Cumberland Pharma Shares Double Premarket on Labeling for Caldolor
November 30 2021 - 6:51AM
Dow Jones News
By Chris Wack
Cumberland Pharmaceuticals Inc. shares doubled to $4.79 in
premarket trading after the company said the U.S. Food and Drug
Administration has approved expanded labeling for Caldolor, an
intravenously delivered formulation of ibuprofen, to now include
use in pre-operative administration.
The specialty pharmaceutical company said the non-narcotic pain
reliever may now be administered just prior to surgery to enable
patients to wake up from their procedure in significantly less
pain.
The newly FDA-approved label includes information regarding the
product's indications and usage, appropriate patient populations,
clinical study results, potential side effects, patient safety
details, and instructions for use in pregnant women, children and
other populations.
Cumberland said the expanded use of Caldolor is supported by a
study of orthopedic surgical pain, which confirmed the significant
pain reduction when the product was administered every six hours
with supplemental morphine available on an as needed basis.
Efficacy was demonstrated as a statistically significant greater
reduction in pain intensity over 24 hours post-operatively for
patients treated with Caldolor as compared with those receiving
placebo.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
November 30, 2021 06:36 ET (11:36 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
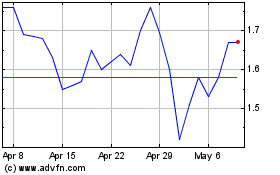
Cumberland Pharmaceutical (NASDAQ:CPIX)
Historical Stock Chart
From Mar 2024 to Apr 2024
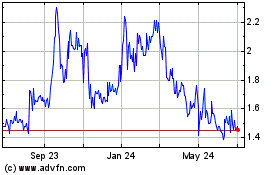
Cumberland Pharmaceutical (NASDAQ:CPIX)
Historical Stock Chart
From Apr 2023 to Apr 2024