As filed with the Securities and Exchange Commission
on May 13, 2022.
Registration No.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
Agile Therapeutics,
Inc.
(Exact name of Registrant as specified in its charter)
Delaware
(State or Other Jurisdiction of
Incorporation or Organization) |
2834
(Primary Standard Industrial
Classification Code Number) |
23-2936302
(I.R.S. Employer
Identification Number) |
500 College Road East, Suite 310
Princeton, New Jersey 08540
(609) 683-1880
(Address, including zip code, and telephone number,
including
area code, of Registrant’s principal executive
offices)
Alfred Altomari
Chief Executive Officer
Agile Therapeutics, Inc.
500 College Road East, Suite 310
Princeton, New Jersey 08540
(609) 683-1880
(Name, address, including zip code, and telephone
number, including area code, of agent for service)
| Please send copies of all communications to: |
|
Steven M. Cohen
Bryan S. Keighery
Morgan, Lewis & Bockius LLP
502 Carnegie Center
Princeton, New Jersey 08540
(609) 919-6600 |
Robert
F. Charron
Ellenoff Grossman & Schole LLP
1345 Avenue of the Americas
New York, NY 10105
(212) 370-1300
|
Approximate date of commencement
of the proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being
registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the
following box. x
If this Form is filed to register
additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities
Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective
amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective
amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration
statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether
the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging
growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting
company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o |
Accelerated filer o |
Non-accelerated filer x
|
Smaller reporting company x
Emerging growth company ¨
|
If an emerging growth company,
indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided pursuant to Section 7(a)(2)(b) of the Securities Act. o
The Registrant hereby amends
this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a
further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a)
of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and
Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary
prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities
and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting offers
to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED MAY
13, 2022
Preliminary Prospectus

Agile Therapeutics, Inc.
$20,000,000
Up to Shares
of Common Stock and
Warrants to Purchase up
to Shares of Common Stock
or
Up to Pre-funded Warrants
to Purchase up to Shares of Common Stock and
Warrants to Purchase up
to Shares of Common Stock
Placement Agent Warrants to Purchase up to Shares
of Common Stock
We are offering up
to shares of common stock, together with warrants (the “Series A
warrants”) to purchase up to shares of common stock at an assumed combined
public offering price of $ per share and Series A warrant, which is equal
to the last reported sale price per share of our common stock on the Nasdaq Capital Market
on May , 2022, (and the shares issuable from time to time upon exercise of
the Series A warrants) pursuant to this prospectus. The shares of common stock and Series A warrant will be separately issued, but
the shares of common stock and Series A warrants will be issued to purchasers in the ratio of one to one. Each Series A warrant
will have an exercise price of $ per share, will be exercisable upon issuance
and will expire years from the date of issuance.
We are also offering up to pre-funded warrants
(the “Series B pre-funded warrants” and collectively with the Series A warrants, the “warrants”) to those purchasers
whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related
parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock following
the consummation of this offering in lieu of the shares of our common stock that would result in ownership in excess of 4.99% (or, at
the election of the purchaser, 9.99%). Each Series B pre-funded warrant will be exercisable for one share of common stock at an exercise
price of $0.001 per share. Each Series B pre-funded warrant is being issued together with the same Series A warrant described above being
issued with each share of common stock. The assumed combined public offering price for each such Series B pre-funded warrant, together
with the Series A warrant, is $ , which is equal to the last reported sale price of our common stock on the Nasdaq Capital Market on
May , 2022 less the $0.001 per share exercise price of each such Series B pre-funded warrant. Each Series B pre-funded warrant will be
exercisable upon issuance and may be exercised at any time until all of the pre-funded warrants are exercised in full. The Series B
pre-funded warrants and Series A warrants are immediately separable and will be issued separately in this offering.
There is no established public trading market for
the warrants, and we do not expect a market to develop. We do not intend to apply for listing of the warrants on any securities exchange
or other nationally recognized trading system. Without an active trading market, the liquidity of the warrants will be limited.
We have engaged H.C. Wainwright & Co., LLC,
or the placement agent, to act as our exclusive placement agent in connection with this offering. The placement agent has agreed to use
its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing
or selling any of the securities we are offering and the placement agent is not required to arrange the purchase or sale of any specific
number of securities or dollar amount. We have agreed to pay to the placement agent the placement agent fees set forth in the table below,
which assumes that we sell all of the securities offered by this prospectus. There is no arrangement for funds to be received in escrow,
trust or similar arrangement. There is no minimum offering requirement. We will bear all costs associated with the offering. See “Plan
of Distribution” on page 28 of this prospectus for more information regarding these arrangements.
Our common stock is listed on the Nasdaq
Capital Market under the symbol “AGRX.” On May , 2022, the last
reported sale price of our common stock on the Nasdaq Capital Market was $ per share. All
share, Series A warrant, and Series B pre-funded warrant numbers are based on an assumed combined public offering price of
$ per share or Series B pre-funded warrant, as applicable, and Series A warrant.
The combined public offering price per share and
Series A warrant and the combined public offering price per Series B pre-funded warrant and Series A warrant will be determined between
us and investors based on market conditions at the time of pricing, and may be at a discount to the current market price of our common
stock. Therefore, the recent market price used throughout this prospectus may not be indicative of the actual public offering price.
Investing in the offered securities involves
a high degree of risk. See “Risk Factors” beginning on page 9 of this prospectus and the section entitled “Risk Factors”
included in our most recent Annual Report on Form 10-K, as revised or supplemented by our subsequent Quarterly Reports on Form 10-Q,
which are incorporated herein by reference, for a discussion of information that you should consider before investing in our securities.
| | |
Per Share
and
Series A
Warrant | |
Per Series B Pre-Funded
Warrant and Series A
Warrant | |
| Total | |
| Public offering price | |
$ | |
$ | |
| $ | |
| Placement Agent’s fees(1) | |
$ | |
$ | |
| $ | |
| Proceeds, before expenses, to us(2) | |
$ | |
$ | |
| $ | |
| (1) | We
have agreed to pay the placement agent a total cash fee equal to 7.0% of the gross proceeds raised in this offering. We have also agreed
to pay the placement agent a management fee equal to 1.0% of the gross proceeds raised in this offering and to reimburse the placement
agent for its non-accountable expenses in the amount of $50,000 and for its legal fees and expenses and other out-of-pocket expenses
in an amount up to $100,000, and for its clearing expenses in the amount of $15,950. In addition, we have agreed to issue to the placement
agent, or its designees, warrants to purchase a number of shares of our common stock equal to 5.0% of the aggregate number of shares
of common stock and Series B pre-funded warrants being offered at an exercise price equal to 125% of the combined public offering price
per share of common stock and Series A warrant. We refer you to “Plan of Distribution” on page 28 of this prospectus for
additional information regarding placement agent compensation.
|
| |
(2) |
Because there
is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual public offering
amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less than the total
maximum offering amounts set forth above. We refer you to “Plan of Distribution” on page 28 of this prospectus for additional
information regarding placement agent compensation. |
The placement agent expects to deliver the shares
and warrants to purchasers in the offering on or about , 2022, subject to satisfaction of certain conditions.
Neither the Securities
and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus
is truthful or complete. Any representation to the contrary is a criminal offense.
H.C. Wainwright & Co.
Prospectus dated , 2022
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
The registration statement
we filed with the Securities and Exchange Commission (the “SEC”) includes exhibits that provide more detail of the matters
discussed in this prospectus. You should read this prospectus, the related exhibits filed with the SEC, and the documents incorporated
by reference herein before making your investment decision. You should rely only on the information provided in this prospectus and the
documents incorporated by reference herein or any amendment thereto. In addition, this prospectus contains summaries of certain provisions
contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the
summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed,
will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you
may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
We have not, and the placement
agent has not, authorized anyone to provide any information or to make any representations other than those contained in this prospectus,
the documents incorporated by reference herein or in any free writing prospectuses prepared by or on behalf of us or to which we have
referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others
may give you. The information contained in this prospectus, the documents incorporated by reference herein or in any applicable free writing
prospectus is current only as of its date, regardless of its time of delivery or any sale of our securities. Our business, financial condition,
results of operations and prospects may have changed since that date.
This prospectus is an offer
to sell only the securities offered hereby, and only under circumstances and in jurisdictions where it is lawful to do so. We are not,
and the placement agent is not, making an offer to sell these securities in any state or jurisdiction where the offer or sale is not permitted.
All other trademarks, trade
names and service marks appearing in this prospectus or the documents incorporated by reference herein are the property of their respective
owners. Use or display by us of other parties’ trademarks, trade dress or products is not intended to and does not imply a relationship
with, or endorsements or sponsorship of, us by the trademark or trade dress owner. Solely for convenience, trademarks, tradenames and
service marks referred to in this prospectus appear without the ® and ™ symbols, but those references are not intended to indicate,
in any way, that we will not assert, to the fullest extent under applicable law, our rights or that the applicable owner will not assert
its rights, to these trademarks and trade names.
FORWARD-LOOKING STATEMENTS
This prospectus, including the
information incorporated by reference into this prospectus, contains “forward-looking statements” within the meaning of Section
27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended,
or the Exchange Act. You can identify these forward-looking statements by the use of words such as “outlook,” “believes,”
“expects,” “potential,” “continues,” “may,” “will,” “should,”
“seeks,” “approximately,” “predicts,” “intends,” “plans,” “estimates,”
“anticipates” or the negative version of these words or other comparable words. These statements relate to future events or
to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors which may cause
our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed
or implied by the forward-looking statements.
Some of the factors that we believe
could cause actual results to differ from those anticipated or predicted include:
| • | our ability to successfully enhance the commercialization and increase the uptake for Twirla, our only approved product; |
| | | |
| • | the rate and degree of market acceptance of Twirla by physicians, patients, third-party payors and others in the healthcare community; |
| | | |
| • | our ability to obtain adequate coverage and reimbursement for Twirla in the United States from private and public third-party payors; |
| | | |
| • | the size and growth of the markets for Twirla and our ability to serve those markets; |
| | | |
| • | the effects of the ongoing COVID-19 pandemic on our commercialization efforts, clinical trials, supply chain, operations and the operations
of third parties we rely on for services such as manufacturing, marketing support and sales support, as well as the effects of the COVID-19
pandemic on our potential customer base; |
| | | |
| • | regulatory and legislative developments in the United States and foreign countries, which could include, among other things, a government
shutdown; |
| | | |
| • | our available cash and our ability to obtain additional funding to fund our business plan without delay and to continue as a going
concern; |
| | | |
| • | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; |
| | | |
| • | growth in demand for Twirla and our ability to manage the levels of Twirla inventory, which could result in our having to write off
inventory and our inability to meet the minimum requirements under our supply agreement with our third-party manufacturer, Corium; |
| | | |
| • | our ability to timely obtain from Corium sufficient quantities or quality of Twirla or other materials required for a clinical trial
or other tests and studies; |
| | | |
| • | the ability of Corium to produce commercial supply in quantities and quality sufficient to satisfy market demand for Twirla; |
| | | |
| • | the performance and financial condition of Corium or any of the suppliers; |
| | | |
| • | our ability to design and successfully complete a post-marketing long-term, prospective observational safety study comparing risks
for venous thromboembolism, or VTE, and arterial thromboembolism, or ATE, in new users of Twirla to new users of oral combined hormonal
contraceptives, or CHCs, and new users of Xulane in U.S. women of reproductive age using CHCs and successfully complete a post-marketing
commitment, or PMC, to assess the residual drug content of Twirla after use; |
| | | |
| • | our ability to maintain regulatory approval of Twirla and the labeling under any approval we obtain; |
| | | |
| • | our ability to obtain and maintain intellectual property protection for Twirla and our product candidates; |
| • | the success and timing of our clinical trials or other studies, including post-marketing studies for Twirla; |
| | | |
| • | development of unexpected safety or efficacy concerns related to Twirla; |
| | | |
| • | our ability to continue to develop and maintain successful sales and marketing capabilities, including our ability to maintain an
effective sales force or failure to build-out and implement an effective health care compliance program; |
| | | |
| • | our ability to maintain compliance with the listing requirements of the Nasdaq Capital Market; |
| | | |
| • | our ability to retain key employees and recruit the additional personnel we will need to support our commercialization plan for Twirla; |
| | | |
| • | our ability to successfully implement our strategy; and |
| | | |
| • | our use of the proceeds from this offering. |
We may not actually achieve the
plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking
statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking
statements we make. We have included important cautionary statements in this prospectus or in the documents incorporated by reference
in this prospectus, particularly in the “Risk Factors” section, that we believe could cause actual results or events to differ
materially from the forward-looking statements that we make. For a summary of such factors, please refer to the section entitled “Risk
Factors” in this prospectus, as updated and supplemented by the discussion of risks and uncertainties under “Risk Factors”
contained in any further supplements to our prospectus and in our most recent Annual Report on Form 10-K, as revised or supplemented by
our subsequent Quarterly Reports on Form 10-Q or our Current Reports on Form 8-K, as well as any amendments thereto, as filed with the
SEC and which are incorporated herein by reference. The information contained in this document is believed to be current as of the date
of this document. We do not intend to update any of the forward-looking statements after the date of this document to conform these statements
to actual results or to changes in our expectations, except as required by law.
In light of these assumptions,
risks and uncertainties, the results and events discussed in the forward- looking statements contained in this prospectus or in any document
incorporated herein by reference might not occur. Investors are cautioned not to place undue reliance on the forward-looking statements,
which speak only as of the date of this prospectus or the date of the document incorporated by reference in this prospectus. We are not
under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result
of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person acting on
our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
PROSPECTUS SUMMARY
This summary highlights information contained
in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before
investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed
information appearing elsewhere in this prospectus, any applicable free writing prospectus and the documents incorporated by reference
herein and therein. You should read all such documents carefully, especially the risk factors and our financial statements and the related
notes included or incorporated by reference herein or therein, before deciding to buy shares of our common stock. Unless the context requires
otherwise, references in this prospectus to “Agile,” “we,” “us” and “our” refer to Agile
Therapeutics, Inc..
Company Overview
We are a women’s healthcare company dedicated
to fulfilling the unmet health needs of today’s women. We are committed to innovating in women’s healthcare where there continues
to be unmet needs — not only in contraception — but also in other meaningful women’s health therapeutic areas.
Our first and only commercial product,
Twirla, which was approved in February 2020 and launched in early December 2020, is a once-weekly prescription combination hormonal
contraceptive patch. It delivers a dose of estrogen that is consistent with commonly prescribed combined hormonal contraceptives, or
CHCs, and is lower than the estrogen dose found in other marketed contraceptive patches. We believe there is a market need for a
contraceptive patch that is designed to deliver 30 mcg of estrogen and 120 mcg of progestin in a convenient, once-weekly dosage form
that may support compliance in a noninvasive fashion. Twirla leverages our proprietary transdermal patch technology called
Skinfusion®. Skinfusion is designed to allow drug delivery through the skin while optimizing patch adhesion and patient comfort
and wearability, which may help support compliance.
