for the treatment of Cytokine Release Syndromes, including Acute Respiratory Distress Syndrome, in COVID-19 patients. The Company pledged the related intellectual property as security for the notes. The notes are convertible at a rate of $0.05 per share at the lender's request. To date, the Company has issued $625,000 in convertible notes under the offering to three investors. $375,000 of the notes mature between January 31, 2021 and July 1, 2022, and $250,000 mature in December 2023. Payment of accrued interest and principal is due at maturity. The Company received cash of $543,750, net of brokerage fees, and recorded discounts on the convertible notes totaling $81,250 related to the issuance costs. Notes totaling $425,000 were issued with in-the-money conversion features, and the Company recorded beneficial conversion feature discounts totaling $347,000 on the related notes. During the year ended March 31, 2022, the Company received $50,000 related to the note offering and recorded a discount on convertible notes payable of $6,500 related to issuance costs.
At March 31, 2022, notes with a carrying amount of $373,235 were included in short term notes and convertible notes payable, net of unamortized discounts of $1,765. Notes with a carrying amount of $176,765 were included in long term notes and convertible notes payable, net of unamortized discounts of $73,235. Interest expense related to the notes was $378,777 for the year ended March 31, 2022, which includes $342,033 from amortization of the note discounts.
Variables and Trends
We have limited operating history with respect to the current business plan. In the event we are able to obtain the necessary financing to move forward with the business plan, we expect business expenses to increase significantly as we go operational. Accordingly, the comparison of the financial data for the periods presented may not be a meaningful indicator of future performance and must be considered in light these circumstances.
Off Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
Critical Accounting Policies
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to allowances for doubtful accounts, collectibility of notes receivable, inventory valuation and standard cost allocations, valuation of initial right-of-use assets and corresponding lease liabilities, valuation of beneficial conversion features in convertible debt, valuation of the assets and liabilities of discontinued operations, stock-based compensation expense, purchased intangible asset valuations, deferred income tax asset valuation allowances, uncertain tax positions, litigation, other loss contingencies, and impairment of long lived assets. These estimates and assumptions are based on current facts, historical experience and various other factors that the Company believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of costs and expenses that are not readily apparent from other sources. The actual results the Company experiences may differ materially and adversely from these estimates.
Discontinued Operations
Discontinued operations comprise those activities that were disposed of during the period or which were classified as held for sale at the end of the period and represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes. The Company has included its former subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC in discontinued operations due to the sale of the Company's Nevada Subsidiaries.
Inventory
We value our inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out method, or its current estimated market value. We periodically review our physical inventory for excess, obsolete, and potentially impaired items and reserve accordingly. Our reserve estimate for excess and obsolete inventory is based on expected future use. Indirect costs, which primarily relate to the lease and operation costs of the Teco Facility, are allocated based on square footage of the facility used in the production of inventory.
Indefinite and Definite-Lived Intangible Assets
Our indefinite-lived intangible assets primarily represent the value of our patents pending and includes the costs paid to draft and file patent applications. Upon issuance of the patents, the indefinite-lived intangible assets will have finite lives. Intangible assets also included the acquisition cost of a cannabis production license with an indefinite life (in prior periods).
We amortize our finite-lived intangible assets, which consist of granted patents, over their estimated useful lives using the straight-line method, and we periodically evaluate the remaining useful lives of our finite-lived intangible assets to determine whether events or circumstances warrant a revision to the remaining period of amortization.
We review all of our intangible assets for impairment indicators throughout the year. Impairment testing for indefinite-lived intangible assets is performed at least annually and we perform testing for definite-lived intangible assets whenever impairment indicators are present. If we determine that the fair value is less than the carrying value of these assets during testing, we record impairment losses equal to the difference between the carrying value of the asset and the fair market value of the asset.
Long-Lived Assets
Property and equipment comprised a significant portion of our total assets from discontinued operations as of March 31, 2021. We evaluate the carrying value of property and equipment if impairment indicators are present or if other circumstances indicate that impairment may exist under authoritative guidance. The annual testing date is March 31. When management believes impairment indicators may exist, projections of the undiscounted future cash flows associated with the use of and eventual disposition of property and equipment are prepared. If the projections indicate that the carrying value of the property and equipment are not recoverable, we reduce the carrying values to fair value. These impairment tests are heavily influenced by assumptions and estimates that are subject to change as additional information becomes available.
Beneficial Conversion Feature of Convertible Notes Payable
The Company accounts for convertible notes payable in accordance with the guidelines established by the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 470-20, Debt with Conversion and Other Options and Emerging Issues Task Force (“EITF”) 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. A beneficial conversion feature (“BCF”) exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. In accordance with this guidance, the BCF of a convertible note is measured by allocating a portion of the note's proceeds to the warrants, if applicable, and as a reduction of the carrying amount of the convertible note equal to the intrinsic value of the conversion feature, both of which are credited to additional paid-in-capital. The Company calculates the fair value of warrants issued with the convertible notes using the Black-Scholes valuation model and uses the same assumptions for valuing any employee options in accordance with ASC Topic 718 Compensation – Stock Compensation. The only difference is that the contractual life of the warrants is used.
The value of the proceeds received from a convertible note is then allocated between the conversion features and warrants on a relative fair value basis. The allocated fair value is recorded in the financial statements as a debt discount (premium) from the face amount of the note and such discount is amortized over the expected term of the convertible note (or to the conversion date of the note, if sooner) and is charged to interest expense.
Equity-Based Compensation
The Company accounts for equity instruments issued to employees in accordance with the provisions of ASC 718 Stock Compensation (ASC 718) and Equity-Based Payments to Non-employees pursuant to ASC 505-50 (ASC 505-50). The computation of the expense associated with stock-based compensation requires the use of a valuation model. The FASB-issued accounting guidance requires significant judgment and the use of estimates, particularly surrounding Black-Scholes assumptions such as stock price volatility, expected option lives, and expected option forfeiture rates, to value equity-based compensation. We currently use a Black-Scholes option pricing model to calculate the fair value of our stock options. We primarily use historical data to determine the assumptions to be used in the Black-Scholes model and have no reason to believe that future data is likely to differ materially from historical data. However, changes in the assumptions to reflect future stock price volatility and future stock award exercise experience could result in a change in the assumptions used to value awards in the future and may result in a material change to the fair value calculation of stock-based awards. This accounting guidance requires the recognition of the fair value of stock compensation in net income. Although every effort is made to ensure the accuracy of our estimates and assumptions, significant unanticipated changes in those estimates, interpretations and assumptions may result in recording stock option expense that may materially impact our financial statements for each respective reporting period.
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in financial statements or tax returns. Deferred tax items are reflected at the enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Due to the uncertainty regarding the success of future operations, management has valued the deferred tax asset allowance at 100% of the related deferred tax assets.
Because the Company operated in the State-licensed cannabis industry until the December 31, 2021 disposition of the Nevada Subsidiaries, revenue from those activities were subject to the limitations of Internal Revenue Code Section 280E (“280E”) for U.S. income tax purposes. Under 280E, the Company is allowed to deduct expenses that are directly related to the production of its products, i.e. cost of goods sold, but is allowed no further deductions for ordinary and necessary business expenses from its gross profit. The Company believes that the deductions disallowed include the deduction of net operating loss carryforwards ("NOLs"). The unused NOLs will continue to carry forward and those that do not expire or become subject to other limitations may be used by the Company to offset future taxable income that is not subject to the limitations of 280E.
Recent Accounting Pronouncements
Standards Recently Adopted
In May 2021, the FASB issued ASU No. 2021-04, Issuer's Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. This guidance clarifies and reduces diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options due to a lack of explicit guidance in the FASB Codification. The ASU 2021-04 is effective for The Company's fiscal year beginning April 1, 2022. The Company adopted the standard on April 1, 2022 and it did not have a material impact on its financial statements.
Standards Not Yet Adopted
On June 16, 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments. The standard requires the use of an “expected loss” model on certain types of financial instruments. The standard also amends the impairment model for available-for-sale debt securities and requires estimated credit losses to be recorded as allowances instead of reductions to amortized cost of the securities. The amendments in this ASU are effective for the Company's fiscal year beginning April 1, 2023. The Company is currently evaluating the impact of ASU 2016-13 on its financial statements.
In June 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity's Own Equity. The guidance simplifies the current guidance for convertible instruments and the derivatives scope exception for contracts in an entity’s own equity. Additionally, the amendments affect the diluted EPS calculation for instruments that may be settled in cash or shares and for convertible instruments. This ASU will be effective for the Company's fiscal year beginning April 1, 2024. Early adoption is permitted. The amendments in this update must be applied on either full retrospective basis or modified retrospective basis through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The Company is currently evaluating the impact of ASU 2020-06 on its consolidated financial statements and related disclosures, as well as the timing of adoption.
All other newly issued accounting pronouncements have been deemed either immaterial or not applicable.
ITEM 7A QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a "smaller reporting company" as defined by Item 10 of Regulation S-K, the Company is not required to provide information required by this Item.
ITEM 8. FINANCIAL STATEMENTS
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
1

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of
GB Sciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of GB Sciences, Inc. (the Company) as of March 31, 2022 and 2021, and the related consolidated statements of operations, stockholders’ deficit and cash flows for each of the years in the two-year period ended March 31, 2022 and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2022 and 2021, and the results of its operations and its cash flows for each of the years in the two-year period ended March 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph- Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has sustained net losses since inception, which have caused an accumulated deficit of $104,580,122 at March 31, 2022. The Company also had a working capital deficit of $3,607,638 and consumed cash in its operating activities of $1,866,154 including $87,772 used in discontinued operations for the year ended March 31, 2022. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
2
Critical Audit Matters
The critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
Collectability of Note Receivable
Description of the Matter
The Company has recorded a $3,025,000 note receivable (“Note”) with AJE Management, LLC (“AJE”) from the sale of the Teco Subsidiaries which is payable as quarterly, interest only payments of $60,500 for the first year, followed by seven quarterly payments of interest and principal of $201,774 beginning March 31, 2023, with a final payment of principal and interest totaling $2,014,225 on December 31, 2024. The note contains a provision that allows payments of principal and interest due prior to the maturity date to be postponed to the next quarterly payment date if cash flow from the operations of the facility is insufficient to cover the amount of the payment. Several days prior to the first interest payment due date of April 1, 2022, AJE notified the Company that it would be postponing the payment of interest of $60,500 due on April 1, 2022 due to insufficient cash flow to make the payment. AJE also notified the Company that it will be unable to make the interest payment due July 1, 2022 due to insufficient cash flow.
We determined based on the fact pattern noted above the long term collectability of the remaining Note balance was as a critical audit matter. The Note is not collateralized and the Company cannot recover any of the assets sold.
How We Addressed the Matter in Our Audit
We evaluated management’s assessment of collectability in accordance with guidance and given there was substantial uncertainty around the collectability of the Note, an impairment charge against the full balance of the Note was recorded as of March 31, 2022.
| /s/ Assurance Dimensions |
| |
|
|
We have served as the Company’s auditor since 2020.
|
| |
|
|
Margate, Florida
June 30, 2022
|
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| |
|
As of March 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
CURRENT ASSETS:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$ |
233,893 |
|
|
$ |
793,040 |
|
|
Prepaid expenses and other current assets
|
|
|
93,933 |
|
|
|
256,251 |
|
|
Current assets from discontinued operations
|
|
|
- |
|
|
|
2,494,564 |
|
|
TOTAL CURRENT ASSETS
|
|
|
327,826 |
|
|
|
3,543,855 |
|
| |
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
- |
|
|
|
25,022 |
|
|
Intangible assets, net of accumulated amortization of $104,201 and $43,096 at March 31, 2022 and 2021, respectively
|
|
|
2,222,074 |
|
|
|
1,706,762 |
|
|
Long term assets from discontinued operations
|
|
|
- |
|
|
|
5,530,415 |
|
|
TOTAL ASSETS
|
|
$ |
2,549,900 |
|
|
$ |
10,806,054 |
|
| |
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$ |
1,657,008 |
|
|
$ |
1,412,459 |
|
|
Accrued interest
|
|
|
384,769 |
|
|
|
493,741 |
|
|
Accrued liabilities
|
|
|
9,627 |
|
|
|
957,946 |
|
|
Notes and convertible notes payable, net of unamortized discount of $1,765 and $296,504 at March 31, 2022 and 2021, respectively
|
|
|
987,565 |
|
|
|
3,594,804 |
|
|
Indebtedness to related parties
|
|
|
- |
|
|
|
84,913 |
|
|
Income tax payable
|
|
|
896,495 |
|
|
|
761,509 |
|
|
Current liabilities from discontinued operations
|
|
|
- |
|
|
|
1,293,076 |
|
|
TOTAL CURRENT LIABILITIES
|
|
|
3,935,464 |
|
|
|
8,598,448 |
|
| |
|
|
|
|
|
|
|
|
|
Convertible notes payable, net of unamortized discount of $99,489 and $154,590 at March 31, 2022 and 2021, respectively
|
|
|
397,308 |
|
|
|
292,410 |
|
|
Long term liabilities from discontinued operations
|
|
|
- |
|
|
|
3,389,124 |
|
|
TOTAL LIABILITIES
|
|
|
4,332,772 |
|
|
|
12,279,982 |
|
|
Commitments and contingencies (Note 11)
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
STOCKHOLDERS' EQUITY/(DEFICIT):
|
|
|
|
|
|
|
|
|
|
Common Stock, $0.0001 par value, 600,000,000 shares authorized, 325,037,557 and 315,340,411 outstanding at March 31, 2022 and 2021, respectively
|
|
|
32,504 |
|
|
|
31,534 |
|
|
Additional paid-in capital
|
|
|
102,764,746 |
|
|
|
102,380,770 |
|
|
Accumulated deficit
|
|
|
(104,580,122 |
) |
|
|
(103,886,232 |
) |
|
TOTAL STOCKHOLDERS' EQUITY/(DEFICIT)
|
|
|
(1,782,872 |
) |
|
|
(1,473,928 |
) |
|
TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT
|
|
$ |
2,549,900 |
|
|
$ |
10,806,054 |
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| |
|
For the Years Ended
|
|
| |
|
March 31,
|
|
| |
|
2022
|
|
|
2021
|
|
| |
|
|
|
|
|
|
|
|
|
Sales revenue
|
|
$ |
- |
|
|
$ |
- |
|
|
Cost of goods sold
|
|
|
- |
|
|
|
- |
|
|
Gross profit (loss)
|
|
|
- |
|
|
|
- |
|
|
General and administrative expenses
|
|
|
1,868,734 |
|
|
|
2,001,617 |
|
|
LOSS FROM OPERATIONS
|
|
|
(1,868,734 |
) |
|
|
(2,001,617 |
) |
|
OTHER INCOME (EXPENSE)
|
|
|
|
|
|
|
|
|
|
Gain on extinguishment
|
|
|
22,405 |
|
|
|
467,872 |
|
|
Gain on settlement of accounts payable
|
|
|
- |
|
|
|
422,414 |
|
|
Gain on deconsolidation
|
|
|
5,206,208 |
|
|
|
- |
|
|
Interest expense
|
|
|
(474,768 |
) |
|
|
(1,285,460 |
) |
|
Loss on modification of line of credit
|
|
|
- |
|
|
|
(650,000 |
) |
|
Loss on impairment of note receivable
|
|
|
(3,025,000 |
) |
|
|
- |
|
|
Debt default penalty
|
|
|
- |
|
|
|
(286,059 |
) |
|
Loss on disposal
|
|
|
(15,639 |
) |
|
|
- |
|
|
Other income
|
|
|
9,000 |
|
|
|
- |
|
|
Total other income/(expense)
|
|
|
1,722,206 |
|
|
|
(1,331,233 |
) |
|
LOSS BEFORE INCOME TAXES
|
|
|
(146,528 |
) |
|
|
(3,332,850 |
) |
|
Income tax expense (Note 8)
|
|
|
- |
|
|
|
- |
|
|
LOSS FROM CONTINUING OPERATIONS
|
|
|
(146,528 |
) |
|
|
(3,332,850 |
) |
|
Net loss from discontinued operations (Note 4)
|
|
|
(384,345 |
) |
|
|
(392,177 |
) |
|
NET LOSS
|
|
$ |
(530,873 |
) |
|
$ |
(3,725,027 |
) |
| |
|
|
|
|
|
|
|
|
|
Net loss attributable to common stockholders of GB Sciences, Inc. - basic and diluted
|
|
|
|
|
|
|
|
|
|
Continuing operations
|
|
$ |
(146,528 |
) |
|
$ |
(3,332,850 |
) |
|
Discontinued operations
|
|
|
(384,345 |
) |
|
|
(392,177 |
) |
|
Net loss
|
|
$ |
(530,873 |
) |
|
$ |
(3,725,027 |
) |
| |
|
|
|
|
|
|
|
|
|
Net loss per common share – basic and diluted
|
|
|
|
|
|
|
|
|
|
Continuing operations
|
|
$ |
(0.00 |
) |
|
$ |
(0.01 |
) |
|
Discontinued operations
|
|
$ |
(0.00 |
) |
|
$ |
(0.00 |
) |
|
Net loss
|
|
$ |
(0.00 |
) |
|
$ |
(0.01 |
) |
| |
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding - basic and diluted
|
|
|
317,621,942 |
|
|
|
285,190,729 |
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY/(DEFICIT)
| |
|
|
|
|
|
|
|
|
|
Additional Paid-
|
|
|
Accumulated
|
|
|
|
|
|
| |
|
Shares
|
|
|
Amount
|
|
|
In Capital
|
|
|
Deficit
|
|
|
Total
|
|
|
Balance at March 31, 2020
|
|
|
275,541,602 |
|
|
$ |
27,554 |
|
|
$ |
97,271,157 |
|
|
$ |
(97,387,205 |
) |
|
$ |
(88,494 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Issuance of stock for debt conversion
|
|
|
4,000,000 |
|
|
|
400 |
|
|
|
159,600 |
|
|
|
- |
|
|
|
160,000 |
|
|
Exercise of warrants for stock, net of issuance costs
|
|
|
35,798,809 |
|
|
|
3,580 |
|
|
|
964,443 |
|
|
|
- |
|
|
|
968,023 |
|
|
Share based compensation expense
|
|
|
- |
|
|
|
- |
|
|
|
436,349 |
|
|
|
- |
|
|
|
436,349 |
|
|
Beneficial conversion feature on notes payable
|
|
|
- |
|
|
|
- |
|
|
|
543,886 |
|
|
|
- |
|
|
|
543,886 |
|
|
Compensation warrants
|
|
|
- |
|
|
|
- |
|
|
|
231,335 |
|
|
|
- |
|
|
|
231,335 |
|
|
Inducement dividend from warrant exercises
|
|
|
- |
|
|
|
- |
|
|
|
2,774,000 |
|
|
|
(2,774,000 |
) |
|
|
- |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
(3,725,027 |
) |
|
|
(3,725,027 |
) |
|
Balance at March 31, 2021
|
|
|
315,340,411 |
|
|
$ |
31,534 |
|
|
$ |
102,380,770 |
|
|
$ |
(103,886,232 |
) |
|
$ |
(1,473,928 |
) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock issued for warrant exercises, net of brokerage fees
|
|
|
2,095,333 |
|
|
|
210 |
|
|
|
56,184 |
|
|
|
- |
|
|
|
56,394 |
|
|
Issuance of shares upon exercise of compensation warrants
|
|
|
7,601,813 |
|
|
|
760 |
|
|
|
75,308 |
|
|
|
- |
|
|
|
76,068 |
|
|
Inducement dividend from warrant exercises
|
|
|
- |
|
|
|
- |
|
|
|
163,017 |
|
|
|
(163,017 |
) |
|
|
- |
|
|
Share-based compensation expense
|
|
|
- |
|
|
|
- |
|
|
|
60,667 |
|
|
|
- |
|
|
|
60,667 |
|
|
Stock options issued as compensation for drafting and filing patents
