We are hereby qualifying for distribution
the issuance of: (i) up to 265,757 common shares (“Common Shares”) of the Corporation, issuable from time to
time, on exercise of up to 265,757 Common Share purchase warrants issued by us on August 16, 2019 pursuant to the Unit Offering
(as defined herein); and (ii) such indeterminate number of additional Common Shares that may be issuable by reason of the anti-dilution
provisions contained in the Warrant Agency Agreement (as defined herein). The Common Shares issuable upon exercise of Warrants,
including any Warrants issued pursuant to the Over-Allotment Option are referred to herein as the “Warrant Shares”.
See “Terms of Warrants” for additional information on the terms of the Warrants.
The exercise price of the Warrants was
determined by negotiation between us and the underwriter for the Unit Offering. See “Plan of Distribution” for
additional information on the Unit Offering.
Our Common Shares are listed on Nasdaq
under the symbol “ONCY” and on the Toronto Stock Exchange (“TSX”) under the symbol “ONC”.
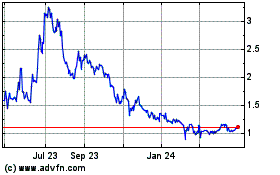
On June 23, 2020, the closing price of our Common Shares on Nasdaq was US$2.05 per Common Share and on the TSX was C$2.78 per Common
Share.
Messrs. Wayne Pisano and Leonard Kruimer
and Drs. William G. Rice and Bernd R. Seizinger are directors of the Corporation who reside outside of Canada. Messrs. Pisano and
Kruimer and Drs. Rice and Seizinger have appointed the Corporation, at its principal place of business, as agent for service of
process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person
that resides outside of Canada, even if the party has appointed an agent for service of process.
The financial information of the Corporation
incorporated by reference in the Prospectus is presented in Canadian dollars. Unless otherwise noted herein, all references to
“US$”, “United States dollars” or “US dollars” are to United States dollars and all references
to “C$” are to Canadian dollars. See “Currency and Exchange Rate Information”.
Our head office and principal place of
business is located at 210, 1167 Kensington Crescent N.W., Calgary, Alberta, T2N 1X7. Our registered office is located at 4000,
421 - 7th Avenue S.W., Calgary, Alberta, T2P 4K9.
Base
Shelf Prospectus dated June 12, 2020
IMPORTANT NOTICE
ABOUT INFORMATION IN THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first
part is this Prospectus Supplement, which describes the specific terms of the Warrant Shares and also supplements and updates information
regarding Oncolytics Biotech Inc. contained and incorporated by reference in the Prospectus. The second part is the accompanying
Prospectus, which gives more general information, some of which may not apply to the Warrant Shares. Both documents contain important
information you should consider when making your investment decision. If the description of the Warrant Shares and Warrants varies
between this Prospectus Supplement and the accompanying Prospectus, investors should rely on the information in this Prospectus
Supplement. This Prospectus Supplement is deemed to be incorporated by reference into the Prospectus solely for the purpose of
the offering of Warrant Shares issuable from time to time on the exercise of the Warrants. If information in this Prospectus Supplement
is inconsistent with the Prospectus or the information incorporated by reference in the Prospectus, you should rely on this Prospectus
Supplement. You should read both this Prospectus Supplement and the accompanying Prospectus, together with the additional information
about us to which we refer you in the section of this Prospectus Supplement entitled “Where You Can Find Additional Information.”
You should rely only on the information
contained in this Prospectus Supplement, the Prospectus and the documents incorporated by reference in the Prospectus. The Corporation
has not authorized anyone to provide you with different information. If anyone provides you with any different or inconsistent
information, you should not rely on it. The Corporation is offering the Warrant Shares only in jurisdictions where such offers
are permitted by law.
You should assume that the information
contained in this Prospectus Supplement, the Prospectus and the documents incorporated by reference in the Prospectus is accurate
only as of their respective dates, regardless of the time of delivery of this Prospectus Supplement and the accompanying Prospectus.
Our business, financial condition, results of operations and prospects may have changed since those dates.
Market data and certain industry forecasts
used in this Prospectus Supplement, the Prospectus and the documents incorporated by reference in the Prospectus were obtained
from market research, publicly available information and industry publications. We believe that these sources are generally reliable,
but the accuracy and completeness of this information is not guaranteed. We have not independently verified such information, and
we do not make any representation as to the accuracy of such information.
In this Prospectus Supplement, “Oncolytics,”
the “Corporation,” “we,” “us,” and “our” refer to Oncolytics
Biotech Inc. and its subsidiaries.
FORWARD-LOOKING STATEMENTS
This Prospectus Supplement, the Prospectus
and the documents incorporated by reference in the Prospectus contain certain statements relating to future events or the Corporation’s
future performance which constitute forward-looking statements. Such forward-looking statements involve known and unknown risks,
uncertainties and other factors which may cause the actual results, performance or achievements of the Corporation, or industry
results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking
statements. Forward-looking statements are statements that are not historical facts, and include, but are not limited to, estimates
and their underlying assumptions; statements regarding plans, objectives and expectations with respect to the efficacy of our technologies;
the timing and results of clinical studies related to our technologies; future operations, products and services; the impact of
regulatory initiatives on our operations; the size of and opportunities related to the markets for our technologies; general industry
and macroeconomic growth rates; expectations related to possible joint and/or strategic ventures and statements regarding future
performance. Forward-looking statements generally, but not always, are identified by the words “expects,” “anticipates,”
“believes,” “intends,” “estimates,” “projects”, “potential”, “possible”
and similar expressions, or that events or conditions “will,” “may,” “could” or “should”
occur.
The forward-looking statements in this
Prospectus Supplement, the Prospectus and the documents incorporated by reference in the Prospectus are subject to various risks
and uncertainties, most of which are difficult to predict and generally beyond the Corporation’s control, including without
limitation:
|
|
·
|
risks related to all of our products, including pelareorep, being in the research and development
stage and requiring further development and testing before they can be marketed commercially;
|
|
|
·
|
risks inherent in pharmaceutical research and development;
|
|
|
·
|
risks related to timing and possible delays in our clinical trials;
|
|
|
·
|
risks related to some of our clinical trials being conducted in, and subject to the laws of, foreign
countries;
|
|
|
·
|
risks related to our pharmaceutical products being subject to intense regulatory approval processes
in the United States and other foreign jurisdictions;
|
|
|
·
|
risks related to being subject to government manufacturing and testing regulations;
|
|
|
·
|
risks related to the extremely competitive biotechnology industry and our competition with larger
companies with greater resources;
|
|
|
·
|
risks related to our reliance on patents and proprietary rights to protect our technology;
|
|
|
·
|
risks related to potential product liability claims;
|
|
|
·
|
risks related to our limited manufacturing experience and reliance on third parties to commercially
manufacture our products, if and when developed;
|
|
|
·
|
risks related to our new products not being accepted by the medical community or consumers;
|
|
|
·
|
risks related to our technologies becoming obsolete;
|
|
|
·
|
risks related to our dependence on third party relationships for research and clinical trials;
|
|
|
·
|
risks related to our license, development, supply and distribution agreement with Adlai Nortye
Biopharma Co. Ltd.;
|
|
|
·
|
risks related to our lack of operating revenues and history of losses;
|
|
|
·
|
uncertainty regarding our ability to obtain third-party reimbursement for the costs of our product;
|
|
|
·
|
risks related to other third-party arrangements;
|
|
|
·
|
risks related to our ability to obtain additional financing to fund future research and development
of our products and to meet ongoing capital requirements;
|
|
|
·
|
risks related to potential increases in the cost of director and officer liability insurance;
|
|
|
·
|
risks related to our dependence on key employees and collaborators;
|
|
|
·
|
risks related to Barbados law, including those relating to the enforcement of judgments obtained
in Canada or the United States;
|
|
|
·
|
risks related to the effect of changes in the law on our corporate structure;
|
|
|
·
|
risks related to expenses in foreign currencies and our exposure to foreign currency exchange rate
fluctuations;
|
|
|
·
|
risks related to data privacy laws;
|
|
|
·
|
risks related to our information technology systems and security breaches;
|
|
|
·
|
risks related to our compliance with the Sarbanes-Oxley Act of 2002, as amended;
|
|
|
·
|
risks related to our status as a foreign private issuer;
|
|
|
·
|
risks related to possible “passive foreign investment company” status;
|
|
|
·
|
risks related to fluctuations in interest rates;
|
|
|
·
|
risks related to information technology systems;
|
|
|
·
|
risks related to business interruptions resulting from pandemics and public health emergencies,
including those related to COVID-19 coronavirus, geopolitical actions, including war and terrorism or natural disasters including
earthquakes, typhoons, floods and fires; and
|
|
|
·
|
risks related to our Common Shares.
|
This list is not exhaustive of the factors
that may affect any of the Corporation’s forward-looking statements. Some of the important risks and uncertainties that could
affect forward-looking statements are described further under the heading “Risk Factors” in this Prospectus
Supplement, in the Prospectus and in the Corporation’s Annual Report (as defined below). If one or more of these risks or
uncertainties materializes, or if underlying assumptions prove incorrect, our actual results may vary materially from those expected,
estimated or projected. Forward-looking statements in this document are not a prediction of future events or circumstances, and
those future events or circumstances may not occur. Given these uncertainties, users of the information included herein, including
investors and prospective investors, are cautioned not to place undue reliance on such forward-looking statements. Investors should
consult our quarterly and annual filings with the securities commissions or similar regulatory authorities in Canada and the SEC
for additional information on risks and uncertainties relating to forward-looking statements.
The Corporation cautions that the foregoing
list of factors that may affect future results is not exhaustive. The forward-looking information contained in this Prospectus
Supplement, the Prospectus and the documents incorporated by reference in the Prospectus is made as of the date of such documents.
The forward-looking information contained in this Prospectus Supplement, the Prospectus and in the documents incorporated by reference
in the Prospectus is expressly qualified by this cautionary statement. The Corporation does not undertake any obligation to publicly
update or revise any forward-looking information except as required pursuant to applicable securities laws.
DOCUMENTS INCORPORATED
BY REFERENCE
This Prospectus Supplement is deemed
to be incorporated by reference into the Prospectus solely for the purposes of the offering of Warrant Shares issuable from time
to time on the exercise of the Warrants.
Information has been incorporated by
reference in the Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the
documents incorporated herein by reference may be obtained on request without charge from our Corporate Secretary at 210, 1167
Kensington Crescent N.W., Calgary, Alberta, T2N 1X7 telephone (403) 670-7377, and are available electronically under the Corporation’s
profile on SEDAR (www.sedar.com) and on EDGAR (www.sec.gov/edgar.shtml).
The following documents, filed with the
securities commissions or similar regulatory authorities in each of the provinces of Canada and filed with, or furnished to, the
SEC are specifically incorporated by reference into, and form an integral part of, the Prospectus:
|
|
·
|
our annual report on Form 20-F (“Annual Report”) dated March 6, 2020, for the
year ended December 31, 2019 (filed in Canada with certain Canadian securities regulatory authorities as our annual information
form for the year ended December 31, 2019);
|
|
|
·
|
our audited consolidated financial statements, together with the notes thereto, as at December
31, 2019 and 2018, which comprise the consolidated statements of financial position as at December 31, 2019 and 2018, and the consolidated
statements of loss and comprehensive loss, changes in equity, and cash flows for the years ended December 31, 2019, 2018 and 2017,
together with the independent auditors’ report thereon; and
|
|
|
·
|
our unaudited interim consolidated financial statements, together with the notes thereto, as at
March 31, 2020, which comprise the interim consolidated statements of financial position as at March 31, 2020 and December 31,
2019, and the interim consolidated statements of income (loss) and comprehensive income (loss), changes in equity, and cash flows
for the three months ended March 31, 2020 and 2019; and
|
Any documents of the type required by National
Instrument 44-101 - Short Form Prospectus Distributions to be incorporated by reference in a short form Prospectus, including
any annual information form, annual report on Form 20-F, comparative annual consolidated financial statements and the auditors’
report thereon, comparative interim consolidated financial statements, management’s discussion and analysis of financial
condition and results of operations, material change report (except a confidential material change report), business acquisition
report and information circular, if filed by us with the securities commissions or similar authorities in Canada after the date
of this Prospectus Supplement and prior to the date on which the offering of Warrants Shares issuable from time to time on the
exercise of the Warrants under this Prospectus Supplement ends, shall be deemed to be incorporated by reference in the Prospectus.
In addition, to the extent that any document
or information incorporated by reference in the Prospectus is included in any report filed with or furnished to the SEC pursuant
to the United States Securities Exchange Act of 1934, as amended (the “U.S. Exchange Act”), after the date of
this Prospectus Supplement and prior to the date on which the offering of Warrants Shares issuable from time to time on the exercise
of the Warrants under this Prospectus Supplement ends, such document or information shall be deemed to be incorporated by reference
as an exhibit to the registration statement of which this Prospectus Supplement and the Prospectus forms a part (in the case of
documents or information deemed furnished on Form 6-K or Form 8-K, only to the extent specifically stated therein).
