Acadia Pharmaceuticals: FDA Accepts New Drug Application for Trofinetide
September 12 2022 - 8:21AM
Dow Jones News
By Mary de Wet
Acadia Pharmaceuticals Inc. said Monday the U.S. Food and Drug
Administration accepted its new drug application of trofinetide for
the treatment of Rett syndrome.
The FDA has granted a priority review and assigned a
Prescription Drug User Fee Act action date of March 12, the
biopharmaceutical company said. The FDA told Acadia that it isn't
planning to hold an advisory committee meeting, the company
said.
Trofinetide has already been granted fast track status and
orphan drug designation for the treatment of Rett syndrome in the
U.S. and the rare pediatric disease designation by the FDA.
Rett syndrome is a neurodevelopmental disorder that includes a
period of normal development followed by regression with loss of
language and hand function skills, impaired gait and development of
hand stereotypes. It occurs worldwide in about one of every 10,000
to 15,000 female births, Acadia said.
The company has Nuplazid on the market to treat Parkinson's
disease psychosis. Sales of the drug were $134.6 million in the
three months ended June 30. Trofinetide is the next drug candidate
in Acadia's pipeline.
Write to Mary de Wet at mary.dewet@dowjones.com
(END) Dow Jones Newswires
September 12, 2022 08:06 ET (12:06 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
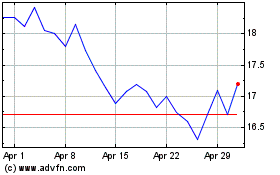
Acadia Pharmaceuticals (NASDAQ:ACAD)
Historical Stock Chart
From Mar 2024 to Apr 2024
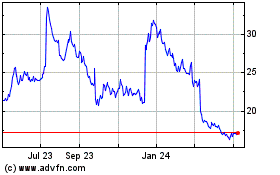
Acadia Pharmaceuticals (NASDAQ:ACAD)
Historical Stock Chart
From Apr 2023 to Apr 2024