Roche Gets FDA Emergency Use OK for Test to Measure Level of SARS-CoV-2 Antibodies
December 01 2020 - 12:13PM
Dow Jones News
By Colin Kellaher
Roche Holding AG on Tuesday said the U.S. Food and Drug
Administration granted emergency-use authorization for its Elecsys
anti-SARS-CoV-2 S antibody test.
The Swiss pharmaceutical company said the semi-quantitative
blood test can be used to measure the level of antibodies in people
who have been exposed to the SARS-CoV-2 virus.
Roche said the test specifically detects antibodies against the
SARS-CoV-2 spike protein, which is the target of many Covid-19
vaccines in development.
Roche said it will begin shipping the Elecsys test in the next
week, adding that Laboratory Corp. of America will be the first lab
to offer the testing option in the U.S.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
December 01, 2020 11:58 ET (16:58 GMT)
Copyright (c) 2020 Dow Jones & Company, Inc.
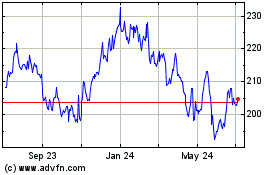
Laboratory Corporation o... (NYSE:LH)
Historical Stock Chart
From Mar 2024 to Apr 2024
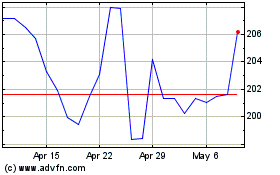
Laboratory Corporation o... (NYSE:LH)
Historical Stock Chart
From Apr 2023 to Apr 2024