Report of Foreign Issuer Pursuant to Rule 13a-16 or 15d-16 (6-k)
August 30 2022 - 4:02PM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF
FOREIGN PRIVATE ISSUER
PURSUANT TO RULE 13a-16 OR
15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the month of August 2022
Commission File Number: 001-40212
Connect Biopharma Holdings Limited
(Translation of registrant’s name into English)
12265 El
Camino Real, Suite 350
San Diego, CA 92130
(Address of principal executive office)
Indicate by check mark whether
the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form
20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On August 30, 2022, Connect Biopharma Holdings Limited (the “Company”) announced the completion of its Phase 1 clinical trial of CBP-174, a highly selective, peripherally acting H3 receptor (H3R) antagonist drug candidate. The Company is developing CBP-174 for oral administration to treat chronic
pruritus associated with allergic and inflammatory skin conditions, including atopic dermatitis.
The Phase 1 clinical trial was a randomized,
double-blind, placebo-controlled, single ascending dose study to assess safety, tolerability, and pharmacokinetics of CBP-174 in healthy adults. The study included eight dose escalation cohorts and one
extension cohort with administration of a single oral dose of up to a maximum of 16 mg of CBP-174 or placebo. A total of 72 subjects were dosed with 8 subjects randomized in a 3:1 ratio to receive CBP-174 or placebo in each cohort.
CBP-174 was generally well-tolerated with no
serious adverse events reported and no dose-limiting toxicities identified. Adverse events were predominantly mild in severity. Other safety parameters, including vital signs, ECGs, and laboratory results showed no clinically notable safety
findings. Pharmacokinetics of CBP-174 exhibited rapid absorption with dose proportional increases in exposure followed by linear elimination.
This report on Form 6-K shall be deemed to be incorporated by reference into the registration statements on Form F-3 (Registration No. 333-264340) and Form S-8 (Registration
Nos. 333-254524 and 333-266006) of the Company and to be a part thereof from the date on which this report is furnished, to the extent not superseded by documents
or reports subsequently filed or furnished.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
|
|
|
|
|
| Dated: August 30, 2022 |
|
CONNECT BIOPHARMA HOLDINGS LIMITED |
|
|
|
|
|
By |
|
/s/ Steven Chan |
|
|
|
|
Name: |
|
Steven Chan |
|
|
|
|
Title: |
|
Chief Financial Officer |
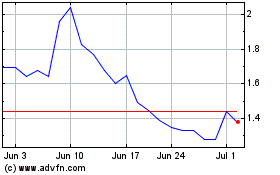
Connect Biopharma (NASDAQ:CNTB)
Historical Stock Chart
From Mar 2024 to Apr 2024
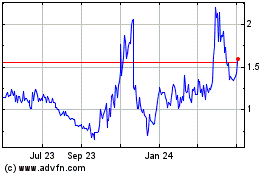
Connect Biopharma (NASDAQ:CNTB)
Historical Stock Chart
From Apr 2023 to Apr 2024