Reata Pharmaceuticals Gets Negative Vote From FDA Advisory Panel on Bardoxolone Methyl
December 08 2021 - 6:43PM
Dow Jones News
By Josh Beckerman
Reata Pharmaceuticals Inc. received a negative opinion from a
U.S. Food and Drug Administration advisory committee for its New
Drug Application for bardoxolone methyl for patients with chronic
kidney disease caused by Alport syndrome.
Reata shares were halted all day Wednesday.
The panel "voted no on the question of whether the provided
evidence demonstrated that bardoxolone is effective in slowing the
progression of CKD in patients with Alport syndrome and that its
benefits outweigh its risks."
Reata said it is disappointed with the vote and will continue to
work closely with the FDA to provide additional information and
data before the Feb. 25 Prescription Drug User-Fee Act date.
The FDA isn't required to follow the advice of its advisory
panels, but generally does so.
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
December 08, 2021 18:28 ET (23:28 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
Reata Pharmaceuticals (NASDAQ:RETA)
Historical Stock Chart
From Mar 2024 to Apr 2024
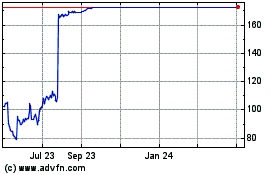
Reata Pharmaceuticals (NASDAQ:RETA)
Historical Stock Chart
From Apr 2023 to Apr 2024