FDA Issues Warning Letter to Emergent Biosolutions After February Inspection
August 12 2022 - 6:01PM
Dow Jones News
By Kathryn Hardison
Emergent Biosolutions Inc. said the Food and Drug Administration
has issued a warning letter to the company regarding deficiencies
it found in a February inspection of its Camden manufacturing
facility in Baltimore, Maryland.
The letter warned of issues related to Emergent's practices for
aseptic processing and systems for cleaning and maintaining
equipment to prevent contamination of drug product, the company
said in a Friday filing with the Securities and Exchange
Commission.
The FDA also recommended the company review and assess its
quality management system and work with a consultant on
manufacturing practices.
Emergent said Friday that it has retained a third party for
additional review of the facility while it waited for the FDA's
response. The company said it has made "significant investment to
upgrade (its) physical capabilities."
"This is a process that doesn't happen overnight, but one to
which we are committed," Emergent said.
Shares fell 4.8% to $29.01 in after-hours trading.
Write to Kathryn Hardison at kathryn.hardison@wsj.com
(END) Dow Jones Newswires
August 12, 2022 17:46 ET (21:46 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
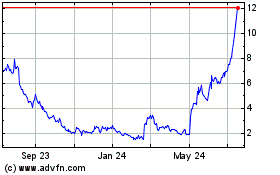
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
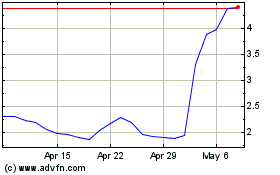
Emergent Biosolutions (NYSE:EBS)
Historical Stock Chart
From Apr 2023 to Apr 2024