|
|
|
- |
|
|
|
- |
|
|
|
28,800 |
|
|
|
- |
|
|
|
28,800 |
|
|
Net loss
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(530,873 |
) |
|
|
(530,873 |
) |
|
Balance at March 31, 2022
|
|
|
325,037,557 |
|
|
$ |
32,504 |
|
|
$ |
102,764,746 |
|
|
$ |
(104,580,122 |
) |
|
$ |
(1,782,872 |
) |
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| |
|
Year Ended March 31,
|
|
| |
|
2022
|
|
|
2021
|
|
|
OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Net loss
|
|
$ |
(530,873 |
) |
|
$ |
(3,725,027 |
) |
|
Loss from discontinued operations
|
|
|
(384,345 |
) |
|
|
(392,177 |
) |
|
Net loss from continuing operations
|
|
|
(146,528 |
) |
|
|
(3,332,850 |
) |
| |
|
|
|
|
|
|
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
70,488 |
|
|
|
47,353 |
|
|
Stock-based compensation
|
|
|
60,667 |
|
|
|
436,349 |
|
|
Compensation warrants
|
|
|
- |
|
|
|
231,335 |
|
|
Amortization of debt discount and beneficial conversion feature
|
|
|
356,340 |
|
|
|
776,122 |
|
|
Debt default penalty
|
|
|
- |
|
|
|
286,059 |
|
|
Loss on modification of line of credit
|
|
|
- |
|
|
|
650,000 |
|
|
Gain on extinguishment
|
|
|
(22,405 |
) |
|
|
(467,872 |
) |
|
Gain on settlement of accounts payable
|
|
|
- |
|
|
|
(422,414 |
) |
|
Loss on disposal of assets
|
|
|
15,639 |
|
|
|
- |
|
|
Loss on impairment of note receivable
|
|
|
3,025,000 |
|
|
|
- |
|
|
Gain on deconsolidation
|
|
|
(5,206,208 |
) |
|
|
- |
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Prepaid expenses and other current assets
|
|
|
162,318 |
|
|
|
(237,475 |
) |
|
Accounts payable
|
|
|
821,253 |
|
|
|
(248,115 |
) |
|
Accrued expenses
|
|
|
(948,319 |
) |
|
|
166,828 |
|
|
Accrued interest
|
|
|
118,286 |
|
|
|
549,703 |
|
|
Indebtedness to related parties
|
|
|
(84,913 |
) |
|
|
(501,599 |
) |
|
Net cash used in operating activities of continuing operations
|
|
|
(1,778,382 |
) |
|
|
(2,066,576 |
) |
|
Net cash used in operating activities of discontinued operations
|
|
|
(87,772 |
) |
|
|
(118,644 |
) |
|
Net cash used in operating activities
|
|
|
(1,866,154 |
) |
|
|
(2,185,220 |
) |
|
INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from sale of Nevada Subsidiaries
|
|
|
1,648,772 |
|
|
|
- |
|
|
Proceeds of note receivable
|
|
|
- |
|
|
|
5,051,923 |
|
|
Acquisition of intangible assets
|
|
|
(200,000 |
) |
|
|
(292,675 |
) |
|
Net cash provided by investing activities of continuing operations
|
|
|
1,448,772 |
|
|
|
4,759,248 |
|
|
Net cash provided by/(used in) investing activities of discontinued operations
|
|
|
1,567 |
|
|
|
(103,729 |
) |
|
Net cash provided by investing activities
|
|
|
1,450,339 |
|
|
|
4,655,519 |
|
|
FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from warrant exercises
|
|
|
138,728 |
|
|
|
1,075,396 |
|
|
Proceeds from convertible notes payable
|
|
|
50,000 |
|
|
|
725,000 |
|
|
Proceeds from line of credit
|
|
|
- |
|
|
|
375,000 |
|
|
Principal payment on notes payable
|
|
|
(575,000 |
) |
|
|
(3,156,014 |
) |
|
Principal payment on related party note
|
|
|
- |
|
|
|
(151,923 |
) |
|
Brokerage fees from warrant exercises and stock issuances
|
|
|
(6,266 |
) |
|
|
(107,373 |
) |
|
Fees for issuance of convertible notes
|
|
|
- |
|
|
|
(74,750 |
) |
|
Net cash used in financing activities of continuing operations
|
|
|
(392,538 |
) |
|
|
(1,314,664 |
) |
|
Net cash used in financing activities of discontinued operations
|
|
|
(103,387 |
) |
|
|
(161,768 |
) |
|
Net cash used in financing activities
|
|
|
(495,925 |
) |
|
|
(1,476,432 |
) |
|
NET CHANGE IN CASH AND CASH EQUIVALENTS
|
|
|
(911,740 |
) |
|
|
993,867 |
|
|
CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR
|
|
|
1,145,633 |
|
|
|
151,766 |
|
|
CASH AND CASH EQUIVALENTS AT END OF YEAR
|
|
|
233,893 |
|
|
|
1,145,633 |
|
|
Less: cash and cash equivalents classified as discontinued operations
|
|
|
- |
|
|
|
(352,593 |
) |
|
CASH AND CASH EQUIVALENTS AT END OF YEAR FROM CONTINUING OPERATIONS
|
|
$ |
233,893 |
|
|
$ |
793,040 |
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
| |
|
Year Ended March 31,
|
|
| |
|
2022
|
|
|
2021
|
|
| |
|
|
|
|
|
|
|
|
|
Cash paid for interest
|
|
$ |
- |
|
|
$ |
241,014 |
|
|
Cash paid for income tax
|
|
$ |
- |
|
|
$ |
- |
|
| |
|
|
|
|
|
|
|
|
|
Noncash investing and financing transactions:
|
|
|
|
|
|
|
|
|
|
Note receivable from sale of Nevada Subsidiaries
|
|
$ |
3,025,000 |
|
|
$ |
- |
|
|
Extinguishment of debt and accrued interest owed to purchasers of Nevada Subsidiaries and purchasers' affiliates
|
|
$ |
2,612,854 |
|
|
$ |
- |
|
|
Extinguishment of accrued management fees payable to purchaser of Nevada Subsidiaries
|
|
$ |
850,000 |
|
|
$ |
- |
|
|
Accrued liabilities forgiven in connection with Wellcana Note settlement
|
|
$ |
- |
|
|
$ |
172,500 |
|
|
Depreciation capitalized in inventory (discontinued operations)
|
|
$ |
349,015 |
|
|
$ |
532,785 |
|
|
Accrued interest capitalized in convertible note principal
|
|
$ |
- |
|
|
$ |
223,094 |
|
|
Patent acquisition costs capitalized in intangible assets
|
|
$ |
347,617 |
|
|
$ |
319,939 |
|
|
Stock options issued for preparing capitalized patent applications
|
|
$ |
28,800 |
|
|
$ |
168,000 |
|
|
Stock issued upon conversion of notes payable
|
|
$ |
- |
|
|
$ |
160,000 |
|
|
Inducement dividend from warrant exercises
|
|
$ |
163,017 |
|
|
$ |
2,774,000 |
|
|
Beneficial conversion feature on notes payable
|
|
$ |
- |
|
|
$ |
543,886 |
|
The accompanying notes are an integral part of these consolidated financial statements
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1 - Background and Nature of Operations
Business
GB Sciences, Inc. (“the Company”, “GB Sciences”, “we”, “us”, or “our”) is a phytomedical research and biopharmaceutical drug development company engaged in creating patented formulations of plant-inspired, complex therapeutic mixtures for the prescription drug market that target a variety of medical conditions. The Company is engaged in the research and development of plant-based medicines and plans to produce plant-inspired, complex therapeutic mixtures based on its portfolio of intellectual property.
The Company is engaged in the research and development of plant-based medicines, primarily cannabinoid-inspired medicines, with virtual operations in North America and Europe. GBSGB’s assets include a portfolio of intellectual property containing both proprietary cannabinoid-containing formulations and our AI-enabled drug discovery platform, as well as critical research contracts and key supplier arrangements. GBSGB’s intellectual property covers a range of medical conditions and several programs are in the pre-clinical animal stage of development including Parkinson’s disease, neuropathic pain, and cardiovascular therapeutic programs. GBSGB runs a lean drug development program and takes effort to minimize expenses, including personnel, overhead, and fixed capital expenses through strategic partnerships with Universities and Contract Research Organizations (“CROs”). GBSGB’s intellectual property portfolio includes five USPTO issued patents, nine USPTO nonprovisional patent applications pending in the US, and one provisional patent application in the US. In addition to the USPTO patents and patent applications, the company has filed 35 patent applications internationally to protect its proprietary technology. We recently filed a provisional USPTO patent application to further protect aspects of our proprietary drug discovery engine, “Phytomedical Analytics for Research Optimization at Scale," or PhAROS™.
We were incorporated in the State of Delaware on April 4, 2001, under the name “Flagstick Venture, Inc.” On March 28, 2008, stockholders owning a majority of our outstanding common stock approved changing our then name “Signature Exploration and Production Corp.” as our business model had changed.
On April 4, 2014, we changed our name from Signature Exploration and Production Corporation to Growblox Sciences, Inc. Effective December 12, 2016, the Company amended its Certificate of Corporation pursuant to shareholder approval, and the Company’s name was changed from Growblox Sciences, Inc. to GB Sciences, Inc.
Effective April 8, 2018, Shareholders of the Company approved the change in corporate domicile from the State of Delaware to the State of Nevada and increase in the number of authorized capital shares from 250,000,000 to 400,000,000. Effective August 15, 2019, Shareholders of the Company approved an increase in authorized capital shares from 400,000,000 to 600,000,000.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Recent Developments
Intellectual Property Portfolio
On October 14th, 2020, GB Sciences filed a provisional patent application to protect its machine learning algorithm for the prediction of novel active ingredients from traditional, plant-based medical preparations. The new provisional patent application is entitled “In Silico Meta-Pharmacopeia Assembly from Non-Western Medical Systems Using Advanced Data Analytic Techniques to Identify and Design Phytotherapeutic Strategies”. GBSGB’s proprietary data analytics tool uses in silico convergence analysis to deconvolve modes of action and predict desirable components of plant-based formulations established in traditional medical practice based on computational consensus analysis across cultures and medical systems.
On September 23rd, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting their Cannabinoid Containing Complex Mixtures (CCCMs) for the Treatment of Mast Cell Activation Syndrome (MCAS). The patent is owned by GBSGB. MCAS is a severe immunological condition in which mast cells inappropriately and excessively release inflammatory mediators, resulting in a range of severe chronic hyperinflammatory symptoms and life-threatening anaphylaxis attacks. There is no single recommended treatment for MCAS patients. Instead, patients, with their doctor’s guidance, attempt to manage MCAS symptoms primarily by avoiding ‘triggers’ and using rescue medicines for their severe hyperinflammatory attacks. Therefore, MCAS patients need new therapeutic options to control their mast cell related symptoms, and the Company’s CCCM™ were designed to simultaneously control multiple inflammatory pathways within mast cells as a comprehensive treatment option. The application, entitled “Cannabinoid-Containing Complex Mixtures for the Treatment of Mast Cell-Associated or Basophil-Mediated Inflammatory Disorders” was originally filed on January 31, 2018 and describes CCCMs that can be used for the treatment of Crohn's disease, Inflammatory Bowel Disease (IBD), Irritable Bowel Syndrome (IBS), rheumatoid arthritis, osteoarthritis, allergic asthma, Chronic Obstructive Pulmonary Disease (COPD), psoriasis, eczema, urticarias, dermatitis, mastocytosis, or anaphylactic sting. Claims for these additional indications will be examined by the USPTO in the future. On December 8, 2020, the patent was issued as United States Patent 10,857,107.
On April 7th, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting Cannabinoid Containing Complex Mixtures ("CCCMs") for the Treatment of Parkinson’s disease (PD), which is owned by GBSGB. On May 19, 2020, the patent was issued as United States Patent 10,653,640.
On May 12th, 2020, GB Sciences received a Notice of Allowance from the United States Patent and Trademark Office (USPTO) for claims protecting Myrcene Containing Complex Mixtures ("MCCMs") for the Treatment of Neuropathic Pain. Intellectual property rights to this application and the MCCM contained within it are owned by GBSGB. The Company's MCCMs are protected for use in the treatment of pain related to arthritis, shingles, irritable bowel syndrome, sickle cell disease, and endometriosis. The patent was issued on July 14, 2020, as United States Patent 10,709,670.
Divestiture of Nevada Cannabis Operations
On March 24, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC. Pursuant to the Teco MIPA, the Company agreed to sell 100% of its membership interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC (the "Teco Subsidiaries") for approximately $8 million, which amount includes a cash payment at closing, the extinguishment and/or repayments of certain liabilities owed to the purchaser and affiliates of the purchaser, and an 8% promissory note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with 483 Management, LLC. Pursuant to the Nopah MIPA, the Company agreed to sell its 100% membership interest in GB Sciences Nopah, LLC ("Nopah"), which holds a Nevada medical marijuana cultivation certificate. As consideration, the Company would receive $312,315 in consideration in the form of a $237,668 reduction to the outstanding principal and accrued interest balances of the 0% Note payable dated October 23, 2017 (Note 5), and extinguishment of accounts payable of $74,647, which were owed to an affiliate of the purchaser.
The closing of the Teco and Nopah sales was contingent upon the successful transfer of the Nevada cultivation and production licenses. On December 14, 2021, the Company received approval from the Nevada Cannabis Compliance Board for the transfer of cannabis cultivation and extraction licenses held by its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC (the "Nevada Subsidiaries"). Consequently, all conditions to closing the sales of the 100% membership interests in the Nevada Subsidiaries were satisfied, and the transactions formally closed on December 31, 2021. After the closing date, the Company retains no ownership interest in the Nevada Subsidiaries.
As consideration for the membership interests, the Company received cash payments of $1,648,772 (including $400,000 in advance payments received during the nine months ended December 31, 2021), the extinguishment $3,462,854 of debt and current liabilities owed to affiliates of the purchaser, and a $3,025,000 8% note receivable (Note 13).
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 2 - Going Concern
The Company’s consolidated financial statements have been prepared assuming the Company will continue as a going concern. The Company has sustained net losses since inception, which have caused an accumulated deficit of $104,580,122 at March 31, 2022. The Company had a working capital deficit of $3,607,638 as of March 31, 2022, compared to a working capital deficit of $5,054,593, net of working capital of $1,201,488 from discontinued operations at March 31, 2021. In addition, the Company has consumed cash in its operating activities of $1,866,154 including $87,772 used in discontinued operations for the year ended March 31, 2022, compared to $2,185,220 including $118,644 used in discontinued operations for the year ended March 31, 2021. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern for one year from the issuance of the consolidated financial statements.
Management has been able, thus far, to finance the losses through a public offering, private placements of debt and equity, and obtaining operating funds from stockholders. The Company is continuing to seek sources of financing. There are no assurances that the Company will be successful in achieving its goals.
In view of these conditions, the Company’s ability to continue as a going concern is dependent upon its ability to obtain additional financing or capital sources, to meet its financing requirements, and ultimately to achieve profitable operations. Management believes that its current and future plans provide an opportunity to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classification of liabilities that may be necessary in the event the Company is unable to continue as a going concern.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 3 - Basis of Presentation and Summary of Significant Accounting Policies
Principles of Consolidation
We prepare our consolidated financial statements in accordance with generally accepted accounting principles (GAAP) for the United States of America. Our consolidated financial statements include all operating divisions and majority-owned subsidiaries, reported as a single operating segment, for which we maintain controlling interests.
The subsidiaries of the Company are:
Continuing Operations:
GBS Global Biopharma, Inc.
ECRX, Inc.
The PhAROS Institute, LLC
GB Sciences Texas, LLC
Discontinued Operations:
GB Sciences Nevada, LLC
GB Sciences Las Vegas, LLC
GB Sciences Nopah, LLC
Intercompany accounts and transactions have been eliminated in consolidation. The ownership interest of non-controlling participants in subsidiaries that are not wholly owned is included as a separate component of equity. The non-controlling participants’ share of the net loss is included as “Net loss attributable to non-controlling interest” on the consolidated statements of operations.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to allowances for doubtful accounts, collectibility of notes receivable, inventory valuation and standard cost allocations, valuation of initial right-of-use assets and corresponding lease liabilities, valuation of beneficial conversion features in convertible debt, valuation of the assets and liabilities of discontinued operations, stock-based compensation expense, purchased intangible asset valuations, deferred income tax asset valuation allowances, uncertain tax positions, litigation, other loss contingencies, and impairment of long lived assets. These estimates and assumptions are based on current facts, historical experience and various other factors that the Company believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the recording of costs and expenses that are not readily apparent from other sources. The actual results the Company experiences may differ materially and adversely from these estimates.
Reclassifications
Certain reclassifications have been made to the comparative period amounts in order to conform to the current period presentation. In particular, income taxes payable were reclassified from current liabilities from discontinued operations to income taxes payable from discontinued operations, to reflect that this liability, while related to discontinued operations, remains an obligation of GB Sciences, Inc. and was not deconsolidated upon the sale of the Nevada Subsidiaries on December 31, 2021 (Note 13). The reclassifications had no effect on the reported financial position, results of operations or cash flows of the Company.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Discontinued Operations
See Note 4.
Fair Value of Financial Instruments
The Company adopted ASC 820, Fair Value Measurements and Disclosures (ASC 820). ASC 820 defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosure requirements for fair value measures. The three levels are defined as follows:
|
-
|
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
|
|
-
|
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument.
|
|
-
|
Level 3 inputs to valuation methodology are unobservable and significant to the fair measurement.
|
The carrying value of cash, accounts receivable, accounts payable and accrued expenses are estimated by management to approximate fair value, primarily due to the short-term nature of the instruments.
Cash and Cash Equivalents
The Company considers all short-term investments with an original maturity of three months or less when purchased to be cash equivalents. The Company had no short-term investments classified as cash equivalents at March 31, 2022 and 2021.
Indefinite and Definite-Lived Intangible Assets
Our indefinite-lived intangible assets primarily represent the value of our patents pending and includes the costs paid to draft and file patent applications. Upon issuance of the patents, the indefinite-lived intangible assets will have finite lives. Intangible assets also included the acquisition cost of a cannabis production license with an indefinite life as of March 31, 2021.
We amortize our finite-lived intangible assets, which consist of granted patents, over their estimated useful lives using the straight-line method, and we periodically evaluate the remaining useful lives of our finite-lived intangible assets to determine whether events or circumstances warrant a revision to the remaining period of amortization.
We review all of our intangible assets for impairment indicators throughout the year. Impairment testing for indefinite-lived intangible assets is performed at least annually and we perform testing for definite-lived intangible assets whenever impairment indicators are present. If we determine that the fair value is less than the carrying value of these assets during testing, we record impairment losses equal to the difference between the carrying value of the asset and the fair market value of the asset.
At March 31, 2022, the Company had six patents that have been granted in the United States, including two licensed patents and four patents assigned to the Company's subsidiary, GBS Global Biopharma, Inc. The patents owned by the Company expire between January 2038 and May 2039. Amortization expense for the years ended March 31, 2022 and 2021, was $61,105 and $34,555, respectively. The carrying amount of definite-lived intangible assets was $1,278,318 at March 31, 2022.
There were 18 United States patent applications that are pending as of March 31, 2022, and the corresponding patent assets are treated as indefinite-lived intangible assets. The carrying amount of the indefinite-lived patent assets was $928,667 at March 31, 2022. In addition, the Company had $15,089 of indefinite-lived trademark assets at March 31, 2022.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Operating Lease Right-of-Use Asset and Liability
The Company determines if an arrangement is a lease at inception and had lease agreements for office facilities, equipment, and other space and assets with non-cancelable lease terms which were primarily components of discontinued operations. Certain real estate and property leases, and various other operating leases are measured on the balance sheet with a lease liability and right-of-use asset ("ROU").