Since the approval of Twirla we have focused on
our advancement as a commercial company. Over the course of 2021, the first year of Twirla’s commercial launch, we have seen consistent
growth in Twirla prescriptions and a broadening of reimbursement and patient access. We have designed our commercial plan to attempt to
account for the impact of the COVID-19 pandemic and market conditions, including a challenging reimbursement environment, and continue
to implement tactics that we believe will further accelerate growth of the Twirla brand. Our ultimate goal remains to become a contraceptive
market leader, while pursuing opportunities to broaden our portfolio to address areas of unmet medical need in additional areas of women’s
health.
It should be noted that current public health threats
could adversely affect our ongoing or planned business operations. In particular, the ongoing COVID-19 pandemic has resulted in federal,
state and local governments and private entities mandating various restrictions, including travel restrictions, access restrictions, restrictions
on public gatherings, and stay at home orders. The most significant impacts to our business were encountered by sales representatives
promoting Twirla in the field, as some offices limited opportunities for face-to-face interactions with healthcare providers. In many
cases COVID-19 restrictions have recently eased, but re-implementation of such restrictions if necessary in the future may disrupt our
business and/or could adversely affect our commercialization plans and results. We cannot presently predict the scope and severity of
any potential business shutdowns or disruptions, but if we or any of the third parties with whom we engage, including personnel at third-party
manufacturing facilities and other third parties with whom we conduct business, were to experience shutdowns or other business disruptions,
our ability to conduct our business in the manner and on the timeline presently planned could be materially and adversely impacted. It
is unknown how long these conditions will last and what the complete effect will be on us. While to date we have been able to continue
to execute our overall business plan, some of our business activities slowed and took longer to complete as we adjusted to the challenges
of operating in a largely remote setting with our employees. While we have acclimated to a hybrid work model with our employees, another
shut down necessitating work in a completely remote environment could result in delays to our business activities and commercialization
plan. Overall, we recognize the challenges of commercializing a new product in a pandemic, will continue to closely monitor events as
they develop and plan for alternative and mitigating measures that we can implement if needed.
Recent Developments
Amendment to Amended and Restated Certificate
of Incorporation — Reverse Stock Split
At a special meeting of stockholders held on April
21, 2022, our stockholders approved an amendment to our Amended and Restated Certificate of Incorporation to effect a reverse stock split
of our outstanding shares of common stock by a ratio of any whole number between 1-for-10 and 1-for-40, at any time prior to December
31, 2022, with the exact ratio to be set within that range at the discretion of our board of directors, without further approval or authorization
of our stockholders. Following the meeting, our board of directors determined the appropriate ratio for the reverse stock split to be
1-for-40. The amendment became effective at 5:00 p.m. Eastern Time upon filing with the Secretary of State of the State of Delaware on
April 26, 2022. Our common stock opened for trading on the Nasdaq Market on Wednesday, April 27, 2022 on a split-adjusted basis under
the current trading symbol “AGRX.”
All information included in
this prospectus has been adjusted to reflect a 1-for-40 reverse stock split of our common stock effective on April 27, 2022.
The table below details the pre- and post-split
share and per share amounts for certain key amounts presented in the Company's Annual Report on Form 10-K for the year ended December
31, 2021. Post-split share amounts and per share amounts for all other share-related items in the 10-K are not reflected in the table
below. The number of authorized shares of common stock and the par value per share remains unchanged.
| Item | |
Pre-Split
Amount | | |
Post-Split
Amount | |
| Shares outstanding, 12/31/18 | |
| 34,377,329 | | |
| 859,433 | |
| Shares outstanding, 12/31/19 | |
| 69,810,305 | | |
| 1,745,257 | |
| Shares outstanding, 12/31/20 | |
| 87,563,753 | | |
| 2,189,093 | |
| Shares outstanding, 12/31/21 | |
| 121,396,033 | | |
| 3,034,900 | |
| Net loss per share (basic and diluted), Year ended 12/31/19 | |
$ | (0.38 | ) | |
$ | (15.20 | ) |
| Net loss per share (basic and diluted), Year ended 12/31/20 | |
$ | (0.61 | ) | |
$ | (24.40 | ) |
| Net loss per share (basic and diluted), Year ended 12/31/21 | |
$ | (0.77 | ) | |
$ | (30.80 | ) |
| Common stock warrants outstanding, 12/31/19 | |
| 180,274 | | |
| 4,506 | |
| Common stock warrants outstanding, 12/31/20 | |
| 1,400,000 | | |
| 35,000 | |
| Common stock warrants outstanding, 12/31/21 | |
| 15,183,324 | | |
| 379,583 | |
| Unvested restricted stock units outstanding, 12/31/19 | |
| 0 | | |
| 0 | |
| Unvested restricted stock units outstanding, 12/31/20 | |
| 159,795 | | |
| 3,994 | |
| Unvested restricted stock units outstanding, 12/31/21 | |
| 333,290 | | |
| 8,332 | |
| Common stock options outstanding, 12/31/19 | |
| 7,192,357 | | |
| 179,808 | |
| Common stock options outstanding, 12/31/20 | |
| 8,519,086 | | |
| 212,977 | |
| Common stock options outstanding, 12/31/21 | |
| 10,367,442 | | |
| 259,186 | |
April 2022 ATM Program with H.C. Wainwright & Co.
On April 27, 2022, we terminated our previous at-the-market
(ATM) offering program and entered into a new ATM program with H.C. Wainwright & Co., which we refer to as the April 2022 ATM, under
which we may, from time to time in our sole discretion, issue and sell through H.C. Wainwright & Co., acting as our agent, up to $12,841,000
of shares of our common stock. We will pay H.C. Wainwright & Co. a commission of up to 3.0% of the gross sales proceeds of shares
sold under the April 2022 ATM. Through May 12, 2022, 874,680 shares had been sold under the ATM for gross proceeds of $2.0 million.
Perceptive Credit Agreement Amendment
On May 11, 2022, we entered into a fourth amendment
to the Credit Agreement and Guarantee dated February 10, 2020, as amended on February 26, 2021, January 7, 2022 and March
10, 2022, between us and Perceptive Credit Holdings III, LP, or Perceptive, which we may refer to as the Amended Perceptive Credit Agreement.
The Amended Perceptive Credit Agreement waived our obligations to comply with certain financial covenants relating to minimum revenue
requirements through September 30, 2022, conditioned upon the satisfaction of certain conditions, including us raising additional
capital and prepaying a portion of our outstanding debt by May 31, 2022.
Nasdaq Listing Compliance
As previously disclosed, on November 9, 2021, we received a deficiency letter from the Listing Qualifications
Department, or the Staff, of the Nasdaq Stock Market, or Nasdaq, notifying us that, for 30 consecutive business days preceding the date
of the letter, the closing bid price for our common stock was below the minimum $1.00 per share requirement for continued inclusion on
The Nasdaq Capital Market pursuant to Nasdaq Listing Rule 5550(a)(2).
On May 11, 2022, we received a letter from the Staff notifying us
that the Staff has determined that for 10 consecutive business days, from April 26, 2022 to May 10, 2022, the minimum closing bid price
for our common stock was at least $1.00 per share. Accordingly, the Staff has determined that we have regained compliance with Listing
Rule 5550(a)(2) and it has indicated that the matter is now closed.
Senior Management Bonuses
On May 12, 2022, the compensation committee of our board of directors, or the Compensation Committee, was notified by the named executive
officers of the Company, Al Altomari, Geoffrey P. Gilmore and Paul Korner, that they, along with other corporate officers and two other
members of the senior management team, were voluntarily forgoing the cash bonuses that they had earned for their performance in 2021 and
that had been granted by the Compensation Committee in January 2022, which we refer to as the Senior Management Bonuses. The Senior Management
Bonuses, which are approximately $700,000, are planned to be used for general corporate purposes.
Risks Associated with this Offering
This offering is subject to numerous risks and
uncertainties, including those highlighted in the section entitled “Risk Factors” immediately following this prospectus summary
and the section entitled “Risk Factors” included in our Annual Report on Form 10-K for the fiscal year ended December 31,
2021 as revised or supplemented by our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, which are incorporated herein
by reference. These risks include, but are not limited to, the following:
| · | the ongoing outbreak of the novel strain of coronavirus, or COVID-19, or other similar public health crises, could have a material
adverse impact on our business, financial condition and results of operations, including our ability to successfully produce, market,
and distribute Twirla®; |
| · | the price of our common stock may be volatile and fluctuate substantially, and you may not be able to resell your shares at
or above the price you paid for them; |
| · | there is no public market for the warrants being offered in this offering; |
| · | holders of warrants purchased in this offering will have no rights as common stockholders until such holders exercise their
warrants and acquire our common stock; |
| · | the warrants being offered may not have value; |
| · | certain of our outstanding common stock purchase warrants contain price protection provisions (anti-dilution protection) in
the event that we sell our securities at prices lower than the current exercise price of such warrants, which may have a negative impact
on the trading price of our common stock or impair our ability to raise capital; |
| · | management will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively;
and |
| · | we have incurred operating losses in each year since our inception and expect to continue to incur substantial losses for the
foreseeable future. Management has concluded that these factors raise substantial doubt about our ability to continue as a going concern. |
Corporate Information
Information concerning our business is contained
in the documents that we file with the SEC as a reporting company under the Exchange Act, which are accessible at www.sec.gov, and on
our website at www.agiletherapeutics.com. The information contained on, or that can be accessed through, our website is not a part of
this prospectus. Investors should not rely on any such information in deciding whether to purchase our common stock. We have included
our website address in this prospectus solely as an inactive textual reference.
Our principal executive offices are located at
500 College Road East, Suite 310, Princeton, New Jersey 08540, and our telephone number is (609) 683-1880.
THE OFFERING
| Securities we are offering |
Up
to shares of our common stock and Series A warrants to
purchase shares of common stock, or up to Series B
pre-funded warrants to purchase shares of common stock and Series A warrants to
purchase shares of common stock, in each case, assuming a
combined offering price of $ per share and Series A warrant and per
Series B pre-funded warrant and Series A warrant, which is equal to the last reported sale price per share of our common
stock on the Nasdaq Capital Market
on ,
2022. The shares, or Series B pre-funded warrants, and Series A warrants will be separately transferable immediately upon
issuance, but the shares, or Series B pre-funded warrants, and Series A warrants will be issued to purchasers in the ratio of one to
one. |
| |
|
| Description of Series A warrants |
Each Series A warrant will have an exercise price of $ per share, will be exercisable upon issuance and will expire years from the date of issuance. |
| |
|
| Description of Series B pre-funded warrants |
If the issuance of shares of our common stock to a purchaser in this offering would result in such purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock following the consummation of this offering, then such purchaser may purchase, if they so choose, in lieu of the shares of our common stock that would result in such excess ownership, a Series B pre-funded warrant to purchase shares of our common stock for a purchase price per share of common stock subject to such Series B pre-funded warrant equal to the per share public offering price for the common stock in this offering less $0.001. Each Series B pre-funded warrant will have an exercise price of $0.001 per share, will be exercisable upon issuance and may be exercised at any time until all of the pre-funded warrants are exercised in full. Purchasers of Series B pre-funded warrants will also receive Series A warrants as if such purchasers were buying shares of our common stock in this offering. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of these Series B pre-funded warrants. |
| |
|
| Common stock outstanding before offering: |
shares of common stock. |
| |
|
| Common stock outstanding after this offering |
shares
of common stock, assuming no sale of Series B pre-funded warrants in this offering and no exercise of the Series A warrants being
issued in this offering and assuming a combined offering price of $ per share
and Series A warrant, which is equal to the last reported sale price per share of our common stock on the Nasdaq Capital Market on
May , 2022. |
| |
|
| Use of proceeds |
We intend to use the net proceeds from this offering for working capital, repayment
of a portion of our debt, business development activities, and general corporate purposes. See “Use of Proceeds” on page
14 of this prospectus. |
| |
|
| Risk factors |
See “Risk Factors” beginning on page 9 and the section entitled
“Risk Factors” included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2021 as revised or supplemented
by our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, which are incorporated herein by reference, and the other
information included or incorporated by reference elsewhere in this prospectus for a discussion of factors you should carefully consider
before deciding to invest in our securities. |
| Nasdaq Capital Market Symbol |
Our common stock is listed on the Nasdaq Capital Market under the symbol “AGRX.” There is no established trading market for the Series A warrants or the Series B pre-funded warrants, and we do not expect a trading market to develop. We do not intend to list the Series A warrants or the Series B pre-funded warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the Series A warrants or Series B pre-funded warrants will be extremely limited. |
The number of shares of our
common stock to be outstanding after this offering is based on 3,668,546 shares of our common stock outstanding as of April 22, 2022,
and excludes:
| • | 304,761 shares of common stock issuable upon the exercise of outstanding options to purchase common stock
as of April 22, 2022 at a weighted average exercise price of $96.53 per share; |
| • | 6,553 shares of common stock issuable upon the vesting of outstanding restricted stock units as of April
22, 2022; |
| • | 37,145 shares of common stock reserved for future issuance under our 2014 Amended and Restated Incentive
Compensation Plan as of April 22, 2022; and |
| • | 1,622,396 shares of common stock issuable upon the exercise of outstanding warrants as of April 22,
2022 at a weighted average exercise price of $18.04 per share. |
Unless otherwise indicated,
all information contained in this prospectus assumes:
| • | no exercise of the outstanding options or warrants described in the bullets above; |
| | | |
| | • | no exercise of the Series A warrants or the placement agent warrants issued in this offering; and |
| • | no sale of Series B pre-funded warrants in this offering and no exercise of the warrants being issued
in this offering. |
All information included in
this prospectus has been adjusted to reflect a 1-for-40 reverse stock split of our common stock effective on April 27, 2022.
RISK FACTORS
An investment in our common stock involves a high
degree of risk. Before deciding whether to invest in our common stock, you should carefully consider the risks described below and those
discussed under the Section captioned “Risk Factors” contained in our Annual Report on Form 10-K for the year ended December
31, 2021, as revised or supplemented by our subsequent quarterly reports on Form 10-Q or our current reports on Form 8-K, each as filed
with the SEC and which are incorporated by reference in this prospectus, together with other information in this prospectus, the information
and documents incorporated by reference herein and therein, and in any free writing prospectus that we have authorized for use in connection
with this offering. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could
be seriously harmed. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment.
Please also read carefully the section above entitled “Forward-Looking Statements.”
Risks Related to This Offering
The ongoing outbreak of the novel strain of coronavirus,
or COVID-19, or other similar public health crises, could have a material adverse impact on our business, financial condition and results
of operations, including our ability to successfully produce, market, and distribute Twirla®.
In December 2019, a novel strain
of coronavirus (SARS-CoV-2), now referred to as COVID-19, surfaced in Wuhan, China. Since then, the virus has spread globally to multiple
countries, including the United States. The impact of this pandemic has been and will likely continue to be extensive, affecting many
aspects of society, and it has resulted in and will likely continue to result in significant disruptions to global business activities
and capital markets around the world, including as emerging variants of the virus, such as the delta variant, are detected and continue
to spread.
