Insulet Gets FDA OK for Omnipod 5 Automated Insulin-Delivery System
January 28 2022 - 6:54AM
Dow Jones News
By Colin Kellaher
Insulet Corp. on Friday said it received U.S. Food and Drug
Administration approval for its Omnipod 5 automated
insulin-delivery system for individuals ages 6 and older with type
1 diabetes.
The Acton, Mass., medical-device company said the Omnipod 5 is
the first tubeless automated delivery system that integrates with
DexCom Inc.'s G6 continuous glucose monitoring system and a
compatible smartphone to automatically adjust insulin and help
protect against highs and lows.
Insulet said that it will launch Omnipod 5 through the pharmacy
channel, and that it expects the device will be broadly available
shortly after a limited market release.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
January 28, 2022 06:39 ET (11:39 GMT)
Copyright (c) 2022 Dow Jones & Company, Inc.
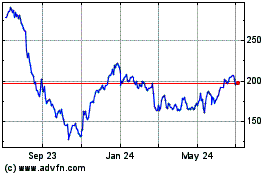
Insulet (NASDAQ:PODD)
Historical Stock Chart
From Mar 2024 to Apr 2024
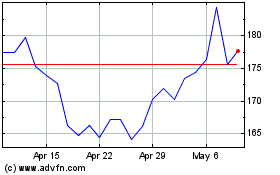
Insulet (NASDAQ:PODD)
Historical Stock Chart
From Apr 2023 to Apr 2024