ROU assets represent the Company's right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make scheduled lease payments. ROU assets and liabilities are recognized on the lease commencement date based on the present value of lease payments over the lease term. The present value of lease payments is calculated using the incremental borrowing rate at lease commencement, which takes into consideration recent debt issuances as well as other applicable market data available.
Lease payments include fixed payments, variable payments based on an index or rate, reasonably certain purchase options, termination penalties, and others as required by the New Lease Standard. Lease payments do not include variable lease payments other than those that depend on an index or rate, any guarantee by the lessee of the lessor’s debt, or any amount allocated to non-lease components.
Lease terms include options to extend when it is reasonably certain that the option will be exercised. Leases with a term of twelve months or less are not recorded on the balance sheet. Additionally, lease and non-lease components are accounted for as a single lease component for real estate agreements.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets: 3-8 years for machinery and equipment, and leasehold improvements are amortized over the shorter of the estimated useful lives or the underlying lease term. Property under finance leases and related obligations are initially recorded at an amount equal to the present value of future minimum lease payments computed on the basis of the Company’s incremental borrowing rate, and depreciation is recorded on a straight-line basis and is included within depreciation and amortization expense. Repairs and maintenance expenditures which do not extend the useful lives of related assets are expensed as incurred.
Long-Lived Assets
Property and equipment comprised a significant portion of our total assets from discontinued operations as of March 31, 2021. We evaluate the carrying value of property and equipment if impairment indicators are present or if other circumstances indicate that impairment may exist under authoritative guidance. The annual testing date is March 31. When management believes impairment indicators may exist, projections of the undiscounted future cash flows associated with the use of and eventual disposition of property and equipment are prepared. If the projections indicate that the carrying value of the property and equipment are not recoverable, we reduce the carrying values to fair value. These impairment tests are heavily influenced by assumptions and estimates that are subject to change as additional information becomes available.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Beneficial Conversion Feature of Convertible Notes Payable
The Company accounts for convertible notes payable in accordance with the guidelines established by the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 470-20, Debt with Conversion and Other Options and Emerging Issues Task Force (“EITF”) 00-27, “Application of Issue No. 98-5 to Certain Convertible Instruments”. A beneficial conversion feature (“BCF”) exists on the date a convertible note is issued when the fair value of the underlying common stock to which the note is convertible into is in excess of the remaining unallocated proceeds of the note after first considering the allocation of a portion of the note proceeds to the fair value of any attached equity instruments, if any related equity instruments were granted with the debt. In accordance with this guidance, the BCF of a convertible note is measured by allocating a portion of the note's proceeds to the warrants, if applicable, and as a reduction of the carrying amount of the convertible note equal to the intrinsic value of the conversion feature, both of which are credited to additional paid-in-capital. The Company calculates the fair value of warrants issued with the convertible notes using the Black-Scholes valuation model and uses the same assumptions for valuing any employee options in accordance with ASC Topic 718 Compensation – Stock Compensation. The only difference is that the contractual life of the warrants is used.
The value of the proceeds received from a convertible note is then allocated between the conversion features and warrants on a relative fair value basis. The allocated fair value is recorded in the financial statements as a debt discount (premium) from the face amount of the note and such discount is amortized over the expected term of the convertible note (or to the conversion date of the note, if sooner) and is charged to interest expense.
Revenue Recognition
The FASB issued Accounting Standards Codification (“ASC”) 606 as guidance on the recognition of revenue from contracts with customers. Revenue recognition depicts the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also requires disclosures regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented, or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (the cumulative catch-up transition method). The Company adopted the guidance on April 1, 2018 and applied the cumulative catch-up transition method.
The Company’s only material revenue source is part of discontinued operations and derives from sales of cannabis and cannabis products, distinct physical goods. Under ASC 606, the Company is required to separately identify each performance obligation resulting from its contracts from customers, which may be a good or a service. A contract may contain one or more performance obligations. All of the Company’s contracts with customers, past and present, contain only a single performance obligation, the delivery of distinct physical goods. Because fulfillment of the company’s performance obligation to the customer under ASC 606 results in the same timing of revenue recognition as under the previous guidance (i.e. revenue is recognized upon delivery of physical goods), the Company did not record any material adjustment to report the cumulative effect of initial application of the guidance.
Research and Development Costs
Research and development costs are expensed as incurred. During the years ended March 31, 2022 and 2021, the Company recorded $821,321 and $352,274, respectively, in research and development expense, which is included in general and administrative expense in the Company's consolidated financial statements.
Equity-Based Compensation
The Company accounts for equity instruments issued to employees and non-employees in accordance with the provisions of ASC 718 Stock Compensation (ASC 718). The computation of the expense associated with stock-based compensation requires the use of a valuation model. The FASB-issued accounting guidance requires significant judgment and the use of estimates, particularly surrounding Black-Scholes assumptions such as stock price volatility, expected option lives, and expected option forfeiture rates, to value equity-based compensation. We currently use a Black-Scholes option pricing model to calculate the fair value of our stock options. We primarily use historical data to determine the assumptions to be used in the Black-Scholes model and have no reason to believe that future data is likely to differ materially from historical data. However, changes in the assumptions to reflect future stock price volatility and future stock award exercise experience could result in a change in the assumptions used to value awards in the future and may result in a material change to the fair value calculation of stock-based awards. This accounting guidance requires the recognition of the fair value of stock compensation in net income. Although every effort is made to ensure the accuracy of our estimates and assumptions, significant unanticipated changes in those estimates, interpretations and assumptions may result in recording stock option expense that may materially impact our financial statements for each respective reporting period.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in financial statements or tax returns. Deferred tax items are reflected at the enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected reverse. Valuation allowances are established when necessary to reduce deferred tax assets to the amount expected to be realized. Due to the uncertainty regarding the success of future operations, management has valued the deferred tax asset allowance at 100% of the related deferred tax assets.
Because the Company operated in the State-licensed cannabis industry until the December 31, 2021 disposition of the Nevada Subsidiaries (Note 13), revenue from those activities were subject to the limitations of Internal Revenue Code Section 280E (“280E”) for U.S. income tax purposes. Under 280E, the Company is allowed to deduct expenses that are directly related to the production of its products, i.e. cost of goods sold, but is allowed no further deductions for ordinary and necessary business expenses from its gross profit. The Company believes that the deductions disallowed include the deduction of net operating loss carryforwards ("NOLs"). The unused NOLs will continue to carry forward and those that do not expire or become subject to other limitations may be used by the Company to offset future taxable income that is not subject to the limitations of 280E.
Loss per Share
The Company’s basic loss per share has been calculated using the weighted average number of common shares outstanding during the year. The Company had 118,594,624 and 164,049,941 potentially dilutive common shares at March 31, 2022 and 2021, respectively. Such common stock equivalents were not included in the computation of diluted net loss per share, as their inclusion would have been anti-dilutive.
Recent Accounting Pronouncements
Standards Recently Adopted
In May 2021, the FASB issued ASU No. 2021-04, Issuer's Accounting for Certain Modifications or Exchanges of Freestanding Equity-Classified Written Call Options. This guidance clarifies and reduces diversity in an issuer’s accounting for modifications or exchanges of freestanding equity-classified written call options due to a lack of explicit guidance in the FASB Codification. The ASU 2021-04 is effective for The Company's fiscal year beginning April 1, 2022. The Company adopted the standard on April 1, 2022 and it did not have a material impact on its financial statements.
Standards Not Yet Adopted
On June 16, 2016, the FASB issued ASU No. 2016-13, Measurement of Credit Losses on Financial Instruments. The standard requires the use of an “expected loss” model on certain types of financial instruments. The standard also amends the impairment model for available-for-sale debt securities and requires estimated credit losses to be recorded as allowances instead of reductions to amortized cost of the securities. The amendments in this ASU are effective for the Company's fiscal year beginning April 1, 2023. The Company is currently evaluating the impact of ASU 2016-13 on its financial statements.
In June 2020, the FASB issued ASU No. 2020-06, Accounting for Convertible Instruments and Contracts in an Entity's Own Equity. The guidance simplifies the current guidance for convertible instruments and the derivatives scope exception for contracts in an entity’s own equity. Additionally, the amendments affect the diluted EPS calculation for instruments that may be settled in cash or shares and for convertible instruments. This ASU will be effective for the Company's fiscal year beginning April 1, 2024. Early adoption is permitted. The amendments in this update must be applied on either full retrospective basis or modified retrospective basis through a cumulative-effect adjustment to retained earnings/(deficit) in the period of adoption. The Company is currently evaluating the impact of ASU 2020-06 on its consolidated financial statements and related disclosures, as well as the timing of adoption.
All other newly issued accounting pronouncements have been deemed either immaterial or not applicable.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 4 - Discontinued Operations
Discontinued Operations
Discontinued operations comprise those activities that were disposed of during the period, or which were classified as held for sale at the end of the period and represent a separate major line of business or geographical area that can be clearly distinguished for operational and financial reporting purposes. The Company has included its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC in discontinued operations due to the sale of the Company's Nevada Subsidiaries (Note 13).
The assets and liabilities associated with discontinued operations included in our consolidated balance sheets as of March 31, 2022 and 2021 were as follows:
| |
|
March 31, 2022
|
|
|
March 31, 2021
|
|
| |
|
Continuing
|
|
|
Discontinued Nevada Subsidiaries
|
|
|
Total
|
|
|
Continuing
|
|
|
Discontinued Nevada Subsidiaries
|
|
|
Total
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$ |
233,893 |
|
|
$ |
- |
|
|
$ |
233,893 |
|
|
$ |
793,040 |
|
|
$ |
352,593 |
|
|
$ |
1,145,633 |
|
|
Accounts receivable, net
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
400,175 |
|
|
|
400,175 |
|
|
Inventory, net
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
1,689,304 |
|
|
|
1,689,304 |
|
|
Prepaid and other current assets
|
|
|
93,933 |
|
|
|
- |
|
|
|
93,933 |
|
|
|
256,251 |
|
|
|
52,492 |
|
|
|
308,743 |
|
|
TOTAL CURRENT ASSETS
|
|
|
327,826 |
|
|
|
- |
|
|
|
327,826 |
|
|
|
1,049,291 |
|
|
|
2,494,564 |
|
|
|
3,543,855 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
25,022 |
|
|
|
4,876,247 |
|
|
|
4,901,269 |
|
|
Intangible assets, net
|
|
|
2,222,074 |
|
|
|
- |
|
|
|
2,222,074 |
|
|
|
1,706,762 |
|
|
|
571,264 |
|
|
|
2,278,026 |
|
|
Deposits and other noncurrent assets
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
82,904 |
|
|
|
82,904 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$ |
2,549,900 |
|
|
$ |
- |
|
|
$ |
2,549,900 |
|
|
$ |
2,781,075 |
|
|
$ |
8,024,979 |
|
|
$ |
10,806,054 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$ |
1,657,008 |
|
|
$ |
- |
|
|
$ |
1,657,008 |
|
|
$ |
1,412,459 |
|
|
$ |
509,477 |
|
|
$ |
1,921,936 |
|
|
Accrued interest
|
|
|
384,769 |
|
|
|
- |
|
|
|
384,769 |
|
|
|
493,741 |
|
|
|
49,211 |
|
|
|
542,952 |
|
|
Accrued liabilities
|
|
|
9,627 |
|
|
|
- |
|
|
|
9,627 |
|
|
|
957,946 |
|
|
|
105,421 |
|
|
|
1,063,367 |
|
|
Notes and convertible notes payable, net
|
|
|
987,565 |
|
|
|
- |
|
|
|
987,565 |
|
|
|
3,594,804 |
|
|
|
485,000 |
|
|
|
4,079,804 |
|
|
Indebtedness to related parties
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
84,913 |
|
|
|
- |
|
|
|
84,913 |
|
|
Income tax payable
|
|
|
896,495 |
|
|
|
- |
|
|
|
896,495 |
|
|
|
761,509 |
|
|
|
- |
|
|
|
761,509 |
|
|
Finance lease obligations, current
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
143,967 |
|
|
|
143,967 |
|
|
TOTAL CURRENT LIABILITIES
|
|
|
3,935,464 |
|
|
|
- |
|
|
|
3,935,464 |
|
|
|
7,305,372 |
|
|
|
1,293,076 |
|
|
|
8,598,448 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Convertible notes payable, net
|
|
|
397,308 |
|
|
|
- |
|
|
|
397,308 |
|
|
|
292,410 |
|
|
|
- |
|
|
|
292,410 |
|
|
Finance lease obligations, long term
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
3,389,124 |
|
|
|
3,389,124 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
$ |
4,332,772 |
|
|
$ |
- |
|
|
$ |
4,332,772 |
|
|
$ |
7,597,782 |
|
|
$ |
4,682,200 |
|
|
$ |
12,279,982 |
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The revenues and expenses associated with discontinued operations included in our consolidated statements of operations for the years ended March 31, 2022 and 2021, were as follows:
| |
|
For the Year Ended March 31,
|
|
|
For the Year Ended March 31,
|
|
| |
|
2022
|
|
|
2021
|
|
| |
|
Continuing
|
|
|
Discontinued - Nevada
|
|
|
Total
|
|
|
Continuing
|
|
|
Discontinued - Nevada
|
|
|
Total
|
|
|
Sales revenue
|
|
$ |
- |
|
|
$ |
3,369,812 |
|
|
$ |
3,369,812 |
|
|
$ |
- |
|
|
$ |
4,110,456 |
|
|
$ |
4,110,456 |
|
|
Cost of goods sold
|
|
|
- |
|
|
|
(3,072,622 |
) |
|
|
(3,072,622 |
) |
|
|
- |
|
|
|
(3,506,722 |
) |
|
|
(3,506,722 |
) |
|
Gross profit (loss)
|
|
|
- |
|
|
|
297,190 |
|
|
|
297,190 |
|
|
|
- |
|
|
|
603,734 |
|
|
|
603,734 |
|
|
General and administrative expenses
|
|
|
1,868,734 |
|
|
|
264,515 |
|
|
|
2,133,249 |
|
|
|
2,001,617 |
|
|
|
276,986 |
|
|
|
2,278,603 |
|
|
LOSS FROM OPERATIONS
|
|
|
(1,868,734 |
) |
|
|
32,675 |
|
|
|
(1,836,059 |
) |
|
|
(2,001,617 |
) |
|
|
326,748 |
|
|
|
(1,674,869 |
) |
|
OTHER INCOME/(EXPENSE)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gain on extinguishment
|
|
|
22,405 |
|
|
|
- |
|
|
|
22,405 |
|
|
|
467,872 |
|
|
|
- |
|
|
|
467,872 |
|
|
Gain on settlement of accounts payable
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
422,414 |
|
|
|
54,958 |
|
|
|
477,372 |
|
|
Gain on deconsolidation
|
|
|
5,206,208 |
|
|
|
- |
|
|
|
5,206,208 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Interest expense
|
|
|
(474,768 |
) |
|
|
(302,923 |
) |
|
|
(777,691 |
) |
|
|
(1,285,460 |
) |
|
|
(486,481 |
) |
|
|
(1,771,941 |
) |
|
Loss on modification of line of credit
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(650,000 |
) |
|
|
- |
|
|
|
(650,000 |
) |
|
Loss on impairment of note receivable
|
|
|
(3,025,000 |
) |
|
|
- |
|
|
|
(3,025,000 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Debt default penalty
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(286,059 |
) |
|
|
- |
|
|
|
(286,059 |
) |
|
Loss on disposal
|
|
|
(15,639 |
) |
|
|
- |
|
|
|
(15,639 |
) |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Other income
|
|
|
9,000 |
|
|
|
20,889 |
|
|
|
29,889 |
|
|
|
- |
|
|
|
(118,875 |
) |
|
|
(118,875 |
) |
|
TOTAL OTHER INCOME/(EXPENSE)
|
|
|
1,722,206 |
|
|
|
(282,034 |
) |
|
|
1,440,172 |
|
|
|
(1,331,233 |
) |
|
|
(550,398 |
) |
|
|
(1,881,631 |
) |
|
NET LOSS BEFORE INCOME TAXES
|
|
|
(146,528 |
) |
|
|
(249,359 |
) |
|
|
(395,887 |
) |
|
|
(3,332,850 |
) |
|
|
(223,650 |
) |
|
|
(3,556,500 |
) |
|
Income tax expense
|
|
|
- |
|
|
|
(134,986 |
) |
|
|
(134,986 |
) |
|
|
- |
|
|
|
(168,527 |
) |
|
|
(168,527 |
) |
|
NET LOSS
|
|
$ |
(146,528 |
) |
|
$ |
(384,345 |
) |
|
$ |
(530,873 |
) |
|
$ |
(3,332,850 |
) |
|
$ |
(392,177 |
) |
|
$ |
(3,725,027 |
) |
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Discontinued Operations - Accounts Receivable
Accounts receivable are carried at their estimated collectible amounts. Trade accounts receivable are periodically evaluated for collectability based on aging and subsequent collections. There were no accounts receivable at March 31, 2022 due to the deconsolidation of the Nevada Subsidiaries (Note 13). Accounts receivable are included in current assets from discontinued operations in the Company's consolidated balance sheet at March 31, 2021.
Discontinued Operations - Inventory
We value inventory at the lower of the actual cost of our inventory, as determined using the first-in, first-out method, or its current estimated market value. We periodically review our physical inventory for excess, obsolete, and potentially impaired items and reserve accordingly. Our reserve estimate for excess and obsolete inventory is based on expected future use. Indirect costs, which primarily related to the lease and operation costs of the Teco Facility, are allocated based on square footage of the facility used in the production of inventory.
Raw materials consist of supplies, materials, and consumables used in the cultivation and extraction processes. Work-in-progress consisted of live plants and cannabis in the drying, curing, and trimming processes. Finished goods includes completed cannabis flower, trim, and extracts in bulk and packaged forms. There is no remaining inventory on the Company's consolidated balance sheet as of March 31, 2022, due to the sale and deconsolidation of the Nevada Subsidiairies (Note 13). Inventory is included in current assets from discontinued operations in the Company's consolidated balance sheet at March 31, 2021.
| |
|
March 31, 2022
|
|
|
March 31, 2021
|
|
|
Inventory (discontinued operations)
|
|
|
|
|
|
|
|
|
|
Raw materials
|
|
$ |
- |
|
|
$ |
86,076 |
|
|
Work in progress
|
|
|
- |
|
|
|
743,844 |
|
|
Finished goods
|
|
|
- |
|
|
|
866,195 |
|
|
Subtotal
|
|
|
- |
|
|
|
1,696,115 |
|
|
Allowance to reduce inventory to NRV
|
|
|
- |
|
|
|
(6,811 |
) |
|
Total inventory (discontinued operations)
|
|
$ |
- |
|
|
$ |
1,689,304 |
|
Discontinued Operations - Deposits and Noncurrent Assets
Deposits and noncurrent assets from discontinued operations were $82,904 at March 31, 2021. There were no deposits and noncurrent assets from discontinued operations at March 31, 2022, due to the deconsolidation of the Nevada Subsidiaries. Deposits and noncurrent assets are included in long term assets from discontinued operations in the Company's consolidated balance sheet at March 31, 2021.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Discontinued Operations - Leases
The Company evaluates all finance and operating leases, and they are measured on the balance sheet with a lease liability and right-of-use asset (“ROU”) at inception. ROU assets represent the Company’s right to use an underlying asset for the lease term, and lease liabilities represent the obligation to make scheduled lease payments. Lease terms include options to extend when it is reasonably certain that the option will be exercised. Leases with a term of 12 months or less are not recorded on the consolidated balance sheet. The present value of lease payments is calculated using the incremental borrowing rate at lease commencement, which takes into consideration recent debt issuances as well as other applicable market data available.