As a result of the COVID-19 pandemic,
or similar pandemics, we may experience disruptions that could severely affect our business, including our plans to clinically develop
and commercialize our products. We may not be able to meet expectations with respect to our anticipated commercial launch of Twirla, our
first approved product, which we plan to begin manufacturing on a commercial scale in the second half of 2020.
Global business interruptions resulting
from COVID-19 may adversely impact our third-party manufacturer, Corium, whom we rely upon for the manufacture of Twirla, as well as its
suppliers of raw materials. If Corium or any of its suppliers of raw materials are adversely impacted by the COVID-19 pandemic or the
restrictions resulting from the pandemic, if they cannot obtain the necessary supplies, or if such third parties need to prioritize other
products or customers over us, including under the Defense Production Act, we may experience delays or disruptions in our supply chain,
which could have a material and adverse impact on our business. Third party manufacturers may also need to implement measures and changes,
or deviate from typical requirements because of the COVID-19 pandemic that may otherwise adversely impact our supply chains or the quality
of the resulting products or supplies. Depending on the change, we may need to obtain FDA pre-approval or otherwise provide FDA with a
notification of the change. As a result, we may not be able to obtain sufficient quantities of Twirla, which could impair our ability
to commercialize Twirla and conduct the post-marketing studies requested by the U.S. Food and Drug Administration, or the FDA, in connection
with the approval of Twirla. In addition, if there are continued or future disruptions, our third-party manufacturers may not be able
to supply our other potential product candidates, which would adversely affect our research and development activities.
Further, many jurisdictions
have implemented travel restrictions and expansive social distancing orders. Although many of the restrictions have been relaxed,
these measures, if re-implemented, may have a material adverse impact on the third-party consultants who assist us with our sales and marketing
functions, as well as on our ability to develop our own sales and marketing infrastructure. For example, such social distancing
orders could limit the ability of sales representatives to interact with healthcare providers and also restrict the ability of
patients to interact with their healthcare providers and obtain prescriptions for our products. Patients may also be more reticent
to visit their providers to obtain Twirla prescriptions during the COVID-19 pandemic. This could negatively affect our ability to
commercialize Twirla as well as market our other potential product candidates.
Delays in the ability to
manufacture commercial supplies of Twirla and disruptions in the operation of a sales force for Twirla could also adversely affect
our financial position. Three vaccines for COVID-19 have been granted Emergency Use Authorization by the FDA, and two have
subsequently been granted full FDA approval, and additional booster shots have been authorized for certain populations and more may
be authorized in the future. The resultant demand for vaccines and potential for manufacturing facilities and materials to be
commandeered under the Defense Production Act of 1950, or equivalent foreign legislation, may make it more difficult to obtain
materials or manufacturing slots for the products needed for our clinical trials and/or commercial product, which could lead to
delays in these trials and/or issues with our commercial supply. If the COVID-19 pandemic or other factors impact our current
business plan or our ability to generate revenue from the launch of Twirla, we believe we have the ability to revise our commercial
plans, including curtailing sales and marketing spending, to allow us to continue to fund our operations. While we have established
inventory levels of Twirla in an effort to mitigate risks to our supply chain, significant delays in the timelines to manufacture
commercial supply of Twirla, and/or the ability of a salesforce to engage with healthcare providers could delay, or even prevent,
our ability to generate revenue, which in turn could require us to raise additional capital if the revisions to our commercial plans
are inadequate or management determines that it is necessary.
Additionally, certain of our clinical
activities, including the post-marketing studies requested by the FDA in connection with the approval of Twirla, as well as any product
development activities that we have planned, may be delayed or interrupted, compromising our ability to maintain regulatory approval for
Twirla and our future ability to obtain marketing approval for our other potential product candidates. By example, the pandemic may result
in slower enrollment than we anticipated, the need to suspend enrollment into studies, patient withdrawals, postponement of planned clinical
or preclinical studies, redirection of site resources from studies, study modification, suspension, or termination, the introduction of
remote study procedures and modified informed consent procedures, study site changes, direct delivery of investigational products to patient
homes requiring state licensing, study deviations or noncompliance, and changes or delays in site monitoring. The foregoing may require
that we consult with relevant review and ethics committees, IRBs, and the FDA. The foregoing may also impact the integrity of our study
data. The effects of the COVID-19 pandemic may also increase the need for clinical trial patient monitoring and regulatory reporting of
adverse effects. The pandemic could further impact our ability to interact with the FDA or other regulatory authorities, and may result
in delays in the conduct of inspections or review of pending applications or submissions. Since March 2020, foreign and domestic inspections
by the FDA have largely been on hold with FDA announcing plans in July 2020 to resume prioritized domestic inspections. Should FDA determine
that an inspection is necessary for approval of a marketing application and an inspection cannot be completed during the review cycle
due to restrictions on travel, FDA has stated that it generally intends to issue a complete response letter. Further, if there is inadequate
information to make a determination on the acceptability of a facility, FDA may defer action on the application until an inspection can
be completed. In 2020, several companies announced receipt of complete response letters due to the FDA’s inability to complete required
inspections for their applications. Regulatory authorities outside the U.S. may adopt similar restrictions or other policy measures in
response to the COVID-19 pandemic and may experience delays in their regulatory activities. In 2020, FDA noted it was continuing to ensure
timely reviews of applications for medical products during the COVID-19 pandemic in line with its user fee performance goals; however,
FDA may not be able to continue its current pace and approval timelines could be extended, including where a pre-approval inspection or
an inspection of clinical sites is required and due to the COVID-19 pandemic and travel restrictions FDA is unable to complete such required
inspections during the review period. Due to the potential impact of the COVID-19 pandemic on clinical trials, drug development, and manufacturing,
FDA issued a number of guidances concerning how sponsors and investigators may address these challenges. FDA’s guidance is continually
evolving. Any of these factors could significantly impair our ability to generate revenue in the future and to attain and maintain profitability.
The COVID-19 pandemic may result
in changes in laws and regulations. For example, in March 2020, the CARES Act, which includes various provisions regarding FDA drug shortage
reporting requirements, as well as provisions regarding supply chain security, such as risk management plan requirements, and the promotion
of supply chain redundancy and domestic manufacturing, was implemented. This and any future changes in law may require that we change
our internal processes and procedures to ensure continued compliance.
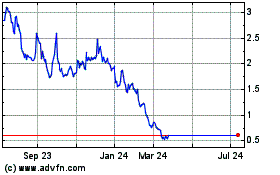
The price of our common stock may be volatile
and fluctuate substantially, and you may not be able to resell your shares at or above the price you paid for them.
The shares sold in this offering,
if any, will be sold from time to time at various prices. The market price for shares of our common stock may be subject to wide fluctuations
in response to many risk factors, including:
| • | Our failure to commercialize Twirla or develop and commercialize additional potential product candidates; |
| | | |
| • | Our ability to successfully enhance the commercialization and increase the uptake for Twirla, our only approved product; |
| | | |
| • | Unanticipated efficacy, safety or tolerability concerns related to the use of Twirla; |
| | | |
| • | Regulatory actions with respect to Twirla; |
| | | |
| • | The growth in demand for Twirla and our ability to manage the levels of Twirla inventory, which could result in our having to write
off inventory and our inability to meet the minimum requirements under our supply agreement with Corium; |
| | | |
| • | Inability to obtain adequate product supply of Twirla or inability to do so at acceptable prices; |
| | | |
| • | Adverse results or delays in our clinical trials for our potential product candidates; |
| | | |
| • | Changes in laws or regulations applicable to Twirla or any future potential product candidates, including but not limited to clinical
trial requirements for approvals, post-approval requirements, and product marketing, advertising, and promotional requirements and limitations; |
| | | |
| | • | Coverage and reimbursement policies with respect to Twirla or our product candidates, if approved; |
| | | |
| • | Our ability to maintain compliance with the listing requirements of the Nasdaq Capital Market; |
| | | |
| • | Actual or anticipated fluctuations in our financial condition and operating results; |
| | | |
| • | Actual or anticipated changes in our growth rate relative to our competitors; |
| | | |
| • | Competition from existing products or new products that may emerge; |
| | | |
| • | Announcements by us, our collaborators or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations
or capital commitments; |
| | | |
| • | Failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| | | |
| • | Issuance of new or updated research or reports by securities analysts; |
| | | |
| • | Fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| | | |
| • | Share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| | | |
| • | Additions or departures of key personnel; |
| | | |
| • | Disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain
and retain patent protection for our technologies; |
| | | |
| • | Announcement or expectation of additional debt or equity financing efforts; |
| | | |
| • | Sales of our common stock by us, our insiders or our other stockholders; and |
| | | |
| • | General economic and market conditions. |
In addition, the stock market
has experienced significant volatility, particularly with respect to pharmaceutical and other life sciences company stocks. The
volatility of such stocks often does not relate to individual company performance. As we operate in a single industry, we are
especially vulnerable to these factors to the extent that they affect our industry or our product candidates or, to a lesser extent,
our markets. In the past, securities class-action litigation has often been instituted against companies following periods of
volatility in their stock price. We may face securities class-action litigation if we fail to commercialize Twirla or cannot obtain
regulatory approvals for our product candidates. Such litigation, if instituted against us, could cause us to incur substantial
costs to defend such claims and divert management’s attention and resources, which could materially harm our financial
condition and results of operations.
There is no public market for the warrants being
offered in this offering.
There is no established trading market for the
warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the
warrants on any securities exchange or nationally recognized trading system, including the Nasdaq Capital Market. Without an active market,
the liquidity of the warrants will be limited.
Holders of warrants purchased in this offering
will have no rights as common stockholders until such holders exercise their warrants and acquire our common stock.
Until holders of the warrants being offered in
this offering acquire shares of our common stock upon exercise of such warrants, the holders will have no rights with respect to the shares
of our common stock underlying such warrants. Upon exercise of the warrants, the holders will be entitled to exercise the rights of a
common stockholder only as to matters for which the record date occurs after the exercise.
The warrants being offered may not have value.
The warrants being offered by us in this offering
have an exercise price of $ per share, subject to certain adjustments, and will expire years from the date of issuance, after which date
any unexercised warrants will have no further value. In the event that the market price of our common stock does not exceed the exercise
price of the warrants during the period when they are exercisable, the warrants may not have any value.
Certain of our outstanding common stock purchase
warrants contain price protection provisions (anti-dilution protection) in the event that we sell our securities at prices lower than
the current exercise price of such warrants, which may have a negative impact on the trading price of our common stock or impair our ability
to raise capital.
As of , 2022, we had 46,250 common stock
purchase warrants outstanding that were issued in connection with the Amended Perceptive Credit Agreement that contain price
protection provisions in the event that we sell securities at a price per share below their respective exercise prices on or before
December 31, 2022, including in this offering (collectively, “Price Protection Warrants”). The current exercise prices
of the Price Protection Warrants are: 17,500 Price Protection Warrants at $104.80, 17,500 Price Protection Warrants at $129.20 and
11,250 Price Protection Warrants at $84.20. Because we sold securities in this offering at a price per share lower than the current
exercise price of the Price Protection Warrants, their exercise prices will be reduced pursuant to a weighted-average anti-dilution
formula. Any future adjustments to the exercise prices of the Price Protection Warrants may have a negative impact on the trading
price of our common stock. Additionally, raising additional capital with new investors may be difficult as a result of the
adjustment feature.
Management will have broad discretion as to the
use of the proceeds from this offering, and we may not use the proceeds effectively.
Our management will have broad
discretion with respect to the use of proceeds of this offering, including for any of the purposes described in the section of this prospectus
entitled “Use of Proceeds.” You will be relying on the judgment of our management regarding the application of the proceeds
of this offering. The results and effectiveness of the use of proceeds are uncertain, and we could spend the proceeds in ways that you
do not agree with or that do not improve our results of operations or enhance the value of our common stock. Our failure to apply these
funds effectively could have a material adverse effect on our business, delay the development of our product candidates and cause the
price of
our common stock to decline.
We have incurred operating losses in each year
since our inception and expect to continue to incur substantial losses for the foreseeable future. Management has concluded that these
factors raise substantial doubt about our ability to continue as a going concern.
We
have incurred losses in each year since our inception in December 1997. Our net loss was $11.8 million for the three months ended
March 31, 2022, and $74.9 million, $51.9 million and $18.6 million for the years ended December 31, 2021, 2020 and 2019,
respectively. As of March 31, 2022, we had an accumulated deficit of approximately $399 million. Our cash and cash equivalents as of
March 31, 2022 will not be sufficient to fund our current and planned operations through the 12 months following the date on which
our Quarterly Report on Form 10-Q for the three months ended March 31, 2022 was filed, which raises substantial doubt about our
ability to continue as a going concern. Substantial doubt about our ability to continue as a going concern may create negative
reactions to the price of our common stock and we may have a more difficult time obtaining financing in the future.
Specialty pharmaceutical product
development is a speculative undertaking, involves a substantial degree of risk and is a capital-intensive business. We expect to incur
expenses without corresponding revenues until we are able to sell Twirla in significant quantities, which may not happen. We have devoted
most of our financial resources to research and development, including our non-clinical development activities and clinical trials. We
will require additional capital to fund our operating needs, including among other items, the commercialization of Twirla and advancing
the development of our other potential product candidates. We may not be able to obtain sufficient additional funding to continue our
operations at planned levels and be forced to reduce, or even terminate, our operations. To date, we have financed our operations primarily
through sales of common stock, convertible preferred stock and convertible promissory notes and to a lesser extent, through term loans
and government grants. Our potential product candidates will also require the completion of regulatory review, significant marketing efforts
and substantial investment before they can provide us with any revenue.
We expect that our expenses will
increase as we commercialize Twirla. As a result, we expect to continue to incur substantial losses for the foreseeable future. We are
uncertain when or if we will be able to achieve or sustain profitability. If we achieve profitability in the future, we may not be able
to sustain profitability in subsequent periods. Any failure to become and remain profitable could impair our ability to sustain operations
and adversely affect the price of our common stock and our ability to raise additional capital. We are significantly dependent on the
success of Twirla, and if we do not achieve the commercial success of Twirla and/or are unable to obtain additional funding, we will need
to reassess our operating capital needs and may be unable to continue our operations at planned levels and be forced to reduce, or even
terminate, our operations.
Our forecast of the period of time
through which our financial resources will be adequate to support our operating requirements is a forward-looking statement and involves
risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed elsewhere in
this “Risk Factors” section and Part I, Item 1A “Risk Factors” of our Annual Report on Form 10-K filed with the
SEC on March 30, 2022, as revised or supplemented by our subsequent quarterly reports on Form 10-Q or our current reports on Form 8-K,
each as filed with the SEC. We have based this estimate on a number of assumptions that may prove to be wrong, and changing circumstances
beyond our control may cause us to consume capital more rapidly than we currently anticipate. Our inability to obtain additional funding
when we need it could seriously harm our business.
Raising additional capital may cause dilution
to our existing stockholders or restrict our operations.
We will need to seek additional capital through
a combination of private and public equity offerings, debt financings and strategic collaborations. The sale of additional equity or convertible
debt securities could result in the issuance of additional shares of our capital stock and could result in dilution to our stockholders.
