Switzerland Agency Approves Moderna and Pfizer/BioNTech Boosters
October 26 2021 - 2:11PM
Dow Jones News
By Josh Beckerman
Switzerland's Swissmedic agency has approved Covid-19 vaccine
booster doses from Moderna Inc. and from Pfizer Inc. and BioNTech
SE.
Swissmedic said a third vaccination at least six months after
the second dose may help "older people or at-risk patients"
maintain their level of protection. For immunocompromised people,
such as organ transplant recipients, who have a weak immune
response or none at all after two doses, a third dose can be
administered at least 28 days after the second shot, the agency
said.
On Monday, Moderna said the European Medicines Agency's
Committee for Medicinal Products for Human Use concluded that a 50
ug booster dose of its vaccine may be considered for people aged 18
and older at least six months after completion of primary
vaccination. The EMA's opinion reflected favorable results from a
data analysis of a Phase 2 clinical study, Moderna said.
The U.S. Food and Drug Administration authorized Pfizer-BioNTech
boosters in September. In October, it approved those from Moderna
and Johnson & Johnson, and authorized "mix and match" use of a
different booster vaccine.
In September 2020, Moderna said Switzerland would be the first
country outside of North America with a Moderna regional hub and
commercial organization.
Write to Josh Beckerman at josh.beckerman@wsj.com
(END) Dow Jones Newswires
October 26, 2021 13:56 ET (17:56 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
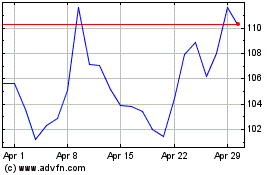
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Mar 2024 to Apr 2024
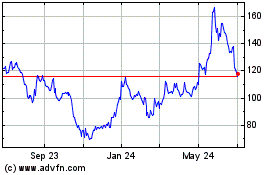
Moderna (NASDAQ:MRNA)
Historical Stock Chart
From Apr 2023 to Apr 2024