The Company's only remaining lease commitment was a finance lease for the Nevada Subsidiaries, which is classified as discontinued operations in the Company's consolidated balance sheet at March 31, 2021. This lease had a remaining non-cancelable term ending December 31, 2025 with an option to extend through December 31, 2030. The rate used to discount this lease was 11.5%.
Finance leases are included in property and equipment (long term assets from discontinued operations), finance lease obligations, short term (current liabilities from discontinued operations), and finance lease obligations, long term (long term liabilities from discontinued operations), on the balance sheet as of March 31, 2021.
The right-of-use asset and lease liability were deconsolidated at December 31, 2021 due to the close of the sale of the Nevada Subsidiaries. The lease costs included in discontinued operations for the years ended March 31, 2022 and 2021 are set forth in the table below:
| |
|
|
March 31,
|
|
|
Lease costs (discontinued operations)
|
Classification on the Statements of Operations
|
|
|
2022 |
|
|
|
2021 |
|
|
Discontinued operations:
|
|
|
|
|
|
|
|
|
|
|
Finance leases - amortization of ROU assets
|
Loss from discontinued operations
|
|
$ |
116,024 |
|
|
$ |
154,699 |
|
|
Finance leases - interest on lease liabilities
|
Loss from discontinued operations
|
|
|
301,796 |
|
|
|
414,993 |
|
|
Operating leases
|
Loss from discontinued operations
|
|
|
- |
|
|
|
3,243 |
|
|
Total lease cost, discontinued operations
|
|
$ |
417,820 |
|
|
$ |
572,935 |
|
Discontinued Operations - 8% Line of Credit dated November 27, 2019
In connection with the Binding Letter of Intent dated November 27, 2019 (Note 13), the Teco Subsidiaries entered into a promissory note and line of credit for up to $470,000 from the purchaser of the membership interests in the Teco Subsidiaries. The purpose of the line of credit was to supply working capital for the Teco Subsidiaries, and the note matures upon the close of the sale of the Teco Subsidiaries. The principal and accrued interest balances outstanding at the time of closing will be considered paid in full upon closing and will not reduce the purchase price received by GB Sciences. In total, the Teco Subsidiaries received $485,000 in advances under the line of credit, reflecting an informal agreement with the lender to increase the line of credit by $15,000. On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility, which provided that no further interest would accrue on the line of credit after November 30, 2020. The balance of the line of credit was $485,000 at December 31, 2021 and accrued interest was $49,211, prior to deconsolidation. The note and related interest expense are included in current liabilities from discontinued operations and loss from discontinued operations on the Company's March 31, 2021 balance sheet.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 5 – Notes Payable and Line of Credit
0% Note Payable dated October 23, 2017
On October 23, 2017, the Company amended the existing Nevada Medical Marijuana Production License Agreement (“Amended Production License Agreement”). Per the terms of the Amended Production License Agreement, GB Sciences purchased the remaining percentage of the production license resulting in the 100% ownership of the license. GB Sciences also received 100% ownership of the cultivation license included in the original Nevada Medical Marijuana Production License Agreement. In exchange, GB Sciences made one-time payment of $500,000 and issued a 0% Promissory Note in the amount of $700,000 payable in equal monthly payments over a three-year period commencing on January 1, 2018. The present value of the note was $521,067 on the date of its issuance based on an imputed interest rate of 20.3% and the Company recorded a discount on notes payable of $178,933 related to the difference between the face value and present value of the note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") for the sale of its interest in GB Sciences Nopah, LLC. The Nopah sale was closed December 31, 2021 after successful transfer of the Nevada Medical Marijuana Cultivation Facility Registration Certificate on December 14, 2021 (Note 13). At close, the principal balance of the note was reduced from $369,445 to $190,272 and accounts payable totaling $74,647 to an affiliate of the purchaser were extinguished.
On March 4, 2022, the Company entered into the Second Promissory Note Modification Agreement, which reduced the total outstanding balance of principal and interest from $201,532 (at the time of the agreement) to $179,127 and modified the terms of the note to provide that the Company would make an immediate payment of $75,000, with $5,000 monthly payments thereafter until the note is repaid in full. The modification also provided that the note would bear interest at 8.0% per annum.
We evaluated the modification under the guidance in ASC 470-50 and determined that the modification represents an extinguishment because the change in the fair value of the note exceeded 10% of the carrying value of the note immediately prior to the modification. As a result, the Company recorded a gain on extinguishment of $22,405 equal to the change in the carrying value of the note resulting from the modification.
The Company made a $75,000 payment pursuant to the terms of the modification on March 4, 2022. At March 31, 2022, the outstanding balance of the note was $104,127 and accrued interest was $616.
8% Line of Credit dated July 24, 2020
On July 24, 2020, the Company entered into the Loan Agreement, 8% Secured Promissory Note, and Security Agreement (together, the "July 24 Note") with AJE Management, LLC, which established a revolving loan of up to $500,000 that the Company may draw on from time to time. The loan was collateralized by the Teco Facility, subject to the pre-existing lien held by CSW Ventures, L.P. in connection with the 8% Senior Secured Convertible Promissory Note dated February 28, 2019. Contemporaneously with the Loan Agreement, the Company and AJE Management entered into the Amendment to the Membership Interest Purchase Agreement with AJE Management. The amendment provides that any balances outstanding under the July 24 Note at the time of the close of the sale of the Teco Facility would be forgiven in exchange for a reduction to the $4,000,000 note receivable that the Company will receive as consideration for the sale of the Teco Facility. The reduction to the note receivable would be equal to 3 times the balance outstanding under the July 24 Note on the date of the close of the sale of the Teco Facility. The balance outstanding under the note plus accrued interest were permitted to be repaid at any time prior to the close of the sale of the Teco facility.
On December 29, 2020, the Company entered into the Omnibus Amendment with the purchaser of the Teco Facility. The Omnibus Amendment reduced the amount of the note receivable that the Company was to receive from the sale of the Teco Facility by $975,000 (three times $325,000 in advances made under the July 24 Note) to $3,025,000. Any advances made to the Company under the July 24 Note in excess of $325,000 were to reduce the amount of cash received upon close of the sale of Teco one-for-one, i.e., such advances would be considered advance payments of the $4,000,000 cash purchase price. No interest would accrue after November 30, 2020. The Company also agreed that it would not repay the balances outstanding under the July 24 Note prior to the closing of the Teco sale. As a result of the Omnibus Amendment, the Company accrued a modification expense of $650,000 during the year ended March 31, 2021. Prior to December 31, 2021, the Company received $50,000 in additional advances above $325,000 during the fiscal year ended March 31, 2021, bringing the total balance to $1,025,000, and accrued interest was $12,510. Upon close of the Teco sale on December 31, 2021, the note and accrued interest balances were forgiven and the Company has no further obligations related to the line of credit (Note 13).
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Summary of Notes Payable
As of March 31, 2022, the following notes payable were recorded in the Company’s consolidated balance sheet:
| |
|
As of March 31, 2022
|
|
| |
|
Face Value
|
|
|
Discount
|
|
|
Carrying Value
|
|
|
0% Note Payable dated October 23, 2017 (as amended), current portion (Note 5)
|
|
$ |
54,330 |
|
|
$ |
- |
|
|
$ |
54,330 |
|
|
6% Convertible promissory notes payable (Note 6)
|
|
|
560,000 |
|
|
|
- |
|
|
|
560,000 |
|
|
6% Convertible notes payable due January 18, 2022 (Note 6)
|
|
|
325,000 |
|
|
|
- |
|
|
|
325,000 |
|
|
6% Convertible note payable due July 1, 2022 (Note 6)
|
|
|
50,000 |
|
|
|
(1,765 |
) |
|
|
48,235 |
|
|
Total short-term notes and convertible notes payable
|
|
$ |
989,330 |
|
|
$ |
(1,765 |
) |
|
$ |
987,565 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
6% Convertible promissory notes payable due September 30, 2023 (Note 6)
|
|
$ |
197,000 |
|
|
$ |
(26,254 |
) |
|
$ |
170,746 |
|
|
6% Convertible note payable due December 31, 2023 (Note 6)
|
|
|
250,000 |
|
|
|
(73,235 |
) |
|
|
176,765 |
|
|
0% Note Payable dated October 23, 2017 (as amended), long term portion (Note 5)
|
|
|
49,797 |
|
|
|
- |
|
|
|
49,797 |
|
|
Total long term convertible notes payable classified as continuing operations
|
|
$ |
496,797 |
|
|
$ |
(99,489 |
) |
|
$ |
397,308 |
|
As of March 31, 2021, the following notes payable were recorded in the Company’s consolidated balance sheet:
| |
|
As of March 31, 2021
|
|
| |
|
Face Value
|
|
|
Discount
|
|
|
Carrying Value
|
|
|
0% Note Payable dated October 23, 2017 (Note 5)
|
|
$ |
369,445 |
|
|
$ |
- |
|
|
$ |
369,445 |
|
|
8% Line of Credit dated November 27, 2019 (Note 5)
|
|
|
485,000 |
|
|
|
- |
|
|
|
485,000 |
|
|
8% Line of Credit dated July 24, 2020 (Note 5)
|
|
|
1,025,000 |
|
|
|
- |
|
|
|
1,025,000 |
|
|
6% Convertible promissory notes payable (Note 6)
|
|
|
1,060,000 |
|
|
|
- |
|
|
|
1,060,000 |
|
|
8% Convertible Secured Promissory Note dated February 28, 2019, as amended (Note 6)
|
|
|
1,111,863 |
|
|
|
- |
|
|
|
1,111,863 |
|
|
6% Convertible notes payable due January 18, 2022 (Note 6)
|
|
|
325,000 |
|
|
|
(296,504 |
) |
|
|
28,496 |
|
|
Total short-term notes and convertible notes payable
|
|
|
4,376,308 |
|
|
|
(296,504 |
) |
|
|
4,079,804 |
|
|
Less: Notes payable classified as discontinued operations
|
|
|
(485,000 |
) |
|
|
- |
|
|
|
(485,000 |
) |
|
Total short term notes and convertible notes payable classified as continuing operations
|
|
$ |
3,891,308 |
|
|
$ |
(296,504 |
) |
|
$ |
3,594,804 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
6% Convertible promissory notes payable due September 30, 2023 (Note 6)
|
|
$ |
197,000 |
|
|
$ |
(40,561 |
) |
|
$ |
156,439 |
|
|
6% Convertible note payable due December 31, 2023 (Note 6)
|
|
|
250,000 |
|
|
|
(114,029 |
) |
|
|
135,971 |
|
|
Total long term convertible notes payable classified as continuing operations
|
|
$ |
447,000 |
|
|
$ |
(154,590 |
) |
|
$ |
292,410 |
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 6 – Convertible Notes
March 2017 and July 2017 Convertible Note Offerings
In March 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.60 per share for the period of three years. Between March 2017 and May 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $2,000,000. The Notes are payable within three years of issuance and are convertible into 8,000,000 shares of the Company’s common stock. The Company also issued 8,000,000 common stock warrants to the Noteholders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $1,933,693, which included $904,690 related to the relative fair value of beneficial conversion features and $1,029,003 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
In July 2017, the Company entered into a Placement Agent’s Agreement with a third-party brokerage firm to offer units consisting of a $1,000 6% promissory note convertible into 4,000 shares of the Company’s common stock at $0.25 per share and 4,000 warrants to purchase shares of the Company’s’ common stock at an exercise price of $0.65 per share for the period of three years. Between July 2017 and December 2017, the Company issued short-term Promissory Notes (“Notes”) to various holders with combined face value of $7,201,000. The Notes are payable within three years of issuance and are convertible into 28,804,000 shares of the Company’s common stock. The Company also issued 28,804,000 common stock warrants to the Note holders. The warrants are exercisable at any time and from time to time before maturity at the option of the holder. Each warrant gives the Noteholder the right to purchase one share of common stock of the Company at an exercise price of $0.60 per share for a period of three years. The Company recorded an aggregate discount on convertible notes of $7,092,796, which included $3,142,605 related to the relative fair value of beneficial conversion features and $3,950,191 for the relative fair value of the warrants issued with each note. The fair value of warrants was derived using the Black-Scholes valuation model.
All notes from the March and July 2017 offerings have passed their maturity dates. During the year ended March 31, 2022, the Company agreed to extensions with the holders of a total of $197,000 of the $1,257,000 that remains outstanding. For the $197,000 of extended notes, the Company agreed to reduce the conversion price to $0.10 per share and issued a total of 788,000 additional warrants to the holders of the notes with a term of three years and an exercise price of $0.10 per share. In exchange, the maturity date of the notes was extended to September 30, 2023. Using the Black-Scholes model, the Company valued the warrants at $13,396 and the change in the fair value of the conversion feature at $33,490. Because the change in the fair value of the conversion feature exceeded 10% of the carrying amount of the notes, the Company accounted for the modification of the notes as an extinguishment and recorded a discount on the new convertible notes of $46,886 related to the fair value of the new warrants issued and the change in the fair value of the conversion feature. The Company recorded interest expense of $26,127 on the new notes during the year ended March 31, 2022, of which $14,306 represented amortization of the note discounts. Accrued interest on the $197,000 extended notes is $56,152 at March 31, 2022, which includes $38,438 accrued prior to the extinguishments.
Three convertible notes totaling $1,060,000 held by the same investor are past maturity and are currently in default. On January 20, 2022, the Company repaid $500,000 of the principal balances owed to the investor, and one convertible note in the amount of $560,000 remains outstanding plus accrued interest on all three notes totaling $286,119. The Company intends to negotiate the terms of an extension of the remaining note and accrued interest with the note holder. The notes do not provide for a default penalty or penalty interest rate. Interest expense for the notes was $57,846 during the year ended March 31, 2022.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
8% Senior Secured Convertible Promissory Note dated February 28, 2019
On February 28, 2019, the Company issued a $1,500,000 8% Senior Secured Convertible Promissory Note and entered into the Note Purchase Agreement and Security Agreement with CSW Ventures, L.P. (together, “CSW Note”). The note matured on August 28, 2020, and was convertible at any time until maturity into 8,823,529 shares of the Company’s common stock at $0.17 per share. Collateral pledged as security for the note includes all of the Company’s 100% membership interests in GB Sciences, Nevada, LLC and GB Sciences Las Vegas, LLC, which together represent substantially all of the Company’s cannabis cultivation and production operations and assets located at the Teco facility in Las Vegas, Nevada.
On December 29, 2020, the Company entered into the Omnibus Amendment, and the note holder agreed to cease interest accrual on the CSW Note after November 30, 2020. After conversions, the remaining principal balance and carrying amount of the note was $1,111,863 as of December 31, 2021. Accrued interest was $144,994.
Upon close of the Teco sale on December 31, 2021, the note and accrued interest balances were extinguished in exchange for a reduction of 110% of the balances of accrued interest and principal outstanding to the $4 million cash payment (Note 13). The 10% increase to the balances owed under the note totaled $125,686, and the Company recorded that amount as a reduction of the gain on deconsolidation.
8% Convertible Promissory Note dated April 23, 2019
On April 23, 2019, the Company entered into the Note Purchase Agreement with Iliad Research and Trading, L.P. ("Iliad") and issued an 8% Convertible Promissory Note with a face value of $2,765,000. The Note was issued with original issue discount of $265,000 and is convertible into shares of the Company’s common stock at a price of $0.17 per share at the option of the note holder at any time until the Note is repaid. The Note matured on April 22, 2020. A total discount of $440,000 was recorded on the note, which includes $265,000 of original issue discount and $175,000 in fees paid to brokers.
During the year ended March 31, 2020, the Company honored the conversion of a total of a total of $125,000 of accrued interest on the Iliad Note at reduced conversion rates. On October 30, 2019, the Company received notice of the conversion of $75,000 at $0.06 per share and issued 1,250,000 shares of its common stock. The fair value of the shares issued exceeded the fair value of the shares issuable under the original terms of the Note by $64,706, and the Company recorded an induced conversion expense. On November 18, 2019, the Company received notice of the conversion of $50,000 of the note balance at $0.0375 per share and issued 1,333,333 shares of its common stock.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On April 22, 2020, the Company failed to make payment of the principal and accrued interest due under the Iliad Note, resulting in a default. Upon the occurrence of the default, the principal and accrued interest balances outstanding increased by 10%. As the result of the default, Company recorded an expense of $9,559 related to a 10% increase in the accrued interest balance and $276,500 related to the 10% increase in the principal balance, totaling $286,059 which is recorded as debt default penalty on the statement of operations for the year ended March 31, 2021.
On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. The lawsuit further sought to compel the Company to participate in arbitration pursuant to the arbitration provisions contained within the Note Purchase Agreement and to prohibit the Company to raise funds through the issuance of its common stock unless the note is paid in full simultaneously with such issuance. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594 plus reasonable attorney's fees and costs and accrued post-judgment interest at the default rate of 15% per annum.
On November 20, 2020, the Company, Iliad, and Wellcana Plus, LLC entered into the Judgment Settlement Agreement, whereby Iliad agreed to discharge all amounts owed to it by the Company upon receipt of payment totaling $3,006,015 directly from the proceeds of the Wellcana Note Receivable on or before December 8, 2020. On December 8, 2020, Wellcana failed to make payment to the Company. On December 9, 2020, the Company entered into a letter agreement with Iliad extending the Judgment Settlement agreement in exchange for payment of $25,000 plus $25,000 per week until the payment totaling $3,006,015 is received by Iliad, with such payments not reducing the amount owed under the Judgment Settlement Agreement. On December 16, 2020, Wellcana made payment of the full amount owed to the Company, of which $3,006,015 was paid directly to Iliad in full satisfaction of the Judgment Settlement Agreement. On December 18, 2020, Iliad filed a Satisfaction of Judgment in the Third Judicial District Court of Salt Lake County in the State of Utah, and the lawsuit was dismissed. The Company has no further obligations to Iliad.
December 2020 $625,000 6% Convertible Notes
On December 18, 2020, the Company began an offering of 6.0% convertible notes for the purpose of funding a pre-clinical study of the Company's patent-pending Cannabinoid-Containing Complex Mixtures for the treatment of Cytokine Release Syndromes, including Acute Respiratory Distress Syndrome, in COVID-19 patients. The Company pledged the related intellectual property as security for the notes. The notes are convertible at a rate of $0.05 per share at the lender's request. To date, the Company has issued $625,000 in convertible notes under the offering to three investors. $375,000 of the notes mature between January 31, 2021 and July 1, 2022, and $250,000 mature in December 2023. Payment of accrued interest and principal is due at maturity. The Company received cash of $543,750, net of brokerage fees, and recorded discounts on the convertible notes totaling $81,250 related to the issuance costs. Notes totaling $425,000 were issued with in-the-money conversion features, and the Company recorded beneficial conversion feature discounts totaling $347,000 on the related notes. During the year ended March 31, 2022, the Company received $50,000 related to the note offering and recorded a discount on convertible notes payable of $6,500 related to issuance costs.
At March 31, 2022, notes with a carrying amount of $373,235 were included in short term notes and convertible notes payable, net of unamortized discounts of $1,765. Notes with a carrying amount of $176,765 were included in long term notes and convertible notes payable, net of unamortized discounts of $73,235. Interest expense related to the notes was $378,777 for the year ended March 31, 2022, which includes $342,033 from amortization of the note discounts.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 7 - Property and Equipment
Property and equipment is recorded at cost and depreciated using the straight-line method over the estimated useful life of the asset or, in the case of leasehold improvements amortized over the lesser of the useful life of the asset or the underlying lease term. We recorded depreciation expense of $25,775 and $34,654 on a consolidated basis for the years ended year ended March 31, 2022 and 2021, respectively, net of depreciation capitalized in inventory of $349,015 and $532,785. Discontinued operations included $16,391 and $21,855 for the years ended March 31, 2022 and 2021, respectively. At March 31, 2022, there was no remaining property and equipment as the result of the completed disposition of the Nevada Subsidiaries (Note 13).