The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants,
such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights
and other operating restrictions that could adversely impact our ability to conduct our business. We cannot guarantee that future financing
will be available in sufficient amounts or on terms acceptable to us, if at all. If we are unable to raise additional capital in sufficient
amounts or on terms acceptable to us, we will be prevented from pursuing research and development efforts, and could be forced to limit
funding of our efforts to commercialize Twirla. This could harm our business, operating results and financial condition and cause the
price of our common stock to fall.
This is a best efforts offering, no minimum amount
of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business plans, including
our near-term business plans.
The placement agent has agreed to use its reasonable
best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities
from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum
number of securities that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required
as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently
determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the securities offered
hereby, which may significantly reduce the amount of proceeds received by us, and investors in this offering will not receive a refund
in the event that we do not sell an amount of securities sufficient to support our continued operations, including our near-term continued
operations. Thus, we may not raise the amount of capital we believe is required for our operations in the short-term and may need to raise
additional funds to complete such short-term operations. Such additional fundraises may not be available or available on terms acceptable
to us.
USE OF PROCEEDS
We estimate that the net proceeds from this offering
will be approximately $ million based on the sale of shares of common stock and Series A warrants to purchase shares of common stock at
an assumed combined public offering price of $ per share of common stock and Series A warrant, which is equal to the last reported sale
price per share of our common stock on the Nasdaq Capital Market on , 2022, after deducting the placement agent fees and estimated offering
expenses payable by us, and assuming no sale of Series B pre-funded warrants in this offering and no exercise of the Series A warrants
being issued in this offering. However, because this is a best efforts offering and there is no minimum offering amount required as a
condition to the closing of this offering, the actual offering amount, the placement agent’s fees and net proceeds to us are not
presently determinable and may be substantially less than the maximum amounts set forth on the cover page of this prospectus.
These estimates exclude the proceeds, if any, from
the exercise of Series A warrants issued in this offering. If all of the Series A warrants issued in this offering were to be exercised
in cash at an assumed exercise price of $ per share of common stock, we would receive additional proceeds of approximately $ million.
We cannot predict when or if these Series A warrants will be exercised. It is possible that these Series A warrants may expire and may
never be exercised. Additionally, the Series A warrants contain a cashless exercise provision that permit exercise of Series A warrants
on a cashless basis at any time where there is no effective registration statement under the Securities Act of 1933, as amended, covering
the issuance of the underlying shares.
We intend to use the net proceeds of this offering
for working capital and other general corporate purposes, and may also use them for the repayment of a portion of the Amended Perceptive
Credit Agreement. Borrowings under the Amended Perceptive Credit Agreement will accrue interest at an annual rate equal to the London
Interbank Offered Rate for one-month deposits, or LIBOR, plus 10.25%, provided that LIBOR shall not be less than 1.5%. The rate of interest
in effect as of February 10, 2022 and at March 31, 2022 was 11.75%. Upon the occurrence and during the continuance of any event of default
under the Amended Perceptive Credit Agreement, the interest rate automatically increases by 3.0% per annum.The Perceptive Credit Agreement,
as amended, will mature on February 10, 2024.
We may also use a portion of the net proceeds to
invest in or acquire businesses or technologies that we believe are complementary to our own, although we have no current plans, commitments
or agreements with respect to any acquisitions as of the date of this prospectus supplement. Pending these uses, we plan to invest these
net proceeds in investment-grade, interest bearing securities.
These expected uses represent our intentions based
upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts
and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development
as a commercial company, the implementation of our commercial plan for Twirla, the status of and results from future clinical trials,
and any unforeseen cash needs. As a result, our management will have broad discretion in the application
of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the
net proceeds from this offering. The timing and amount of our actual expenditures will be based on many factors, including cash flows
from operations and the anticipated growth of our business.
DIVIDEND POLICY
We currently intend to retain all available funds
and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the
foreseeable future. In addition, our Credit Agreement and Guaranty, as amended, among us, the guarantors from time to time party thereto,
the lenders from time to time party thereto and Perceptive Credit Holdings III, LP, as a lender and as Administrative Agent for the lenders,
contains, and any other loan facilities that we may enter into may contain, restrictions on our ability to pay dividends. Subject to such
restrictions, any future determination to declare dividends will be made at the discretion of our board of directors and will depend on
our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors
may deem relevant.
DESCRIPTION
OF CAPITAL STOCK
The following description is a general summary
of the terms of the shares of common stock or shares of preferred stock, does not include all of the terms of the shares of common stock
or shares of preferred stock and should be read together with our Amended and Restated Certificate of Incorporation and Amended and Restated
Bylaws, copies of which have been filed previously with the SEC. For more information on how you can obtain copies of our Amended and
Restated Certificate of Incorporation and Amended and Restated Bylaws, see “Where You Can Find More Information.”
Common Stock
General
Our Amended and Restated Certificate of Incorporation
provides the authority to issue 300,000,000 shares of common stock, par value $0.0001 per share. At April 22, 2022, there were 3,668,546
shares of common stock outstanding. Each share of our common stock has the same relative rights and is identical in all respects to each
other share of our common stock. The rights, preferences and privileges of holders of our common stock are subject to the rights, preferences
and privileges of the holders of shares of any series of preferred stock that we have issued or may issue in the future.
Voting Rights
The holders of our common stock are entitled to
one vote per share on any matter to be voted upon by our stockholders. Our Amended and Restated Certificate of Incorporation does not
permit cumulative voting in connection with the election of directors.
Dividends
The holders of our common stock are entitled to
dividends, if any, as our board of directors may declare from time to time from funds legally available for that purpose, subject to the
holders of other classes of stock, if any, at the time outstanding having prior rights as to dividends, if any.
Liquidation Rights
Upon any voluntary or involuntary liquidation,
dissolution, or winding up of our affairs, the holders of our common stock are entitled to share ratably in all assets remaining after
the payment of creditors, subject to any prior liquidation distribution rights of holders of other classes of stock, if any, at the time
outstanding.
Miscellaneous
Holders of our common stock have no preemptive,
conversion, redemption or sinking fund rights. The outstanding shares of our common stock are, and the shares of common stock to be offered
hereby when issued will be, validly issued, fully paid and non-assessable.
Nasdaq Listing
Our common stock is listed on the Nasdaq Capital
Market under the symbol “AGRX.”
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is Broadridge Corporate Issuer Solutions, Inc. whose address is 51 Mercedes Way, Edgewood, New York 11717.
Preferred Stock
Our Amended and Restated Certificate of
Incorporation authorizes the issuance of up to 10,000,000 shares of preferred stock, par value $0.0001 per share, none of which are
issued and outstanding as of the date of this prospectus. We may issue, from time to time in one or more series, the terms of which
may be determined at the time of issuance by our board of directors, without further action by our stockholders, shares of preferred
stock and such shares may include voting rights, preferences as to dividends and liquidation, conversion rights, redemption rights
and sinking fund provisions. The shares of each series of preferred stock shall have preferences, limitations and relative rights,
including voting rights, identical with those of other shares of the same series and, except to the extent provided in the
description of such series, of those of other series of preferred stock.
Delaware Law and Certain Amended and Restated Certificate of Incorporation
and Amended and Restated Bylaws Provisions
Our Amended and Restated Certificate of Incorporation
and Amended and Restated Bylaws to be in effect upon completion of this offering contain provisions that could delay or prevent a change
of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions:
• Authorize
the issuance of preferred stock which can be created and issued by the board of directors without prior stockholder approval, with rights
senior to those of our common stock;
• Provide
for a classified board of directors, with each director serving a staggered three-year term;
• Prohibit
our stockholders from filling board vacancies, calling special stockholder meetings or taking action by written consent;
• Provide
for the removal of a director only with cause and by the affirmative vote of the holders of 75% or more of the shares then entitled to
vote at an election of our directors;
• Require
advance written notice of stockholder proposals and director nominations; and
• Require
any action instituted against our officers or directors in connection with their service to the Company to be brought in the state of
Delaware.
In addition, we are subject to the provisions of
Section 203 of the Delaware General Corporation Law, which may prohibit certain business combinations with stockholders owning 15% or
more of our outstanding voting stock. These and other provisions in our Amended and Restated Certificate of Incorporation, Amended and
Restated Bylaws and Delaware law could make it more difficult for stockholders or potential acquirors to obtain control of our board of
directors or initiate actions that are opposed by our then-current board of directors, including a merger, tender offer or proxy contest
involving our company. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired
by or beneficial to our stockholders. Any delay or prevention of a change of control transaction or changes in our board of directors
could cause the market price of our common stock to decline.
Indemnification
Our Amended and Restated Certificate of Incorporation
contains provisions permitted under the General Corporation Law of Delaware relating to the liability of directors. The provisions eliminate,
to the extent legally permissible, a director’s liability for monetary damages for a breach of fiduciary duty, except in circumstances
involving wrongful acts, such as the breach of a director’s duty of loyalty or acts or omissions that involve intentional misconduct
or a knowing violation of law. The limitation of liability described above does not alter the liability of our directors and officers
under federal securities laws. Furthermore, our Amended and Restated Certificate of Incorporation contains provisions to indemnify our
directors and officers to the fullest extent permitted by the General Corporation Law of Delaware. These provisions do not limit or eliminate
our right or the right of any stockholder of ours to seek non-monetary relief, such as an injunction or rescission in the event of a breach
by a director or an officer of his or her duty of care to us. We believe that these provisions assist us in attracting and retaining qualified
individuals to serve as directors.
DESCRIPTION OF SECURITIES WE
ARE OFFERING
We are offering
up to shares of common stock, together with warrants (the “Series A
warrants”) to purchase shares of common stock. We are also offering up to
pre-funded warrants (the “Series B pre-funded warrants” and collectively with the Series A warrants, the
“warrants”) to those purchasers, whose purchase of shares of common stock in this offering would result in the
purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the
purchaser, 9.99%) of our outstanding common stock following the consummation of this offering in lieu of the shares of our common
stock that would result in ownership in excess of 4.99% (or, at the election of the purchaser, 9.99%). Each Series B pre-funded
warrant will be exercisable for one share of common stock. Each Series B pre-funded warrant is being issued together with the same
Series A warrant described above being issued with each share common stock. The shares of common stock or Series B pre-funded
warrants, as the case may be, and the Series A warrants, can only be purchased together in this offering, but the Series B
pre-funded warrants and Series A warrants are immediately separable and will be issued separately in this offering. We are also
registering the shares of common stock and the shares of common stock issuable from time to time upon exercise of the Series B
pre-funded warrants and Series A warrants offered hereby.
Common Stock
The description of our common stock under the section
“Description of Our Capital Stock” in this prospectus is incorporated herein by reference.
Series B Pre-Funded Warrants
The following summary of certain terms and provisions
of the Series B pre-funded warrants that are being issued hereby is not complete and is subject to, and qualified in its entirety by,
the provisions of the Series B pre-funded warrant, the form of which will be filed as an exhibit to the registration statement of which this
prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of Series B pre-funded warrant
for a complete description of the terms and conditions of the Series B pre-funded warrants.
Duration and Exercise Price
Each Series B pre-funded warrant offered hereby
will have an initial exercise price per share equal to $0.001. The Series B pre-funded warrants will be immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full. The exercise price and number of shares of common stock issuable upon
exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting
our common stock and the exercise price. The Series B pre-funded warrants will be issued separately from the accompanying Series A warrants
with Series B pre-funded warrants.
Exercisability
The Series B pre-funded warrants will be exercisable,
at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full
for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below).
A holder (together with its affiliates) may not exercise any portion of the Series B pre-funded warrant to the extent that the holder
would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice
from the holder to us, the holder may increase the amount of beneficial ownership of outstanding stock after exercising the holder’s
Series B pre-funded warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the
exercise, as such percentage ownership is determined in accordance with the terms of the Series B pre-funded warrants and in accordance
with the rules and regulations of the SEC. Purchasers of Series B pre-funded warrants in this offering may also elect prior to the issuance
of the Series B pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding common stock.
Cashless Exercise
In lieu of making the cash payment otherwise
contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive
upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth
in the Series B pre-funded warrants.
Transferability
Subject to applicable laws, a Series B pre-funded
warrant may be transferred at the option of the holder upon surrender of the Series B pre-funded warrant to us together with the appropriate
instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the Series B pre-funded warrants. Rather, the number of shares of common stock to be issued will be rounded to the
nearest whole number.
Trading Market
There is no trading market available for the Series
B pre-funded warrants on any securities exchange or nationally recognized trading system, and we do not expect a trading market to develop.
We do not intend to list the Series B pre-funded warrants on any securities exchange or other trading market. Without a trading market,
the liquidity of the Series B pre-funded warrants will be extremely limited. The common stock issuable upon exercise of the Series B pre-funded
warrants is currently listed on the Nasdaq Capital Market.
Right as a Stockholder
Except as otherwise provided in the Series B pre-funded
warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the Series B pre-funded warrants
do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Series B pre-funded
warrants. The Series B pre-funded warrants will provide that holders have the right to participate in distributions or dividends paid
on our common stock.
Fundamental Transaction
In the event of a fundamental transaction, as described
in the Series B pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock,
the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into
another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner
of 50% of the voting power represented by our outstanding common stock, the holders of the Series B pre-funded warrants will be entitled
to receive upon exercise of the Series B pre-funded warrants the kind and amount of securities, cash or other property that the holders
would have received had they exercised the Series B pre-funded warrants immediately prior to such fundamental transaction.
Series A Warrants
The following summary of certain terms and provisions
of the Series A warrants included with the shares of common stock and the Series B pre-funded warrants that are being issued hereby is
not complete and is subject to, and qualified in its entirety by, the provisions of the Series A warrants, the form of which will be filed
as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms
and provisions of the form of Series A warrant for a complete description of the terms and conditions of the Series A warrants.
Duration and Exercise Price
Each Series A warrant offered hereby will have
an initial exercise price equal to $ per share of common stock. The Series A warrants will be
immediately exercisable and will expire years from the date of issuance.
The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock
dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. The Series A warrants will
be issued separately from the common stock, or the Series B pre-funded warrants, as the case may be.
Exercisability
The Series A warrants will be exercisable, at the
option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the
number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder
(together with its affiliates) may not exercise any portion of the Series A warrant to the extent that the holder would own more than
4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder
to us, the holder may increase the amount of beneficial ownership of outstanding stock after exercising the holder’s Series A warrants
up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage
ownership is determined in accordance with the terms of the Series A warrants and in accordance with the rules and regulations of the
SEC.
Cashless Exercise
If, at the time a holder exercises its Series A
warrants, a registration statement registering the issuance of the shares of common stock underlying the Series A warrants under the Securities
Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated
to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise
(either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Series A warrants.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the Series A warrants. Rather, the number of shares of common stock to be issued will be rounded to the nearest whole
number.
Transferability
Subject to applicable laws, a Series A warrant
may be transferred at the option of the holder upon surrender of the Series A warrant to us together with the appropriate instruments
of transfer.
Trading Market
There is no trading market available for the Series
A warrants on any securities exchange or nationally recognized trading system, and we do not expect a trading market to develop. We do
not intend to list the Series A warrants on any securities exchange or other trading market. Without a trading market, the liquidity of
the Series A warrants will be extremely limited. The common stock issuable upon exercise of the Series A warrants is currently listed
on the Nasdaq Capital Market.