Any statement contained in this Prospectus
Supplement, the Prospectus or in a document incorporated or deemed to be incorporated by reference in the Prospectus shall be deemed
to be modified or superseded for the purposes of this Prospectus Supplement and the Prospectus to the extent that a statement contained
herein or in any other subsequently filed document which also is, or is deemed to be, incorporated by reference in the Prospectus
or therein modifies or supersedes such statement. The modifying or superseding statement need not state that it has modified or
superseded a prior statement or include any other information set forth in the document that it modifies or supersedes. The making
of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement,
when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that
was required to be stated or that was necessary to make a statement not misleading in light of the circumstances in which it was
made. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of
this Prospectus Supplement or the Prospectus.
DOCUMENTS FILED AS
PART OF THE REGISTRATION STATEMENT
The following documents have been or will
be filed with the SEC as part of the registration statement of which this Prospectus Supplement and the Prospectus forms a part:
(i) the documents set out under the heading “Documents Incorporated by Reference” in this Prospectus Supplement
and the Prospectus; (ii) the consents of the Corporation’s auditor; (iii) the powers of attorney from the directors and certain
officers of the Corporation and (iv) the Warrant Agency Agreement described in this Prospectus Supplement.
CURRENCY AND EXCHANGE
RATE INFORMATION
In this Prospectus Supplement and the accompanying
Prospectus, unless otherwise indicated, all dollar amounts and references to “$” and “US$” are to U.S.
dollars and references to “C$” are to Canadian dollars. This Prospectus Supplement and the accompanying Prospectus
and the documents incorporated by reference in the Prospectus contain translations of some Canadian dollar amounts into U.S. dollars
solely for your convenience.
The following table sets forth, for the periods indicated, the
high, low, average and period-end rates of exchange for US$1.00, expressed in Canadian dollars, posted by the Bank of Canada:
|
|
|
Year
Ended December 31(1)
|
|
|
|
|
2019
|
|
|
2018
|
|
|
2017
|
|
|
Highest rate during the period
|
|
C$
|
1.3600
|
|
|
C$
|
1.3642
|
|
|
C$
|
1.3743
|
|
|
Lowest rate during the period
|
|
C$
|
1.2988
|
|
|
C$
|
1.2288
|
|
|
C$
|
1.2128
|
|
|
Average rate for the period
|
|
C$
|
1.3269
|
|
|
C$
|
1.2957
|
|
|
C$
|
1.2986
|
|
|
Rate at the end of the period
|
|
C$
|
1.2988
|
|
|
C$
|
1.3642
|
|
|
C$
|
1.2545
|
|
Note:
|
|
(1)
|
Data from the Bank of Canada reflects the daily average
rates.
|
On June 23, 2020, the daily average exchange
rate posted by the Bank of Canada for conversion of U.S. dollars into Canadian dollars was US$1.00 = C$1.3516. Unless otherwise
indicated, currency translation in this Prospectus Supplement reflect the June 23, 2020 rate.
RISK FACTORS
Prospective purchasers of Warrant Shares
should consider carefully the risk factors set out in this Prospectus Supplement, the Prospectus and the documents incorporated
by reference in the Prospectus. Discussions of certain risks affecting Oncolytics in connection with its business are set forth
under “Risk Factors” in the Prospectus and in our annual disclosure documents filed with the various securities
regulatory authorities which are incorporated by reference in the Prospectus.
Volatility of market price of the Common
Shares
The market price of the Common Shares may
be volatile. The volatility may affect the ability of holders of Common Shares to sell the Common Shares at an advantageous price.
Market price fluctuations in the Common Shares may be due to the Corporation’s operating results failing to meet the expectations
of securities analysts or investors in any quarter, downward revision in securities analysts’ estimates, governmental regulatory
action, adverse change in general market conditions or economic trends, acquisitions, dispositions or other material public announcements
by the Corporation or its competitors, along with a variety of additional factors, including, without limitation, those set forth
under “Forward-Looking Statements” in this Prospectus Supplement. In addition, the market price for securities
in the stock markets, including the NASDAQ and the TSX, recently experienced significant price and trading fluctuations. These
fluctuations have resulted in volatility in the market prices of securities that often has been unrelated or disproportionate to
changes in operating performance. These broad market fluctuations may adversely affect the market price of the Common Shares.
The Corporation will have broad discretion
over the use of the net proceeds from the offering of the Warrant Shares issuable on the exercise of Warrants and the Corporation
may not use these proceeds in a manner desired by the Corporation’s shareholders
Management will have broad discretion with
respect to the use of the proceeds from the exercise of the Warrants and investors will be relying on the judgment of management
regarding the application of these proceeds. Management could spend most of the proceeds from the exercise of the Warrants in ways
that the Corporation’s shareholders may not desire or that do not yield a favorable return. You will not have the opportunity,
as part of your investment in the Common Shares, to influence the manner in which the proceeds from the exercise of the Warrants
are used. At the date of this Prospectus Supplement, the Corporation intend to use the proceeds from the exercise of the Warrants
as described under the heading “Use of Proceeds”. However, the Corporation’s needs may change as the business
and the industry the Corporation addresses evolve. As a result, the proceeds to be received from the exercise of the Warrants may
be used in a manner significantly different from the Corporation’s current expectations.
The Corporation does not currently intend
to pay any cash dividends on the Common Shares; therefore, the Corporation’s shareholders may not be able to receive a return
on their Common Shares until they sell them
The Corporation has not declared or paid
any dividends since its incorporation. The Corporation intends to retain earnings, if any, to finance the growth and development
of its business and does not intend to pay cash dividends on the Common Shares in the foreseeable future. Any return on an investment
in the Warrant Shares will come from the appreciation, if any, in the value of the Common Shares. The payment of future cash dividends,
if any, will be reviewed periodically by our board of directors and will depend upon, among other things, conditions then existing
including earnings, financial condition and capital requirements, restrictions in financing agreements, business opportunities
and conditions and other factors.
Positive return on an investment in
the Warrant Shares is not guaranteed
There is no guarantee that an investment
in the Warrant Shares will earn any positive return in the short term or long term. A purchase of Warrant Shares upon the exercise
of Warrants involves a high degree of risk and should be undertaken only by purchasers whose financial resources are sufficient
to enable them to assume such risks and who have no need for immediate liquidity in their investment. An investment in the Warrant
Shares is appropriate only for purchasers who have the capacity to absorb a loss of some or all of their investment.
You may be unable to enforce actions
against us, certain of our directors and officers, or the experts named in this Prospectus Supplement under U.S. federal securities
laws.
We are a company continued under the laws
of the Province of Alberta, Canada. Most of our directors and officers as well as the certain of the experts named in this Prospectus
Supplement and the accompanying Prospectus, reside principally in Canada. Because all or a substantial portion of our assets and
the assets of these persons are located outside of the United States, it may not be possible for you to effect service of process
within the United States upon us or those persons. Furthermore, it may not be possible for you to enforce against us or those persons
in the United States, judgments obtained in U.S. courts based upon the civil liability provisions of the U.S. federal securities
laws or other laws of the United States. There is doubt as to the enforceability, in original actions in Canadian courts, of liabilities
based upon U.S. federal securities laws and as to the enforceability in Canadian courts of judgments of U.S. courts obtained in
actions based upon the civil liability provisions of the U.S. federal securities laws. Therefore, it may not be possible to enforce
those actions against us, certain of our directors and officers or certain of the experts named in this Prospectus Supplement.
The Corporation is likely a “passive
foreign investment company” which may have adverse U.S. federal income tax consequences for U.S. shareholders
U.S. holders of Common Shares should be
aware that the Corporation believes it was classified as a passive foreign investment company (“PFIC”) during
the tax year ended December 31, 2019, and based on current business plans and financial expectations, the Corporation expects that
it will be a PFIC for the current tax year and may be a PFIC in future tax years. If the Corporation is a PFIC for any year during
a U.S. shareholder’s holding period of the Common Shares, then such U.S. shareholder generally will be required to treat
any gain realized upon a disposition of Common Shares, or any “excess distribution” received on its Common Shares,
as ordinary income, and to pay an interest charge on a portion of such gain or distribution, unless the shareholder makes a timely
and effective “qualified electing fund” election (“QEF Election”) or a “mark-to-market”
election with respect to the Common Shares. A U.S. shareholder who makes a QEF Election generally must report on a current basis
its share of the Corporation’s net capital gain and ordinary earnings for any year in which the Corporation is a PFIC, whether
or not the Corporation distributes any amounts to its shareholders. A U.S. shareholder who makes a mark-to-market election generally
must include as ordinary income each year the excess of the fair market value of the Common Shares over the taxpayer’s adjusted
tax basis therein. This paragraph is qualified in its entirety by the discussion below under the heading “Material United
States Federal Income Tax Considerations.” Each U.S. shareholder should consult its own tax advisors regarding the PFIC
rules and the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares.
THE CORPORATION
Oncolytics Biotech Inc. was incorporated
pursuant to the ABCA on April 2, 1998 as 779738 Alberta Ltd. On April 8, 1998, we amended our articles of incorporation
(the “Articles”) and changed our name to Oncolytics Biotech Inc. On July 29, 1999, we amended our Articles
by removing the private company restrictions included therein and subdivided the 2,222,222 Common Shares issued and outstanding
into 6,750,000 Common Shares. On February 9, 2007, we amended our Articles to permit shareholder meetings to be held at any
place in Alberta or at any other location as determined by our board of directors (the “Board”). On May 22,
2018, we amended our Articles of Incorporation to effect a consolidation (the “Consolidation”) of the Common
Shares on the basis of 9.5 pre-Consolidation Common Shares for each one post-Consolidation Common Share.
We have two material operating subsidiaries:
Oncolytics Biotech (Barbados) Inc. and Oncolytics Biotech (US) Inc., a Delaware corporation. Oncolytics Biotech (Barbados) Inc.
is incorporated pursuant to the laws of Barbados and is a wholly-owned direct subsidiary of the Corporation. Oncolytics Biotech
(U.S.) Inc. is incorporated pursuant to the laws of Delaware and is a wholly-owned direct subsidiary of Oncolytics Biotech (Barbados)
Inc.
Our head office and principal place of
business is located at 210, 1167 Kensington Crescent N.W., Calgary, Alberta, T2N 1X7. Our registered office is located at
4000, 421 - 7th Avenue S.W., Calgary, Alberta, T2P 4K9.
RECENT DEVELOPMENTS
On June 23, 2020, we announced the first
patient has been dosed in the Corporation's phase 2 study of pelareorep-based combination therapies in HR+/HER2- metastatic breast
cancer (“mBC”). The study, known as BRACELET-1, is being conducted under a co-development agreement with Merck
KGaA, Darmstadt, Germany, which operates its biopharmaceutical business in the US and Canada as EMD Serono, and Pfizer Inc. (NYSE:
PFE). Participants in the study receive paclitaxel, pelareorep in combination with paclitaxel alone, or pelareorep in combination
with paclitaxel and Merck KGaA, Darmstadt, Germany and Pfizer's anti-PD-L1 checkpoint inhibitor, avelumab (Bavencio®). The
randomized BRACELET-1 study is designed to support the results of a prior successful phase 2 trial (IND-213) that showed a near
doubling of overall survival with pelareorep treatment, by demonstrating pelareorep's ability to induce a robust anti-tumor immune
response in an identical patient population (patients with HR+/HER2- mBC). The ability of pelareorep-induced immune responses to
enhance anti- PD-L1 therapy will also be evaluated through the inclusion of the paclitaxel-pelareorep-avelumab combination therapy
cohort. Importantly, the trial also aims to validate peripheral T cell clonality as a biomarker of pelareorep response in HR+/HER2-
mBC, which may aid in future registrational trial study design and patient selection.
BUSINESS OF THE CORPORATION
General
Since our inception in April of 1998, Oncolytics
Biotech Inc. has been a development stage company and we have focused our research and development efforts related to pelareorep,
a systemically administered immuno-oncology (“I-O”) viral agent with the potential to treat a variety of cancers.
We have not been profitable since our inception and expect to continue to incur substantial losses as we continue research and
development efforts. We do not expect to generate significant revenues until, if and when, pelareorep becomes commercially viable.
Our potential product for human use, pelareorep,
an unmodified reovirus, is a first in class systemically administered I-O viral agent for the treatment of solid tumors and hematological
malignancies.
