Exelixis in Clinical Trial Collaboration with Bristol Myers
June 14 2021 - 9:16AM
Dow Jones News
By Michael Dabaie
Exelixis Inc. said it is in a clinical trial collaboration and
supply agreement with Bristol Myers Squibb Co. for a Phase 1b trial
evaluating XL092 in combination with immuno-oncology therapies in
advanced solid tumors.
The objective of the study is to evaluate the safety,
tolerability and efficacy of Exelixis' XL092, in combination with:
nivolumab, or Opdivo; nivolumab and ipilimumab, or Yervoy; and
nivolumab and bempegaldesleukin.
Exelixis said it is sponsoring the trial and Bristol Myers
Squibb will provide nivolumab, ipilimumab and bempegaldesleukin for
use in the trial.
Nektar Therapeutics will supply bempegaldesleukin to Bristol
Myers Squibb through their existing global development and
commercialization collaboration.
The STELLAR-002 study will begin with a dose-escalation phase to
determine the recommend dose for each of the combination
therapies.
Expansion cohorts to include patients with advanced kidney,
prostate and bladder cancers, Exelixis said.
Write to Michael Dabaie at michael.dabaie@wsj.com
(END) Dow Jones Newswires
June 14, 2021 09:01 ET (13:01 GMT)
Copyright (c) 2021 Dow Jones & Company, Inc.
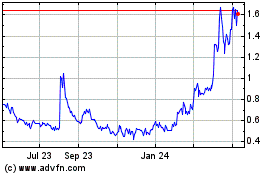
Nektar Therapeutics (NASDAQ:NKTR)
Historical Stock Chart
From Mar 2024 to Apr 2024
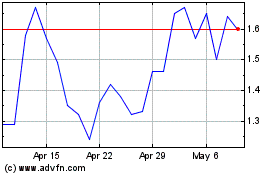
Nektar Therapeutics (NASDAQ:NKTR)
Historical Stock Chart
From Apr 2023 to Apr 2024