Right as a Stockholder
Except as otherwise provided in the Series A warrants
or by virtue of such holder’s ownership of shares of our common stock, the holders of the Series A warrants do not have the rights
or privileges of holders of our common stock, including any voting rights, until they exercise their Series A warrants.
Fundamental Transaction
In the event of a fundamental transaction, as
described in the Series A warrants and generally including any reorganization, recapitalization or reclassification of our common
stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger
with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the
beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the Series A warrants will
be entitled to receive upon exercise of the Series A warrants the kind and amount of securities, cash or other property that the
holders would have received had they exercised the Series A warrants immediately prior to such fundamental transaction. In addition,
in the event of a fundamental transaction which is approved by our Board, the holders of the Series A warrants have the right to
require us or a successor entity to redeem the Series A warrant for cash in the amount of the Black-Scholes value of the unexercised
portion of the Series A warrant on the date of the consummation of the fundamental transaction. In the event of a fundamental
transaction which is not approved by our Board, the holders of the Series A warrants have the right to require us or a successor
entity to redeem the Series A warrant for the consideration paid in the fundamental transaction in the amount of the Black Scholes
value of the unexercised portion of the Series A warrant on the date of the consummation of the fundamental transaction.
Placement Agent Warrants
The following summary of certain terms and
provisions of the Placement Agent Warrants that are being issued hereby is not complete and is subject to, and qualified in its
entirety by, the provisions of the Placement Agent Warrants, the form of which will be filed as an exhibit to the registration
statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form
of Placement Agent Warrant for a complete description of the terms and conditions of the Placement Agent Warrant.
Duration and Exercise Price
Each Placement Agent Warrant offered hereby
will have an initial exercise price equal to $ per share of common stock (125% of the combined public offering price per share of common stock and Series A warrant). The Placement
Agent Warrants will be immediately exercisable and will expire years from the commencement of sales in this offering.
The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of
stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
The Placement Agent Warrants will be exercisable,
at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full
for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below).
A holder (together with its affiliates) may not exercise any portion of the Placement Agent Warrant to the extent that the holder would
own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice
from the holder to us, the holder may increase the amount of beneficial ownership of outstanding stock after exercising the holder’s
Placement Agent Warrant up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise,
as such percentage ownership is determined in accordance with the terms of the Placement Agent Warrants and in accordance with the rules
and regulations of the SEC.
Cashless Exercise
If, at the time a holder exercises its Placement
Agent Warrants, a registration statement registering the issuance of the shares of common stock underlying the Placement Agent Warrants
under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise
contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon
such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the
Placement Agent Warrants.
Fractional Shares
No fractional shares of common stock will be issued
upon the exercise of the Placement Agent Warrants. Rather, the number of shares of common stock to be issued will be rounded to the nearest
whole number.
Transferability
Subject to applicable laws, a Placement Agent Warrant
may be transferred at the option of the holder upon surrender of the Placement Agent Warrant to us together with the appropriate instruments
of transfer.
Trading Market
There is no trading market available for the
Placement Agent Warrants on any securities exchange or nationally recognized trading system, and we do not expect a trading market
to develop. We do not intend to list the Placement Agent Warrants on any securities exchange or other trading market. Without a
trading market, the liquidity of the Placement Agent Warrants will be extremely limited. The common stock issuable upon exercise of
the Placement Agent Warrants is currently listed on the Nasdaq Capital Market.
Right as a Stockholder
Except as otherwise provided in the Placement Agent
Warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the Placement Agent Warrants do not
have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Placement Agent Warrants.
Fundamental Transaction
In the event of a fundamental transaction, as described
in the Placement Agent Warrants and generally including any reorganization, recapitalization or reclassification of our common stock,
the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into
another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner
of 50% of the voting power represented by our outstanding common stock, the holders of the Placement Agent Warrants will be entitled to
receive upon exercise of the Placement Agent Warrants the kind and amount of securities, cash or other property that the holders would
have received had they exercised the Placement Agent Warrants immediately prior to such fundamental transaction. In addition, in the event
of a fundamental transaction which is approved by our Board, the holders of the Placement Agent Warrants have the right to require us
or a successor entity to redeem the Placement Agent Warrant for cash in the amount of the Black-Scholes value of the unexercised portion
of the Placement Agent Warrant on the date of the consummation of the fundamental transaction. In the event of a fundamental transaction
which is not approved by our Board, the holders of the Placement Agent Warrants have the right to require us or a successor entity to
redeem the Placement Agent Warrants for the consideration paid in the fundamental transaction in the amount of the Black Scholes value
of the unexercised portion of the Placement Agent Warrant on the date of the consummation of the fundamental transaction.
MATERIAL U.S. FEDERAL TAX CONSIDERATIONS
FOR HOLDERS OF OUR COMMON STOCK, SERIES B PRE-FUNDED WARRANTS AND SERIES A WARRANTS
The following discussion describes certain material
U.S. federal income consequences of the acquisition, ownership and disposition of our common stock, Series B pre-funded warrants
and Series A warrants acquired in this offering. This discussion is based on the current provisions of the Internal Revenue Code
of 1986, as amended (referred to as the “Code”), existing and proposed U.S. Treasury regulations promulgated thereunder, and
administrative rulings and court decisions in effect as of the date hereof, all of which are subject to change at any time, possibly with
retroactive effect. No ruling has been or will be sought from the Internal Revenue Service, or IRS, with respect to the matters discussed
below, and there can be no assurance the IRS will not take a contrary position regarding the tax consequences of the acquisition, ownership
or disposition of our common stock, Series B pre-funded warrants or Series A warrants, or that any such contrary position would
not be sustained by a court.
We assume in this discussion that the shares of
our common stock, Series B pre-funded warrants and Series A warrants will be held as capital assets (generally, property held
for investment). This discussion does not address all aspects of U.S. federal income taxes, does not discuss the potential application
of the Medicare contribution tax or the alternative minimum tax and does not deal with state or local taxes or U.S. federal gift and estate
tax laws, or any non-U.S. tax consequences that may be relevant to holders in light of their particular circumstances. This discussion
also does not address the special tax rules applicable to particular holders, such as:
| • | a bank, insurance company, or other financial institution; |
| • | a tax-exempt entity, organization, or arrangement; |
| • | a government or any agency, instrumentality, or controlled entity thereof; |
| • | a real estate investment trust; |
| • | an S corporation or other pass-through entity (or an investor in an S corporation or other pass-through
entity); |
| • | a regulated investment company; |
| • | a “controlled foreign corporation” or a “passive foreign investment company”; |
| • | a dealer or broker in stocks and securities, or currencies; |
| • | a trader in securities that elects mark-to-market treatment or any other holder subject to mark-to-market
treatment; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that is liable
for the alternative minimum tax; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that received
such security through the exercise of options, warrants, or similar derivative securities or otherwise as compensation; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that holds
such security in a tax-deferred account (such as an individual retirement account or a plan qualifying under Section 401(k) of
the Code); |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that has a
functional currency other than the U.S. dollar; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that holds
such security as part of a hedge, straddle, constructive sale, conversion or other integrated transaction; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants required to
accelerate the recognition of any item of gross income with respect to such security, as a result of such income being recognized on an
applicable financial statement; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that is a U.S.
expatriate or former citizen or long-term resident of the United States; |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that does not
hold such security as a capital asset within the meaning of Section 1221 of the Code (generally, for investment purposes); |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants whose security
may constitute “qualified small business stock” under Section 1202 of the Code or “Section 1244 stock”
for purposes of Section 1244 of the Code; or |
| • | a holder of our common stock, Series B pre-funded warrants, or Series A warrants that acquired
such security in a transaction subject to the gain rollover provisions of Section 1045 of the Code; |
In addition, this discussion does not address the
tax treatment of partnerships or other pass-through entities or persons who hold our common stock, Series B pre-funded warrants or
Series A warrants through partnerships or other entities which are pass-through entities for U.S. federal income tax purposes. A
partner in a partnership or other pass-through entity that will hold our common stock, Series B pre-funded warrants or Series A
warrants should consult his, her or its own tax advisor regarding the tax consequences of the ownership and disposition of our common
stock, Series B pre-funded warrants or Series A warrants through a partnership or other pass-through entity, as applicable.
The discussion of U.S. federal income tax considerations
is for information purposes only and is not tax advice. Investors should consult their own tax advisors regarding the U.S. federal, state,
local and non-U.S. income and other tax considerations of acquiring, holding and disposing of our common stock, Series B pre-funded
warrants and Series A warrants.
For the purposes of this discussion, a “U.S.
Holder” means a beneficial owner of our common stock, Series B pre-funded warrants or Series A warrants that is for U.S.
federal income tax purposes (a) an individual citizen or resident of the United States, (b) a corporation (or other entity treated
as a corporation for U.S. federal income tax purposes), organized in or under the laws of the United States, any state thereof or the
District of Columbia, (c) an estate the income of which is includable in gross income for U.S. federal income tax purposes regardless
of its source, or (d) a trust if it (1) is subject to the primary supervision of a court within the United States and one or
more U.S. persons (within the meaning of Section 7701(a)(30) of the Code) has the authority to control all substantial decisions
of the trust or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. A “Non-U.S.
Holder” is, for U.S. federal income tax purposes, a beneficial owner of common stock, Series B pre-funded warrants or Series A
warrants (other than a partnership or other entity or arrangement classified as a partnership for U.S. federal income tax purposes) that
is not a U.S. Holder.
Treatment of Series B Pre-Funded Warrants
Although it is not entirely free from doubt, we believe a Series B
pre-funded warrant should be treated as a share of our common stock for U.S. federal income tax purposes and a holder of Series B
pre-funded warrants should generally be taxed in the same manner as a holder of common stock as described below. Accordingly, for U.S.
federal income tax purposes, no gain or loss should be recognized upon the exercise of a Series B pre-funded warrant, and upon exercise,
the holding period of the share of common stock received should include the holding period of the Series B pre-funded warrant. Similarly,
the tax basis of a share of common stock received upon exercise of a Series B pre-funded warrant should include the tax basis of
the Series B pre-funded warrant (discussed below) increased by the exercise price of $0.0001. Each holder should consult his, her
or its own tax advisor regarding the risks associated with the acquisition of an investment unit pursuant to this offering (including
potential alternative characterizations). The balance of this discussion generally assumes that the characterization described above is
respected for U.S. federal income tax purposes.
Allocation of Purchase Price to Common Stock, Series B Pre-Funded
Warrants and Series A Warrants
For U.S. federal income tax purposes, a holder’s
acquisition of the Series A warrants and common stock or Series B pre-funded warrants, as applicable, will be treated as the
acquisition of an “investment unit” consisting of one share of common stock (or one Series B pre-funded warrant, as applicable)
and a Series A warrant to acquire one share of our common stock, subject to adjustment. The purchase price for each investment unit
will be allocated between these two components in proportion to their relative fair market values at the time the investment unit is purchased
by the holder. This allocation of the purchase price for each investment unit will establish the holder’s initial tax basis for
U.S. federal income tax purposes in the common stock (or Series B pre-funded warrant, as applicable) and the Series A warrant
included in each investment unit. The separability of the share of common stock (or Series B pre-funded warrant, as applicable) and
the Series A warrant included in each investment unit should not in itself result in the recognition of income or gain for U.S. federal
income tax purposes. Each holder should consult his, her or its own tax advisor regarding the allocation of the purchase price for an
investment unit.
Tax Considerations Applicable to U.S. Holders
Exercise and Expiration of Series A Warrants
In general, a U.S. Holder will not recognize gain
or loss for U.S. federal income tax purposes upon exercise of a Series A warrant. The U.S. Holder will take a tax basis in the shares
acquired on the exercise of a Series A warrant equal to the exercise price of the Series A warrant, increased by the U.S. Holder’s
adjusted tax basis in the Series A warrant exercised (as determined pursuant to the rules discussed above). The U.S. Holder’s
holding period in the shares of our common stock acquired on exercise of the Series A warrant will begin on the date of exercise
of the Series A warrant, and will not include any period for which the U.S. Holder held the Series A warrant.
In certain limited circumstances, a U.S. Holder
may be permitted to undertake a cashless exercise of Series A warrants into our common stock. The U.S. federal income tax treatment
of a cashless exercise of Series A warrants into our common stock is unclear, and the tax consequences of a cashless exercise could
differ from the consequences upon the exercise of a Series A warrant described in the preceding paragraph. U.S. Holders should consult
their own tax advisors regarding the U.S. federal income tax consequences of a cashless exercise of Series A warrants.
The lapse or expiration of a Series A warrant
will be treated as if the U.S. Holder sold or exchanged the Series A warrant and recognized a capital loss equal to the U.S. Holder’s
tax basis in the Series A warrant. The deductibility of capital losses is subject to limitations.
Certain Adjustments to and Distributions on the Series A Warrants
Under Section 305 of the Code, an adjustment
to the number of shares of common stock issued on the exercise of the Series A warrants, or an adjustment to the exercise price of
the Series A warrants, may be treated as a constructive distribution to a U.S. Holder of the Series A warrants if, and to the
extent that, such adjustment has the effect of increasing such U.S. Holder’s proportionate interest in our “earnings and profits”
or assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution of cash
or other property to our shareholders). Any such constructive distribution would be taxable whether or not there is an actual distribution
of cash or other property. In addition, if we were to make a distribution in cash or other property with respect to our common stock after
the issuance of the Series A warrants, then we may, in certain circumstances, make a corresponding distribution to a Series A
warrant holder. The taxation of a distribution received with respect to a Series A warrant is unclear. It is possible such a distribution
would be treated as a distribution (or constructive distribution), although other treatments are possible. For more information regarding
the tax considerations related to distributions, see the discussion below regarding “Distributions.” U.S. Holders should consult
their tax advisors regarding the proper treatment of any adjustments to and distributions on the Series A warrants.
Distributions
As discussed above in the section captioned “Dividend
Policy”, we currently anticipate that we will retain all available funds and any future earnings for use in the operation of
our business and do not anticipate declaring or paying any cash dividends on our common stock for the foreseeable future. In the event
that we do make distributions on our common stock or Series B pre-funded warrants to a U.S. Holder, those distributions generally
will constitute dividends for U.S. tax purposes to the extent paid out of our current or accumulated earnings and profits (as determined
under U.S. federal income tax principles). Distributions to a U.S. Holder that are not derived from our current or accumulated earnings
and profits will constitute a return of capital that is applied against and reduces, but not below zero, the U.S. Holder’s adjusted
tax basis in our common stock or Series B pre-funded warrant, as applicable, and to the extent in excess of such basis, will be treated
as gain realized on the sale or exchange of our common stock or Series B pre-funded warrants, as applicable, as described below under
the section titled “—Disposition of Our Common Stock, Series B Pre-Funded Warrants or Series A Warrants.”