Scientific Background
Pelareorep’s anti-tumor activity
is based on three modes of action which are complementary but not interdependent (see Figure 1, below):
|
|
·
|
Selective viral replication in permissive cancer cells which leads to tumor cell lysis.
|
|
|
·
|
Activation of innate immunity in response to the infection which results in a cascade of chemokines/cytokines
causing natural killer (“NK”) cells to be activated and attack cancer cells.
|
|
|
·
|
A specific adaptive immune response triggered by tumor- and viral-associated antigens displayed
by antigen-presenting cells (“APCs”), infected tumor cells and/or dendritic cells to T cells.
|
Summary of Research and Development
highlights
Preclinical and translational research
to date indicates the following:
|
|
·
|
Pelareorep has anticancer effects in models of metastatic cancers that can prolong survival in
these models when using immuno-competent rodents.
|
|
|
·
|
The survival benefit in animal models can be enhanced when pelareorep is given in combination with
chemotherapy, immunotherapy or radiotherapy.
|
|
|
·
|
A toxic dose of pelareorep has not been reached/established in animal models and infection presents
with minimal side-effects.
|
Clinical data to date indicate the following:
|
|
·
|
More than 1,400 patients have been enrolled in clinical studies conducted in the US, Canada and
the European Union. Of these, more than 1,000 patients received pelareorep, with over 930 via intravenous (“IV”)
administration.
|
|
|
·
|
Pelareorep has been administered as single or multiple doses (intratumoral or intravenous), either
as a mono-therapy or in combination with chemotherapy, immunotherapy (e.g., checkpoint inhibitors), and radiotherapy.
|
|
|
·
|
Pelareorep is generally well-tolerated and has a manageable side effect profile for most patients.
|
|
|
·
|
When combined with chemotherapeutic agents, pelareorep does not appear to enhance either the frequency
or severity of the adverse effects of the chemotherapeutic agents.
|
|
|
·
|
There is emerging evidence that pelareorep may impact overall survival (“OS”)
in mBC and metastatic adenocarcinoma of the pancreas (“MAP”):
|
|
|
·
|
In a randomized, controlled Phase 2 study of paclitaxel with pelareorep versus paclitaxel alone
in mBC (Canadian Cancer Trials Group IND.213) the median OS was greater for subjects treated with paclitaxel and pelareorep (median
17.4 months) than subjects treated with paclitaxel alone (10.4 months, hazard ratio (“HR”) 0.65).
|
|
|
·
|
In a single-arm study with gemcitabine plus pelareorep in first-line MAP (REO 017) the median OS
was 10 months with a 1 year and 2-year survival of 46% and 24%, respectively.
|
|
|
·
|
In a two-arm Phase 2 randomized study (NCI 8601), patients with MAP were randomized to receive
either carboplatin, paclitaxel and pelareorep (test arm) or carboplatin and paclitaxel alone (control arm). The median OS was similar
for both arms, but the probability of survival at Year 2 was 20% in the test arm vs 9% in the control arm.
|
Mechanism of Action
Figure 1. Proposed mechanism of action for pelareorep.

Direct cell lysis - Reovirus Replication in Permissive
Cancer Cells
Selective viral replication and lysis in
cancer cells and not normal cells is mediated by the host cellular protein dsRNA-activated protein kinase (“PKR”).
In non-cancer cells that are infected with reovirus, PKR activates in the presence of the virus which in turn inhibits viral gene
translation. However, in permissive cancer cells, PKR activation is inhibited, allowing for viral gene translation and eventual
cell lysis.
It was originally established that selective
lysis with reovirus was mediated by tumor cells with an activated rat sarcoma virus oncogene (“RAS”) pathway,
since active RAS inhibits PKR activation. However, more recent investigations have revealed that reovirus replication is not just
restricted to cells with an active RAS pathway, oncogenic mutations and amplifications in upstream and downstream mediators of
the RAS-pathway also allow for viral replication and oncolysis. Moreover, active RAS is known to stimulate over 18 downstream effector
proteins, many of which have been shown to facilitate viral replication. Cells bearing dysfunctional or deleted tumor suppressor
genes and or chemo- or radiation-induced cell stress also show increased sensitivity to reovirus replication and lysis.
Induction of Innate Immunity
Preclinical and clinical studies provide
compelling evidence that pelareorep functions as an immunogenic agent. Indeed, preclinical studies demonstrated that cancer cells
infected with pelareorep can produce an innate immune response triggering the release of inflammatory cytokines. This inflammatory
environment promotes a chemotactic response in NK cells, dendritic cells, and cytotoxic T-cells, altering the tumor microenvironment
to support bystander immune-mediated cancer cell death. Intriguingly, preclinical studies have also demonstrated that the beneficial
immunogenic functions of pelareorep can occur independently of viral replication. Pelareorep performs this immunogenic function,
in part, by activating dendritic cells, key regulators of both adaptive and innate immunity. Dendritic cells activated by reovirus,
in turn, stimulate the innate antitumor activity of natural killer (“NK”) cells and aid in the priming of specific
antitumor cytotoxic lymphocyte, demonstrating that dendritic cell recognition of reovirus may trigger a beneficial innate immune
response.
A clinical trial with pelareorep (REO 013)
provided an opportunity to study human NK cell activation in a controlled manner. Ten colorectal cancer patients with liver metastases
received between one and five doses of pelareorep prior to surgical resection of their tumor. NK cell activation peaked 24-48 hours
post-infection, coincident with a peak of pro-inflammatory cytokines. NK cells within reovirus-treated blood mononuclear cells
were stimulated to kill tumor targets, but not normal hepatocytes. Moreover, peripheral blood mononuclear cells were able to hand-off
virus to tumors for direct oncolytic killing. Similarly, NK cells within liver mononuclear cells became selectively cytotoxic towards
tumor cells when activated by reovirus. These results showed that reovirus modulates human NK cell activity in vivo and suggest
that this may contribute to the therapeutic effect of pelareorep.
Induction of Adaptive Immunity
Adaptive anti-tumor immunity allows for
elimination of existing cancer cells and performs constant surveillance, preventing relapse, and increases OS. An adaptive immune
response requires two signals: a signal from an APC, as well as a co-stimulation signal in the form of cytokines. In the absence
of both signals, the adaptive immune response fails. Therapy with pelareorep has the potential to activate both signals. Following
its therapeutic administration, pelareorep enhances the expression of ‘foreign’ antigens/markers on tumor cells. Oncolysis
of tumor cells exposes tumor-associated antigens (“TAAs”) and viral-associated antigens (“VAAs”)
for processing and presentation by APCs, such as dendritic cells. Through the combined actions of these immunological events, pelareorep
facilitates the display of novel ‘foreign’ antigens on the surface of infected tumor cells and APCs. Simultaneously,
pelareorep induces an inflammatory response promoting the expression of co-stimulatory molecules and inflammatory cytokines. Together,
pelareorep mediated immunological events over-rule tumor antigen presentation impairments and initiate adaptive anti-tumor immunity.
By promoting the expression of novel antigens
and the release of inflammatory cytokines, pelareorep, promotes an inflamed tumor phenotype. An inflamed tumor phenotype is characterized
by NK and T-cell infiltration, increased expression of chemokines/ cytokines, and increased expression of checkpoint ligands. This
phenotype correlates with an increase in OS and has a positive prognostic value for early stage cancers. In patients with metastatic
cancer, an inflamed tumor phenotype is associated with better clinical outcomes when treated with immunotherapies, including immune
checkpoint blockade inhibitors, cancer vaccines, and adoptive T-cell therapies. By promoting an inflamed tumor phenotype, pelareorep
primes an anti-cancer immune response (see Figure 2, below).
Figure 2. Pelareorep primes an anti-cancer immune response

Clinical Development Plan
The ultimate objective of our clinical
development plan is to obtain regulatory approval for pelareorep as quickly as possible and is based on the compelling efficacy
data from previous studies in breast, multiple myeloma, and selected gastrointestinal cancers. Our clinical development program
centers on key immunotherapy combinations. Specifically, immunotherapy combinations in which pelareorep has the potential to provoke
a specific innate and adaptive immune responses when combined with checkpoint blockade therapy, chemotherapy and/or targeted therapies.
In 2017, we reported clinical results from
our randomized clinical program which includes clinical study collaborations with the Canadian Cancer Trials Group (formerly known
as the National Cancer Institute of Canada). Specifically, subgroup analysis in the IND 213 trial in mBC revealed a significant
improvement in the OS of patients that are hormone receptor-positive (HR+) / human epidermal growth factor receptor 2 negative
(HER2-). In HR+/HER2- patients, pelareorep therapy, in combination with paclitaxel, more than doubled the OS from 10.8 month with
paclitaxel therapy alone, to 21.8 months with pelareorep plus paclitaxel. This increase in OS is consistent with previous survival
data reported from our U.S. NCI pancreatic trial which suggests a long-term survival benefit when comparing test and control arms
at 24 months, as well as test to historical data.
In 2019, we published our first data set
from a clinical study that combined pelareorep with chemotherapy and pembrolizumab (Keytruda®) in patients with pancreatic
cancer. This study, published in the journal of Clinical Cancer Research, demonstrated the safety and tolerability of this combination
treatment regime and showed encouraging clinical efficacy with one patient achieving a partial response (six-month duration) and
two patients with stable disease (lasting 126 and 221 days). In early 2019 we announced the regulatory approval of the AWARE-1
study which examines the use of pelareorep and atezolizumab (Tecentriq®) in a breast cancer window-of -opportunity study. Preliminary
results from AWARE-1 were presented at the Society for Immunotherapy of Cancer (SITC) meeting in November 2019 and demonstrated
pelareorep mediated priming of the tumor microenvironment for checkpoint blockade therapy. In the second half of 2019, we initiated
start-up activities for the BRACELET-1 (BReast cAnCEr with the Oncolytic Reovirus PeLareorEp in CombinaTion with anti-PD-L1 and
Paclitaxel) study which will essentially repeat IND 213 in HR+/HER2- patients, with the addition of a third arm that includes pelareorep,
paclitaxel and avelumab (Bavencio®) in mBC. This study is expected to begin enrollment in the first quarter of 2020. Additional
studies in gastrointestinal indications are now being evaluated.
Business Strategy
Our business strategy is to develop and
market pelareorep in an effective and timely manner, and access additional technologies at a time and in a manner that we believe
is best for our development. We intend to achieve our business strategy by focusing on these key areas:
|
|
·
|
Develop pelareorep through our clinical development plan assessing the safety and efficacy in human
subjects;
|
|
|
·
|
Establish collaborations with experts to assist us with scientific and clinical developments of
this new potential pharmaceutical product;
|
|
|
·
|
Implement strategic alliances with select biopharmaceutical companies and laboratories, at a time
and in a manner whereby such alliances may complement and expand our own research and development efforts. Such alliances may also
result in an eventual expansion to include providing additive sales and marketing capabilities;
|
|
|
·
|
Utilize our broadening patent base and collaborator network as a mechanism to meet our strategic
objectives; and
|
|
|
·
|
Develop relationships with companies that could be instrumental in assisting us to access other
innovative therapeutics.
|
Our business strategy is based on attaining
a number of commercial objectives, which, in turn, are supported by a number of product development goals. In this Prospectus Supplement,
statements of our “belief” are based primarily upon our results derived to date from our research and development program
with animals, early stage human trials and our most recent data in HR+/HER2- mBC patients, upon which we believe that we have a
reasonable scientific basis to expect the particular results to occur. It is not possible to predict, based upon studies in animals,
or early stage human trials, whether a new therapeutic will ultimately prove to be safe and effective in humans. There are no assurances
that the particular result expected by us will occur.
As of the date hereof, we do not intend
to become a fully integrated pharmaceutical company with substantial in-house research and development, marketing and distribution
or manufacturing capabilities. We are pursuing a strategy of establishing relationships with larger companies as strategic partners.
It is anticipated that future clinical development into large international or pivotal trials would generally occur in conjunction
with a strategic partner or partners, who would contribute expertise and financial assistance. In exchange for certain product
rights and commitments to market our products, the strategic partners would be expected to share in proceeds from the sale of our
product or products.
USE OF PROCEEDS
From time to time, when the Warrants are
exercised, we may receive proceeds equal to the aggregate exercise price of such Warrants. Assuming that all of the Warrants are
exercised prior to their expiry time and that no adjustment based on the anti-dilution provisions contained in the Warrant certificate
has taken place, the gross proceeds to us from the exercise of all of the Warrants will be approximately US$239,181.
The net proceeds from the exercise of the
Warrants are currently intended to be used to advance our clinical development program, our manufacturing activities in support
of the program and general corporate and administrative purposes.
The amounts actually expended for the purposes
described above may vary significantly depending upon a number of factors, including those listed under the heading “Risk
Factors” in this Prospectus Supplement.
COMMON SHARES
We are authorized to issue an unlimited
number of Common Shares. Each Common Share entitles the holder to one vote per share held at meetings of shareholders, to receive
such dividends as declared by us and to receive our remaining property and assets upon dissolution or winding up. Our Common Shares
are not subject to any future call or assessment and there are no pre-emptive, conversion or redemption rights attached to such
shares. See “Consolidated Capitalization” in this Prospectus Supplement for additional information on our outstanding
Common Shares.
TERMS OF WARRANTS
The Warrants issued under the Unit Offering
are governed by a Warrant Agency Agreement (the “Warrant Agency Agreement”) entered into between the Corporation
and American Stock Transfer & Trust Company, LLC, as agent for the holders of the Warrants (the “Warrant Agent”).
The following description is subject to the detailed provisions of the Warrant Agency Agreement. Reference should be made to the
Warrant Agency Agreement for the full text of attributes of the Warrants, which is available under our profile on SEDAR at www.sedar.com.
Exercisability. The
Warrants are exercisable immediately upon issuance and at any time up to the date that is five years from the date of issuance.
The Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice
accompanied by payment in full for the number of Warrant Shares purchased upon such exercise (except in the case of a cashless
exercise as discussed below). Unless otherwise specified in the Warrant, the holder will not have the right to exercise any portion
of the Warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% (or, upon election by a holder
prior to the issuance of any Warrants, 9.99%) of the number of Common Shares outstanding immediately after giving effect to the
exercise, as such percentage ownership is determined in accordance with the terms of the Warrants.
