Kymera Therapeutics Appoints Juliet Williams, PhD, as Senior Vice President, Head of Biology
May 05 2021 - 7:00AM

Kymera Therapeutics, Inc. (NASDAQ: KYMR), a clinical-stage
biopharmaceutical company advancing targeted protein degradation to
deliver novel small molecule protein degrader medicines, today
announced the appointment of Juliet Williams, PhD, as Senior Vice
President, Head of Biology. Dr. Williams joins Kymera with 20 years
of experience in the biopharmaceutical industry, including
leadership roles in drug discovery and translational development.
“I’m very excited to welcome Juliet to the leadership team at
Kymera,” said Nello Mainolfi, PhD, Co-Founder, President and CEO,
Kymera Therapeutics. “Juliet has been a successful drug hunter in
both biotech and large pharma. Her expertise and leadership in
target identification, drug discovery, and translational drug
development across small molecule and innovative drug classes will
be invaluable as we continue to expand Kymera’s capabilities to
build a best-in-class degrader medicines company.”
“I am excited to join Kymera during a transformational time as
the Company has initiated a first-in-class development program in
immunology and inflammation and expects to advance its two lead
oncology degrader programs into clinical development later this
year,” said Dr. Williams. “I look forward to working with the team
to continue to advance our platform and science and I am inspired
by the opportunity to invent and deliver new medicines for patients
in need.”
Dr. Williams joins Kymera with 20 years of drug development
experience, including service at Novartis, Sanofi, Millennium, and
Curis. Prior to Kymera, she led oncology small molecule drug
discovery at Novartis Institutes for Biomedical Research and served
in Head of Oncology Pharmacology roles at both Novartis and Sanofi.
She holds a degree in Natural Sciences (Biochemistry) from the
University of Cambridge and a PhD in Developmental Biology from
University College London. Dr. Williams completed a Wellcome
Postdoctoral Fellowship at University College London in
Developmental Biology.
About Kymera TherapeuticsKymera Therapeutics
(Nasdaq: KYMR) is a clinical-stage biopharmaceutical company
founded with the mission to discover, develop, and commercialize
transformative therapies while leading the evolution of targeted
protein degradation, a transformative new approach to address
previously intractable disease targets. Kymera’s Pegasus™ platform
enables the discovery of novel small molecule degraders designed to
harness the body’s natural protein recycling machinery to degrade
disease-causing proteins, with a focus on undrugged nodes in
validated pathways currently inaccessible with conventional
therapeutics. Kymera’s initial programs are IRAK4, IRAKIMiD, and
STAT3, which each address high impact targets within the IL-1R/TLR
or JAK/STAT pathways, providing the opportunity to treat a broad
range of immune-inflammatory diseases, hematologic malignancies,
and solid tumors. Kymera’s goal is to be a fully integrated
biopharmaceutical company at the forefront of this new class of
protein degrader medicines, with a pipeline of novel degrader
medicines targeting disease-causing proteins that were previously
intractable.
Founded in 2016, Kymera is headquartered in Watertown, Mass.
Kymera has been named a “Fierce 15” biotechnology company by
FierceBiotech and has been recognized by the Boston Business
Journal as one of Boston’s “Best Places to Work.” For more
information about our people, science, and pipeline, please visit
www.kymeratx.com or follow us on Twitter or LinkedIn.
Cautionary Note Regarding Forward-Looking
StatementsThis press release contains forward-looking
statements within the meaning of the Private Securities Litigation
Reform Act of 1995, as amended, including, without limitation,
implied and express statements regarding its: beliefs regarding its
program in immunology and inflammation and that it is
first-in-class; plans to continue to expand capabilities to build a
best-in-class degrader medicines company; expectations for Dr.
Williams; strategy, business plans and objectives for the IRAK4,
IRAKIMiD and STAT3 degrader programs and the clinical development
of Kymera Therapeutics' product candidates, including the
therapeutic potential and clinical benefits thereof. The words
"may," “might,” "will," "could," "would," "should," "expect,"
"plan," "anticipate," "intend," "believe," “expect,” "estimate,"
“seek,” "predict," “future,” "project," "potential," "continue,"
"target" and similar words or expressions are intended to identify
forward-looking statements, although not all forward-looking
statements contain these identifying words. Any forward-looking
statements in this press release are based on management's current
expectations and beliefs and are subject to a number of risks,
uncertainties and important factors that may cause actual events or
results to differ materially from those expressed or implied by any
forward-looking statements contained in this press release,
including, without limitation, risks associated with: the impact of
COVID-19 on countries or regions in which Kymera Therapeutics has
operations or does business, as well as on the timing and
anticipated results of its current preclinical studies and future
clinical trials, strategy and future operations; the delay of any
current preclinical studies or future clinical trials or the
development of Kymera Therapeutics' drug
candidates; the risk that the results of current preclinical
studies may not be predictive of future results in connection with
future clinical trials; Kymera Therapeutics' ability to
successfully demonstrate the safety and efficacy of its drug
candidates; the timing and outcome of Kymera Therapeutics’ planned
interactions with regulatory authorities, including the resolution
of the current partial clinical hold for KT-474; and obtaining,
maintaining and protecting its intellectual property. These
and other risks and uncertainties are described in greater detail
in the section entitled "Risk Factors" in the Annual Report on Form
10-K for the period ended December 31, 2020, filed on March 11,
2021, as well as discussions of potential risks, uncertainties, and
other important factors in Kymera Therapeutics' subsequent filings
with the Securities and Exchange Commission. In addition, any
forward-looking statements represent Kymera Therapeutics' views
only as of today and should not be relied upon as representing its
views as of any subsequent date. Kymera Therapeutics explicitly
disclaims any obligation to update any forward-looking statements.
No representations or warranties (expressed or implied) are made
about the accuracy of any such forward-looking statements.
Investor Contact:Paul CoxVP, Investor Relations
and Communicationspcox@kymeratx.com917-754-0207
Media Contact:Lissette L. SteeleVerge
Scientific Communications for Kymera
Therapeuticslsteele@vergescientific.com 202-930-4762
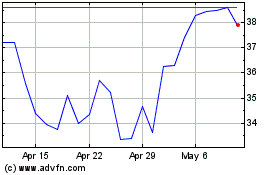
Kymera Therapeutics (NASDAQ:KYMR)
Historical Stock Chart
From Mar 2024 to Apr 2024
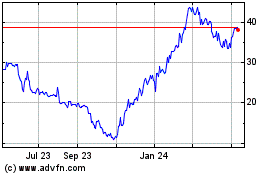
Kymera Therapeutics (NASDAQ:KYMR)
Historical Stock Chart
From Apr 2023 to Apr 2024