Acadia Pharmaceuticals Rise 16% on Resubmission Plan for Pimavanserin
December 20 2021 - 5:03PM
Dow Jones News
By Mary de Wet
Acadia Pharmaceuticals Inc. shares rose 16% to $31.40 in
post-market trade Monday after the company said it plans to
resubmit its supplemental new drug application for pimavanserin to
treat hallucinations and delusions associated with Alzheimer's
disease psychosis.
Acadia plans to resubmit the application in the first quarter of
2022, after the Food and Drug Administration rejected it in
April.
The company is trying to expand the label for pimavanserin,
which is already approved to treat hallucinations and delusions
associated with Parkinson's disease psychosis, and is marketed as
Nuplazid.
"Following our recent meetings with the FDA, we plan to resubmit
our sNDA for pimavanserin, narrowing the proposed indication from
dementia-related psychosis to Alzheimer's disease psychosis," said
Chief Executive Steve Davis.
Write to Mary de Wet at mary.dewet@dowjones.com
(END) Dow Jones Newswires
December 20, 2021 16:48 ET (21:48 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
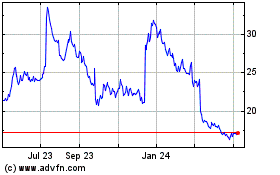
Acadia Pharmaceuticals (NASDAQ:ACAD)
Historical Stock Chart
From Mar 2024 to Apr 2024
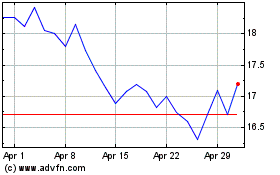
Acadia Pharmaceuticals (NASDAQ:ACAD)
Historical Stock Chart
From Apr 2023 to Apr 2024