Cashless Exercise.
In the event that a registration statement covering the Warrant Shares underlying the Warrants, or an exemption from registration,
is not available for the resale of such Warrant Shares underlying the Warrants, the holder may, in its sole discretion, exercise
the Warrant in whole or in part and, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise
in payment of the aggregate exercise price, elect instead to receive upon such exercise the net number of Warrant Shares determined
according to the formula set forth in the Warrant. In no event shall we be required to make any cash payments or net cash settlement
to the registered holder in lieu of issuance of Warrant Shares underlying the Warrants.
Exercise Price. The
initial exercise price per share of Warrants Shares purchasable upon exercise of the Warrants is US$0.90. The exercise price is
subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations,
reclassifications or similar events affecting our Common Shares and also upon any distributions of assets, including cash, shares
or other property to our shareholders.
Certain Adjustments.
The exercise price and the number of Warrant Shares purchasable upon the exercise of the Warrants are subject to adjustment upon
the occurrence of specific events, including stock dividends, stock splits, combinations and reclassifications of our Common Shares.
Transferability. Subject
to applicable laws, the Warrants may be transferred at the option of the holders upon surrender of the Warrants to us together
with the appropriate instruments of transfer.
Warrant Agent and Exchange Listing.
The Warrants were issued in registered form under the Warrant Agency Agreement between the Warrant Agent, as warrant agent, and
the Corporation. There is no market through which the Warrants may be sold and purchasers may not be able to resell the Warrants.
This may affect the pricing of the Warrants in the secondary market, the transparency and availability of trading prices and the
liquidity of the Warrants. See “Risk Factors”.
Fundamental Transaction.
If, at any time while the Warrants are outstanding, (1) we consolidate or merge with or into another corporation and we are not
the surviving corporation, (2) we sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all
of our assets, (3) any purchase offer, tender offer or exchange offer (whether by us or another individual or entity) is completed
pursuant to which holders of our Common Shares are permitted to sell, tender or exchange their Common Shares for other securities,
cash or property and has been accepted by the holders of 50% or more of our outstanding Common Shares, (4) we effect any reclassification
or recapitalization of our Common Shares or any compulsory share exchange pursuant to which our Common Shares are converted into
or exchanged for other securities, cash or property, or (5) we consummate a stock or share purchase agreement or other business
combination with another person or entity whereby such other person or entity acquires more than 50% of our outstanding Common
Shares, each, a “Fundamental Transaction,” then upon any subsequent exercise of the Warrants, the holders thereof
will have the right to receive the same amount and kind of securities, cash or property as it would have been entitled to receive
upon the occurrence of such Fundamental Transaction if it had been, immediately prior to such Fundamental Transaction, the holder
of the number of Warrant Shares then issuable upon exercise of the Warrant, and any additional consideration payable as part of
the Fundamental Transaction. Notwithstanding the foregoing, in the event of a fundamental transaction (other than certain fundamental
transactions where the Company remains the surviving company) as described above, the holder may, subject to certain conditions,
require the Company or a successor entity to purchase the warrant from the holder by paying to the holder an amount in cash equal
to the Black-Scholes value of the remaining unexercised portion of the warrant on the effective date of such change of control;
provided, however, that, if the change of control is not within the Company’s control, including not approved by the Company’s
board of directors, the holder will only be entitled to receive from the Company or any successor entity, as of the date of consummation
of such change of control, the same type or form of consideration (and in the same proportion), at the Black-Scholes value of the
unexercised portion of the warrant, that is being offered and paid to the holders of our common stock in connection with the change
of control, whether that consideration is in the form of cash, stock or any combination thereof, or whether the holders of common
stock are given the choice to receive from among alternative forms of consideration in connection with the change of control.
Rights as a Shareholder.
Except as otherwise provided in the Warrants or by virtue of such holder’s ownership of Common Shares, the holder of a Warrant
does not have the rights or privileges of a holder of our Common Shares, including any voting rights, until the holder exercises
the Warrant.
No fractional Warrant Shares will be
issuable upon the exercise of any Warrants, and no cash or other consideration will be paid in lieu of fractional shares.
CONSOLIDATED CAPITALIZATION
Except for the issuance of Common Shares
of the Corporation as set forth under the heading “Prior Sales” in this Prospectus Supplement, there has not
been any material change in the share and loan capital of the Corporation, on a consolidated basis, since the Corporation’s
most recently filed financial statements for the three months ended March 31, 2020.
TRADING PRICE AND
VOLUME
The Common Shares are listed and posted
for trading on the TSX under the trading symbol “ONC” and on the NASDAQ under the trading symbol “ONCY”.
On June 23, 2020, the closing bid price of our Common Shares on the NASDAQ was US$2.05 and the closing price of our Common Shares
on the TSX was C$2.78.
The following table sets forth the market
price ranges and the aggregate volume of trading of the Common Shares on the TSX and NASDAQ for the periods indicated:
|
|
|
TSX
|
|
|
NASDAQ
|
|
|
|
|
High
|
|
|
Low
|
|
|
Close
|
|
|
Volume
|
|
|
High
|
|
|
Low
|
|
|
Close
|
|
|
Volume
|
|
|
Period
|
|
(C$)
|
|
|
(C$)
|
|
|
(C$)
|
|
|
(Shares)
|
|
|
(US$)
|
|
|
(US$)
|
|
|
(US$)
|
|
|
(Shares)
|
|
|
2019
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June
|
|
|
2.61
|
|
|
|
2.18
|
|
|
|
2.26
|
|
|
|
546,095
|
|
|
|
1.94
|
|
|
|
1.61
|
|
|
|
1.70
|
|
|
|
1,205,291
|
|
|
July
|
|
|
2.26
|
|
|
|
1.75
|
|
|
|
1.79
|
|
|
|
348,974
|
|
|
|
1.78
|
|
|
|
1.31
|
|
|
|
1.34
|
|
|
|
644,978
|
|
|
August
|
|
|
1.75
|
|
|
|
0.74
|
|
|
|
0.81
|
|
|
|
2,218,683
|
|
|
|
1.33
|
|
|
|
0.53
|
|
|
|
0.60
|
|
|
|
8,536,025
|
|
|
September
|
|
|
0.99
|
|
|
|
0.74
|
|
|
|
0.76
|
|
|
|
639,265
|
|
|
|
0.75
|
|
|
|
0.56
|
|
|
|
0.57
|
|
|
|
3,363,096
|
|
|
October
|
|
|
1.75
|
|
|
|
0.48
|
|
|
|
1.61
|
|
|
|
3,323,534
|
|
|
|
1.33
|
|
|
|
0.35
|
|
|
|
1.25
|
|
|
|
13,774,816
|
|
|
November
|
|
|
1.92
|
|
|
|
1.10
|
|
|
|
1.46
|
|
|
|
3,241,718
|
|
|
|
1.47
|
|
|
|
0.82
|
|
|
|
1.12
|
|
|
|
14,214,362
|
|
|
December
|
|
|
7.84
|
|
|
|
1.40
|
|
|
|
6.15
|
|
|
|
10,754,714
|
|
|
|
6.02
|
|
|
|
1.05
|
|
|
|
4.76
|
|
|
|
50,413,513
|
|
|
2020
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
January
|
|
|
6.26
|
|
|
|
3.16
|
|
|
|
3.42
|
|
|
|
13,737,433
|
|
|
|
4.82
|
|
|
|
2.38
|
|
|
|
2.38
|
|
|
|
40,177,410
|
|
|
February
|
|
|
4.07
|
|
|
|
2.45
|
|
|
|
2.71
|
|
|
|
5,260,826
|
|
|
|
3.06
|
|
|
|
1.83
|
|
|
|
2.02
|
|
|
|
16,936,244
|
|
|
March
|
|
|
3.81
|
|
|
|
1.35
|
|
|
|
1.98
|
|
|
|
7,982,156
|
|
|
|
2.85
|
|
|
|
0.94
|
|
|
|
1.38
|
|
|
|
17,768,154
|
|
|
April
|
|
|
2.71
|
|
|
|
1.81
|
|
|
|
2.34
|
|
|
|
4,490,898
|
|
|
|
1.92
|
|
|
|
1.26
|
|
|
|
1.65
|
|
|
|
10,112,601
|
|
|
May
|
|
|
4.02
|
|
|
|
2.02
|
|
|
|
3.34
|
|
|
|
8,413,279
|
|
|
|
2.92
|
|
|
|
1.44
|
|
|
|
2.44
|
|
|
|
34,431,369
|
|
|
June 1 to 24
|
|
|
3.22
|
|
|
|
2.50
|
|
|
|
2.78
|
|
|
|
3,760,892
|
|
|
|
2.36
|
|
|
|
1.83
|
|
|
|
2.05
|
|
|
|
11,020,986
|
|
PRIOR SALES
Common Shares
During the twelve month period prior to
the date of this Prospectus Supplement, the Corporation has issued:
|
|
(a)
|
an aggregate of 9,633,129 Common Shares pursuant to an equity distribution agreement dated October
25 2018 and prospectus supplements dated October 25, 2018, January 3, 2020 and March 6, 2020 to the 2018 Prospectus, at prices
ranging from US$0.89 to US$4.37 per Common Share, with the weighted average price being US$2.18 per Common Share;
|
|
|
(b)
|
an aggregate of 1,104,571 Common Shares pursuant to a purchase agreement dated September 28, 2018
between the Corporation and Lincoln Park Capital Fund and a prospectus supplement dated September 28, 2018 to the 2018 Prospectus,
at prices ranging from US$0.90 to US$1.64 per Common Share, with the weighted average price being US$1.27 per Common Share; and
|
|
|
(c)
|
an aggregate of 4,619,773 Common Shares and 4,619,773 Warrants pursuant to the Unit Offering;
|
|
|
(d)
|
an aggregate of 4,354,016 Common Shares upon exercise of Warrants at a price of US$0.90 per Common
Share;
|
|
|
(e)
|
an aggregate of 398,817 Common Shares issued on the vesting of restricted share awards and performance
share awards granted pursuant to its amended and restated incentive share award plan; and
|
|
|
(f)
|
an aggregate of 45,120 Common Shares issued on the exercise of stock options granted pursuant to
its amended and restated stock option plan, particulars of which are set forth in the following table:
|
|
Date
of Issue
|
|
Number
of Common Shares Issued
|
|
|
Price
per Common Share (C$)
|
|
|
January 8, 2020
|
|
|
10,000
|
|
|
|
1.45
|
|
|
January 10, 2020
|
|
|
22,796
|
|
|
|
2.78
|
|
|
January 13, 2020
|
|
|
5,000
|
|
|
|
1.45
|
|
|
June 4, 2020
|
|
|
7,324
|
|
|
|
2.41
|
|
Stock Options
During the twelve month period preceding
the date of this Prospectus Supplement, the Corporation granted stock options pursuant to its amended and restated stock option
plan exercisable for an aggregate of 1,080,000 Common Shares. The particulars of such grants are set forth in the following table:
|
Date of Grant
|
|
Number of Options Granted
|
|
|
Exercise Price (C$)
|
|
|
July 16, 2019
|
|
|
20,000
|
|
|
|
2.12
|
|
|
October 2, 2019
|
|
|
50,000
|
|
|
|
0.54
|
|
|
November 12, 2019
|
|
|
50,000
|
|
|
|
1.38
|
|
|
December 13, 2019
|
|
|
900,000
|
|
|
|
1.45
|
|
|
January 9, 2020
|
|
|
60,000
|
|
|
|
5.23
|
|
Share Awards
During the twelve month period preceding
the date of this Prospectus Supplement, the Corporation granted restricted share awards pursuant to its amended and restated incentive
share award plan which, upon vesting, will entitle the holders thereof to receive up to an aggregate of 281,645 Shares. The particulars
of such grants are set forth in the following table:
|
Date of Grant
|
|
Number of Share Awards Granted
|
|
|
June 30, 2019
|
|
|
10,148
|
|
|
September 30, 2019
|
|
|
26,702
|
|
|
October 1, 2019
|
|
|
224,135
|
|
|
January 6, 2020
|
|
|
6,430
|
|
|
March 31, 2020
|
|
|
14,230
|
|
PLAN OF DISTRIBUTION
This prospectus supplement relates to the
qualification for distribution of: (i) up to 265,757 Warrant Shares issuable from time to time, on exercise of up to 265,757 Warrants
issued by us on August 16, 2019 pursuant to the Unit Offering; and (ii) such indeterminate number of additional Warrant Shares
that may be issuable by reason of the anti-dilution provisions contained in the Warrant Agency Agreement. See “Terms of
Warrants” for additional information on the terms of the Warrants.
On August 14, 2019, we filed the 2019 Supplement
to the 2018 Prospectus with the ASC and a registration statement on Form F-10 (File No. 333-224432), principally filed on April
25, 2018, as amended May 4, 2018, with the SEC relating to the Unit Offering, being the offering by us of up to 4,619,773 Units,
each Unit consisting of one Common Share and one Warrant, at a price of US$0.80 per Unit. Each Warrant entitles the holder to purchase
one Warrant Share upon payment of US$0.90, subject to adjustment, at any time until 5:00 p.m. (New York City time) on August 16,
2024. On June 12, 2020 we filed the Prospectus with the ASC and a registration statement on Form F-10 (File No. 333-239025), principally
filed on June 5, 2020, as amended June 12, 2020, with the SEC.
