NovaBay Pharmaceuticals Now Included in EPA N Listing of Products That Kill SARS-CoV-2
December 14 2020 - 6:50AM
Business Wire
NovaBay’s pure hypochlorous formulation safely
eliminates 99.9% of viruses and bacteria on nonporous hard surfaces
without cytotoxic chemicals
NovaBay® Pharmaceuticals, Inc. (NYSE American: NBY) announces
its proprietary hypochlorous acid solution has received U.S.
Environmental Protection Agency (EPA) approval for a kill claim
against SARS-CoV-2, the virus that causes COVID-19. This official
listing follows the July 2020 announcement that independent,
third-party laboratory results demonstrated that NovaBay’s
proprietary formulation kills SARS-CoV-2 on hard surfaces.
“The FDA requires that any product containing hypochlorous acid
(HOCl) used by patients in healthcare is registered as a medical
device and may be marketed as either a prescription (Rx) or
over-the-counter (OTC) product following its intended use label
guidelines. However, it is the EPA that regulates disinfectants on
surfaces and requires formal testing of such products to make any
disinfectant claims, even if the ingredients are the same as the
FDA-registered product,” said Justin Hall, NovaBay’s CEO.
“Obtaining EPA Registration Number 98003-1 for NovaBay Hard
Nonporous Surface Pro is a validation that our HOCl solution is
effective against the virus and can be used by consumers to safely
disinfect commonly used personal items like eyeglasses, facial
coverings, and cell phones.”
Hypochlorous acid has been used for more than 100 years by
medical professionals for disinfecting medical equipment and
dressing wounds. Until recently, all HOCl solutions contained toxic
chemicals like sodium hypochlorite, or were very unstable and could
not be stored for long periods of time. In 2015, NovaBay
revolutionized the HOCl marketplace with a pure and stable
formulation, devoid of bleach particles and toxic impurities.
“The special characteristics of NovaBay’s proprietary HOCl, plus
state-of-the-art manufacturing in the USA, set it apart from all
other hypochlorous acid products,” added Mr. Hall. “We are highly
pleased that our years of research and development, independent
laboratory testing, and our interaction with the EPA have
culminated in NovaBay Hard Nonporous Surface Pro’s recognition for
use in combating the spread of the coronavirus.”
About NovaBay Pharmaceuticals, Inc.: Going Beyond
Antibiotics®
NovaBay Pharmaceuticals, Inc. is a biopharmaceutical company
focusing on commercializing and developing its non-antibiotic
anti-infective products to address the unmet therapeutic needs of
the global, topical anti-infective market with its two distinct
product categories: the NEUTROX® family of products and the
AGANOCIDE® compounds. The Neutrox family of products includes
AVENOVA® for the eye care market, CELLERX® for the aesthetic
dermatology market and NEUTROPHASE® for the wound care market. The
Aganocide compounds, still under development, have target
applications in the dermatology and urology markets.
Forward-Looking Statements
Except for historical information herein, matters set forth in
this press release are forward-looking within the meaning of the
“safe harbor” provisions of the Private Securities Litigation
Reform Act of 1995, including statements about the commercial
progress and future financial performance of NovaBay
Pharmaceuticals, Inc. This release contains forward-looking
statements that are based upon management’s current expectations,
assumptions, estimates, projections and beliefs. These statements
include, but are not limited to, statements regarding the
regulatory clearance of any of our products or future products, and
any future revenue that may result from selling these products, as
well as generally the Company’s expected future financial results.
These statements involve known and unknown risks, uncertainties and
other factors that may cause actual results or achievements to be
materially different and adverse from those expressed in or implied
by the forward-looking statements. Factors that might cause or
contribute to such differences include, but are not limited to,
risks and uncertainties relating to the size of the potential
market for our products, the possibility that the available market
for the Company’s products will not be as large as expected, the
Company’s products will not be able to penetrate one or more
targeted markets, revenues will not be sufficient to meet the
Company’s cash needs, and any potential regulatory problems. Other
risks relating to NovaBay’s business, including risks that could
cause results to differ materially from those projected in the
forward-looking statements in this press release, are detailed in
NovaBay’s latest Form 10-Q/K filings with the Securities and
Exchange Commission, especially under the heading “Risk Factors.”
The forward-looking statements in this release speak only as of
this date, and NovaBay disclaims any intent or obligation to revise
or update publicly any forward-looking statement except as required
by law.
Socialize and Stay informed on
NovaBay’s progress
Like us on Facebook Follow us on Twitter Connect
with NovaBay on LinkedIn Visit NovaBay’s
Website
Avenova Purchasing
Information For NovaBay Avenova purchasing information:
Please call 800-890-0329 or email sales@avenova.com.
www.Avenova.com
View source
version on businesswire.com: https://www.businesswire.com/news/home/20201214005255/en/
NovaBay Contact Justin Hall
Chief Executive Officer and General Counsel 510-899-8800
jhall@novabay.com
Investor Contact LHA
Investor Relations Jody Cain 310-691-7100 jcain@lhai.com
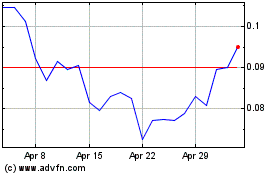
NovaBay Pharmaceuticals (AMEX:NBY)
Historical Stock Chart
From Mar 2024 to Apr 2024
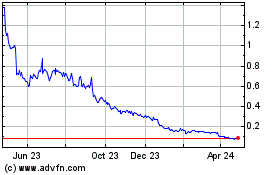
NovaBay Pharmaceuticals (AMEX:NBY)
Historical Stock Chart
From Apr 2023 to Apr 2024