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 8 – Income Taxes
The Company files income tax returns in the U.S. federal jurisdiction. The Company operates in the state of Nevada, which does not levy an income tax. The Company has analyzed filing positions for all open tax years in the federal jurisdiction where it is required to file income tax returns. The Company identified its federal tax return as its “major” tax jurisdiction, as defined under generally accepted accounting principles.
The Company’s effective tax rate was 1.8% and-3.6% for the years ended March 31, 2022 and 2021, respectively.
Income tax expense was $134,986 for the year ended March 31, 2022, which includes $53,955 in penalties and $22,754 in accrued interest related to a $506,145 tax liability from the March 31, 2018 tax year, as well as a $178,727 tax liability from the March 31, 2021 tax tear. Income tax payable at March 31, 2022 was $896,495 including accrued penalties and interest of $207,945. Income tax expense was $168,527 for the year ended March 31, 2021. This amount represents penalties and interest on the March 31, 2018 tax liability. Income tax payable was $592,982 as of March 31, 2021. Income tax expense and income tax payable are included in discontinued operations in the Company's financial statements for the years ended March 31, 2022 and 2021.
Because the Company operates in the State-licensed cannabis industry, it is subject to the limitations of Internal Revenue Code Section 280E (“280E”) for U.S. income tax purposes. Under 280E, the Company is allowed to deduct expenses that are directly related to the production of its products, i.e., cost of goods sold, but is allowed no further deductions for ordinary and necessary business expenses from its gross profit. The Company believes that the deductions disallowed include the deduction of NOLs. The unused NOLs will continue to carry forward and may be used by the Company to offset future taxable income that is not subject to the limitations of 280E.
At March 31, 2022 and 2021 respectively, the Company had net operating loss carryforwards (“NOLs”) for income tax purposes of $51,507,562 and $51,063,886. $34,481,122 of the Company's NOL carryforwards are expected to expire at various times from 2025 through 2039. $17,026,440 of the NOL carryforwards generated in tax years ending March 31, 2019 to present have no expiration date. These NOLs have the potential to be used to offset future ordinary taxable income and reduce future cash tax liabilities. Utilization of the Company’s net operating losses may be subject to substantial annual limitation if the Company experiences a 50% change in ownership, as provided by the Internal Revenue Code. Such an ownership change would substantially increase the possibility of net operating losses expiring before complete utilization.
The provision for income taxes included in discontinued operations is different than would result from applying the U.S. statutory rate to profit before taxes for the reasons set forth in the following reconciliation:
| |
|
2022
|
|
|
2021
|
|
|
Tax expense/(benefit) computed at U.S. statutory rates
|
|
$ |
691,042 |
|
|
$ |
(697,040 |
) |
|
Increases (decreases) in taxes resulting from:
|
|
|
|
|
|
|
|
|
|
IRC Section 280E
|
|
|
132,063 |
|
|
|
173,045 |
|
|
Other permanent items
|
|
|
5,526 |
|
|
|
14,407 |
|
|
Change in valuation allowance
|
|
|
579,861 |
|
|
|
26,720 |
|
|
Adjustments to valuation of deferred tax assets
|
|
|
(1,408,492 |
) |
|
|
603,319 |
|
|
Tax return true-up
|
|
|
58,277 |
|
|
|
- |
|
|
Total provision for income taxes
|
|
|
58,277 |
|
|
|
120,451 |
|
|
Penalties and interest on prior year tax liabilities
|
|
|
76,709 |
|
|
|
48,076 |
|
|
Total income tax expense
|
|
$ |
134,986 |
|
|
$ |
168,527 |
|
The tax effects of the primary temporary differences giving rise to the Company’s deferred tax assets and liabilities are as follows for the year ended March 31, 2022 and 2021:
| |
|
2022
|
|
|
2021
|
|
|
Deferred tax assets:
|
|
|
|
|
|
|
|
|
|
Stock based compensation
|
|
$ |
3,144,084 |
|
|
$ |
3,131,344 |
|
|
Net operating loss carryforward
|
|
|
10,816,588 |
|
|
|
10,460,788 |
|
|
Impairment of long-lived assets
|
|
|
- |
|
|
|
975,461 |
|
|
Depreciation and Amortization expense
|
|
|
(1,369 |
) |
|
|
(458,938 |
) |
|
Other temporary items
|
|
|
(209,714 |
) |
|
|
220,795 |
|
|
Total deferred tax assets
|
|
|
13,749,589 |
|
|
|
14,329,450 |
|
|
Less valuation allowance
|
|
|
(13,749,589 |
) |
|
|
(14,329,450 |
) |
|
Net deferred tax asset
|
|
$ |
- |
|
|
$ |
- |
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Deferred tax assets are evaluated by considering historical levels of income, estimates of future taxable income and the impact of tax planning strategies. The Company continues to evaluate its deferred tax asset valuation allowance on a quarterly basis. The Company concluded that, as of March 31, 2022, it is more likely than not that the Company will not have sufficient taxable income within the applicable net operating loss carry-forward period to realize any portion of its deferred tax assets.
The Company believes that the tax positions taken in its tax returns would be sustained upon examination by taxing authorities. The Company files income tax returns in the U.S. federal jurisdiction and other required state jurisdictions. The Company's periodic tax returns filed in 2019 and thereafter are subject to examination by taxing authorities under the normal statutes of limitations in the applicable jurisdictions.
Note 9 – Capital Transactions
Year Ended March 31, 2022
On April 1, 2020, the Company entered into the Advisory Agreement with its brokers and effected a temporary decrease in the exercise price of the Company's outstanding warrants to $0.03-$.05 per share. On July 18, 2021, the Company entered into an amendment to the Advisory Agreement extending the temporary decrease through September 30, 2021 and agreed that each exercising warrant holder will receive an equal number of replacement warrants to purchase one share of the Company's common stock at $0.10 for three years. During the year ended March 31, 2022, the Company received notice of the exercise of 2,095,333 warrants at $0.03 per share and received proceeds of $56,394, net of brokerage fees of $6,266. As the result of the exercises, the Company recorded an inducement dividend of $163,017, which includes $62,660 related to the intrinsic value of the exercised warrants at the dates of exercise and $100,357 related to the Black-Scholes fair value of the 2,088,667 replacement warrants issued to the exercising investors.
On March 1, 2022, the Company received notices for the exercise of 7,601,813 compensation warrants previously issued for services, at an exercise price of $0.01 per share, and received proceeds of $76,068.
During the year ended March 31, 2022, the Company issued 600,000 options to purchase one share of common stock at $0.05 per share for ten years to a researcher as compensation for drafting and filing six provisional USPTO patent applications related to the Company's PhAROS platform. The options were valued at $0.048 per share using the Black-Scholes model, and the Company recorded an addition of $28,800 to the related patent assets and equity.
During the year ended March 31, 2022, the Company recorded $60,667 expense related to unvested employee options issued in prior periods. Remaining unrecognized compensation expense related to the options was $17,333 at March 31, 2022.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Year Ended March 31, 2021
On April 1, 2020, the Company entered into the Advisory Agreement with its brokers and effected a temporary decrease in the exercise price of the Company's outstanding warrants to $0.03-$.05 per share. As a result of the price reduction, the Company received notice of the exercise of 35,798,809 warrants during the year ended March 31, 2021, and received proceeds of $968,023, net of brokerage fees of $107,373. The Company recorded inducement dividends totaling $1,591,080 as the difference between the reduced exercise price of the warrants and the stock price on the date of exercise.
During the year ended March 31, 2021, the Company granted 3,500,000 immediately vesting options to purchase one share of the Company's Common Stock at the price of $0.05 per share for a period of ten years, as compensation to a scientist and researcher for drafting and filing U.S. and international patents. The options were valued at $168,000 using the Black-Scholes model.
During the year ended March 31, 2021, the Company issued a total of 788,000 warrants to convertible note holders with a term of three years and an exercise price of $0.10 per share in exchange for a three-year extension of notes having an aggregate principal balance of $197,000 (Note 6). Using the Black-Scholes model, the Company valued the warrants at $13,396.
On November 16, 2020, the Company entered into a Severance Agreement with Leslie Bocskor, a former member of the Company's board of directors, and re-priced 450,000 options held by the director to that day's closing share price of $0.03. The term of the options was extended to November 16, 2025, from June 1, 2023. Using the Black-Scholes Model, the Company valued the options at $4,950 immediately prior to the modification and at $11,250 immediately after the modification, and the Company recorded share-based compensation expense of $6,300.
On December 7, 2020, the board of directors approved the issuance of warrants to purchase a total of 3,500,000 shares of the Company's common stock at $0.04 per share for a term of ten years to current employees and directors. The Company valued the warrants at $133,000 using the Black-Scholes Model and recorded share-based compensation expense of $133,000 related to the warrants.
On December 15, 2020, the board of directors approved the issuance of options to purchase a total of 3,250,000 shares of the Company's common stock at $0.05 per share for a term of ten years to current employees and directors. The options vest one-third upon grant, one-third after one year of service, and one-third after two years of service. The Company valued the options at $156,000 using the Black-Scholes Model and recorded share-based compensation expense of $62,000 related to the options for the year ended March 31, 2021. Remaining unrecognized compensation cost related to the options was $78,000 at March 31, 2021.
On December 15, 2020, the board of directors approved the re-pricing of 6,050,000 options held by current employees to an exercise price of $0.05, the closing stock price on that date. All of the options subject to the modification were fully vested. Using the Black-Scholes Model, the Company valued the options at $199,600 immediately prior to the modification and at $250,650 immediately after the modification, and the Company recorded share-based compensation expense of $51,050.
On January 2, 2021, the board of directors approved the re-pricing and extension of 9,424,613 outstanding compensation warrants issued to the Company's brokers. The exercise price of the warrants was reduced to $0.01, and the warrants were extended to the expiration date of January 2, 2024. Prior to the extension and re-pricing, the warrants had expiration dates ranging from December 11, 2020 through October 1, 2023, and had exercise prices ranging from $0.25 to $1.00. The company valued the modified warrants at $367,196 using the Black-Scholes model. The Black-Scholes value of the warrants immediately prior to the modification was $135,861, and the Company recorded compensation expense of $231,335.
During the quarter ended March 31, 2021, the Company received notice of the conversion of $160,000 total principal balance of the note payable to CSW Ventures, L.P. at $0.04 per share and issued 4,000,000 shares of common stock to the note holder (Note 6).
On February 8, 2021, the board of directors approved the issuance of 42,705,809 replacement warrants to investors who had exercised warrants at prices that were near or at-the-money beginning in December of 2019 in order to provide working capital to the Company. The replacement warrants expire three years from the date of the initial warrant exercise and have a strike price of $0.10 per share. The Company valued the warrants at $1,182,920 using the Black-Scholes model and recorded the value of the warrants as an inducement dividend.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Warrants Outstanding
Presented below is a summary of the Company’s warrant activity, exclusive of warrants held by employees (Note 10), for the years ended March 31, 2022 and 2021:
| |
|
Warrants Outstanding
|
|
| |
|
Number of Shares
|
|
|
Exercise Price
|
|
| |
|
|
|
|
|
|
|
|
Outstanding at March 31, 2020
|
|
|
84,538,161 |
|
|
|
|
|
Warrants issued
|
|
|
43,493,809 |
|
|
$0.10
|
|
|
Warrants exercised
|
|
|
(35,798,809) |
|
|
$0.030-$0.035
|
|
|
Warrants expired/cancelled
|
|
|
(6,390,125) |
|
|
$0.60-$0.90 |
|
|
Outstanding at March 31, 2021
|
|
|
85,843,036 |
|
|
|
|
| Warrants issued |
|
|
2,095,333 |
|
|
$0.10 |
|
|
Warrants exercised
|
|
|
(9,697,146) |
|
|
$0.01- $0.03
|
|
|
Warrants expired/cancelled
|
|
|
(3,116,550) |
|
|
$0.60
|
|
| Outstanding at March 31, 2022 |
|
|
75,124,673 |
|
|
|
|
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 10 – Employee Benefit Plan
Share-Based Employee Compensation
On February 6, 2008, the board of directors adopted the GB Sciences, Inc. 2007 Amended Stock Option Plan (“2007 Plan”). Under the 2007 Plan, 4,500,000 shares of the Company’s restricted common stock may be issuable upon the exercise of options issued to employees, advisors and consultants. The Company revised the plan, and the board of directors adopted the new 2014 Equity Compensation Plan. On June 30, 2015, GB Sciences filed a Form S-8 Registration Statement with the SEC to register 8,500,000 shares of common stock issuable under stock options to grant to employees and consultants. At the Company’s special meeting of the shareholders held on April 6, 2018, the adoption by the board of directors of the 2014 Equity Compensation Plan was ratified by a majority of shareholders present at the meeting, either in person or by proxy and the Company adopted the GB Sciences, Inc 2018 Stock Plan. On October 25, 2018, GB Sciences filed a Form S-8 Registration Statement with the SEC to register 10,000,000 shares of common stock issuable under the 2018 Plan.
On September 17, 2021, the Board of Directors adopted the 2021 Equity Incentive Plan ("2021 Plan"), and the Company filed a Registration Statement with the SEC on Form S-8 to register 20,000,000 shares of common stock issuable under the 2021 Plan. All 20,000,000 shares registered under the 2021 Plan were available for issuance at March 31, 2022.
Compensation Expense
For the years ended March 31, 2022 and 2021, the Company recorded share-based compensation expense of $60,667 and $436,349, respectively, which includes $60,667 and $211,000, respectively, related to employee stock options and warrants. There was no expense for restricted stock. Unrecognized compensation cost for non-vested awards was $17,333 as of March 31, 2022.
Fair Value
The closing price of the Company's stock on the date of grant is used as the fair value for issuances of restricted stock. The fair value of stock options granted is estimated as of the grant date using the Black-Scholes option pricing model.
The following range of assumptions in the Black-Scholes option pricing model was used to determine fair value:
| |
|
Year Ended
|
|
| |
|
March 31, 2022
|
|
|
March 31, 2021
|
|
|
Weighted-average volatility
|
|
|
131 |
% |
|
|
127 |
% |
|
Expected term (in years)
|
|
|
10 |
% |
|
|
10 |
|
|
Risk-free interest rate
|
|
|
1.42 |
% |
|
|
0.93 |
% |
Expected volatilities used for award valuation are based on historical volatility of the Company's common stock. The risk-free interest rate for periods equal to the expected term of an award is based on Federal Reserve yields for U.S. Treasury securities.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Stock Options
A summary of employee option activity, including warrants issued to employees, as of March 31, 2022 and 2021, and changes during the years then ended, is presented below:
| |
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
| |
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Aggregate
|
|
| |
|
|
|
|
|
Exercise
|
|
|
Contractual
|
|
|
Intrinsic
|
|
|
Employee options and warrants
|
|
Options
|
|
|
Price $
|
|
|
Life (years)
|
|
|
Value ($)
|
|
|
Outstanding at March 31, 2020
|
|
|
10,983,334 |
|
|
$ |
0.28 |
|
|
|
6.02 |
|
|
$ |
- |
|
|
Granted
|
|
|
6,750,000 |
|
|
$ |
0.05 |
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2021
|
|
|
17,733,334 |
|
|
$ |
0.11 |
|
|
|
6.80 |
|
|
$ |
172,000 |
|
|
Granted
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2022
|
|
|
17,733,334 |
|
|
$ |
0.11 |
|
|
|
5.80 |
|
|
$ |
- |
|
|
Fully vested and expected to vest at March 31, 2022
|
|
|
17,733,334 |
|
|
$ |
0.11 |
|
|
|
|
|
|
|
|
|
|
Exercisable at March 31, 2022
|
|
|
15,566,668 |
|
|
$ |
0.12 |
|
|
|
|
|
|
|
|
|
The table below sets forth nonemployee option activity for the years ended March 31, 2022 and 2021:
| |
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
| |
|
|
|
|
|
Weighted
|
|
|
Average
|
|
|
|
|
|
| |
|
|
|
|
|
Average
|
|
|
Remaining
|
|
|
Aggregate
|
|
| |
|
|
|
|
|
Exercise
|
|
|
Contractual
|
|
|
Intrinsic
|
|
|
Nonemployee options
|
|
Options
|
|
|
Price $
|
|
|
Life (years)
|
|
|
Value ($)
|
|
|
Outstanding at March 31, 2020
|
|
|
2,383,000 |
|
|
$ |
0.27 |
|
|
|
6.75 |
|
|
$ |
- |
|
|
Granted
|
|
|
3,500,000 |
|
|
$ |
0.05 |
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2021
|
|
|
5,883,000 |
|
|
$ |
0.14 |
|
|
|
8.11 |
|
|
$ |
- |
|
|
Granted
|
|
|
600,000 |
|
|
$ |
0.05 |
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at March 31, 2022
|
|
|
6,483,000 |
|
|
$ |
0.13 |
|
|
|
7.31 |
|
|
$ |
- |
|
|
Fully vested and expected to vest at March 31, 2022
|
|
|
6,483,000 |
|
|
$ |
0.13 |
|
|
|
|
|
|
|
|
|
|
Exercisable at March 31, 2022
|
|
|
6,483,000 |
|
|
$ |
0.13 |
|
|
|
|
|
|
|
|
|
Restricted stock awards
No restricted stock awards were granted during the years ended March 31, 2022 and 2021.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 11 – Commitments and Contingencies
On April 11, 2022, the Company was served notice of a lawsuit filed in the Eighth Judicial District Court in Clark County, Nevada by an individual who alleges he was shot by a security guard at the Teco Facility in May of 2020. The alleged incident occurred after the claimant broke into the Teco Facility during closing hours. GB Sciences, Inc. and its former subsidiaries GB Sciences Nevada, LLC and GB Sciences Las Vegas, LLC, along with the security provider, Protective Force International, Inc., were named as defendants in the lawsuit. The Company holds a certificate of insurance with the insurer for Protective force International and believes it may have coverage under that policy in the event the Company is found liable for damages, however, the Company denies any liability and intends to vigorously defend the lawsuit. We are unable to make any determination at this time as to the likelihood or amount of damages.
On April 22, 2020, the Company failed to repay any of the outstanding balance of the Convertible Promissory Note Payable to Iliad Research and Trading, L.P., resulting in a default. On May 20, 2020, Iliad filed a lawsuit against the Company in the Third Judicial District Court of Salt Lake County in the State of Utah demanding repayment of the note. On July 14, 2020, the Court entered judgment in favor of Iliad in the amount of $3,264,594. The Company's obligation to Iliad was satisfied in full on December 16, 2020 upon payment of $3,006,015 pursuant to the Judgment Settlement Agreement (Note 6).
On April 22, 2020, the Company was served notice of a lawsuit filed in the Eighth Judicial District Court in Clark County, Nevada by a contractor who had been hired to perform architectural and design services. The lawsuit demanded payment of $73,050 for the services provided. On September 17, 2020, the Company entered into a Mutual Compromise, Settlement, and Release Agreement with the contractor and made payment of $25,000 in full satisfaction of the alleged debt and reduced the cost of the related fixed asset by $48,050.
From time to time, the Company may become involved in certain legal proceedings and claims which arise in the ordinary course of business. In management’s opinion, based on consultations with outside counsel, the results of any of these ordinary course matters, individually and in the aggregate, are not expected to have a material effect on our results of operations, financial condition, or cash flows. As more information becomes available, if management should determine that an unfavorable outcome is probable on such a claim and that the amount of such probable loss that it will incur on that claim is reasonably estimable, the Company would record a reserve for the claim in question. If and when the Company records such a reserve, it could be material and could adversely impact its results of operations, financial condition, and cash flows.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 12 - Related Party Transactions
During the year ended March 31, 2022, the Company repaid its indebtedness to executive officers for unpaid compensation from prior year periods totaling $84,913. There was no remaining indebtedness to related parties at March 31, 2022.
