Rigel Pharma Shares Down Sharply After WAIHA Treatment Trial Data
June 08 2022 - 2:53PM
Dow Jones News
By Michael Dabaie
Shares of Rigel Pharmaceuticals Inc. fell sharply Wednesday
after the company said data from the Forward Phase 3 clinical trial
of fostamatinib in warm autoimmune hemolytic anemia, or wAIHA,
didn't demonstrate statistical significance in the primary efficacy
objective.
Shares fell 53% to 82 cents in afternoon trading.
The company said the study didn't demonstrate statistical
significance in the primary efficacy endpoint of durable hemoglobin
response in the overall study population.
In a post-hoc regional analysis of U.S., Canadian, Australian,
and Western European trial sites, patients treated with
fostamatinib had a favorable durable hemoglobin response compared
with placebo, the company said. In the Eastern European trial
sites, patients didn't, it said.
Chief Medical Officer Wolfgang Dummer said during a conference
call after the results that the company believes the results were
negatively impacted by a large placebo response rate from patients
treated in Eastern European trial sites. The Eastern European
patients seemed to differ in some baseline characteristics from
other patients, Dr. Dummer said.
Chief Executive Raul Rodriguez said in the call that the study
was based on positive results from the company's Phase 2 study,
which was conducted solely in the U.S. and "showed a compelling
response in its patient population."
"We were surprised, therefore, that the trial did not
demonstrate statistical significance in the primary endpoint of
durable hemoglobin response in the overall patient population," Mr.
Rodriguez said.
"Overall, the study results appear to be negatively impacted by
a large placebo response rate in Eastern European clinical sites,"
H.C. Wainwright analysts Joseph Pantginis and Emanuela Branchetti
said in a research note. "Geographic impacts notwithstanding, we
remain encouraged by the totality of the results and look forward
to the outcome of company discussions with the FDA which management
anticipates taking place by 3Q/YE22."
Wainwright maintained its buy recommendation and lowered its
price target to $7 from $11.
Rigel said it plans to continue analyzing the data to understand
the geographical differences in patient disease characteristics and
outcomes and discuss these findings with the U.S. Food and Drug
Administration.
The safety and tolerability profile in the Forward trial was
consistent with the existing fostamatinib safety database, the
company said.
Autoimmune hemolytic anemia is a rare, serious blood disorder in
which the immune system produces antibodies that lead to the
destruction of the body's own red blood cells. Warm antibody AIHA,
the most common form, is characterized by the presence of
antibodies that react with the red blood cell surface at body
temperature.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
June 08, 2022 14:38 ET (18:38 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
Rigel Pharmaceuticals (NASDAQ:RIGL)
Historical Stock Chart
From Mar 2024 to Apr 2024
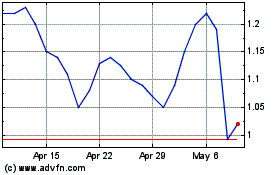
Rigel Pharmaceuticals (NASDAQ:RIGL)
Historical Stock Chart
From Apr 2023 to Apr 2024