The exercise price of the Warrants was
determined by negotiation between us and the underwriter for the Unit Offering.
This Prospectus Supplement registers the
offering of the securities to which it relates under the U.S. Securities Act in accordance with MJDS. This Prospectus Supplement
does not qualify the distribution of the Warrant Shares in any province or territory of Canada.
Holders of Warrants resident in the United
States who acquire Warrant Shares pursuant to the exercise of Warrants in accordance with their terms and under the Prospectus
and this Prospectus Supplement may have a right of action against us for any misrepresentation in the Prospectus and this Prospectus
Supplement. However, the existence and enforceability of such a right of action is not without doubt.
The Warrant Shares to which this Prospectus
Supplement relates will be sold directly by us to holders of Warrants, as the case may be, on the exercise of such Warrants. No
underwriters, dealers or agents will be involved in these sales. No underwriter has been involved in the preparation of, or has
performed any review of, this Prospectus Supplement.
It was a condition of closing of the Unit
Offering that the shelf registration statement remain effective with the SEC and that we file with the SEC a prospectus supplement
registering the offering of the Warrant Shares issuable from time to time on the exercise of the Warrants. No U.S. Person, person
within the United States or person holding Warrants for the account or benefit of a U.S. Person or person within the United States
may exercise the Warrants during any period of time when a registration statement covering such Warrant Shares is not effective
or an exemption from such registration is not otherwise available. If a registration statement under the U.S. Securities Act is
not effective, the Warrants may be exercised on a net cashless basis. See “Terms of Warrants” for additional
information of the terms of the Warrants.
MATERIAL UNITED STATES
FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary of certain
material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the
acquisition, ownership, and disposition of Warrant Shares received upon exercise of the Warrants.
This summary is for general information
purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations
that may apply to a U.S. Holder as a result of the acquisition of Warrant Shares pursuant to this Prospectus Supplement. In addition,
this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the
U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder
under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or
U.S. federal income tax advice with respect to any U.S. Holder. This summary does not address the U.S. alternative minimum, U.S.
federal net investment income, U.S. federal alternative minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S.
tax consequences to U.S. Holders of the acquisition, ownership, and disposition of Warrant Shares. In addition, except as specifically
set forth below, this summary does not discuss applicable tax reporting requirements. Each U.S. Holder should consult its own tax
advisors regarding the U.S. federal alternative minimum, U.S. federal net investment income, U.S. federal estate and gift, U.S.
state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Warrant Shares.
No legal opinion from legal counsel or
ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the
U.S. federal income tax consequences of the acquisition, ownership, and disposition of Warrant Shares. This summary is not binding
on the IRS, and the IRS is not precluded from taking a position that is different from, and contrary to, the positions taken in
this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS
and the U.S. courts could disagree with one or more of the positions taken in this summary.
Scope of this Summary
Authorities
This summary is based on the Internal Revenue
Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed), published
rulings of the IRS, published administrative positions of the IRS, the Convention Between Canada and the United States of America
with Respect to Taxes on Income and on Capital, signed September 26, 1980, as amended (the “Canada-U.S. Tax Convention”),
and U.S. court decisions, that are applicable, and, in each case, as in effect and available, as of the date of this document.
Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such
change could be applied retroactively. This summary does not discuss the potential effects, whether adverse or beneficial, of any
proposed legislation.
U.S. Holders
For purposes of this summary, the term
“U.S. Holder” means a beneficial owner of Warrant Shares acquired pursuant to this Prospectus Supplement that
is for U.S. federal income tax purposes:
|
|
•
|
an individual that is a citizen or resident of the United States;
|
|
|
•
|
a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized
under the laws of the United States, any state thereof or the District of Columbia;
|
|
|
•
|
an estate whose income is subject to U.S. federal income taxation regardless of its source; or
|
|
|
•
|
a trust that (1) is subject to the primary supervision of a court within the U.S. and the control
of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations
to be treated as a U.S. person.
|
U.S. Holders Subject to Special U.S.
Federal Income Tax Rules Not Addressed
This summary does not address the U.S.
federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including U.S.
Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred
accounts; (b) are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment
companies; (c) are brokers or dealers in securities or currencies or U.S. Holders that are traders in securities that elect to
apply a mark-to-market accounting method; (d) have a “functional currency” other than the U.S. dollar; (e) own Warrants
or Warrant Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other integrated transaction;
(f) acquired Warrants or Warrant Shares in connection with the exercise of employee stock options or otherwise as compensation
for services; (g) hold Warrants or Warrant Shares other than as a capital asset within the meaning of Section 1221 of the Code
(generally, property held for investment purposes); (h) are partnerships and other pass-through entities (and investors in such
partnerships and entities); (i) are required to accelerate the recognition of any item of gross income with respect to Warrants
or Warrant Shares as a result of such income being recognized on an applicable financial statement; or (i) own, have owned or will
own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of the Corporation’s
outstanding shares. This summary also does not address the U.S. federal income tax considerations applicable to U.S. Holders who
are (a) U.S. expatriates or former long-term residents of the U.S., or (b) subject to taxing jurisdictions other than, or in addition
to, the United States. U.S. Holders that are subject to special provisions under the Code, including U.S. Holders described immediately
above, should consult their own tax advisors regarding the U.S. federal, U.S. federal net investment income, U.S. federal alternative
minimum, U.S. federal estate and gift, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership
and disposition of Warrant Shares.
If an entity or arrangement that is classified
as a partnership for U.S. federal income tax purposes holds Warrants or Warrant Shares, the U.S. federal income tax consequences
to such entity or arrangement and the owners of such entity or arrangement generally will depend on the activities of such entity
or arrangement and the status of such owners. This summary does not address the tax consequences to any such entity or arrangement
or owner. Owners of entities or arrangements that are classified as partnerships for U.S. federal income tax purposes should consult
their own tax advisor regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership,
and disposition of Warrant Shares.
Passive Foreign Investment Company Rules
If the Corporation were to constitute a
“passive foreign investment company” under the meaning of Section 1297 of the Code (a “PFIC”, as
defined below) for any year during a U.S. Holder’s holding period then certain potentially adverse rules would affect the
U.S. federal income tax consequences to a U.S. Holder as a result of the acquisition, ownership, and disposition of Warrant Shares.
The Corporation believes that it was classified
as a PFIC during the tax year ended December 31, 2019, and based on current business plans and financial expectations, the Corporation
expects that it will be a PFIC for the current tax year and may be a PFIC in future tax years. No opinion of legal counsel or ruling
from the IRS concerning the status of the Corporation as a PFIC has been obtained or is currently planned to be requested. The
determination of whether any corporation was, or will be, a PFIC for a tax year depends, in part, on the application of complex
U.S. federal income tax rules, which are subject to differing interpretations. In addition, whether any corporation will be a PFIC
for any tax year depends on the assets and income of such corporation over the course of each such tax year and, as a result, cannot
be predicted with certainty as of the date of this document. Accordingly, there can be no assurance that the IRS will not challenge
any PFIC determination made by the Corporation (or any subsidiary of the Corporation) concerning its PFIC status. Each U.S. Holder
should consult its own tax advisors regarding the PFIC status of the Corporation and each subsidiary of the Corporation.
In any year in which the Corporation is
classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury
Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may
result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors
regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form
8621 annually.
The Corporation generally will be a PFIC
if, for a tax year , (a) 75% or more of the gross income of the Corporation is passive income (the “PFIC income test”)
or (b) 50% or more of the value of the assets of the Corporation either produce passive income or are held for the production of
passive income, based on the quarterly average of the fair market value of such assets (the “PFIC asset test”).
“Gross income” generally includes all sales revenues less the cost of goods sold, plus income from investments and
from incidental or outside operations or sources, and “passive income” generally includes, for example, dividends,
interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities
transactions.
For purposes of the PFIC income test and
PFIC asset test described above, if the Corporation owns, directly or indirectly, 25% or more of the total value of the outstanding
shares of another corporation, the Corporation will be treated as if it (a) held a proportionate share of the assets of such other
corporation and (b) received directly a proportionate share of the income of such other corporation. In addition, for purposes
of the PFIC income test and PFIC asset test described above, and assuming certain other requirements are met, “passive income”
does not include certain interest, dividends, rents, or royalties that are received or accrued by the Corporation from certain
“related persons” (as defined in Section 954(d)(3) of the Code) also organized in Canada, to the extent such items
are properly allocable to the income of such related person that is not passive income.
Under certain attribution rules, if the
Corporation is a PFIC, U.S. Holders will generally be deemed to own their proportionate share the Corporation’s direct or
indirect equity interest in any company that is also a PFIC (a “Subsidiary PFIC”), and will generally be subject
to U.S. federal income tax on their proportionate share of any (a) any “excess distributions,” as described below,
on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by the Corporation
or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders
may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition
of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax under the PFIC rules even if no distributions
are received and no redemptions or other dispositions of Warrant Shares are made.
Default PFIC Rules Under Section 1291
of the Code
If the Corporation is a PFIC for any tax
year during which a U.S. Holder owns Common Shares, the U.S. federal income tax consequences to such U.S. Holder of the acquisition,
ownership, and disposition of Warrant Shares will depend on whether and when such U.S. Holder makes a “qualified electing
fund” or “QEF” under Section 1295 of the Code (a “QEF Election”) or makes a mark-to-market
election under Section 1296 of the Code (a “Mark-to-Market Election”). A U.S. Holder that does not make either
a QEF Election or a Mark-to-Market Election will be referred to in this summary as a “Non-Electing U.S. Holder.”
A Non-Electing U.S. Holder will be subject
to the rules of Section 1291 of the Code (as described below) with respect to (a) any gain recognized on the sale or other taxable
disposition of Warrant Shares and (b) any excess distribution received on the Warrant Shares. A distribution generally will be
an “excess distribution” to the extent that such distribution (together with all other distributions received in the
current tax year) exceeds 125% of the average distributions received during the three preceding tax years (or during a U.S. Holder’s
holding period for the Warrant Shares, if shorter).
Under Section 1291 of the Code, any gain
recognized on the sale or other taxable disposition of Warrant Shares (including an indirect disposition of the stock of a Subsidiary
PFIC), and any “excess distribution” received on such Warrant Shares or with respect to the stock of a Subsidiary PFIC,
must be ratably allocated to each day in a Non-Electing U.S. Holder’s holding period for the respective Warrant Shares. The
amount of any such gain or excess distribution allocated to the tax year of disposition or distribution of the excess distribution
and to years before the entity became a PFIC, if any, would be taxed as ordinary income (and not eligible for certain preferred
rates). The amounts allocated to any other tax year would be subject to U.S. federal income tax at the highest tax rate applicable
to ordinary income in each such year, and an interest charge would be imposed on the tax liability for each such year, calculated
as if such tax liability had been due in each such year. A Non-Electing U.S. Holder that is not a corporation must treat any such
interest paid as “personal interest,” which is not deductible.
If the Corporation is a PFIC for any tax
year during which a Non-Electing U.S. Holder holds Warrant Shares or Warrants, the Corporation will continue to be treated as a
PFIC with respect to such Non-Electing U.S. Holder, regardless of whether the Corporation ceases to be a PFIC in one or more subsequent
tax years. A Non-Electing U.S. Holder may terminate this deemed PFIC status by electing to recognize gain (which will be taxed
under the rules of Section 1291 of the Code discussed above) but not loss, as if such Warrant Shares were sold on the last day
of the last tax year for which the Corporation was a PFIC. No such election, however, may be made with respect to the Warrants.
Under proposed Treasury Regulations, if
a U.S. holder has an option, warrant, or other right to acquire stock of a PFIC (such as the Warrants), such option, warrant or
right is considered to be PFIC stock subject to the default rules of Section 1291 of the Code. Under rules described below, the
holding period for the Warrant Shares will begin on the date a U.S. Holder acquires the Warrants. This will impact the availability
of the QEF Election and Mark-to-Market Election with respect to the Warrant Shares.
QEF Election
As discussed above, under proposed Treasury
Regulations, if a U.S. holder has an option, warrant or other right to acquire stock of a PFIC (such as the Warrants), such option,
warrant or right is considered to be PFIC stock subject to the default rules of Section 1291 of the Code. However, a U.S. Holder
of an option, warrant or other right to acquire stock of a PFIC may not make a QEF Election that will apply to the option, warrant
or other right to acquire PFIC stock. In addition, under proposed Treasury Regulations, if a U.S. Holder holds an option, warrant
or other right to acquire stock of a PFIC, the holding period with respect to shares of stock of the PFIC acquired upon exercise
of such option, warrant or other right will include the period that the option, warrant or other right was held.