Disposition of Our Common Stock, Series B Pre-Funded Warrants
or Series A Warrants
Upon a sale or other taxable disposition of our
common stock, Series B pre-funded warrants or Series A warrants, a U.S. Holder generally will recognize capital gain or loss
in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the applicable common
stock, Series B pre-funded warrants or Series A warrants. Capital gain or loss will constitute long-term capital gain or loss
if the U.S. Holder’s holding period for the applicable common stock, Series B pre-funded warrant or Series A warrant exceeds
one year. The deductibility of capital losses is subject to certain limitations. U.S. Holders who recognize losses with respect to a disposition
of our common stock, Series B pre-funded warrants or Series A warrants should consult their own tax advisors regarding the tax
treatment of such losses.
Information Reporting and Backup Withholding
Information reporting requirements generally will
apply to payments of dividends (including constructive dividends) on our common stock, Series B pre-funded warrants and Series A
warrants and to the proceeds of a sale or other disposition of common stock, Series A warrants and Series B pre-funded warrants
by a U.S. Holder unless such U.S. Holder is an exempt recipient, such as a corporation. Backup withholding will apply to those payments
if the U.S. Holder fails to provide the holder’s taxpayer identification number, or certification of exempt status, or if the holder
otherwise fails to comply with applicable requirements to establish an exemption. Backup withholding is not an additional tax. Rather,
amounts withheld as backup withholding may be credited against a person’s U.S. federal income tax liability, and a holder generally
may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for
refund with the IRS and furnishing any required information.
Tax Considerations Applicable to Non-U.S. Holders
Exercise and Expiration of Series A Warrants
In general, a Non-U.S. Holder will not be subject
to U.S. federal income tax on the exercise of the Series A warrants into shares of common stock. As described under “—
U.S. Holders — Exercise and Expiration of Series A Warrants,” the U.S. federal income tax treatment
of a cashless exercise of Series A warrants into our common stock is unclear. A Non-U.S. Holder should consult his, her, or its own
tax advisor regarding the U.S. federal income tax consequences of a cashless exercise of Series A warrants.
The expiration of a Series A warrant will
be treated as if the Non-U.S. Holder sold or exchanged the Series A warrant and recognized a capital loss equal to the Non-U.S. Holder’s
tax basis in the Series A warrant. However, a Non-U.S. Holder will not be able to utilize a loss recognized upon expiration of a
Series A warrant against the Non-U.S. Holder’s U.S. federal income tax liability unless the loss is effectively connected with
the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if an income tax treaty applies, is attributable
to a permanent establishment or fixed base in the United States) or is treated as a U.S.-source loss and the Non-U.S. Holder is an individual
nonresident and present 183 days or more in the taxable year of disposition in the United States and certain other conditions are met.
Certain Adjustments to and Distributions on the Series A Warrants
As described under “— U.S. Holders — Certain
Adjustments to and Distributions on the Series A Warrants,” an adjustment to the Series A warrants could result in
a constructive distribution to a Non-U.S. Holder, which would be treated as described under “Distributions” below,
and the tax treatment of a distribution on a Series A warrant is unclear. Any resulting withholding tax attributable to deemed dividends
would be collected from other amounts payable or distributable to the Non-U.S. Holder. Non-U.S. Holders should consult their tax advisors
regarding the proper treatment of any adjustments to or distributions on the Series A warrants.
Distributions
As discussed above in the section captioned “Dividend
Policy”, we currently anticipate that we will retain all available funds and any future earnings for use in the operation of
our business and do not anticipate declaring or paying any cash dividends on our common stock for the foreseeable future. In the event
that we do make distributions on our common stock or Series B pre-funded warrants to a Non-U.S. Holder, those distributions generally
will be treated as dividends, as return of capital or as gain on the sale or exchange of common stock or Series B pre-funded warrants
for U.S. federal income tax purposes as described in “— U.S. Holders — Distributions.”
Subject to the discussions below under the sections
titled “— Information Reporting and Backup Withholding” and “— Foreign Accounts,” any
distribution (including constructive distributions) on our common stock or Series B pre-funded warrants that is treated as a dividend
paid to a Non-U.S. Holder that is not effectively connected with the holder’s conduct of a trade or business in the United States
will generally be subject to withholding tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between
the United States and the Non-U.S. Holder’s country of residence. To obtain a reduced rate of withholding under a treaty, a Non-U.S.
Holder generally will be required to provide the applicable withholding agent with a properly executed IRS Form W-8BEN, IRS
Form W-8BEN-E or other appropriate form, certifying the Non-U.S. Holder’s entitlement to benefits under that treaty. Such form
must be provided prior to the payment of dividends and must be updated periodically.
We generally are not required to withhold tax on
dividends paid (or constructive dividends deemed paid) to a Non-U.S. Holder that are effectively connected with such holder’s conduct
of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent
establishment or fixed base that the holder maintains in the United States) if a properly executed IRS Form W-8ECI, stating that
the dividends are so connected, is furnished to us. In general, such effectively connected dividends will be subject to U.S. federal income
tax on a net income basis at the regular rates applicable to U.S. persons. A corporate Non-U.S. Holder receiving effectively connected
dividends may also be subject to an additional “branch profits tax,” which is imposed, under certain circumstances, at a rate
of 30% (or such lower rate as may be specified by an applicable treaty) on the corporate Non-U.S. Holder’s effectively connected
earnings and profits, subject to certain adjustments.
Distributions to a Non-U.S.
Holder that are not derived from our current or accumulated earnings and profits generally will be treated as a return of capital that
will be applied against and reduce (but not below zero) the Non-U.S. Holder’s basis in its common stock or Series B pre-funded
warrants, as applicable, and to the extent in excess of such basis, will be treated as gain from the sale or exchange of such common stock
or Series B pre-funded warrants, as applicable, as described under “— Disposition of Our Common Stock, Series B
Pre-Funded Warrants or Series A Warrants” below.
If a Non-U.S. Holder holds
stock through a financial institution or other agent acting on the holder’s behalf, the holder will be required to provide appropriate
documentation to such agent. The holder’s agent may then be required to provide certification to the applicable withholding agent,
either directly or through other intermediaries.
Disposition of Our Common Stock, Series B
Pre-Funded Warrants or Series A Warrants
Subject to the discussions below under the sections
titled “— Information Reporting and Backup Withholding” and “— Foreign Accounts,” a
Non-U.S. Holder generally will not be subject to U.S. federal income or withholding tax with respect to gain realized on a sale or other
disposition of our common stock, Series B pre-funded warrants or Series A warrants unless:
| • | the gain is effectively connected with the Non-U.S. Holder’s
conduct of a trade or business in the United States (and, if required by an applicable income tax treaty between the United States and
such Non-U.S. Holder’s country of residence, the gain is attributable to a permanent establishment or fixed base maintained by
the Non-U.S. Holder in the U.S.), in which case the Non-U.S. Holder will be taxed on a net income basis at the regular rates and in the
manner applicable to U.S. persons, and if the Non-U.S. Holder is a corporation, an additional branch profits tax at a rate of 30%, or
a lower rate as may be specified by an applicable income tax treaty, may also apply; |
| • | the Non-U.S. Holder is a nonresident alien present in the United
States for 183 days or more in the taxable year of the disposition and certain other requirements are met, in which case the Non-U.S.
Holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States
and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S.-source
capital losses of the Non-U.S. Holder, if any, provided that the Non-U.S. Holder has timely filed U.S. federal income tax returns reporting
those losses; or |
| • | we are, or have been, a “United States real property holding
corporation,” or USRPHC, for U.S. federal income tax purposes during the five-year period preceding such disposition (or the Non-U.S.
Holder’s holding period, if shorter). We do not believe that we are or have been a USRPHC and, even if we are or were
a USRPHC, dispositions will not be subject to tax for a Non-U.S. Holder that has not held more than 5% of our common stock, actually
or constructively, during the five-year period preceding such Non-U.S. Holder’s disposition (or the Non-U.S. Holder’s holding
period, if shorter). Special rules may apply to the determination of the 5% threshold in the case of a holder of a Series B
pre-funded warrant or Series A warrant. |
See the sections titled “— Information
Reporting and Backup Withholding” and “— Foreign Accounts” below for additional information regarding withholding
rules that may apply to proceeds of a disposition of our common stock, Series B pre-funded warrants or Series A warrants
paid to foreign financial institutions or non-financial foreign entities.
Information Reporting and Backup Withholding
We must report annually to the IRS and to each
Non-U.S. Holder the gross amount of the distributions (including constructive distributions) on our common stock, Series B pre-funded
warrants or Series A warrants paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. Holders
may have to comply with specific certification procedures to establish that the holder is not a U.S. person (as defined in the Code) in
order to avoid backup withholding at the applicable rate, currently 24%. Generally, a Non-U.S. Holder will comply with such procedures
if it provides a properly executed applicable IRS Form W-8 or by otherwise establishing an exemption. Dividends paid to Non-U.S.
Holders subject to withholding of U.S. federal income tax, as described above under the heading “— Distributions,”
will generally be exempt from U.S. backup withholding.
Information reporting and backup withholding generally
will apply to the proceeds of a disposition of our common stock, Series B pre-funded warrants or Series A warrants by a Non-U.S.
Holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the Non-U.S. Holder certifies its status as a Non-U.S.
Holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding
will not apply to a payment of disposition proceeds to a Non-U.S. Holder where the transaction is effected outside the United States through
a non-U.S. office of a non-U.S. broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of
a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through
a U.S. office of a broker. Non-U.S. Holders should consult their own tax advisors regarding the application of the information reporting
and backup withholding rules to them.
Copies of information returns may be made available
to the tax authorities of the country in which the Non-U.S. Holder resides or is incorporated under the provisions of a specific treaty
or agreement.
Backup withholding is not an additional tax. Any
amounts withheld under the backup withholding rules from a payment to a Non-U.S. Holder can be refunded or credited against the Non-U.S.
Holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Foreign Accounts
Legislation commonly referred to as the “Foreign
Account Tax Compliance Act,” or “FATCA,” generally imposes a 30% withholding tax on dividends on common stock, Series B
pre-funded warrants and Series A warrants if paid to a non-U.S. entity unless (i) if the non-U.S. entity is a “foreign
financial institution,” the non-U.S. entity undertakes certain due diligence, reporting, withholding, and certification obligations,
(ii) if the non-U.S. entity is not a “foreign financial institution,” the non-U.S. entity identifies certain of its U.S.
investors, if any, or (iii) the non-U.S. entity is otherwise exempt under FATCA.
Intergovernmental agreements between the United
States and foreign countries with respect to FATCA may significantly modify the requirements described in this section for Non-U.S. Holders.
Holders should consult their own tax advisors regarding the possible implications of FATCA on their investment in our common stock, Series B
pre-funded warrants or Series A warrants.
The preceding discussion of material U.S. federal
tax considerations is for information only. It is not tax advice. Prospective investors should consult their own tax advisors regarding
the particular U.S. federal, state, local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, Series B
pre-funded warrants or Series A warrants, including the consequences of any proposed changes in applicable laws.
PLAN OF DISTRIBUTION
We engaged H.C. Wainwright & Co., LLC
(“H.C. Wainwright” or the “placement agent”) to act as our exclusive placement agent to solicit offers to
purchase the securities offered by this prospectus on a reasonable best efforts basis. H.C. Wainwright is not purchasing or selling
any securities, nor are they required to arrange for the purchase and sale of any specific number or dollar amount of securities,
other than to use their “reasonable best efforts” to arrange for the sale of the securities by us. Therefore, we may not
sell the entire amount of securities being offered. There is no minimum amount of proceeds that is a condition to closing of this
offering. The placement agent does not guarantee that it will be able to raise new capital in this offering. We will enter into a
securities purchase agreement directly with the institutional investors, at the investor’s option, who purchase our securities
in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection
with the purchase of our securities in this offering. H.C. Wainwright may engage one or more sub-placement agents or selected
dealers to assist with the offering.
Fees and Expenses
The following table shows the per share and Series
A warrant and Series B pre-funded warrant and Series A warrant placement agent fees and total placement agent fees we will pay in connection
with the sale of the securities in this offering, assuming the purchase of all of the securities we are offering.
| Per share and Series A warrant placement agent cash fees |
$ |
| Per Series B pre-funded warrant and Series A warrant placement agent cash fees |
$ |
| Total |
$ |
We have agreed to pay the placement agent a total
cash fee equal to 7.0% of the gross proceeds of this offering and a management fee equal to 1.0% of the gross proceeds raised in this
offering. We will also pay the placement agent a non-accountable expense allowance of $50,000, $15,950 for the expenses of its clearing
firm, and will reimburse the placement agent’s legal fees and expenses in an amount up to $100,000.
We estimate the total offering expenses of this offering that will be payable by us, excluding the placement agent fees and expenses,
will be approximately $ million. After deducting the placement agent fees and our estimated offering expenses, we expect the net proceeds
from this offering to be approximately $ million.
Placement Agent Warrants
We have agreed to grant placement agent warrants to
H.C. Wainwright (the “Placement Agent Warrant”) to purchase a number of shares of our common stock equal to 5.0% of the aggregate
number of shares of common stock and Series B pre-funded warrants sold to the investors in this offering. The placement agent warrants will
have an exercise price of $ (125% of the combined public offering price per share of common stock
and Series A warrant) and will terminate on the year anniversary
of commencement of sales in this offering. The Placement Agent Warrants are registered on the registration statement of which this prospectus
is a part. The form of the Placement Agent Warrant will be included as an exhibit to this registration statement of which this prospectus forms a
part.
Tail
We have also agreed to pay the placement agent a tail fee equal to the cash and warrant compensation in this offering, if any investor,
who was contacted or introduced to us by the placement agent during the term of its engagement, provides us with capital in any public
or private offering or other financing or capital raising transaction during the 12-month period following expiration or termination of
our engagement of the placement agent.
Right of First Refusal
In addition, we have granted a right of first
refusal to the placement agent pursuant to which it has the right to act as the sole book-running manager, sole underwriter or sole
placement agent, as applicable, if the Company or its subsidiaries sell or acquire a business, finance any indebtedness using an
agent, or raise capital through a public offering (including any new at-the-market) facility or private placement or any other capital-raising financing of equity, equity-linked or debt
securities using an underwriter or placement agent, subject to certain exceptions, during the six-month period following the consummation of this offering.
Other Relationships
The placement agent acted as the placement agent
in connection with our previous offering consummated in March 2022, for which it has received customary fees and expenses, and has served
as sales agent for our at the market offerings pursuant to the sales agreements dated April 27, 2022, January 10, 2022 and January
23, 2019. The placement agent may, from time to time, engage in transactions with or perform services for us in the ordinary course of
its business and may continue to receive compensation from us for such services.
Determination of Offering Price
The combined public offering price per share and
Series A warrant and the combined public offering price per Series B pre-funded warrant and Series A warrant we are offering and the exercise
prices and other terms of the warrants were negotiated between us and the investors, in consultation with the placement agent based on
the trading of our common stock prior to this offering, among other things. Other factors considered in determining the public offering
prices of the securities we are offering and the exercise prices and other terms of the warrants include the history and prospects of
our company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented,
an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were
deemed relevant.
