Eiger BioPharmaceuticals Gets Breakthrough Therapy Designation for Avexitide
August 05 2021 - 8:51AM
Dow Jones News
By Chris Wack
Eiger BioPharmaceuticals Inc. said the U.S. Food and Drug
Administration granted breakthrough therapy designation for
avexitide for the treatment of congenital hyperinsulism.
The designation is a process designed to speed up the
development and review of drugs that are intended to treat a
serious condition and where preliminary clinical evidence indicates
that the drug may demonstrate substantial improvement over
available therapy on a clinically significant endpoint.
Eiger said its application was supported by data from three
completed Phase 2 studies in 39 neonates, children and adolescents
with congenital hyperinsulinism. Avexitide is a targeted GLP-1
antagonist in development for the treatment of metabolic disorders,
including congential hyperinsulinism, an ultra-rare,
life-threatening, pediatric disorder of persistent hypoglycemia
that results in irreversible brain damage in up to 50% of
children.
Eiger shares were up 9% to $8.24 in premarket trade.
Write to Chris Wack at chris.wack@wsj.com
(END) Dow Jones Newswires
August 05, 2021 08:45 ET (12:45 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
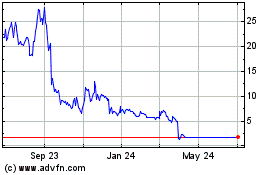
Eiger BioPharmaceuticals (NASDAQ:EIGR)
Historical Stock Chart
From Mar 2024 to Apr 2024
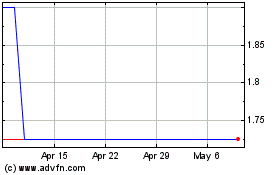
Eiger BioPharmaceuticals (NASDAQ:EIGR)
Historical Stock Chart
From Apr 2023 to Apr 2024