Philips Gets FDA Clearance to Expand Patient Monitors Across U.S.
December 08 2021 - 9:57AM
Dow Jones News
By Kimberly Chin
Koninklijke Philips N.V. said the U.S. Food and Drug
Administration has given it a 501(k) clearance that allows it to
expand its acute-care patient monitors to more people across the
U.S.
The company said the advanced patient monitors support
scalability, alarm management, cybersecurity and infection
protection within a hospital setting. The monitors are
interoperable with other devices and applications and can allow
hospitals to standardize and customize patient care, it said.
Philips Patient Monitors IntelliVue MX750 and MX850 received
emergency use authorization by the FDA in 2020.
Write to Kimberly Chin at kimberly.chin@wsj.com
(END) Dow Jones Newswires
December 08, 2021 09:42 ET (14:42 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
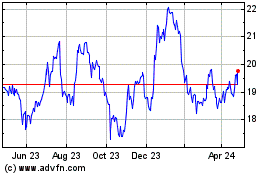
Koninklijke Philips NV (EU:PHIA)
Historical Stock Chart
From Mar 2024 to Apr 2024
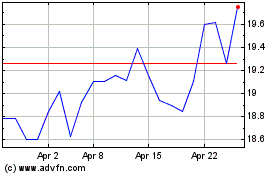
Koninklijke Philips NV (EU:PHIA)
Historical Stock Chart
From Apr 2023 to Apr 2024