Lock-up Agreements
We and each of our officers and directors have
agreed with the placement agent to be subject to a lock-up period of days following the date of this prospectus. This means that, during
the applicable lock-up period, we may not offer for sale, contract to sell, or sell any shares of our common stock or any securities convertible
into, or exercisable or exchangeable for, shares of our common stock subject to certain customary exception such as issuing stock options
to directors, officers, employees and consultants under our existing plans. The placement agent may, in its sole discretion and without
notice, waive the terms of any of these lock-up agreements. In addition, we have agreed to not issue any shares of common stock or securities
exercisable or convertible into shares of common stock for a period of days following the closing date of this offering, subject
to certain exceptions, and to not issue any securities that are subject to a price reset based on trading prices of our common stock or
upon a specified or contingent event in the future, or enter into an agreement to issue securities at a future determined price, until
the date that no warrants are outstanding.
Transfer Agent and Registrar
The transfer agent and registrar for our common
stock is Broadridge Corporate Issuer Solutions, Inc. whose address is 51 Mercedes Way, Edgewood, New York 11717.
Nasdaq Listing
Our common stock is currently listed on the Nasdaq
Capital Market under the symbol “AGRX.” On , 2022, the
reported closing price per share of our common stock was $ . We do not plan to list the warrants
on the Nasdaq Capital Market or any other securities exchange or trading market.
Indemnification
We have agreed to indemnify the placement agent
against certain liabilities, including liabilities under the Securities Act of 1933, or to contribute to payments the placement agent
may be required to make with respect to any of these liabilities.
Regulation M
The placement agent may be deemed to be an underwriter
within the meaning of Section 2(a)(11) of the Securities Act and any fees received by it and any profit realized on the sale of the securities
by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. The placement agent
will be required to comply with the requirements of the Securities Act and the Exchange Act of 1934, as amended (the “Exchange Act”),
including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of
purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement agent may not (i) engage
in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities or attempt to induce
any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed their participation
in the distribution.
Electronic Offer, Sale and Distribution of
Securities
A prospectus in electronic format may be made available on the websites maintained
by the placement agent, if any, participating in this offering and the placement agent may distribute prospectuses electronically. Other
than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement
of which this prospectus forms a part, has not been approved or endorsed by us or the placement agent, and should not be relied upon by
investors.
LEGAL MATTERS
The validity of the securities being offered in
this offering will be passed upon for us by Morgan, Lewis & Bockius LLP, Princeton, New Jersey. The placement agent is being represented
by Ellenoff Grossman & Schole LLP, New York, New York, in connection with this offering.
EXPERTS
The financial statements of Agile Therapeutics,
Inc. appearing in Agile Therapeutics, Inc.’s Annual Report (Form 10 K) for the year ended December 31, 2021, have been audited by
Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon included therein, and incorporated
herein by reference which contains an explanatory paragraph describing conditions that raise substantial doubt about the Company’s
ability to continue as a going concern as described in Note 1 to the financial statements. Such financial statements are, and audited
financial statements to be included in subsequently filed documents will be, incorporated herein in reliance upon the report of Ernst
& Young LLP pertaining to such financial statements (to the extent covered by consents filed with the Securities and Exchange Commission)
given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus forms part of a registration statement
on Form S-1 that we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement
and the exhibits to the registration statement or the documents incorporated by reference herein and therein. For further information
with respect to us and the securities that we are offering under this prospectus, we refer you to the registration statement and the exhibits
and schedules filed as a part of the registration statement and the documents incorporated by reference herein and therein. You should
rely only on the information contained in this prospectus or incorporated by reference herein or therein. We have not authorized anyone
else to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted.
You should not assume that the information in this prospectus is accurate as of any date other than the date on the front page of this
prospectus, regardless of the time of delivery of this prospectus or any sale of the securities offered hereby. We file annual, quarterly
and current reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy statements
and other information regarding issuers that file electronically with the SEC, including Agile Therapeutics. The address of the SEC website
is www.sec.gov.
We maintain a website at www.agiletherapeutics.com.
Information contained in or accessible through our website does not constitute a part of this prospectus.
INFORMATION INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference”
information into this prospectus, which means that we can disclose important information to you by referring you to another document filed
separately with the SEC. The SEC file number for the documents incorporated by reference in this prospectus is 001-36464. The documents
incorporated by reference into this prospectus contain important information that you should read about us.
The following documents are incorporated by reference
into this document:
| · | our
Current Reports on Form 8-K filed with the SEC on January
10, 2022, January
11, 2022, March
11, 2022, March
15, 2022, April
26, 2022 , April
27, 2022 and May 12, 2022; (other than Item 2.02) |
We also incorporate by reference into this prospectus
all documents (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are
related to such items) that are filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the
date of this prospectus but prior to the termination of the offering. These documents include periodic reports, such as Annual Reports
on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy statements.
We will provide to each person, including any beneficial
owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the documents that are
incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits which are specifically incorporated
by reference into such documents. Requests should be directed to: Agile Therapeutics, Inc., Attn: Investor Relations, 500 College Road
East, Suite 310, Princeton, New Jersey 08540. Our telephone number is (609) 683-1880.
Any statement contained herein or in a document
incorporated or deemed to be incorporated by reference into this document will be deemed to be modified or superseded for purposes of
the document to the extent that a statement contained in this document or any other subsequently filed document that is deemed to be incorporated
by reference into this document modifies or supersedes the statement.

$20,000,000
Up
to Shares of Common Stock and
Warrants
to Purchase up to Shares of Common Stock
or
Pre-funded
Warrants to Purchase up to Shares of Common Stock and
Warrants
to Purchase up to Shares of Common Stock
Placement Agent Warrants to Purchase up to Shares of Common Stock
Preliminary Prospectus
H.C. Wainwright & Co.
, 2022
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and
Distribution.
The following table sets forth
the fees and expenses in connection with the issuance and distribution of the securities being registered (excluding the underwriting
discount). Except for the Securities and Exchange Commission registration fee and the FINRA filing fee, all amounts are estimates.
| |
|
|
Amount Paid
or to be Paid |
| SEC registration fee |
|
$ |
1,854 |
| FINRA filing fee |
|
$ |
3,500 |
| Legal fees and expenses |
|
|
* |
| Accounting fees and expenses |
|
|
* |
| Printing expenses |
|
|
* |
| Transfer agent fees and expenses |
|
|
* |
| Miscellaneous |
|
|
* |
| Total |
|
|
* |
*
To be completed by amendment
Each of the amounts set forth
above, other than the SEC registration fee and FINRA filing fee, is an estimate.
Item 14. Indemnification of Directors
and Officers.
Section 145 of the Delaware
General Corporation Law authorizes a corporation’s board of directors to grant, and authorizes a court to award, indemnity to officers,
directors, and other corporate agents.
As permitted by Delaware law,
our certificate of incorporation provides that, to the fullest extent permitted by Delaware law, no director will be personally liable
to us or our stockholders for monetary damages for breach of fiduciary duty as a director. Pursuant to Delaware law such protection would
be not available for liability:
| • | for any breach of a duty of loyalty to us or our stockholders; |
| • | for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation
of law; |
| • | for any transaction from which the director derived an improper benefit; or |
| • | for an act or omission for which the liability of a director is expressly provided by an applicable statute,
including unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware
General Corporation Law. |
Our amended and restated certificate
of incorporation also provides that if Delaware law is amended after the approval by our stockholders of the amended and restated certificate
of incorporation to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability
of our directors will be eliminated or limited to the fullest extent permitted by Delaware law.
Our amended and restated bylaws
further provide that we must indemnify our directors and officers to the fullest extent permitted by Delaware law. The amended and restated
bylaws also authorize us to indemnify any of our employees or agents and permit us to secure insurance on behalf of any officer, director,
employee or agent for any liability arising out of his or her action in that capacity, whether or not Delaware law would otherwise permit
indemnification.
In addition, our amended and
restated bylaws provide that we are required to advance expenses to our directors and officers as incurred in connection with legal proceedings
against them for which they may be indemnified and that the rights conferred in the amended and restated bylaws are not exclusive.
At present, there is no pending
litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware
of any threatened litigation that may result in claims for indemnification.
We have entered into indemnification
agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director
and officer to the fullest extent permitted by Delaware law, the amended and restated certificate of incorporation and amended and restated
bylaws, for expenses such as, among other things, attorneys’ fees, judgments, fines, and settlement amounts incurred by the director
or executive officer in any action or proceeding, including any action by or in our right, arising out of the person’s services
as our director or executive officer or as the director or executive officer of any subsidiary of ours or any other company or enterprise
to which the person provides services at our request. We also maintain directors’ and officers’ liability insurance.
The SEC has taken the position
that personal liability of directors for violation of the federal securities laws cannot be limited and that indemnification by us for
any such violation is unenforceable. The limitation of liability and indemnification provisions in our amended and restated certificate
of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against our directors and officers
for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even
though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected
to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification
provisions.
Item 15. Recent Sales of Unregistered
Securities
Set forth below is information
regarding securities we have issued within the past three years that were not registered under the Securities Act:
Set forth below is information
regarding securities we have issued within the past three years that were not registered under the Securities Act:
On February
10, 2020, in connection with the Perceptive Credit Agreement, we issued to Perceptive an aggregate of 35,000 warrants to purchase shares
of our common stock. Of these warrants, 17,500 are exercisable at a
price per share of $104.80 for a term expiring on February 10, 2027 and 17,500 are exercisable at a
price per shares of $129.20 for a term ending on February 10, 2027. On February 26, 2021, in connection with
the first amendment to the Perceptive Credit Agreement, we issued to Perceptive 11,250 warrants to purchase shares of our common
stock exercisable at a price per share of $84.20 for a term expiring
February 26, 2028.
Our issuances of the warrants were deemed to be
exempt from registration under the Securities Act pursuant to Section 4(a)(2) of the Securities Act in that such issuances did not
involve a public offering. The recipient of securities in each of these transactions acquired the securities for investment only
and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities
issued in these transactions. The recipient of securities in these transactions was an accredited investor within the meaning of Rule
501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships, to information
about us. No underwriters were involved in these transactions.
Item 16. Exhibits and Financial Statement
Schedules.
Exhibit
Number |
|
|
| |
|
|
| 3.3 |
|
Certificate
of Amendment to Amended and Restated Certificate of Incorporation filed with the Secretary of State of Delaware on April 26, 2022
(Incorporated by reference, Exhibit 3.1 to
Company’s Current Report on Form 8-K, file number 001-36464, filed on April 27,
2022). |
| |
|
|
| 3.4 |
|
Amended and Restated Bylaws of the Registrant. (Incorporated by reference, Exhibit 3.2 to Company’s Current Report on Form 8-K, file number 001-36464, filed on May 30, 2014.) |
| |
|
|
| 3.5 |
|
Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock filed with the Secretary of State of the State of Delaware on March 14, 2022 (Incorporated by
reference, Exhibit 3.1 to Company’s Current Report on Form 8-K, file number 001-36464, filed on March 15, 2022.) |
| |
|
|
| 3.6 |
|
Certificate
of Designation of Preferences, Rights and Limitations of Series B Convertible Preferred Stock filed with the Secretary of State of
the State of Delaware on March 14, 2022
(Incorporated by reference, Exhibit 3.2 to Company’s Current Report on Form 8-K,
file number 001-36464, filed on March 15, 2022.) |
| |
|
|
| 4.1 |
|
Specimen Certificate evidencing shares of Registrant’s common stock (Incorporated by reference, Exhibit 4.1 to Company’s Third Amendment of Registration Statement on Form S-1, file number 333-194621, filed on May 9, 2014.) |
| |
|
|
| 4.2 |
|
Warrant Agreement between Agile Therapeutics, Inc. and Perceptive Credit Holdings III, LP, dated as of February 10, 2020 (Incorporated by reference, Exhibit 4.1 to Company’s Current Report on Form 8-K, file number 001-36464, filed on February 12, 2020.) |
| |
|
|
| 4.3 |
|
Warrant Agreement between Agile Therapeutics, Inc. and Perceptive Credit Holdings III, LP, dated as of February 10, 2020 (Incorporated by reference, Exhibit 4.2 to Company’s Current Report on Form 8-K, file number 001-36464, filed on February 12, 2020.) |
| |
|
|
| 4.4 |
|
Warrant Agreement between Agile Therapeutics, Inc. and Perceptive Credit Holdings III, LP, dated as of February 26, 2021 (Incorporated by reference, Exhibit 4.4 to Company’s Annual Report on Form 10-K, file number 001-36464, filed on March 1, 2021.) |
| |
|
|
| 4.5 |
|
Form of Warrant (Incorporated by reference, Exhibit 4.2 to Company’s Current Report on Form 8-K, file number 001-36464, filed on October 8, 2021.) |
| |
|
|
| 4.6 |
|
Form of Series A Warrant (Incorporated by reference, Exhibit 4.1 to Company’s Current Report on Form 8-K, file number 001-36464, filed on March 15, 2022.) |
| |
|
|
| 4.7 |
|
Form of Series B Warrant (Incorporated by reference, Exhibit 4.2 to Company’s Current Report on Form 8-K, file number 001-36464, filed on March 15, 2022.) |
| |
|
|
| 4.8 |
|
Form of Placement Agent Warrant (Incorporated by reference, Exhibit 4.3 to Company’s Current Report on Form 8-K, file number 001-36464, filed on March 15, 2022.) |
| |
|
|
| 4.9 |
|
Description of Capital Stock (Incorporated by reference, Exhibit 4.4 to Company’s Annual Report on Form 10-K, file number 001-36464, filed on February 20, 2020.) |
| |
|
|
| 4.10 ++ |
|
Form of Series A Warrant |
| |
|
|
| 4.11 ++ |
|
Form of Series B Pre-Funded Warrant |
| |
|
|
| 4.12 ++ |
|
Form of Placement Agent Warrant |
| |
|
|
| 5.1++ |
|
Opinion of Morgan, Lewis & Bockius LLP |
| |
|
|
| 10.1+ |
|
Form of Indemnification Agreement. (Incorporated by reference, Exhibit 10.1 to Company’s Second Amendment of Registration Statement on Form S-1, file number 333-194621, filed on May 5, 2014.) |
Exhibit
Number |
|
|
| |
|
|
| 10.2+ |
|
Agile
Therapeutics, Inc. Amended and Restated 1997 Equity Incentive Plan, as amended, and form of Stock Option Agreement thereunder.
(Incorporated by reference, Exhibit 10.2 to Company’s Registration Statement on Form S-1, file number 333-194621,
filed on March 17, 2014.) |
| |
|
|
| 10.3+ |
|
Agile Therapeutics, Inc.