On September 1, 2019, the Company and its former CFO and COO, Ksenia Griswold, terminated their relationship and entered into the Severance Agreement. Pursuant to the Severance Agreement, Ms. Griswold would receive severance payments of $20,000 per month for 9 months following the date of separation and would continue receive health insurance coverage for the same period. On January 2, 2021, the Company entered into the Settlement Agreement with Ms. Griswold, and made payment of $57,000 in full satisfaction of $114,159 in Severance compensation owed to Ms. Griswold. As the result of the settlement, the Company recorded a gain of $57,159, which is included in gain on settlement of accounts payable in the Company's consolidated financial statements.
On November 16, 2020, the Company entered into a Severance Agreement with Leslie Bocskor, a former member of the board of directors. Pursuant to the Severance Agreement, the Company made payments totaling $84,745 to Mr. Bocskor subsequent to his departure consisting of $78,245 in compensation accrued during Mr. Bocskor's service on the board of directors and $6,500 related to health insurance, for which the Company had agreed to reimburse until the compensation was paid in full. Prior to the termination of his board service, Mr. Bocskor was paid $44,192 in director's compensation during the year ended March 31, 2021.
In connection with the sale of membership interest in GB Sciences Louisiana, LLC, the Company issued a note payable in the amount of $151,923 to John Davis, the Company's former General Counsel and President of GB Sciences Louisiana, LLC, for unpaid compensation and bonuses. The note matured upon receipt of the first payment from the Wellcana Note Receivable. The principal balance of the note and accrued interest were repaid in full on August 4, 2020.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 13 - Sale of Membership Interests in Nevada Subsidiaries
On March 24, 2020, the Company entered into the Membership Interest Purchase Agreement ("Teco MIPA") with AJE Management, LLC. Pursuant to the Teco MIPA, the Company agreed to sell 100% of its membership interests in GB Sciences Nevada, LLC, and GB Sciences Las Vegas, LLC (the "Teco Subsidiaries") for approximately $8 million, which amount includes a cash payment at closing, the extinguishment and/or repayments of certain liabilities owed to the purchaser and affiliates of the purchaser, and an 8% promissory note.
On August 10, 2020, the Company entered into the Membership Interest Purchase Agreement ("Nopah MIPA") and Promissory Note Modification Agreement with 483 Management, LLC. Pursuant to the Nopah MIPA, the Company agreed to sell its 100% membership interest in GB Sciences Nopah, LLC ("Nopah"), which holds a Nevada medical marijuana cultivation certificate. As consideration, the Company would receive $312,315 in consideration in the form of a $237,668 reduction to the outstanding principal and accrued interest balances of the 0% Note payable dated October 23, 2017 (Note 5), and extinguishment of accounts payable of $74,647, which were owed to an affiliate of the purchaser.
The closing of the Teco and Nopah sales was contingent upon the successful transfer of the Nevada cultivation and production licenses. On December 14, 2021, the Company received approval from the Nevada Cannabis Compliance Board for the transfer of cannabis cultivation and extraction licenses held by its subsidiaries GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC (the "Nevada Subsidiaries"). Consequently, all conditions to closing the sales of the 100% membership interests in the Nevada Subsidiaries were satisfied, and the transactions formally closed on December 31, 2021. After the closing date, the Company retains no ownership interest in the Nevada Subsidiaries.
As consideration for the membership interests, the Company received cash payments of $1,648,772 (including $400,000 in advance payments received during the nine months ended December 31, 2021), the extinguishment $3,462,854 of debt and current liabilities owed to affiliates of the purchaser (see Note 5 and Note 6), and a $3,025,000 8% note receivable.
As the result of sale of the Nevada Subsidiaries, the Company recorded a gain on deconsolidation of $5,206,208 on December 31, 2021, calculated as follows:
| |
|
December 31, 2021
|
|
|
Cash payments received, including advancements of $400,000
|
|
$ |
1,648,772 |
|
|
8% Note Receivable due December 31, 2024
|
|
|
3,025,000 |
|
|
Extinguishment of debt and accrued interest due to purchasers and purchasers' affiliates
|
|
|
2,612,854 |
|
|
Extinguishment of accrued management fees due to purchaser
|
|
|
850,000 |
|
|
Total consideration
|
|
|
8,136,626 |
|
| |
|
|
|
|
|
Carrying amount of assets
|
|
|
7,130,159 |
|
|
Carrying amount of liabilities
|
|
|
(4,199,741 |
) |
|
Net assets deconsolidated
|
|
|
2,930,418 |
|
|
GAIN ON DECONSOLIDATION
|
|
$ |
5,206,208 |
|
As the result of sale, the income, assets, and cash flows of GB Sciences Nevada, LLC, GB Sciences Las Vegas, LLC, and GB Sciences Nopah, LLC have been reclassified as discontinued operations for all periods presented in the Company's consolidated financial statements prior to the sale.
Note Receivable from Sale of Teco Subsidiaries
The $3,025,000 note receivable from the sale of the Teco Subsidiaries is payable as quarterly, interest only payments of $60,500 for the first year, followed by seven quarterly payments of interest and principal of $201,774 beginning March 31, 2023, with a final payment of principal and interest totaling $2,014,225 on December 31, 2024.
The note contains a provision that allows payments of principal and interest due prior to the maturity date to be postponed to the next quarterly payment date if cash flow from the operations of the facility is insufficient to cover the amount of the payment. Several days prior to the first interest payment due date of April 1, 2022, AJE Management, LLC notified the Company that it would be postponing the payment of interest of $60,500 due on April 1, 2022 due to insufficient cash flow to make the payment. AJE Management, LLC has also notified us that it will be unable to make the interest payment due July 1, 2022 due to insufficient cash flow. As a result, the Company reevaluated the factors relating to the collectibility of the note and determined that an impairment charge in the amount of $3,025,000, equal to the full balance of the note, was warranted as there is substantial uncertainty around the collectibility of the note, and we are unable to make an appropriate estimate of the amount of payments, if any, the Company will ultimately receive. The impairment charge is included on the Company's Statement of Operations as loss on impairment of note receivable.
GB SCIENCES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 14 – Concentrations
We held no cash deposits in excess of FDIC limits as of March 31, 2022 and $513,901 in excess of FDIC limits at March 31, 2021. All of the Company's sales for the year ended March 31, 2022 related to the Nevada Subsidiaries, which were deconsolidated effective December 31, 2021 (Note 13).
Note 15 – Subsequent Events
On May 9, 2022 the Company entered into a Placement Agent's Agreement with its brokers for the private placement of up to $565,000 in units at a price of $0.03 per unit. For each unit purchased, the investor will receive one share of the Company's common stock and one warrant to purchase one share of the Company's common stock at a price of $0.10 for a period of five years. As of the date of this report, the Company has received $125,000 under the private placement and issued 4,166,667 shares of its common stock and 4,166,667 warrants to purchase one share of the Company's common stock at $0.10 for five years.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We conducted an evaluation under the supervision and with the participation of management, including the Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of disclosure controls and procedures. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities and Exchange Act of 1934, as amended (“Exchange Act”), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by the company in the reports it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the Securities and Exchange Commission's rules and forms. Disclosure controls and procedures also include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company's management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure. Based on this evaluation, the Chief Executive Officer and the Chief Financial Officer concluded as of March 31, 2022 that disclosure controls and procedures were not effective at the reasonable assurance level due to the material weaknesses in internal controls over financial reporting discussed below.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as define in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The evaluation of internal control over financial reporting includes using the COSO framework, an integrated framework for the evaluation of internal controls issued by the Committee of Sponsoring Organizations of the Treadway Commission, to identify the risks and control objectives related to the evaluation of the control environment. The internal controls for the Company are provided by executive management’s review and approval of all transactions. Internal control over financial reporting also includes those policies and procedures that:
(1) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of assets;
(2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. GAAP, and that receipts and expenditures are being made only in accordance with the authorization of management; and
(3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of internal control over financial reporting as of March 31, 2022. This annual report does not include an attestation report of registered public accounting firm regarding internal control over financial reporting. Management's report was not subject to attestation by registered public accounting firm pursuant to the rules of the Securities and Exchange Commission that permits us to provide only management's report in this annual report.
Identified Material Weaknesses
A material weakness is a control deficiency, or combination of control deficiencies, that results in more than a remote likelihood that a material misstatement of the financial statements will not be prevented or detected. The matters involving internal controls over financial reporting that management considered to be material weaknesses under the standards of the Public Company Accounting Oversight Board were ineffective controls over period end financial disclosure and reporting processes as no member of our board of directors qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act.
Management’s Remediation Initiatives
As a result of findings, we have begun to remediate the deficiencies. In an effort to remediate the identified material weaknesses and enhance internal controls, we have been evaluating possible candidates meeting definition of an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act. However, due to a lack of resources it may not be possible to timely remediate the deficiencies. We anticipate our initiative will be at least partially implemented by March 31, 2023. Additionally, we plan to test the updated controls in order to remediate the deficiencies by March 31, 2023.
Conclusion
As a result of management's assessment of the effectiveness of internal control over financial reporting as of March 31, 2022, and the identification of the material weakness set forth above, management has concluded that the internal control over financial reporting is not effective. It is reasonably possible that, if not remediated, the material weaknesses noted above, could result in a material misstatement in the reported financial statements that might result in a material misstatement in a future annual or interim period. In light of the identified material weakness and the conclusion that the internal controls over financial reporting are not effective, management will take the remediation initiatives set forth above. In addition, management performed (1) additional review of the area described above, and (2) performed additional analyses, including but not limited to a detailed balance sheet and statement of operations analytical review. These procedures were completed so management could gain assurance that the financial statements and schedules included in this Form 10-K fairly present, in all material respects, the financial position, results of operations and cash flows for the periods presented.
Changes in Internal Control over Financial Reporting
There were no changes made during the most recently completed fiscal quarter that have materially affected or are reasonably likely to materially affect, internal control over financial reporting, as required by Rules 13a-15(d) and 15d-15(d) under the exchange Act.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10 DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
The names of the executive officers and directors of GB Sciences, their ages as of June 30, 2022, and the positions currently held by each are as follows:
|
Name
|
|
Age
|
|
Position
|
|
John Poss
|
|
74
|
|
President, Chief Executive Officer, and Chairman of the Board of Directors
|
|
Dr. Andrea Small-Howard
|
|
53
|
|
Chief Science Officer and Director
|
| Ed DeFrank |
|
55 |
|
Director |
|
Zach Swarts
|
|
34
|
|
Chief Financial Officer
|
Biographies
Set forth below are brief accounts of the business experience of each director an executive officer of the Company.
John Poss, Chief Executive Officer and Chairman of the Board
Effective April 29, 2016, The board of directors elected John Poss to serve as Chief Executive Officer. Mr. Poss served as the CFO of the Company beginning in August 2015, and its COO since December 31, 2015. He resigned his position as CFO on August 4, 2016 and his position as COO on November 10, 2017.
Effective May 8, 2017, following the retirement of Craig Ellins, our Chief Innovation Officer and Chairman of the Board, Mr. Poss, replaced Mr. Ellins as Chairman of the Board.
Mr. Poss has over 30 years of experience working as a consultant to companies facing major transitions and transformations. Mr. Poss began his career in the Washington, D.C. office of Arthur Andersen & Co. and has served as Chief Executive Officer, Chief Operating Officer, Chief Financial Officer and Chief Technology Officer of both public and private companies in such diverse industries as homebuilding, mining, telecommunications, manufacturing, logistics, construction lending and mortgage banking. For the past twenty months prior to joining Growblox, Mr. Poss served as Chief Executive Officer of Experiential Teaching Online Corp., an educational content developer and for four years prior thereto owned and operated his own consulting firm. Mr. Poss has also worked extensively internationally, successfully negotiating agreements in countries throughout Asia, Europe and the Americas. Mr. Poss graduated from the University of Texas in 1974 with a degree in accounting.
Dr. Andrea Small-Howard, PhD, MBA, President, Chief Science Officer, and Director
Effective June 16, 2021, the board of directors appointed Dr. Andrea Small-Howard to the office of president. Dr. Small-Howard was appointed as the Chief Science Officer and as a member of our board of directors on June 10, 2014, and she has served continuously in both positions since that time. As the Chief Science Officer, Dr. Small-Howard’s goal has been to create and maintain a novel plant-inspired therapy pipeline based on the Company's proprietary in silico technology suite, direct research & development efforts, facilitate research and development partnerships, guide product commercialization strategies, develop corporate messaging around our novel drug discovery process, and make public presentations promoting the Company’s drug development programs and unique corporate strategy.
Dr. Andrea Small-Howard has more than 20 years of research experience; as well as executive experience in the biopharmaceutical industry supervising research and development, manufacturing, and quality control divisions in both the US and China. In her biotechnology career, Dr. Small-Howard has taken novel biological products from ideation through commercialization. Dr. Small-Howard has been named an inventor on more than sixty patent applications and taken the lead in obtaining regulatory approvals from the U.S. Food and Drug Administration ("US FDA") and numerous international regulatory agencies. Dr. Small-Howard also created commercialization strategies, advised on distribution relationships, led branding committees, and supervised marketing materials. In one instance, Dr. Small-Howard designed a commercialization strategy for an in-licensed cervical cancer test. To that end, she developed technical product files in Korea with the original manufacturer, sourced US raw materials, hired contract manufacturers, created the US prototypes, and prepared regulatory filings. As VP of Scientific Oversight at Radient Pharmaceuticals Corp., she provided strategic product development and regulatory oversight across multiple international business divisions.
Dr. Small-Howard has directed research efforts on cannabinoids for over 20 years, leading a project group dedicated to the study of cannabinoids in the immune system as an NIH-funded post-doctoral fellow. In this work, she published one of the earliest studies of cannabinoid impacts on pro-inflammatory immunocytes. More recently she has contributed to published studies on consumer protection issues surrounding ‘medicinal’ Cannabis chemovars in Nevada, co-authored scholarly reviews on cannabinoids in heart disease and Parkinson’s disease, co-authored mechanistic studies on cannabinoid and terpene regulation of ion channels, and co-authored an innovative study demonstrating the utility of nanoparticles as delivery vehicles for Cannabis-derived therapeutic compounds.
For a four-year term (2012-2016), Dr. Small-Howard served on the Board of Directors of the Center for Healthcare Innovation, a nonprofit, non-partisan, and independent organization based in Chicago that is committed to serving as a catalyst for stimulating ideas, people, companies, and institutions to collaborate and achieve excellence in healthcare innovation. Her board level responsibilities at CHI included shaping and supporting the evolving mission of this dynamic group. She also served on the planning committee for their annual "Emerging Markets in the Life Sciences" seminar series, which ran for 5 years.
From July 2011 to June 2014, Dr. Small-Howard was the Founder and President of International Biotechnology Solutions, a management consulting firm that created customized, cost-effective commercialization solutions for viable yet abandoned biopharmaceutical products. International Biotechnology Solutions provided management consulting with a focus on assisting US biotech companies with products that could be commercialized within the Asia-Pacific region. Dr. Small-Howard successfully completed projects within the areas of business development, corporate alliance building, product commercialization, due diligence reporting on medical marijuana companies, corporate restructuring, and management of successful fund-raising campaigns.
From June 2011 to March 2013, she served as a Director on the Board of Directors (President for part of that time), for the Ceremax Investment Corporation. The Ceremax Investment Group was established by members of the USC EMBA Class XXV to pool its financial and intellectual resources to identify investment opportunities. During her tenure at Ceremax, Dr. Small Howard reviewed and approved capital and resource investments in promising start-up or scale-up phase private companies.
From November 2008 to July, 2011, Dr. Small-Howard served as the Vice President of Scientific Oversight for the Radient Pharmaceutical Corporation, a vertically-integrated biopharmaceutical research, development, and manufacturing corporation with operations in both the US and China. Dr. Small-Howard provided oversight for global product development in multiple international business divisions. She authored and/or attained 12 patents & 3 trademarks on proprietary cancer tests, cancer (gene) therapies, cosmeceuticals, and novel animal models. She achieved numerous regulatory approvals for cancer tests, cancer therapies, pharmaceuticals, and cosmeceutical products with the United States FDA, Health Canada, and other foreign ministries of health. She initiated and/or nurtured five international, collaborative, cancer research trial programs with universities that yielded 7 publications supporting cancer products and supervised the Quality Management Systems for an ISO 13485/cGMP compliant medical device manufacturing facility in the US; as well as the regulated manufacturing facilities in China. She also led and participated in internal and US FDA, CDPH, CE Mark/ISO 13485, and CMDR audits of Radient’s Quality Management System.
Zach Swarts, Chief Financial Officer, Treasurer
Mr. Swarts was appointed as the Company's CFO on April 16, 2021, and as Treasurer on June 9, 2021, after serving as Interim CFO since September 5, 2019. He began employment with GB Sciences in October 2017 and served as Director of Finance and Accounting prior to his appointment as Interim CFO. Prior to joining GB Sciences, Mr. Swarts worked for a local public accounting firm as Manager of the litigation support department from April 2016 to October 2017. He has provided forensic accounting, expert witness, business valuation, and consulting services to clients in a wide variety of industries. From January 2013 to April 2016, he worked as an auditor in the Las Vegas office of Ernst & Young LLP. His clients consisted primarily of SEC filers in the highly regulated gaming industry. He is a Certified Public Accountant licensed in the State of Nevada.
Edmond DeFrank, Director
Mr. DeFrank was elected to the GB Sciences, Inc. Board of Directors on October 23, 2019. He is a registered U.S. Patent Attorney and intellectual property specialist since 1993 with over 25 years’ experience as a computer engineer and a patent and trademark attorney in the high technology sector. He has written and prosecuted over one thousand trademarks and patent applications and patents for large high technology companies, educational institutions, and government entities. Mr. DeFrank is one of the first patent attorneys in U.S. history to successfully write and prosecute software, e-commerce, and IT business model patents for the World Wide Web in the early 1990s. Mr. DeFrank has protected his clients’ IP by working with state, federal, and foreign governments to combat the importation and sale of counterfeit products violating his clients’ patents and trademarks.
In addition, Mr. DeFrank has worked with small start-up companies and “Fortune 100” companies on strategic patent counseling, including managing and exploiting patent portfolios worth from six figures to billions of dollars through audit, analysis, valuation and licensing; performing due diligence for intellectual property acquisition, licensing, prosecution and litigation; managing, structuring and negotiating relationships between high tech companies, including forming licensing opportunities to generate revenue from intellectual property; negotiating and creating complex licensing, outsourcing, software development, manufacturing, marketing and distribution agreements; and performing due diligence and managing all intellectual property aspects of multi-million-dollar mergers and acquisitions. Over his career, Mr. DeFrank has founded and sold several software companies. He is the named inventor on 5 issued patents and over 30 pending patents.
For the past five years prior to joining the board of directors, Mr. DeFrank has provided legal services in the field of patent and trademark law as the owner of the Law Office of Edmond DeFrank from January 2001 to present. He is the founder of Ergo Sum Healthcare, Inc., a software development company which helps physicians produce better patient outcomes using personalized healthcare software solutions and served as its Chief Financial Officer from September 2013 to August of 2018.
During the past five years none of our directors, executive officers, promoters or control persons was:
| |
1)
|
the subject of any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time;
|
| |
2)
|
convicted in a criminal proceeding or is subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
| |
3)
|
subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or
|
| |
4)
|
found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law.
|
Family Relationships
None.
Audit Committee
On July 6, 2016, the Board established the Audit Committee and approved and adopted a charter (the "Audit Committee Charter") to govern the Audit Committee. The audit committee is comprised of John Poss, and Andrea Small-Howard. No member of the Audit Committee is independent under the rules governing OTC Market. Andrea Small-Howard is the chair of the audit committee. In addition to the enumerated responsibilities of the Audit Committee in the Audit Committee Charter, the primary function of the Audit Committee is to assist the Board in its general oversight of our accounting and financial reporting processes, audits of our financial statements, and internal control and audit functions. The Audit Committee Charter is filed herewith as Exhibit 10.7.