Consequently, under the proposed Treasury
Regulations, if a U.S. Holder of Common Shares makes a QEF Election, such election generally will not be treated as a timely QEF
Election with respect to Warrant Shares and the rules of Section 1291 of the Code discussed above will continue to apply with respect
to such U.S. Holder’s Warrant Shares. However, a U.S. Holder of Warrant Shares should be eligible to make a timely QEF Election
if such U.S. Holder elects in the tax year in which such Warrant Shares are received to recognize gain (which will be taxed under
the rules of Section 1291 of the Code discussed above) as if such Warrant Shares were sold for fair market value on the date such
U.S. Holder acquired them by exercising the corresponding Warrant. In addition, gain recognized on the sale or other taxable disposition
(other than by exercise) of the Warrants by a U.S. Holder will be subject to the rules of Section 1291 of the Code discussed above.
Each U.S. Holder should consult its own tax advisors regarding the application of the PFIC rules to the Warrants and Warrant Shares.
A U.S. Holder that makes a timely and effective
QEF Election under the rules set forth in the preceding paragraph generally will not be subject to the rules of Section 1291 of
the Code discussed above with respect to its Warrant Shares. However, a U.S. Holder that makes a timely and effective QEF Election
will be subject to U.S. federal income tax on such U.S. Holder’s pro rata share of (a) the Corporation’s net capital
gain, which will be taxed as long-term capital gain to such U.S. Holder, and (b) the Corporation’s ordinary earnings, which
will be taxed as ordinary income to such U.S. Holder. Generally, “net capital gain” is the excess of (a) net long-term
capital gain over (b) net short-term capital loss, and “ordinary earnings” are the excess of (a) “earnings and
profits” over (b) net capital gain. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on
such amounts for each tax year in which the Corporation is a PFIC, regardless of whether such amounts are actually distributed
to such U.S. Holder by the Corporation. However, for any tax year in which the Corporation is a PFIC and has no net income or gain,
U.S. Holders that have made a QEF Election would not have any income inclusions as a result of the QEF Election. If a U.S. Holder
that made a QEF Election has an income inclusion, such a U.S. Holder may, subject to certain limitations, elect to defer payment
of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any
such interest paid will be treated as “personal interest,” which is not deductible.
A U.S. Holder that makes a timely and effective
QEF Election as described above generally (a) may receive a tax-free distribution from the Corporation to the extent that such
distribution represents “earnings and profits” of the Corporation that were previously included in income by the U.S.
Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the Warrant Shares to reflect the
amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, a U.S. Holder that makes
a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of Warrant Shares.
A QEF Election will apply to the tax year
for which such QEF Election is timely made and to all subsequent tax years, unless such QEF Election is invalidated or terminated
or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent tax year, the
Corporation ceases to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those tax years
in which the Corporation is not a PFIC. Accordingly, if the Corporation becomes a PFIC in another subsequent tax year, the QEF
Election will be effective and the U.S. Holder will be subject to the QEF rules described above during any subsequent tax year
in which the Corporation qualifies as a PFIC.
The Corporation: (a) will make available
to U.S. Holders, upon their written request, information as to its status as a PFIC and the PFIC status of any subsidiary in which
the Corporation owns more than 50% of such subsidiary’s total aggregate voting power and (b) for each year in which the Corporation
is a PFIC, provide to a U.S. Holder, upon written request, such information and documentation that a U.S. Holder making a QEF Election
with respect to the Corporation and such more than 50% owned subsidiary which constitutes a PFIC is reasonably required to obtain
for U.S. federal income tax purposes. The Corporation may elect to provide such information on its website. With respect to any
Subsidiary PFIC in which the Corporation owns 50% or less of the aggregate voting power, upon the written request of a U.S. Holder
acquiring Warrant Shares, the Corporation will request that such Subsidiary PFIC provide such U.S. Holder with the information
that such U.S. Holder requires to report under the QEF rules; provided, however, the Corporation can provide no assurances that
such Subsidiary PFIC will provide such information.
A U.S. Holder makes a QEF Election by attaching
a completed IRS Form 8621, including a PFIC Annual Information Statement, to a timely filed U.S. federal income tax return. However,
if the Corporation does not provide the required information with regard to the Corporation or any of its Subsidiary PFICs, U.S.
Holders will not be able to make a QEF Election for such entity and will continue to be subject to the rules of Section 1291 of
the Code discussed above that apply to Non-Electing U.S. Holders with respect to the taxation of gains and excess distributions.
Mark-to-Market Election
A U.S. Holder may make a Mark-to-Market
Election with respect to Warrant Shares only if the Warrant Shares are marketable stock. The Warrant Shares generally will be “marketable
stock” if the Warrant Shares are regularly traded on (a) a national securities exchange that is registered with the SEC,
(b) the national market system established pursuant to Section 11A of the U.S. Exchange Act or (c) a foreign securities exchange
that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such
foreign exchange has trading volume, listing, financial disclosure, surveillance requirements, and meets other requirements and
the laws of the country in which such foreign exchange is located, together with the rules of such foreign exchange, ensure that
such requirements are actually enforced and (ii) the rules of such foreign exchange ensure active trading of listed stocks. If
such stock is traded on such a qualified exchange or other market, such stock generally will be considered “regularly traded”
for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar
quarter. Provided that the Warrant Shares are “regularly traded” as described in the preceding sentence, the Warrant
Shares are expected to be marketable stock. However, Each U.S. Holder should consult its own tax advisor in this matter.
A U.S. Holder that makes a Mark-to-Market
Election with respect to its Warrant Shares generally will not be subject to the rules of Section 1291 of the Code discussed above
with respect to such Warrant Shares. However, if a U.S. Holder does not make a Mark-to-Market Election beginning in the first tax
year of such U.S. Holder’s holding period for the Warrant Shares for which the Corporation is a PFIC and such U.S. Holder
has not made a timely QEF Election, the rules of Section 1291 of the Code discussed above will apply to certain dispositions of,
and distributions on, the Warrant Shares.
Any Mark-to-Market Election made by a U.S.
Holder for its Common Shares will also apply to such U.S. Holder’s Warrant Shares. As a result, if a Mark-to-Market Election
has been made by a U.S. Holder with respect to its Common Shares, any Warrant Shares received will automatically be marked-to-market
in the year of exercise. Because, under the proposed Treasury Regulations, a U.S. Holder’s holding period for Warrant Shares
includes the period during which such U.S. Holder held the Warrants, a U.S. Holder will be treated as making a Mark-to-Market Election
with respect to its Warrant Shares after the beginning of such U.S. Holder’s holding period for the Warrant Shares unless
the Warrant Shares are acquired in the same tax year as the year in which the U.S. Holder acquired its Warrants. Consequently,
the default rules under Section 1291 described above generally will apply to the mark-to-market gain realized in the tax year in
which Warrant Shares are received. However, the general mark-to-market rules will apply to subsequent tax years.
A U.S. Holder that makes a Mark-to-Market
Election will include in ordinary income, for each tax year in which the Corporation is a PFIC, an amount equal to the excess,
if any, of (a) the fair market value of the Warrant Shares, as of the close of such tax year over (b) such U.S. Holder’s
adjusted tax basis in the Warrant Shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an
amount equal to the excess, if any, of (i) such U.S. Holder’s adjusted tax basis in the Warrant Shares, over (ii) the fair
market value of such Warrant Shares (but only to the extent of the net amount of previously included income as a result of the
Mark-to-Market Election for prior tax years).
A U.S. Holder that makes a Mark-to-Market
Election generally also will adjust such U.S. Holder’s tax basis in the Warrant Shares to reflect the amount included in
gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition
of Warrant Shares, a U.S. Holder that makes a Mark-to-Market Election will recognize ordinary income or ordinary loss (not to exceed
the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior tax years over
(b) the amount allowed as a deduction because of such Mark-to-Market Election for prior tax years).
A U.S. Holder makes a Mark-to-Market Election
by attaching a completed IRS Form 8621 to a timely filed U.S. federal income tax return. Mark-to-Market Election applies to the
tax year in which such Mark-to-Market Election is made and to each subsequent tax year, unless the Warrant Shares cease to be “marketable
stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisors regarding
the availability of, and procedure for making, a Mark-to-Market Election.
Although a U.S. Holder may be eligible
to make a Mark-to-Market Election with respect to the Warrant Shares, no such election may be made with respect to the stock of
any Subsidiary PFIC that a U.S. Holder is treated as owning because such stock is not marketable. Hence, the Mark-to-Market Election
will not be effective to avoid the application of the default rules of Section 1291 of the Code described above with respect to
deemed dispositions of Subsidiary PFIC stock or excess distributions from a Subsidiary PFIC to its shareholder.
Other PFIC Rules
Under Section 1291(f) of the Code, the
IRS has issued proposed Treasury Regulations that, subject to certain exceptions, would cause a U.S. Holder that had not made a
timely QEF Election to recognize gain (but not loss) upon certain transfers of Warrant Shares that would otherwise be tax-deferred
(e.g., gifts and exchanges pursuant to corporate reorganizations). However, the specific U.S. federal income tax consequences to
a U.S. Holder may vary based on the manner in which Warrants or Warrant Shares are transferred.
If finalized in their current form, the
proposed Treasury Regulations applicable to PFICs would be effective for transactions occurring on or after April 1, 1992. Because
the proposed Treasury Regulations have not yet been adopted in final form, they are not currently effective, and there is no assurance
that they will be adopted in the form and with the effective date proposed. Nevertheless, the IRS has announced that, in the absence
of final Treasury Regulations, taxpayers may apply reasonable interpretations of the Code provisions applicable to PFICs and that
it considers the rules set forth in the proposed Treasury Regulations to be reasonable interpretations of those Code provisions.
The PFIC rules are complex, and the implementation of certain aspects of the PFIC rules requires the issuance of Treasury Regulations
which in many instances have not been promulgated and which, when promulgated, may have retroactive effect. U.S. Holders should
consult their own tax advisors about the potential applicability of the proposed Treasury Regulations.
Certain additional adverse rules may apply
with respect to a U.S. Holder if the Corporation is a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example
under Section 1298(b)(6) of the Code, a U.S. Holder that uses Warrants or Warrant Shares as security for a loan will, except as
may be provided in Treasury Regulations, be treated as having made a taxable disposition of such Warrants or Warrant Shares.
In addition, a U.S. Holder who acquires
Warrants or Warrant Shares from a decedent will not receive a “step up” in tax basis of such Warrants or Warrant Shares
to fair market value.
Special rules also apply to the amount
of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, foreign taxes
paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules
relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should
consult with its own tax advisors regarding the availability of the foreign tax credit with respect to distributions by a PFIC.
The PFIC rules are complex, and each U.S.
Holder should consult its own tax advisors regarding the PFIC rules and how the PFIC rules may affect the U.S. federal income tax
consequences of the acquisition, ownership, and disposition of Warrants and Warrant Shares.
U.S. Federal Income Tax Consequences
of the Ownership, Exercise and Disposition of Warrants
The following discussion describes the
general rules applicable to the ownership, exercise and disposition of the Warrants but is subject in its entirety to the special
rules described above under the heading “Passive Foreign Investment Company Rules.”
Exercise of Warrants
A U.S. Holder should not recognize gain
or loss on the exercise of a Warrant and related receipt of a Warrant Share (unless cash is received in lieu of the issuance of
a fractional Warrant Share). A U.S. Holder’s initial tax basis in the Warrant Share received on the exercise of a Warrant
should be equal to the sum of (a) such U.S. Holder’s tax basis in such Warrant plus (b) the exercise price paid by such U.S.
Holder on the exercise of such Warrant. It is unclear whether a U.S. Holder’s holding period for the Warrant Share received
on the exercise of a Warrant would commence on the date of exercise of the Warrant or the day following the date of exercise of
the Warrant. If, as anticipated, the Corporation is a PFIC, a U.S. Holder’s holding period for the Warrant Share for PFIC
purposes only will begin on the date on which such U.S. Holder acquired its Warrants.
Disposition of Warrants
A U.S. Holder will recognize gain or loss
on the sale or other taxable disposition of a Warrant in an amount equal to the difference, if any, between (a) the amount of cash
plus the fair market value of any property received and (b) such U.S. Holder’s tax basis in the Warrant sold or otherwise
disposed of. Subject to the PFIC rules discussed above, any such gain or loss generally will be a capital gain or loss, which will
be long-term capital gain or loss if the Warrant is held for more than one year. Deductions for capital losses are subject to complex
limitations under the Code.
Expiration of Warrants Without Exercise
Upon the lapse or expiration of a Warrant,
a U.S. Holder will recognize a loss in an amount equal to such U.S. Holder’s tax basis in the Warrant. Any such loss generally
will be a capital loss and will be long-term capital loss if the Warrants are held for more than one year. Deductions for capital
losses are subject to complex limitations under the Code.
Certain Adjustments to the Warrants
Under Section 305 of the Code, an adjustment
to the number of Warrant Shares that will be issued on the exercise of the Warrants, or an adjustment to the exercise price of
the Warrants, may be treated as a constructive distribution to a U.S. Holder of the Warrants if, and to the extent that, such adjustment
has the effect of increasing such U.S. Holder’s proportionate interest in the “earnings and profits” or the Corporation’s
assets, depending on the circumstances of such adjustment (for example, if such adjustment is to compensate for a distribution
of cash or other property to the shareholders). Adjustments to the exercise price of Warrants made pursuant to a bona fide reasonable
adjustment formula that has the effect of preventing dilution of the interest of the holders of the Warrants should generally not
be considered to result in a constructive distribution. Any such constructive distribution would be taxable whether or not there
is an actual distribution of cash or other property. (See more detailed discussion of the rules applicable to distributions made
by the Corporation at “Distributions on Warrant Shares” below).