Amended and Restated 2008 Equity Incentive Plan and form of Nonqualified Stock Option Agreement and form of Incentive Stock Option
Agreement thereunder. (Incorporated by
reference, Exhibit 10.3 to Company’s Registration Statement on Form S-1, file number
333-194621, filed on March 17, 2014.) |
| |
|
|
| 10.4+ |
|
Form of Performance Unit
Issuance Agreement (Incorporated by reference, Exhibit 10.1 to Company’s Current Report on Form 8-K, file number 001-36464,
filed on January 26, 2018.) |
| |
|
|
| 10.5 |
|
Lease Agreement, dated
November 19, 2010, by and between the Registrant and Bunn Farm Associates, LLC, as modified by the Lease Amendment, dated November
20, 2012, by and between the Registrant and Bunn Farm Associates, LLC, and the Second Lease Amendment, dated July 24, 2013, by and
between the Registrant and Bunn Farm Associates, LLC, (Incorporated by reference, Exhibit 10.11 to Company’s Registration Statement
on Form S-1, file number 333-194621, filed on March 17, 2014.) |
| |
|
|
| 10.6 |
|
Third Lease Amendment,
dated August 20, 2015, by and between the Registrant and Bunn Farm Associates, LLC. (Incorporated by reference, Exhibit 10.1 to Company’s
Quarterly Report on Form 10-Q, file number 001-36464, filed on November 9, 2015.) |
| |
|
|
| 10.7 |
|
Fourth Lease Amendment,
dated April 22, 2016, by and between the Registrant and Bunn Farm Associates, LLC and Fifth Lease Amendment dated December 1, 2016, by and between the Registrant
and Bunn Farm Associates, LLC. (Incorporated by reference, Exhibit 10.15 to Company’s Annual
Report on Form 10-K, file number 001-36464, filed on March 12, 2018.) |
| |
|
|
| 10.8 |
|
Sixth
Lease Amendment, dated November 11, 2020, by and between the Registrant and Bunn Farm Associates, LLC (Incorporated by reference,
Exhibit 10.5 to Company’s Quarterly
Report on Form 10-Q, file number 001-36464, filed on November 12, 2020.) |
| |
|
|
| 10.9 |
|
Lease
agreement, dated August 6, 2021 by and between the Registrant and 500 College Road Venture, LLC (Incorporated by reference, Exhibit
10.1 to Company’s Quarterly Report on
Form 10-Q, file number 001-36464, filed on November 2, 2021.) |
| |
|
|
| 10.10 |
|
Common Stock Sales Agreement
dated November 8, 2019 by and between the Registrant and H.C. Wainwright & Co., LLC (Incorporated by reference, Exhibit 1.1 to
Company’s Current Report on Form 8-K, file number 001-36464, filed on November 8, 2019.) |
| |
|
|
| 10.11 |
|
Common Stock Sales Agreement
dated March 18, 2021, by and between Agile Therapetuics, Inc. and H.C. Wainwright & Co., LLC (Incorporated by reference, Exhibit
1.1 to the Company’s Current Report on Form 8-K, file number 001-036464, filed on March 18, 2021.) |
| |
|
|
| 10.12 |
|
Controlled
Equity OfferingSM Sales Agreement dated January 10, 2022 by and among Agile Therapeutics, Inc. and Cantor Fitzgerald &
Co. and H.C. Wainwright & Co., LLC (Incorporated by
reference, Exhibit 1.1 to Company’s Current Report on Form 8-K,
file number 001-36464, filed on January 10, 2022.) |
| |
|
|
| 10.13 |
|
Common
Stock Sales Agreement dated April 27, 2022 by and between Agile Therapeutics and H.C. Wainwright & Co., LLC (Incorproated by
reference, Exhibit 3.1 to
Company’s Current Report on Form 8-K, file number 001-036464) |
| |
|
|
| 10.14 |
|
Credit Agreement and Guaranty
among Agile Therapeutics, Inc., the guarantors from time to time party thereto, the lenders from time to time party thereto and Perceptive
Credit Holdings III, LP, dated as of February 10, 2020 (Incorporated by reference, Exhibit 10.1 to Company’s Current Report
on Form 8-K, file number 001-36464, filed on February 12, 2020.) |
| |
|
|
Exhibit
Number |
|
|
| |
|
|
| 10.15 |
|
Waiver
and First Amendment to Credit Agreement and Guaranty among Agile Therapeutics, Inc., the guarantors from time to time party thereto,
the lenders from time
to time party thereto and Perceptive Credit Holdings III, LP, dated as of February 26, 2021(Incorporated by
reference, Exhibit 10.11
to Company’s Annual Report on Form 10-K, file number 001-36464, filed on March 1, 2021.) |
| |
|
|
| 10.16 |
|
Waiver
and Second Amendment to Credit Agreement and Guaranty among Agile Therapeutics, Inc., the guarantors from time to time party thereto,
the lenders from time to time party
thereto and Perceptive Credit Holdings III, LP, dated as of February 26, 2021 (Incorporated by
reference, Exhibit 10.1 to
Company’s Current Report on Form 8-K, file number 001-36464, filed on March 15, 2022.) |
| |
|
|
| 10.17 |
|
Waiver
and Third Amendment to Credit Agreement and Guaranty among Agile Therapeutics, Inc., the guarantors from time to time party thereto,
the lenders from time to time party
thereto and Perceptive Credit Holdings III, LP, dated as of March 10, 2022 (Incorporated by reference,
Exhibit 10.1 to
Company’s Current Report on Form 8-K, file number 001-36464, filed on March 11, 2022.) |
| |
|
|
| 10.18 |
|
Waiver
and Fourth Amendment to Credit Agreement and Guaranty among Agile Therapeutics, Inc., the guarantors from time to time party thereto,
the lenders from time to time party thereto and Perceptive Credit Holdings III, LP, dated as of May 11, 2022 (Incorporated by reference,
Exhibit 10.1 to Company’s
Current Report on Form 8-K, file number 001-36464, filed on May 13, 2022.) |
| |
|
|
| 10.17 |
|
Form
of Securities Purchase Agreement, dated March 13, 2022, by and between Agile Therapeutics, Inc. and the purchaser signatory thereto
(Incorporated by reference, Exhibit 10.1
to Company’s Current Report on Form 8-K, file number 001-36464, filed on
March 15, 2022.) |
| |
|
|
| 10.18* |
|
Project Agreement, dated
April 30, 2020, by and between the Registrant and inVentiv Commercial Services, LLC (Incorporated by reference, Exhibit 10.1 to Company’s
Quarterly Report on Form 10-Q, file number 001-36464, filed on August 11, 2020.) |
| |
|
|
| 10.19* |
|
First Amendment to Project
Agreement, dated June 1, 2020, by and between the Registrant and inVentiv Commercial Services, LLC (Incorporated by reference, Exhibit
10.13 to Company’s Annual Report on Form 10-K, file number 001-36464, filed on March 1, 2021.) |
| |
|
|
| 10.20* |
|
Master Service Agreement,
dated October 11. 2017, by and between the Registrant and inVentiv Commercial Services, LLC (Incorporated by reference, Exhibit 10.2
to Company’s Quarterly Report on Form 10-Q, file number 001-36464, filed on August 11, 2020.) |
| |
|
|
| 10.21* |
|
First Amendment to Master
Service Agreement, dated April 30, 2020, by and between the Registrant and inVentiv Commercial Services, LLC (Incorporated by reference,
Exhibit 10.3 to Company’s Quarterly Report on Form 10-Q, file number 001-36464, filed on August 11, 2020.) |
| |
|
|
| 10.22* |
|
Second Amendment to Master
Service Agreement, dated January 1, 2021, by and between the Registrant and inVentiv Commercial Services, LLC (Incorporated by reference,
Exhibit 10.2 to Company’s Quarterly Report on Form 10 Q, file number 001 36464, filed on November 2, 2021.) |
| |
|
|
| 10.23* |
|
Third Amendment to Master
Service Agreement, dated July 1, 2021, by and between the Registrant and inVentiv Commercial Services, LLC. (Incorporated by reference,
Exhibit 10.3 to Company’s Quarterly Report on Form 10 Q, file number 001 36464, filed on November 2, 2021.) |
| |
|
|
| 10.24* |
|
Manufacturing and Commercialization
Agreement, dated April 30, 2020, by and between the Registrant and Corium, Inc. (Incorporated by reference, Exhibit 10.4 to Company’s
Quarterly Report on Form 10-Q, file number 001-36464, filed on August 11, 2020.) |
| |
|
|
| 10.25+ |
|
Agile Therapeutics, Inc.
Amended and Restated 2014 Incentive Compensation Plan (Incorporated by reference, Appendix A to Registrant’s Proxy Statement
pursuant to Section 14(a) of the Securities Exchange Act of 1934, file number 001-36464, filed on April 25, 2018.) |
| |
|
|
Exhibit
Number |
|
|
| |
|
|
| 10.26 |
|
Clinical Research Agreement,
dated October 26, 2018, by and between the Registrant and TKL Research, Inc. (Incorporated by reference, Exhibit 10.24 to Company’s
Annual Report on Form 10-K, file number 001-36464, filed on March 12, 2019.) |
| |
|
|
| 10.27 |
|
Amended
and Restated Employment Agreement, dated August 14, 2020 by and between the Registrant and Alfred Altomari (incorporated by
reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, file number 001-36464, filed on August 17,
2020). |
| |
|
|
| 10.28 |
|
Amended and Restated Employment Agreement,
dated August 14, 2020 by and between the Registrant and Robert Conway (incorporated by reference to Exhibit 10.2 to the Company’s
Current Report on Form 8-K, file number 001-36464, filed on August 17, 2020). |
| |
|
|
| 10.29 |
|
Amended and Restated
Employment Agreement, dated August 14, 2020 by and between the Registrant and Geoffrey Gilmore (incorporated by
reference to Exhibit
10.3 to the Company’s Current Report on Form 8-K, file number 001-36464, filed on August 17, 2020). |
| |
|
|
| 10.30 |
|
Amended and Restated
Employment Agreement, dated August 14, 2020 by and between the Registrant and Dennis Reilly (incorporated by
reference to Exhibit
10.4 to the Company’s Current Report on Form 8-K, file number 001-36464, filed on August 17, 2020). |
| |
|
|
| 10.31++ |
|
Form of Securities
Purchase Agreement, by and between Agile Therapeutics, Inc. and the purchasers signatory thereto. |
| |
|
|
| 23.1 |
|
Consent of Independent Registered Public
Accounting Firm. |
| |
|
|
| 23.2++ |
|
Consent of Morgan,
Lewis & Bockius LLP (included in Exhibit 5.1) |
| |
|
|
| 24.1 |
|
Power of Attorney (contained
on the signature page to the Registration Statement). |
| |
|
|
| 107 |
|
Filing Fee Table |
| + | Indicates management contract or compensatory plan or arrangement. |
| ++ | To be filed by amendment. |
| * | Portions of this exhibit have been redacted in accordance with Regulation S-K Item 601(b)(10). |
| (b) | Financial Statement Schedules |
All financial statement schedules
have been omitted because they are not required or because the required information is given in the financial statements or notes to those
statements.
Item 17. Undertakings.
The undersigned registrant
hereby undertakes:
| (a) | (1) To file, during any period in which offers or sales are being made, a post-effective amendment to
this registration statement: |
(i) To include any prospectus
required by Section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”);
(ii) To reflect in the prospectus any
facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof)
which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement.
Notwithstanding the foregoing, any increase or decrease in the volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering
range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes
in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of
Registration Fee” table in the effective registration statement; and
(iii) To include any
material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change
to such information in the registration statement.
provided, however, that paragraphs
(1)(i), (1)(ii) and (1)(iii) do not apply if the information required to be included in a post-effective amendment by those paragraphs
is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities
Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration statement, or
is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
(2) That, for the
purposes of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at the time shall be deemed to be the initial
bona fide offering thereof.
(3) To remove from
registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the
offering.
(4) That, for the
purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities,
the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration
statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to
such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will
be considered to offer or sell such securities to such purchaser:
(i) Any preliminary
prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing
prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of
any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities
provided by or on behalf of the undersigned registrant; and
(iv) Any other communication
that is an offer in the offering made by the undersigned registrant to the purchaser.
(5) That, for purposes
of determining any liability under the Securities Act:
(i) the information
omitted from the form of prospectus filed as part of the registration statement in reliance upon Rule 430A and contained in the form of
prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of
the registration statement as of the time it was declared effective; and
(ii) each post-effective
amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein,
and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(b) The undersigned
registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s
annual report pursuant to Section 13(a) or 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s
annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be
deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time
shall be deemed to be the initial bona fide offering thereof.
(c) Insofar as indemnification
for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant
to the indemnification provisions described herein, or otherwise, the registrant has been advised that in the opinion of the SEC such
indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim
for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer
or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer
or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the
matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification
by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements
of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the
undersigned, thereunto duly authorized, in the City of Princeton, State of New Jersey on May 13, 2022.
| |
Agile
Therapeutics, Inc. |
| |
|
| |
By: |
| |
|
/s/ Alfred Altomari |
| |
|
Alfred Altomari |
| |
|
Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person
whose signature appears below constitutes and appoints Alfred Altomari and Dennis P. Reilly, and each of them, as his or her true and
lawful agent, proxy and attorney-in-fact, each acting alone, with full power of substitution and resubstitution, for him or her and in
his or her name, place and stead, in any and all capacities, to (i) act on, sign, and file with the SEC any and all amendments (including
post-effective amendments) to this registration statement together with all schedules and exhibits thereto, (ii) act on, sign and file
such certificates, instruments, agreements and other documents as may be necessary or appropriate in connection therewith, (iii) act on
and file any supplement to any prospectus included in this registration statement or any such amendment or any subsequent registration
statement filed pursuant to Rule 462(b) under the Securities Act and (iv) take any and all actions which may be necessary or appropriate
to be done, as fully for all intents and purposes as he or she might or could do in person, hereby approving, ratifying and confirming
all that such agent, proxy and attorney-in-fact or any of his substitutes may lawfully do or cause to be done by virtue thereof. This
Power of Attorney may be executed in several counterparts.
Pursuant to the requirements
of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and
on the dates indicated.
| Name |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Alfred Altomari |
|
|
|
|
| Alfred
Altomari |
|
Chief
Executive Officer and Director (Principal Executive Officer) |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
Dennis P. Reilly |
|
|
|
|
| Dennis
P. Reilly |
|
Chief
Financial Officer (Principal Financial Officer) |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
Jason Butch |
|
|
|
|
| Jason
Butch |
|
Chief
Accounting Officer (Principal Accounting Officer) |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
Sharon Barbari |
|
|
|
|
| Sharon
Barbari |
|
Director |
|
May
13, 2022 |
| |
|
|
|
|
| /s/ Sandra
Carson, M.D., FACOG |
|
|
|
|
| Sandra
Carson, M.D., FACOG |
|
Director |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
Seth H.Z. Fischer |
|
|
|
|
| Seth
H.Z. Fischer |
|
Director |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
John Hubbard, Ph.D. |
|
|
|
|
| John
Hubbard, Ph.D. |
|
Director |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
Ajit S. Shetty, Ph.D. |
|
|
|
|
| Ajit
S. Shetty, Ph.D. |
|
Director |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
Josephine Torrente |
|
|
|
|
| Josephine
Torrente |
|
Director |
|
May
13, 2022 |
| |
|
|
|
|
| /s/
James Tursi, M.D. |
|
|
|
|
| James
Tursi, M.D. |
|
Director |
|
May
13, 2022 |
Agile Therapeutics (NASDAQ:AGRX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Agile Therapeutics (NASDAQ:AGRX)
Historical Stock Chart
From Apr 2023 to Apr 2024