Audit Committee Financial Expert
As of the date of filling of this Form 10-K, no member of our board of directors qualifies as an “audit committee financial expert” as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act.
Compensation Committee
On July 6, 2016, the Board established the Compensation Committee and approved and adopted a charter (the "Compensation Committee Charter"). The compensation committee is comprised of John Poss and Ed DeFrank, of whom Ed DeFrank is independent under the rules of the Securities and Exchange Commission standards. Ed DeFrank is designated the chairperson of the committee. In addition to the enumerated responsibilities of the Compensation Committee in the Compensation Committee Charter, the primary function of the Compensation Committee is to oversee the compensation of our executives, produce a report on executive compensation for inclusion in our proxy statement, if and when required by applicable laws or regulations, and advise the Board on the adoption of policies that govern our compensation programs. The Compensation Committee Charter is filed herewith as Exhibit 10.8.
Section 16(a) Beneficial Ownership Reporting Compliance.
Section 16(a) of the Exchange Act requires our executive officers and directors and persons who directly or indirectly beneficially own more than 10% of our equity securities to file reports of ownership on Forms 3, 4 and 5 with the SEC. Executive officers, directors and 10% stockholders are required by the SEC to furnish us with copies of all Forms 3, 4 and 5 they file. Based solely on our review of the copies of such forms we have received, we believe that each of our officers and directors is under a current obligation to file a Form 3.
Code of Ethics
We adopted the GB Sciences, Inc. Code of Ethics for the CEO and Senior Financial Officers (the “finance code of ethics”), a code of ethics that applies to Chief Executive Officer, Chief Financial Officer, Chief Science Officer and other finance organization employees. A copy of the finance code of ethics may be obtained from the Company, free of charge, upon written request delivered to GB Sciences, Inc. 3550 W. Teco Avenue, Las Vegas, NV 89118. If we make any substantive amendments to the finance code of ethics or grant any waiver, including any implicit waiver, from a provision of the code to the Chief Executive Officer or Chief Financial Officer, we will disclose the nature of such amendment or waiver in a report on Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION
The following summary compensation table reflects all compensation awarded to, earned by, or paid to the Chief Executive Officer, Chief Science Officer, and Chief Financial Officer for all services rendered to us in all capacities during each of the years ended March 31, 2022 and 2021.
Summary Compensation Table
| |
|
|
|
|
|
|
|
|
Stock |
|
|
Option |
|
|
|
|
| |
|
|
|
|
|
|
|
|
Awards |
|
|
Awards
|
|
|
|
|
|
Name and Position
|
Year
|
|
Salary |
|
|
Bonus |
|
|
(1)
|
|
|
(2)
|
|
|
Total |
|
|
John Poss, President, CEO and Chairman of the Board
|
2022
|
|
$ |
69,068 |
|
|
$ |
33,748 |
|
|
$ |
- |
|
|
$ |
- |
|
|
$ |
102,816 |
|
| |
2021
|
|
|
74,500 |
|
|
|
- |
|
|
|
- |
|
|
|
19,000 |
|
|
|
93,500 |
|
|
Dr. Andrea Small-Howard, CSO and Director
|
2022
|
|
|
177,846 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
177,846 |
|
| |
2021
|
|
|
99,000 |
|
|
|
- |
|
|
|
- |
|
|
|
67,000 |
|
|
|
166,000 |
|
|
Zach Swarts, CFO
|
2022
|
|
|
128,250 |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
128,250 |
|
| |
2021
|
|
|
127,500 |
|
|
|
- |
|
|
|
- |
|
|
|
43,000 |
|
|
|
170,500 |
|
(1) Represents the grant date fair value of restricted stock awards granted, as calculated in accordance with stock-based compensation accounting standards. The fair value of each of these awards is based on the closing share price of our stock on the grant date. Although the table above indicates the full grant date value of the awards in the year which the compensation is considered, the restricted stock granted vests over a three-year period.
(2) Represents the grant date fair value of option awards granted, as calculated in accordance with stock-based compensation accounting standards. The fair value of these awards is determined under the Black-Scholes option pricing model. For the assumptions used for purposes of determining the value of the awards included in each year's compensation, please refer to Note 10. Although the table above indicates the full grant date value of the awards in the year which the compensation is considered, the options granted vest over a three-year period.
Employment Agreements
John Poss, Chief Executive Officer, President, and Chairman of the Board of Directors
On August 10, 2015, Mr. Poss, entered into an employment agreement with the Company. The term of employment is one-year subject to automatic extensions for additional one-year periods unless either party chooses to terminate such employment. The Company may terminate the Employment Agreement at any time with or without cause. If the Company terminates the Employment Agreement without cause, Mr. Poss is entitled to three months' severance if the termination takes place during the first year of employment, four months' severance if the termination takes place during the second year of employment and six months' severance if the termination takes place during the third year or a subsequent year of employment. No severance payments are due in the case of a termination for cause. Similar severance provisions apply to a termination by Mr. Poss for good reason but not to a termination by Mr. Poss without good reason. Mr. Poss received a monthly salary of $10,000 per month per the agreement. In addition, in August 2015, the Company issued 600,000 options to Mr. Poss under our 2014 Equity Incentive Plan. The options are exercisable upon vesting for a period of 10 years from issuance for the purchase of shares of our common stock at a price of $0.30 per share. The options issue pursuant to the agreement have all vested.
Pursuant to the appointment of Mr. Poss as the Company's President, Chief Executive Officer and Board Member, the Company entered into an Amended and Restated Employment Agreement, effective June 1, 2016. The agreement will end on May 1, 2017, which end date can be extended upon the mutual agreement of the parties. Under the agreement Mr. Poss received an annual salary of not less than $120,000 and quarterly bonuses equal to the value of 125,000 shares of the Company’s common stock. Bonuses are payable in S-8 stock or cash in the discretion of the Company. Under the agreement, Mr. Poss also received options to acquire 1.4 million shares of the Company's common stock subject to certain vesting requirements. The option strike price is the market value of the stock on the date the options were granted. All of the related options are fully vested.
Effective May 8, 2017, following the retirement of Craig Ellins, our Chief Innovation Officer and Chairman of the Board, Mr. Poss, replaced Mr. Ellins as Chairman of the Board.
Effective July 1, 2020, all employees agreed to temporary voluntary pay reductions and Mr. Poss' salary was decreased to $3,000 per month with no quarterly bonuses. On November 12, 2020, the Company and Mr. Poss entered into an Indemnification Agreement. Beginning February 1, 2021, the board of directors approved an increase of Mr. Poss' monthly salary to $5,000. The board of directors granted Mr. Poss a discretionary award of 500,000 warrants to purchase one share of the Company's common stock at $0.04 per share for a period of ten years, with immediate vesting, on December 7, 2020. On December 15, 2020, 3,500,000 existing options held by Mr. Poss were re-priced to $0.05 per share.
Dr. Andrea Small-Howard, PhD, MBA, Chief Science Officer and Director
On June 19, 2014, Dr. Andrea Small-Howard, Chief Science Officer, entered into a three-year employment agreement with the Company. Dr. Small-Howard received a salary at the annual rate of $78,000 and 450,000 shares of restricted common stock that vests over the three-year term of employment. The stock is restricted as defined by the Rules and Regulations promulgated under the Securities Act of 1933, as amended. The Company may terminate the Employment Agreement at any time with or without cause. If the Company terminates the Employment Agreement without cause, Dr. Small-Howard is entitled any unpaid base salary accrued through the effective date of termination notice and pay in a lump sum of an amount equal to the product of the sum of the executive’s-based salary plus the amount of the highest annual bonus or other incentive compensation payment therefore made by the Company to the executive, multiplied by one. In the event of a Change of Control, as such term is defined in the 2014 Equity Incentive Plan, all of the restricted stock granted to Dr. Small-Howard vested immediately. Dr. Small-Howard also received 500,000 of stock options not in connection with her employment agreement, of which 100,000 vested immediately and the remainder vested over three years.
Effective on June 1, 2016, the Company amended its employment agreement with Dr. Small-Howard. Pursuant to the amendment, Ms. Small-Howard surrendered a stock award for 450,000 shares of common stock in exchange for warrants to purchase 1.2 million common shares at the strike price of $0.30 per share.
Effective July 1, 2020, all employees agreed to temporary voluntary pay reductions and Dr. Small-Howard's salary was decreased to $5,000 per month. On November 12, 2020, the Company and Dr. Small-Howard entered into an Indemnification Agreement. Beginning February 1, 2021, the Board of directors approved an increase of Dr. Small-Howard's monthly salary to $12,000. The board of directors granted Dr. Small-Howard a discretionary award of 500,000 warrants to purchase one share of the Company's common stock at $0.04 per share for a period of ten years, with immediate vesting, on December 7, 2020. On December 15, 2020, Dr. Small-Howard was granted a discretionary award of 1,000,000 options to purchase one share of the Company's common stock at $0.05 per share for a period of ten years, vesting over a two-year period, and 1,000,000 existing options and 1,200,000 existing warrants held by Dr. Small-Howard were re-priced to $0.05 per share.
Zach Swarts, Chief Financial Officer and Treasurer
The Company and Mr. Swarts have not entered into a written employment agreement. On November 12, 2020, the Company and Mr. Swarts entered into an Indemnification Agreement. Beginning with his appointment as Interim CFO in September 2019, Mr. Swarts was paid a salary of $160,000 per year. Effective July 1, 2020, all employees agreed to temporary voluntary pay reductions and Mr. Swarts' salary was decreased to $5,000 per month. Beginning October 1, 2021, the board of directors approved an increase of Mr. Swarts' monthly salary to $10,000. The board of directors granted Mr. Swarts a discretionary award of 500,000 warrants to purchase one share of the Company's common stock at $0.04 per share for a period of ten years, with immediate vesting, on December 7, 2020. On December 15, 2020, Mr. Swarts was granted a discretionary award of 500,000 options to purchase one share of the Company's common stock at $0.05 per share for a period of ten years, vesting over a two-year period, and 150,000 existing options held by Mr. Swarts were re-priced to $0.05 per share.
Outstanding Equity Awards
The following table summarizes the number of shares underlying outstanding equity incentive plan awards for each named executive officer as of March 31, 2022:
|
Name
|
|
Number of shares underlying exercisable options/warrants (2)
|
|
|
Number of shares underlying unexercisable options/warrants
|
|
Option exercise price ($)
|
|
Option expiration date
|
|
Market value of shares not vested (1)
|
|
John Poss
|
|
600,000
|
|
|
-
|
|
$ 0.05
|
|
8/10/2025
|
|
-
|
| |
|
1,400,000
|
|
|
-
|
|
$ 0.05
|
|
6/1/2023
|
|
-
|
| |
|
1,500,000
|
|
|
-
|
|
$ 0.05
|
|
11/26/2027
|
|
-
|
| |
|
500,000
|
(4)
|
|
-
|
|
$ 0.04
|
|
12/7/2030
|
|
-
|
|
Andrea Small-Howard
|
|
500,000
|
|
|
-
|
|
$ 0.05
|
|
3/27/2025
|
|
-
|
| |
|
1,200,000
|
(3)
|
|
-
|
|
$ 0.05
|
|
6/1/2026
|
|
-
|
| |
|
500,000
|
|
|
-
|
|
$ 0.05
|
|
11/26/2027
|
|
-
|
| |
|
666,666
|
|
|
333,334
|
|
$ 0.05
|
|
12/15/2030
|
|
$ 9,387
|
| |
|
500,000
|
(4)
|
|
-
|
|
$ 0.04
|
|
12/7/2030
|
|
-
|
|
Zach Swarts
|
|
150,000
|
|
|
-
|
|
$ 0.05
|
|
10/1/2027
|
|
-
|
| |
|
333,333
|
|
|
166,667
|
|
$ 0.05
|
|
12/15/2030
|
|
$ 4,694
|
| |
|
500,000
|
(4)
|
|
-
|
|
$ 0.04
|
|
12/7/2030
|
|
-
|
|
Ed DeFrank
|
|
333,333
|
|
|
166,667
|
|
$ 0.05
|
|
12/15/2030
|
|
$ 4,694
|
| |
|
500,000
|
(4)
|
|
-
|
|
$ 0.04
|
|
12/7/2030
|
|
-
|
(1) Represents the Black-Scholes fair value of unvested awards as of the grant date.
(2) These options and warrants were vested at March 31, 2022.
(3) Represents a warrant to purchase 1,200,000 shares of common stock at an exercise price of $0.05 per share.
(4) Represents warrants to purchase 500,000 shares of common stock at an exercise price of $0.04 per share.
Directors’ Compensation
All directors hold office until the next annual meeting of stockholders and until their successors have been duly elected and qualified. There are no agreements with respect to the election of directors. Officers are appointed annually by the board of directors and each executive officer serves at the discretion of the board of directors. Directors are entitled to be reimbursed for reasonable and necessary expenses incurred on behalf of the Company. Outside directors are paid $2,000 monthly with an additional $1,000 for each meeting attended in person. The compensation is payable in cash or stock at the election of the Company.
ITEM 12 SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table presents information known to us, as of June 15, 2021, relating to the beneficial ownership of common stock by:
| |
●
|
each person who is known by us to be the beneficial holder of more than 5% of outstanding common stock;
|
| |
●
|
each of named executive officers and directors; and
|
| |
●
|
directors and executive officers as a group.
|
We believe that all persons named in the table have sole voting and investment power with respect to all shares beneficially owned by them, except as noted.
Percentage ownership in the following table is based on 329,204,224 shares of common stock outstanding as of the date of this report. A person is deemed to be the beneficial owner of securities that can be acquired by that person within 60 days from the date of this Annual Report upon the exercise of options, warrants or convertible securities. Each beneficial owner’s percentage ownership is determined by dividing the number of shares beneficially owned by that person by the base number of outstanding shares, increased to reflect the shares underlying options, warrants or other convertible securities included in that person’s holdings, but not those underlying shares held by any other person.
| |
|
No. of Shares
|
|
|
|
Percentage of Total Shares
|
|
|
Name of Beneficial Owner (1)
|
|
Owned
|
|
|
|
Owned
|
|
|
Officers and Directors
|
|
|
|
|
|
|
|
|
|
|
John Poss
|
|
|
4,225,000 |
|
(2) |
|
|
1.27% |
|
|
Dr. Andrea Small-Howard
|
|
|
3,482,666 |
|
(3) |
|
|
*(10) |
|
|
Zach Swarts
|
|
|
983,333 |
|
(4) |
|
|
*(10) |
|
| Gary Henrie |
|
|
833,333 |
|
(5) |
|
|
*(10) |
|
|
Ed DeFrank
|
|
|
833,333 |
|
(6) |
|
|
*(10) |
|
|
Directors and officers as a group (four) persons
|
|
|
10,357,665 |
|
|
|
|
3.05% |
|
|
5% Holders:
|
|
|
|
|
|
|
|
|
|
| Robert Moody, Jr. |
|
|
25,544,985 |
|
(7) |
|
|
7.43% |
|
| Lawrence B. Ordower |
|
|
26,226,560 |
|
(8) |
|
|
7.77% |
|
| David Ruggieri |
|
|
24,455,950 |
|
(9) |
|
|
7.18% |
|
(1) Unless otherwise noted, the address of each person listed is GB Sciences, Inc. 3550 W. Teco Avenue, Las Vegas, NV 89118.
(2) Includes (a) 225,000 shares of common stock currently owned of record by Mr. Poss, (b) options to purchase 3,500,000 shares of common stock at $0.05 per share exercisable as of the Record Date or within 60 days thereafter, and (c) warrants to purchase 500,000 shares of common stock at $0.04 per share exercisable as of the Record Date or within 60 days thereafter.
(3) Includes (a) 116,000 shares of common stock currently owned of record by Dr. Small-Howard, (b) options to purchase 1,666,666 shares of common stock and warrants to purchase 1,200,000 shares of common stock at $0.05 per share exercisable as of the Record Date or within 60 days thereafter, and (c) warrants to purchase 500,000 shares of common stock at $0.04 per share exercisable as of the Record Date or within 60 days thereafter.
(4) Includes (a) options to purchase 483,333 shares of common stock at $0.05 per share exercisable as of the Record Date or within 60 days thereafter and (b) warrants to purchase 500,000 shares of common stock at $0.04 per share exercisable as of the Record Date or within 60 days thereafter.
(5) Includes (a) 333,333 options to purchase shares of common stock at $0.05 per share exercisable as of the Record Date or within 60 days thereafter and (b) warrants to purchase 500,000 shares of common stock at $0.04 per share exercisable as of the Record Date or within 60 days thereafter.
(6) Includes (a) 333,333 options to purchase shares of common stock at $0.05 per share exercisable as of the Record Date or within 60 days thereafter and (b) warrants to purchase 500,000 shares of common stock at $0.04 per share exercisable as of the Record Date or within 60 days thereafter.
(7) Address is Robert Moody Jr, 2302 Post Office Street, Suite 601, Galveston, TX 77550. The total consists of 7,762,500 common shares, 8,002,500 shares that may be acquired upon the exercise of warrants, and 9,779,985 shares that may be acquired upon the exercise of convertible notes.
(8) Address is Lawrence B. Ordower, 25 East Washington Street, Suite 1400, Chicago, IL 60602. The total consists of 13,488,560 common shares and 12,738,000 shares of common stock issuable upon exercise of warrants.
(9) Address is David Ruggieri, 1107 West Marion Ave, Unit 116, Punta Gorda, FL 33950. The total consists of 9,016,800 common shares, 9,029,500 shares of common stock issuable upon exercise of warrants, and 6,409,150 shares that may be acquired upon the exercise of convertible notes.
(10) Less than 1%.
ITEM 13. CERTAIN RELATIONSHIPS, RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
At March 31, 2022, the Company had one independent director serving on the board of directors. The definition the Company uses to determine whether a director is independent are the rules governing the OTC market.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Our independent registered public accounting firm is Assurance Dimensions, Inc., Margate, FL, Auditor Firm ID: 5036
| |
|
Fiscal 2022
|
|
|
Fiscal 2021
|
|
|
Audit Fees(1)
|
|
$ |
65,141 |
|
|
$ |
65,000 |
|
|
Audit-Related Fees(2)
|
|
|
3,000 |
|
|
|
1,500 |
|
|
Tax Fees(3)
|
|
|
- |
|
|
|
- |
|
|
Subtotal
|
|
|
68,141 |
|
|
|
66,500 |
|
|
All other Fees(4)
|
|
|
- |
|
|
|
- |
|
|
Total
|
|
$ |
68,141 |
|
|
$ |
66,500 |
|
(1) Audit Fees – Audit fees billed to the Company in FY 2022 and 2021 include fees billed by Assurance Dimensions, Inc. for auditing the Company's annual financial statements and reviewing the financial statements included in the Company's Quarterly Reports on Form 10-Q.
(2) Audit-Related Fees – Fees billed by Assurance Dimensions, Inc. for providing auditor's consent.
(3) Tax Fees – No tax services were provided by the principal accountant during the past two fiscal years.
(4) All Other Fees – There were no other fees billed in the past two fiscal years for products and services provided.
Pre-approval of Audit and Non-Audit Services
It is the policy of the board of directors to pre-approve all audit and non-audit services provided by the independent auditors. These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to 12 months from the date of pre-approval and any pre-approval is detailed as to the particular service or category of services. The Board of Directors may delegate pre-approval authority to one or more of its members when expedition of services is necessary.
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| |
1.
|
GB Sciences, Inc. Consolidated Financial Statements (including related notes to Consolidated Financial Statements) filed in Part II of this report are listed below:
|
Report of Independent Registered Public Accounting Firm – Assurance Dimensions, Inc.
