Sage Therapeutics, Biogen to Seek FDA Approval of Zuranolone in 2nd Half of 2022
October 19 2021 - 7:40AM
Dow Jones News
By Colin Kellaher
Sage Therapeutics Inc. and Biogen Inc. on Tuesday said they plan
to seek U.S. Food and Drug Administration approval for their
antidepressant drug candidate zuranolone in the second half of
2022.
The companies said they plan to start a rolling submission early
next year, and the initial package will seek approval of zuranolone
for the treatment of major depressive disorder.
Sage and Biogen said the decision follows recent discussions
with the FDA, noting that they expect the current efficacy and
safety databases to be adequate for filing.
Sage and Biogen said they plan an additional filing for
postpartum depression in the first half of 2023.
Sage and Biogen, both based in Cambridge, Mass., late last year
signed a global collaboration and license agreement to jointly
develop and commercialize zuranolone for major depressive disorder,
postpartum depression and other psychiatric disorders.
The companies in June said a Phase 3 study of the drug in major
depressive disorder met its primary endpoint, showing statistically
significant improvement in symptoms compared with placebo.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
October 19, 2021 07:25 ET (11:25 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
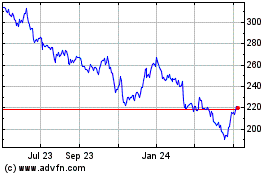
Biogen (NASDAQ:BIIB)
Historical Stock Chart
From Mar 2024 to Apr 2024
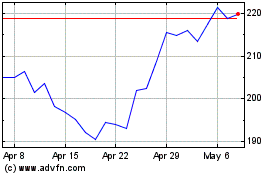
Biogen (NASDAQ:BIIB)
Historical Stock Chart
From Apr 2023 to Apr 2024