Canada, Pfizer Strike Deal on Pediatric Vaccine Doses
October 21 2021 - 1:41PM
Dow Jones News
By Paul Vieira
OTTAWA--Canada said Thursday the country has secured a deal with
Pfizer Inc. to ensure it will have ample supply of the company's
Covid-19 pediatric vaccine, produced in partnership with BioNTech
SE, to provide a first dose to every eligible child in the
country.
The deal is conditional on Canada's drug regulator authorizing
use of the Pfizer-BioNTech vaccine on children aged 5 to 11. Pfizer
submitted this week an application for Canadian authorization.
Anita Anand, Canada's procurement minister, in a statement
posted on social media said the country is scheduled to receive 2.9
million doses of Pfizer-BioNTech's vaccine for pediatric use.
"Pfizer has agreed to an accelerated delivery schedule of its
pediatric vaccine once approved," Ms. Anand said.
The delivery schedule for second doses is conditional on the
vaccine rollout across the country, she said.
Canada's federal government has responsibility for acquiring
Covid-19 vaccines, while provincial authorities are responsible for
ensuring doses are injected.
A Pfizer spokeswoman confirmed the deal, citing Ms. Anand's
statement.
Data from the Canadian government indicates that 82% of people
12 and over are fully vaccinated, one of the highest rates among
major developed-world economies.
Write to Paul Vieira at paul.vieira@wsj.com
(END) Dow Jones Newswires
October 21, 2021 13:26 ET (17:26 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
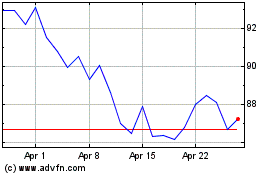
BioNTech (NASDAQ:BNTX)
Historical Stock Chart
From Mar 2024 to Apr 2024
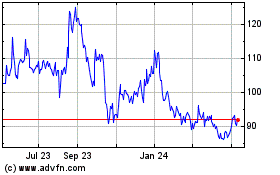
BioNTech (NASDAQ:BNTX)
Historical Stock Chart
From Apr 2023 to Apr 2024