Financial Statements:
Consolidated Balances Sheets as of March 31, 2022 and 2021
Consolidated Statements of Operations – Years ended March 31, 2022 and 2021
Consolidated Statements of Changes in Stockholders’ Equity (Deficit) – Years ended March 31, 2022 and 2021
Consolidated Statements of Cash Flows – Years ended March 31, 2022 and 2021
Notes to the Consolidated Financial Statements
| |
2.
|
All schedules for which provision is made in the applicable accounting regulations of the Securities and Exchange Commission are not required under related instructions or are inapplicable and therefore have been omitted.
|
|
No.
|
|
Description
|
|
3.1
|
|
Articles of Incorporation (Incorporated by reference to an exhibit to Form SB-2 No. 333-82580 filed with the Commission on February 12, 2002)
|
|
3.2
|
|
Amendment to Articles of Incorporation (Incorporated by reference to Exhibit 3.2 to Form S-1/A filed with the Commission on October 6, 2014 and Exhibit 3.2 to Form 10-K filed with the Commission on June 27, 2014)
|
|
3.3
|
|
Articles of Incorporation (Incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020)
|
| 3.4 |
|
Amendment to Articles of Incorporation (Incorporated by reference to Exhibit 3.3 to the Annual Report on Form 10-K filed with the Commission on August 28, 2020) |
| 3.5 |
|
Bylaws (Incorporated by reference to an exhibit to Form SB-2 filed with the Commission on February 12, 2002) |
| 4.6 |
|
Description of Registrant's Securities |
|
10.1
|
|
2005 Restricted Stock Plan (Incorporated by reference to Annex A to Schedule 14A filed with the Commission on June 14, 2005)
|
|
10.2
|
|
2007 Restricted Stock Plan (Incorporated by reference to Exhibit 4.2 to Form S-8/POS filed with the Commission on February 8, 2008)
|
| 10.3 |
|
2014 Equity Incentive Plan (Incorporated by reference to Exhibit 10.6 to Form S-1/A No. 333-198967 filed with the Commission on December 23, 2014) |
|
10.4
|
|
2021 Equity Incentive Plan (Incorporated by reference to Exhibit 4.4 to Form S-8 filed with the Commission on September 17, 2021) |
|
10.5
|
|
Amended Employment Agreement between Registrant and John Poss dated June 1, 2016 (Incorporated by reference to Exhibit 10.23 to Form 10-K filed with the Commission on July 14, 2016)
|
|
10.6
|
|
Amended Employment Agreement between Registrant and Andrea Small-Howard dated June 1, 2016 (Incorporated by reference to Exhibit 10.24 to Form 10-K filed with the Commission on July 14, 2016)
|
|
10.7
|
|
Audit Committee Charter (Incorporated by reference to Exhibit 10.25 to Form 10-K filed with the Commission on July 14, 2016)
|
|
10.8
|
|
Compensation Committee Charter (Incorporated by reference to Exhibit 10.26 to Form 10-K filed with the Commission on July 14, 2016)
|
| 10.9 |
|
Indemnification Agreement between Registrant and Edmond A. DeFrank dated November 16, 2020 (Incorporated by reference to Exhibit 10.38 to Form 10-K filed with the Commission on July 6, 2021). |
| 10.10 |
|
Indemnification Agreement between Registrant and John Poss dated November 16, 2020 (Incorporated by reference to Exhibit 10.38 to Form 10-K filed with the Commission on July 6, 2021). |
| 10.11 |
|
Indemnification Agreement between Registrant and Andrea Small-Howard dated November 16, 2020 (Incorporated by reference to Exhibit 10.38 to Form 10-K filed with the Commission on July 6, 2021). |
| 10.12 |
|
Indemnification Agreement between Registrant and Zach Swarts dated November 16, 2020 (Incorporated by reference to Exhibit 10.38 to Form 10-K filed with the Commission on July 6, 2021). |
| 10.13 |
|
Promissory Note dated December 31, 2021 between AJE Management, LLC and Registrant (Incorporated by reference to Exhibit 10.1 to Form 10-Q filed with the Commission on February 2, 2022). |
| 10.14 |
|
Second Promissory Note Modification Agreement between Registrant and 483 Management, LLC dated March 4, 2022 |
| 10.15 |
|
Promissory Note dated December 31, 2021 made by AJE Management, LLC in favor of Registrant |
|
14.1
|
|
Code of Ethics (Incorporated by reference to Exhibit 14.1 to Form 10-KSB No. 333-82580 filed with the Commission on June 22, 2004)
|
|
21.1
|
|
List of Subsidiaries
|
|
31.1
|
|
Certification of Chief Executive Officer pursuant to Rules 13a-14 and 15d-14 of the Securities Exchange Act of 1934
|
|
31.2
|
|
Certification of Chief Financial Officer pursuant to Rules 13a-14 and 15d-14 of the Securities Exchange Act of 1934
|
|
32.1
|
|
18 U.S.C. Section 1350 Certification of Chief Executive Officer
|
|
32.2
|
|
18 U.S.C. Section 1350 Certification of Chief Financial Officer
|
|
101.INS
|
|
Inline XBRL Instance Document
|
|
101.SCH
|
|
Inline XBRL Taxonomy Extension Schema Document
|
|
101.CAL
|
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document
|
|
101.LAB
|
|
Inline XBRL Taxonomy Extension Labels Linkbase Document
|
|
101.PRE
|
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document
|
|
101.DEF
|
|
Inline XBRL Taxonomy Extension Definition Linkbase Document
|
|
104
|
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101)
|
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized in the city of Las Vegas, NV on June 30, 2022.
|
|
GB Sciences, Inc.
|
|
|
|
|
|
|
|
|
By:
|
/s/ John Poss
|
|
|
|
|
Name: John Poss
|
|
|
|
|
Title: Chief Executive Officer
|
|
|
|
|
|
|
|
|
GB Sciences, Inc.
|
|
|
|
|
|
|
| |
By:
|
/s/ Zach Swarts
|
|
| |
|
Name: Zach Swarts
|
|
|
|
|
Title: Chief Financial Officer
|
|
|
|
|
|
|
Exhibit 4.6
Common Stock
The holders of common stock are entitled to one vote per share on all matters to be voted on by stockholders and are entitled to receive such dividends, if any, as may be declared from time to time by our board of directors from funds legally available therefore, subject to the dividend preferences of the preferred stock, if any. Upon our liquidation or dissolution, the holders of common stock are entitled to share ratably in all assets available for distribution after payment of liabilities and liquidation preferences of the preferred stock, if any. Holders of common stock have no preemptive rights, no cumulative voting rights and no rights to convert their common stock into any other securities. Any action taken by holders of common stock must be taken at an annual or special meeting or by written consent of the holders of over 33% of our capital stock entitled to vote on such action.
Exhibit 10.14
SECOND PROMISSORY NOTE MODIFICATION AGREEMENT
THIS SECOND PROMISSORY NOTE MODIFICATION AGREEMENT (this “Second Modification”), dated this 4th day of March, 2022 (the “Effective Date”), is entered into by GB SCIENCES, INC. (the “Company”), and 483 MANAGEMENT, LLC (“483”). Hereinafter the Company and 483 are sometimes referred to jointly as the “Parties”.
WHEREAS, 483 is the holder of that certain Promissory Note issued by the Company, in the principal amount of $700,000, dated October 23, 2017 (the “Note”);
WHEREAS, on August 10, 2020, the Parties entered into a Promissory Note Modification Agreement (the “First Modification”), which modified certain terms and conditions of the Note. Hereinafter the Note and the First Modification are sometimes referred to jointly as the “Prior Loan Documents”; and
WHEREAS, the Parties now desire to enter into this Second Modification for the purpose of modifying the terms and conditions of the Prior Loan Documents.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants contained herein, the legal sufficiency of which is hereby acknowledged, the Parties agree as follows:
| |
1.
|
Prior to giving effect to this Second Modification, the amount owed by the Company under the Prior Loan Documents as of the Effective Date, including without limitation, all principal and accrued interest, is $199,030.00 (the “Prior Balance Owed”).
|
| |
2.
|
The Prior Balance Owed by the operation of this Second Modification is hereby reduced to $179,127.00 (the “New Balance Owed”).
|
| |
3.
|
The Company shall make a payment in the amount of $75,000.00 on or before March 4, 2022, which shall reduce the amount of the New Balance Owed in the amount of $75,000.00.
|
| |
4.
|
Following the reduction of the New Balance Owed in the amount of $75,000.00 by said payment, interest shall accrue on the New Balance Owed at the rate of 8% per annum.
|
| |
5.
|
The Company shall also make monthly payments on the New Balance Owed in the amount of $5,000.00 per month beginning on April 1, 2022, and continuing on the first day of each month thereafter until the New Balance Owed is paid in full. Each monthly payment shall first be applied to accrued interest and then to principal.
|
| |
6.
|
Unless the Company is more than 30 days in arrears on any monthly payment, it is not reasonable for 483 to believe that the prospect of payment is impaired.
|
| |
7.
|
Any discrepancy between any provision in this Second Modification and any provision in the Prior Loan Documents shall be resolved in favor of the provision in this Second Modification.
|
| |
8.
|
This Second Modification shall be governed by and construed in accordance with the laws of the State of Nevada applicable to contracts entered into and to be performed wholly within the State of Nevada, and any action based upon this Second Modification shall be brought in the United States federal and state courts situated in Nevada only, and the Parties shall submit to the jurisdiction of such courts for all purposes hereunder.
|
IN WITNESS WHEREOF, the undersigned have duly executed and delivered this Second Modification as of the day and year first above written.
GB SCIENCES, INC.
/s/: John Poss
Name: John C. Poss
Title: CEO
483 MANAGEMENT, LLC
/s/: Bill Moore
By: Bill Moore
Title: Member
/s/: Brian Moore
By: Brian Moore
Title: Member
Exhibit 10.15
PROMISSORY NOTE
$3,025,000 December 31, 2021
FOR VALUE RECEIVED, AJE MANAGEMENT, LLC (“Maker”), hereby promises to pay to GB SCIENCES, INC., a Nevada corporation (“Payee”), with an address at 3550 W. Teco Avenue, Las Vegas Nevada 89118, the principal sum of THREE MILLION TWENTY FIVE THOUSAND DOLLARS ($3,025,000), together with all interest that has accrued thereon in accordance with the terms of this Promissory Note (this “Note”).
The outstanding principal amount of this Note shall bear interest at the rate of eight (8%) percent per annum, based on a year of 365 days or 366 days, as applicable, for actual days elapsed, until the date on which the last payment of principal under this Note shall have been paid.
This Note shall be payable as follows: (i) quarterly amortizing payments of principal and interest in the aggregate amount of $201,773.86 each shall be due and payable commencing on March 31, 2023 (the “Amortization Commencement Date”) and on each three-month anniversary of such date, and (ii) a final payment equal to the remaining unpaid principal amount outstanding under this Note, together with all accrued interest thereon, shall be due and payable on December 31, 2024 (the “Maturity Date”).
Prior to the Amortization Commencement Date, payments of interest only in the amount of $60,500 each shall be due and payable quarterly in arrears commencing on March 31, 2022 and on each of the next three three-month anniversaries of such date.
This Note has been issued to Payee under that certain Membership Interest Purchase Agreement (this “Purchase Agreement”), dated as of March 24, 2020, by and among Maker, Payee, GB Sciences Las Vegas, LLC (“GBSLV”), and GB Sciences Nevada LLC (“GBSNV” and, together with GBSLV, the “Teco Subsidiaries”). Maker shall have the right to set off and apply against any and all amounts payable under this Note, in the order of their maturities, any amount owing under the Purchase Agreement from Payee to the Maker.
Notwithstanding any other provision of this Note, Maker shall not be required to make any payment of principal or interest due under this Note prior to the Maturity Date to the extent there is insufficient cash flow from the operations of the Teco Facility, before giving effect to management fees (if any) paid or payable to affiliates of Maker, to make such payment (each, a “Deferred Payment”). Any Deferred Payment not paid when due to the foregoing cash flow limitation shall be deferred to the next applicable payment date under this Note on which the such limitation does not apply.
This Note may be prepaid, in whole or in part, at any time or from time to time, without premium or penalty. All payments made on this Note shall be applied first to interest accrued to the date of the payment and then to the outstanding principal payments due under this Note. All prepayments of the principal due under this Note shall be applied to such maturities as shall be designated by Maker.
All payments or prepayments of principal and interest and other sums due pursuant to this Note shall be made by check to Payee at its address set forth above, or in immediately available funds by wire transfer to Payee’s account at such bank as Payee shall have previously designated to Maker.
Whenever any payment to be made hereunder shall be due on a Saturday, Sunday or legal holiday under the laws of the State of Nevada, such payment may be made on the next succeeding business day, and such extension of time shall be included in the computation of payment of interest hereunder.
The occurrence of any one or more of the following events shall constitute an “Event of Default” under this Note: (i) Maker shall fail to pay when due any amount due under this Note and such failure shall not be cured within fifteen days after the date such payment was due; or (ii) Maker shall commence any case, proceeding or other action under any law relating to bankruptcy, insolvency, reorganization or relief of debtors, seeking to have an order for relief entered with respect to Maker, or seeking to adjudicate Maker a bankrupt or insolvent, or seeking reorganization, arrangement or other relief with respect to Maker or any of its debts, or seeking appointment of a receiver, trustee, custodian or other similar official for Maker or for all or any part of its assets, or Maker shall make a general assignment for the benefit of creditors, or there shall be commenced against Maker any case, proceeding or other action of a nature referred to in this clause (ii), or there shall be commenced against Maker any case, proceeding or other action seeking issuance of a warrant of attachment, execution, restraint or similar process against all or any substantial part of the assets of Maker which results in the entry of an order for any such relief, or Maker shall take any action in furtherance of, or indicating its consent to, approval of, or acquiescence in, any of the acts set forth in this clause (ii), or Maker shall generally not, or shall be unable to, or shall admit in writing its inability to, pay his debts as they become due.
Upon the occurrence and during the continuance of an Event of Default, the holder of this Note may, at its option, by notice in writing to Maker, declare this Note to be, and this Note shall forthwith become, due and payable; provided, however, that upon the occurrence of an Event of Default specified in clause (ii) above, the principal balance of and all accrued interest on this Note shall automatically become due and payable forthwith, without demand or notice of any kind. If an Event of Default occurs, Maker shall pay to the holder of this Note all expenses (including, without limitation, reasonable attorneys’ fees and expenses and court fees and court costs) incurred by the holder in connection with obtaining advice as to its rights and remedies in connection with such default and in connection with enforcing and collecting this Note.
Maker hereby waives presentment, demand for payment, notice of dishonor, protest and notice of protest of this Note. No waiver of any provision of this Note, or any agreement or instrument evidencing or providing security for this Note, made by agreement of Payee and any other person or party, shall constitute a waiver of any other terms hereof, or otherwise release or discharge the liability of Maker under this Note. No failure to exercise and no delay in exercising, on the part of Payee, any right, power or privilege under this Note shall operate as a waiver thereof nor shall simple or partial exercise of any right, power or privilege preclude any other or further exercise thereof, or the exercise of any other power, right or privilege. The rights and remedies herein provided are cumulative and are not exclusive of any rights or remedies provided by law.
In case any provision contained in this Note should be invalid, illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions contained herein shall not in any way be affected or impaired thereby and shall remain in full force and effect.
This Note may not be amended except by an instrument in writing signed by the Maker and the holder of this Note.
This Note shall be governed by and construed in accordance with the laws of the State of Nevada without regard to its doctrine of conflict of laws. Maker, by its execution hereof, and Payee by its acceptance hereof (I) AGREE THAT ANY LEGAL SUIT, ACTION OR PROCEEDING ARISING FROM OR RELATED TO THIS NOTE MAY BE INSTITUTED ONLY IN A STATE OR FEDERAL COURT LOCATED IN CLARK COUNTY, NEVADA; (II) WAIVE ANY OBJECTION WHICH IT MAY NOW OR HEREAFTER HAVE TO THE LAYING OF VENUE OF ANY SUCH SUIT, ACTION OR PROCEEDING OR BASED ON FORUM NON CONVENIENS; AND (III) CONSENT TO AND SUBMITS TO THE EXERCISE OF JURISDICTION OVER ITS PERSON BY ANY SUCH COURT HAVING JURISDICTION OVER THE SUBJECT MATTER.
This Note shall be binding upon the successors and assigns of the Maker and shall inure to the benefit of the successors and assigns of the Payee.
Any notice from the holder of this Note to the Maker shall be deemed given when delivered to the Maker by hand or when deposited in the United States mail and addressed to the Maker at the address of the Maker set forth in the first paragraph of this Note.
AJE MANAGEMENT, LLC
By: /s/: David Weiner
Name: David Weiner
Title: Manager
2
Exhibit 21.1
Subsidiaries of GB Sciences, Inc.
GBS Global Biopharma, Inc.
ECRX, Inc.
The PhAROS Institute, LLC
GB Sciences Texas, LLC
Exhibit 31.1
Certification of Chief Executive Officer
Pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934
I, John Poss, certify that:
|
1.
|
I have reviewed this annual report on Form 10-K for the year ended March 31, 2022 of GB Sciences, Inc.;
|
|
|
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
|
|
|
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
|
|
|
|
|
4.
|
The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
|
|
|
a)
|
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
|
|
|
|
|
b)
|
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
|
|
|
|
c)
|
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
|
|
|
|
|
|
|
d)
|
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
|
|
5.
|
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
|
|
|
a)
|
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
|
|
|
|
|
|
|
b)
|
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
|
|
Date: June 30, 2022
|
By:
|
/s/ John Poss
|
|
|
|
|
John Poss
|
|
|
|
|
President and Chief Executive Officer
|
|
Exhibit 31.2
Certification of Chief Executive Officer
Pursuant to Rule 13a-14(a) under the Securities Exchange Act of 1934
I, Zach Swarts, certify that:
|
1.
|
I have reviewed this annual report on Form 10-K for the year ended March 31, 2022 of GB Sciences, Inc.;
|
|
|
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
|
|
|
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
|
|
|
|
|
4.
|
The registrant's other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
|
|
|
a)
|
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
|
|
|
|
|
b)
|
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
|
|
|
|
c)
|
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
|
|
|
|
|
|
|
d)
|
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
|
|
5.
|
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
|
|
|
a)
|
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
|
|
|
|
|
|
|
b)
|
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
|
|
Date: June 30, 2022
|
By:
|
/s/ Zach Swarts
|
|
|
|
|
Zach Swarts
|
|
|
|
|
Chief Financial Officer
|
|
Exhibit 32.1
Certification Pursuant to 18 U.S.C. Section 1350,
As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act Of 2002
In connection with the annual report of GB Sciences, Inc. (the “Company”) on Form 10-K for the period ended March 31, 2022 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, John Poss, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
1. The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
2. The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
Date: June 30, 2022
|
By:
|
/s/ John Poss
|
|
|
|
|
John Poss
|
|
|
|
|
Chief Executive Officer
|
|
Exhibit 32.2
Certification Pursuant to 18 U.S.C. Section 1350,
As Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act Of 2002
In connection with the annual report of GB Sciences, Inc. (the “Company”) on Form 10-K for the period ended March 31, 2022 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned, Zach Swarts, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, that to the best of my knowledge:
1. The Report fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
2. The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
Date: June 30, 2022
|
By:
|
/s/ Zach Swarts
|
|
|
|
|
Zach Swarts
|
|
|
|
|
Chief Financial Officer
|
|
GB Sciences (PK) (USOTC:GBLX)
Historical Stock Chart
From Mar 2024 to Apr 2024
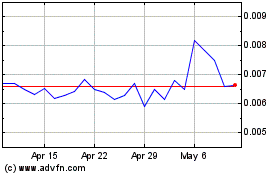
GB Sciences (PK) (USOTC:GBLX)
Historical Stock Chart
From Apr 2023 to Apr 2024