General Rules Applicable to U.S. Federal
Income Tax Consequences of the Acquisition, Ownership, and Disposition of Warrant Shares
The following discussion describes the
general rules applicable to the acquisition, ownership and disposition of the Warrant Shares but is subject in its entirety to
the special rules described above under the heading “Passive Foreign Investment Company Rules.”
Distributions on Warrant Shares
A U.S. Holder that receives a distribution,
including a constructive distribution, with respect to a Warrant Share (as well as any constructive distribution on a Warrant as
described above) will be required to include the amount of such distribution in gross income as a dividend (without reduction for
any Canadian income tax withheld from such distribution) to the extent of the Corporation’s current and accumulated “earnings
and profits”, as computed for U.S. federal income tax purposes. A dividend generally will be taxed to a U.S. Holder at ordinary
income tax rates if the Corporation is a PFIC for the tax year of such distribution or the preceding tax year. To the extent that
a distribution exceeds the current and accumulated “earnings and profits” of the Corporation, such distribution will
be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in the Warrant Shares and thereafter
as gain from the sale or exchange of such Warrant Shares (see “Sale or Other Taxable Disposition of Warrant Shares”
below). However, the Corporation may not maintain the calculations of earnings and profits in accordance with U.S. federal income
tax principles, and each U.S. Holder may be required to assume that any distribution by the Corporation with respect to the Warrant
Shares will constitute ordinary dividend income. Dividends received on Warrant Shares by corporate U.S. Holders generally will
not be eligible for the “dividends received deduction.” Subject to applicable limitations and provided the Corporation
is eligible for the benefits of the Canada-U.S. Tax Convention, or the Common Shares are readily tradable on a United States securities
market, dividends paid by the Corporation to non-corporate U.S. Holders, including individuals, generally will be eligible for
the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions
are satisfied, including that the Corporation not be classified as a PFIC in the tax year of distribution or in the preceding tax
year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisors regarding the application of such
rules.
Sale or Other Taxable Disposition of
Warrant Shares
Upon the sale or other taxable disposition
of Warrant Shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the
U.S. dollar value of cash received plus the fair market value of any property received and such U.S. Holder’s tax basis in
such Warrant Shares sold or otherwise disposed of. A U.S. Holder’s tax basis in Warrant Shares generally will be such holder’s
U.S. dollar cost for such Warrant Shares. Gain or loss recognized on such sale or other disposition generally will be long-term
capital gain or loss if, at the time of the sale or other disposition, the Warrant Shares have been held for more than one year.
Preferential tax rates currently apply
to long-term capital gain of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates
for long-term capital gain of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations
under the Code.
Additional Tax Considerations
Receipt of Foreign Currency
The amount of any distribution paid to
a U.S. Holder in foreign currency or on the sale, exchange or other taxable disposition of Warrants or Warrant Shares generally
will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt (regardless
of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a basis in the foreign currency
equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency
after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and
generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the
accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax
consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Subject to the PFIC rules discussed above,
a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Warrant
Shares (or with respect to any constructive dividend on the Warrants) generally will be entitled, at the election of such U.S.
Holder, to receive either a deduction or a credit for such Canadian income tax. Generally, a credit will reduce a U.S. Holder’s
U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder’s income subject
to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid or accrued (whether
directly or through withholding) by a U.S. Holder during a year. The foreign tax credit rules are complex and involve the application
of rules that depend on a U.S. Holder’s particular circumstances. Accordingly, each U.S. Holder should consult its own U.S.
tax advisors regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law certain
categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation.
For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals that are U.S. Holders that hold
certain specified foreign financial assets in excess of certain thresholds. The definition of specified foreign financial assets
includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained
by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment
that has an issuer or counterparty other than a U.S. person and any interest in a foreign entity. U.S. Holders may be subject to
these reporting requirements unless their Warrants and Warrant Shares are held in an account at certain financial institutions.
Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult with their own
tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the U.S., or by a
U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of the Warrants
and Warrant Shares will generally be subject to information reporting and backup withholding tax, if a U.S. Holder (a) fails to
furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on Form W-9), (b) furnishes an incorrect
U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report
items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that it has furnished its correct U.S.
taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax.
However, certain exempt persons, generally are excluded from these information reporting and backup withholding rules. Backup withholding
is no an additional tax. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a
U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information
to the IRS in a timely manner.
The discussion of reporting requirements
set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder.
A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess
a tax and, under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting
requirement. Each U.S. Holder should consult its own tax advisors regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO
CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP,
AND DISPOSITION OF WARRANTS AND WARRANT SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS
APPLICABLE TO THEM IN THEIR OWN PARTICULAR CIRCUMSTANCES.
CERTAIN CANADIAN
FEDERAL INCOME TAX CONSIDERATIONS
The following is, as of the date hereof,
a general summary of the principal Canadian federal income tax considerations under the Tax Act and the regulations thereunder
(the “Regulations”) generally applicable to a holder who acquires Warrant Shares issuable from time to time
on the exercise of Warrants as beneficial owner and who, for purposes of the Tax Act and at all relevant times: (a) deals at arm’s
length with the Corporation and the Underwriter; (b) is not affiliated with the Corporation or the Underwriter; (c) acquires and
holds the Warrant Shares acquired on the exercise of Warrants as capital property; and (d) is neither resident nor deemed to be
resident in Canada and does not use or hold, and will not be deemed to use or hold, Warrant Shares in carrying on a business in
Canada (each, a “Non-Resident Holder”). The Warrant Shares will generally be considered to be capital property
to a Non-Resident Holder unless the Non-Resident Holder holds or uses the Warrant Shares, or is deemed to hold or use the Warrant
Shares, in the course of carrying on a business of trading or dealing in securities or has acquired them, or is deemed to have
acquired them, in a transaction or transactions considered to be an adventure in the nature of trade. The term “U.S. Holder,”
for the purposes of this summary, means a Non-Resident Holder who, for purposes of Canada-United States Income Tax Convention (1980)
(the “Canada-U.S. Tax Convention”), is at all relevant times a resident of the United States and is a “qualifying
person” within the meaning of the Canada-U.S. Tax Convention. In some circumstances, persons deriving amounts through fiscally
transparent entities (including limited liability companies) may be entitled to benefits under the Canada-U.S. Tax Convention.
U.S. Holders are urged to consult their own tax advisors to determine their entitlement to benefits under the Canada-U.S. Tax Convention
based on their particular circumstances.
Special considerations, which are not discussed
in this summary, may apply to a Non-Resident Holder that is an insurer that carries on an insurance business in Canada and elsewhere
or an authorized foreign bank (as defined in the Tax Act). Such Non-Resident Holders should consult their own tax advisors.
This summary is based upon the current
provisions of the Tax Act and the Regulations in force as of the date hereof, specific proposals to amend the Tax Act and the Regulations
(the “Tax Proposals”) which have been publically announced by or on behalf the Minister of Finance (Canada)
prior to the date hereof, the current provisions of the Canada-U.S. Tax Convention and counsel’s understanding of the current
published administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”). This summary
assumes that the Tax Proposals will be enacted in the form proposed and does not take into account or anticipate any other changes
in law, whether by way of judicial, legislative or governmental decision or action, or changes in the CRA’s administrative
policies and assessing practices, nor does it take into account provincial, territorial or foreign income tax legislation or considerations,
which may differ from the Canadian federal income tax considerations discussed herein. No assurances can be given that the Tax
Proposals will be enacted as proposed or at all, or that legislative, judicial or administrative changes will not modify or change
the statements expressed herein.
This summary is not exhaustive of all
possible Canadian federal income tax considerations applicable to an investment in Warrant Shares. This summary is of a general
nature only and is not intended to be, nor should it be construed to be, legal or income tax advice to any particular Non-Resident
Holder. Non-Resident Holders should consult their own income tax advisors with respect to the tax consequences applicable to them
based on their own particular circumstances.
Exercise of Warrants
No gain or loss will be realized by a Non-Resident
Holder upon the exercise of a Warrant to acquire a Warrant Share. When a Warrant is exercised, the Non-Resident Holder’s
cost of the Warrant Share acquired thereby will be the aggregate of the Non-Resident Holder’s adjusted cost base of such
Warrant and the exercise price paid for the Warrant Share. The Non-Resident Holder’s adjusted cost base of the Warrant Share
so acquired will be determined by averaging such cost with the adjusted cost base to the Non-Resident Holder of all Common Shares
of the Corporation owned by the Non-Resident Holder as capital property immediately prior to such acquisition.
Taxation of Dividends
Dividends paid or credited, or deemed to
be paid or credited, to a Non-Resident Holder on the Warrant Shares will be subject to Canadian withholding tax under the Tax Act
at the rate of 25% of the gross amount of the dividend. Such rate is generally reduced under the Canada-U.S. Tax Convention to
15% if the beneficial owner of such dividend is a U.S. Holder. The rate of withholding tax is further reduced to 5% if the beneficial
owner of such dividend is a U.S. Holder that is a company that owns, directly or indirectly, at least 10% of the voting stock of
the Corporation. In addition, under the Canada-U.S. Tax Convention, dividends may be exempt from such Canadian withholding tax
if paid to certain U.S. Holders that are qualifying religious, scientific, literary, educational or charitable tax-exempt organizations
or qualifying trusts, companies, organizations or arrangements operated exclusively to administer or provide pension, retirement
or employee benefits or benefits for the self-employed under one or more funds or plans established to provide pension or retirement
benefits or other employee benefits that are exempt from tax in the United States and that have complied with specific administrative
procedures.
Disposition of Warrant Shares
A Non-Resident Holder will not be subject
to tax under the Tax Act in respect of any capital gain realized by such Non-Resident Holder on a disposition of Warrant Shares,
unless the Warrant Shares constitute “taxable Canadian property” (as defined in the Tax Act) of the Non-Resident Holder
at the time of the disposition and are not “treaty-protected property” (as defined in the Tax Act) of the Non-Resident
Holder at the time of the disposition.
Generally, as long as the Common Shares
are then listed on a designated stock exchange (which currently includes the TSX), the Warrant Shares will not constitute taxable
Canadian property of a Non-Resident Holder, unless at any time during the 60-month period immediately preceding the disposition
the following two conditions are met concurrently: (a) the Non-Resident Holder, persons with which the Non-Resident Holder does
not deal at arm’s length, partnerships whose members include, either directly or indirectly through one or more partnerships,
the Non-Resident Holder or persons which do not deal at arm’s length with the Non-Resident Holder, or any combination of
them, owned 25% or more of the issued shares of any class or series of shares of the capital stock of the Corporation, and (b)
more than 50% of the fair market value of the Common Shares was derived directly or indirectly, from one or any combination of
real or immovable property situated in Canada, “Canadian resource properties”, “timber resource properties”
(each as defined in the Tax Act) and options in respect of or interests in, or for civil law rights in, any such property (whether
or not such property exists).
In the case of a U.S. Holder, the Common
Shares of such US Holder will generally constitute “treaty-protected property” for purposes of the Tax Act unless the
value of the Common Shares is derived principally from real property situated in Canada. For this purpose, “real property”
has the meaning that term has under the laws of Canada and includes any option or similar right in respect thereof and usufruct
of real property, rights to explore for or to exploit mineral deposits, sources and other natural resources and rights to amounts
computed by reference to the amount or value of production from such resources.
Non-Resident Holders whose Warrant Shares
may be taxable Canadian property should consult their own tax advisors.
LEGAL MATTERS AND
INTEREST OF EXPERTS
The auditors of the Corporation are Ernst
& Young LLP, Chartered Accountants, Calgary City Centre, 2200, 215 – 2nd Street S.W., Calgary, Alberta, T2P
1M4. Ernst & Young LLP is independent of the Corporation in accordance with the Rules of Professional Conduct as outlined by
the Chartered Professional Accountants of Alberta. Ernst & Young LLP is registered with the U.S. Public Corporation Accounting
Oversight Board.
Certain legal matters relating to the issuance
of Warrants Shares upon the exercise of Warrants will be passed upon on our behalf by McCarthy Tétrault LLP with respect
to certain Canadian legal matters and by Dorsey & Whitney LLP with respect to certain U.S. legal matters and on behalf of the
Agent by Goodwin Procter LLP with respect to certain U.S. legal matters. As at the date hereof, the partners and associates of
each of McCarthy Tétrault LLP beneficially own, directly or indirectly, less than 1% of the Common Shares.
AGENT FOR SERVICE
OF PROCESS
Messrs. Wayne Pisano and Leonard Kruimer
and Drs. William G. Rice and Bernd R. Seizinger are directors of the Corporation who reside outside of Canada. Messrs. Pisano and
Kruimer and Drs. Rice and Seizinger have appointed the Corporation, at its principal place of business, as agent for service of
process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person
that resides outside of Canada, even if the party has appointed an agent for service of process.
WHERE YOU CAN FIND
ADDITIONAL INFORMATION
The Corporation has filed with the SEC
a registration statement on Form F-10 relating to, among other securities, the Common Shares. This Prospectus Supplement and the
Prospectus, both of which constitute a part of the registration statement, do not contain all of the information contained in the
registration statement, certain items of which are contained in the exhibits to the registration statement as permitted by the
rules and regulations of the SEC. See “Documents Filed as Part of the Registration Statement” in this Prospectus
Supplement and the Prospectus. Statements contained in this Prospectus Supplement, the Prospectus or a document incorporated by
reference in the Prospectus about the contents of any contract, agreement or other documents referred to are not necessarily complete,
and in each instance you should refer to the exhibits to the registration statement for a more complete description of the matter
involved. The registration statement, and the items of information omitted from this Prospectus Supplement and the Prospectus but
contained in the registration statement, will be available on EDGAR (www.sec.gov/edgar.shtml).
The Corporation is subject to the information
requirements of the U.S. Exchange Act and applicable Canadian securities legislation and, in accordance therewith, files and
furnishes annual and quarterly financial information and material change reports, business acquisition reports and other material
with the securities commission or similar regulatory authority in each of the provinces of Canada and with the SEC. Under MJDS
adopted by the United States and Canada, documents and other information that the Corporation files with the SEC may be prepared
in accordance with the disclosure requirements of Canada, which are different from those of the United States. As a foreign private
issuer within the meaning of rules made under the U.S. Exchange Act, the Corporation is exempt from the rules under the U.S. Exchange
Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders
are exempt from the reporting and shortswing profit recovery provisions contained in Section 16 of the U.S. Exchange
Act. In addition, the Corporation is not required to publish financial statements as promptly as United States companies.
You may read any document that the Corporation
has filed with the SEC on EDGAR at www.sec.gov/edgar.shtml. You may read and download any public document that the Corporation
has filed with the Canadian securities regulatory authorities under the Corporation’s profile on SEDAR (www.sedar.com).
BASE
SHELF PROSPECTUS
JUNE 12, 2020

Cdn.$150,000,000
Common Shares
Subscription Receipts
Warrants
Units
Oncolytics Biotech Inc. (the “Corporation”,
“Oncolytics”, “we”, “our” or “us”) may from time to
time offer and issue the following securities: (i) common shares in the capital of the Corporation (“Common Shares”);
(ii) subscription receipts of the Corporation exchangeable for Common Shares and/or other securities of the Corporation (“Subscription
Receipts”); (iii) warrants exercisable to acquire Common Shares and/or other securities of the Corporation (“Warrants”);
and (iv) securities comprised of more than one of Common Shares, Subscription Receipts and/or Warrants offered together as a unit
(“Units”), or any combination thereof, up to an aggregate offering price of $150,000,000 (or the equivalent
thereof, at the date of issue, in any other currency or currencies, as the case may be) at any time during the 25-month period
that this short form base shelf prospectus (including any amendments hereto, the “Prospectus”) remains valid.
The Common Shares, Subscription Receipts, Warrants and Units (collectively, the “Securities”) offered hereby
may be offered separately or together, in separate series, in amounts, at prices and on terms to be set forth in one or more prospectus
supplements (collectively or individually, as the case may be, “Prospectus Supplements”).
The specific terms of any offering of Securities
will be set forth in the applicable Prospectus Supplement and may include, without limitation, where applicable: (i) in the case
of Common Shares, the number of Common Shares being offered, the offering price (in the event the offering is a fixed price distribution),
the manner of determining the offering price(s) (in the event the offering is not a fixed price distribution) and any other specific
terms; (ii) in the case of Subscription Receipts, the number of Subscription Receipts being offered, the offering price, the terms,
conditions and procedures for the exchange of the Subscription Receipts into or for Common Shares and/or other securities of the
Corporation and any other specific terms; (iii) in the case of Warrants, the number of such Warrants offered, the offering price,
the terms, conditions and procedures for the exercise of such Warrants into or for Common Shares and/or other securities of the
Corporation and any other specific terms; and (iv) in the case of Units, the number of Units being offered, the offering price,
the terms of the Common Shares, Subscription Receipts and/or Warrants, as the case may be, underlying the Units, and any other
specific terms.
An investment in Securities involves
significant risks that should be carefully considered by prospective investors before purchasing Securities. The risks outlined
in this Prospectus and in the documents incorporated by reference herein, including the applicable Prospectus Supplement, should
be carefully reviewed and considered by prospective investors in connection with any investment in Securities. See “Risk
Factors”.
This offering is made by a Canadian
issuer that is permitted, under a multijurisdictional disclosure system adopted by the United States and Canada (“MJDS”),
to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors in the United States should
be aware that such requirements are different from those of the United States. Financial statements included or incorporated by
reference herein have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued
by the International Accounting Standards Board (“IASB”) and may not be comparable to financial statements of United
States companies. Such financial statements are subject to Canadian generally accepted auditing standards and auditor independence
standards, in addition to the standards of the Public Company Accounting Oversight Board (United States) and the United States
Securities and Exchange Commission (“SEC”) independence standards.
Prospective
investors should be aware that the acquisition of the Securities described herein may have tax consequences both in the United
States and in Canada. This Prospectus may not describe these tax consequences fully. You should read the tax discussion
in the applicable Prospectus Supplement and consult with your own tax advisor with respect to your own particular circumstances.
The enforcement by investors of civil
liabilities under the United States federal securities laws may be affected adversely by the fact that the Corporation is incorporated
under the laws of Alberta, Canada, that the majority of its officers and directors are residents of Canada, that many of the experts
named in this Prospectus are not residents of the United States, and that a substantial portion of the assets of the Corporation
and said persons are located outside the United States.
NEITHER THE SEC NOR ANY STATE OR CANADIAN
SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED THE SECURITIES OFFERED HEREBY OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR
COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENCE.
All shelf information permitted under applicable
securities legislation to be omitted from this Prospectus including, without limitation, the information disclosed in the specific
terms of any offering of Securities, as discussed above, will be contained in one or more Prospectus Supplements that will be delivered
to purchasers together with this Prospectus, except in cases where an exemption from such delivery requirements has been obtained.
Each Prospectus Supplement will be incorporated by reference into this Prospectus for the purposes of securities legislation as
of the date of such Prospectus Supplement and only for the purposes of the distribution of the Securities to which that Prospectus
Supplement pertains.
We may sell the Securities to or through
one or more underwriters or dealers purchasing as principals and may also sell the Securities to one or more purchasers directly,
through applicable statutory exemptions, or through one or more agents designated by us from time to time. The Securities may be
sold from time to time in one or more transactions at fixed prices or not at fixed prices, such as market prices prevailing at
the time of sale, prices related to such prevailing market prices or prices to be negotiated with purchasers, which prices may
vary as between purchasers and during the period of distribution of the Securities. The Prospectus Supplement relating to a particular
offering of Securities will identify each underwriter, dealer or agent engaged in connection with the offering and sale of such
Securities, as well as the method of distribution and the terms of the offering of such Securities, including the initial offering
price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the event
the offering is not a fixed price distribution), the net proceeds to us and, to the extent applicable, any fees, discounts or any
other compensation payable to underwriters, dealers or agents and any other material terms. See “Plan of Distribution”.
In connection with any offering of the
Securities other than an “at-the-market distribution” (as defined under applicable Canadian legislation) (unless otherwise
specified in the relevant Prospectus Supplement), the underwriters or agents may over-allot or effect transactions that stabilize
or maintain the market price of the offered Securities at a level above that which might otherwise prevail on the open market.
Such transactions, if commenced, may be interrupted or discontinued at any time. See “Plan of Distribution”.
No underwriter or dealer involved in an
“at-the-market distribution” under this Prospectus, no affiliate of such an underwriter or dealer and no person or
company acting jointly or in concert with such underwriter or dealer will over-allot securities in connection with such distribution
or effect any other transactions that are intended to stabilize or maintain the market price of the Securities.
Owning the Securities may subject you to
tax consequences. This Prospectus and any applicable Prospectus Supplement may not describe the tax consequences fully. You should
read the tax discussion in any applicable Prospectus Supplement and consult with your own tax advisor with respect to your own
particular circumstances.
Unless otherwise specified in the applicable
Prospectus Supplement, the Subscription Receipts, Warrants and Units will not be listed on any securities exchange. There is no
market through which these securities may be sold and purchasers may not be able to resell such securities purchased under this
Prospectus. This may affect the pricing of such securities in the secondary market, the transparency and availability of trading
prices, the liquidity of such securities, and the extent of issuer regulation. See “Forward-Looking Statements”
and “Risk Factors”.
Our outstanding Common Shares are listed
for trading on the Toronto Stock Exchange under the trading symbol “ONC” and on the Nasdaq Capital Market under the
trading symbol “ONCY”. On June 11, 2020, the closing price of our Common Shares on the Toronto Stock Exchange and
Nasdaq Capital Market was $2.80 and US$1.95 per Common Share, respectively.
Messrs. Wayne Pisano and Leonard Kruimer
and Drs. William G. Rice and Bernd R. Seizinger are directors of the Corporation who reside outside of Canada. Messrs. Pisano and
Kruimer and Drs. Rice and Seizinger have appointed the Corporation, at its principal place of business, as agent for service of
process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against any person
that resides outside of Canada, even if the party has appointed an agent for service of process.
No underwriter, agent or dealer has
been involved in the preparation of this Prospectus or performed any review of the contents of this Prospectus.
Our head office and principal place of
business is located at 210, 1167 Kensington Crescent N.W., Calgary, Alberta, T2N 1X7. Our registered office is located at
4000, 421 - 7th Avenue S.W., Calgary, Alberta, T2P 4K9.
TABLE
OF CONTENTS
ABOUT THIS PROSPECTUS
AND OTHER MATTERS
In this Prospectus and any Prospectus Supplement,
unless otherwise indicated, references to “we”, “us”, “our”, “issuer”,
“Oncolytics” or the “Corporation” are to Oncolytics Biotech Inc., including, where the context
requires, its subsidiaries and affiliates.
In this Prospectus and in any Prospectus
Supplement, unless otherwise specified or the context otherwise requires, all references to “dollars”, “Cdn.$”
or “$” are to Canadian dollars and all references to “US$” are to United States dollars.
Unless otherwise indicated, all financial
information included and incorporated by reference in this Prospectus and any Prospectus Supplement is determined using IFRS as
issued by IASB and adopted by the Accounting Standards Board of Canada.
This Prospectus provides you with a general
description of the Securities that the Corporation may offer. Each time the Corporation sells Securities under this Prospectus,
the Corporation will file and deliver, except in cases where an exemption from such delivery requirement has been obtained, a Prospectus
Supplement that will contain specific information about the terms of that offering of Securities. The Prospectus Supplement also
may add, update or change information contained in this Prospectus. Before investing, investors should read both this Prospectus
and any applicable Prospectus Supplement together with additional information described under the heading “Documents Incorporated
by Reference”.
You should rely only on the information
contained in or incorporated by reference in this Prospectus or any applicable Prospectus Supplement. The Corporation has not authorized
anyone to provide you with different or additional information. The Corporation is not making an offer of these Securities in any
jurisdiction where the offer is not permitted by law.
FORWARD-LOOKING STATEMENTS
This Prospectus and the documents incorporated
by reference herein contain certain statements relating to future events or the Corporation’s future performance which constitute
forward-looking statements within the meaning of applicable Canadian securities laws and within the meaning of the United States
Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve known and unknown risks, uncertainties
and other factors which may cause the actual results, performance or achievements of the Corporation, or industry results, to be
materially different from any future results, performance or achievements expressed or implied by such forward-looking statements.
Forward-looking statements are statements that are not historical facts, and include, but are not limited to, estimates and their
underlying assumptions; statements regarding plans, objectives and expectations with respect to the efficacy of our technologies;
the timing and results of clinical studies related to our technologies; future operations, products and services; the impact of
regulatory initiatives on our operations; the size of and opportunities related to the markets for our technologies; general industry
and macroeconomic growth rates; expectations related to possible joint and/or strategic ventures and statements regarding future
performance. Forward-looking statements generally, but not always, are identified by the words “expects,” “anticipates,”
“believes,” “intends,” “estimates,” “projects”, “potential”, “possible”
and similar expressions, or that events or conditions “will,” “may,” “could” or “should”
occur.
The forward-looking statements in this
Prospectus are subject to various risks and uncertainties, most of which are difficult to predict and generally beyond our control,
including without limitation:
|
|
·
|
risks related to all of our products, including pelareorep, being in the research and development
stage and requiring further development and testing before they can be marketed commercially;
|
|
|
·
|
risks inherent in pharmaceutical research and development;
|
|
|
·
|
risks related to timing and possible delays in our clinical trials;
|
|
|
·
|
risks related to some of our clinical trials being conducted in, and subject to the laws of, foreign
countries;
|
|
|
·
|
risks related to our pharmaceutical products being subject to intense regulatory approval processes
in the United States and other foreign jurisdictions;
|
|
|
·
|
risks related to being subject to government manufacturing and testing regulations;
|
|
|
·
|
risks related to the extremely competitive biotechnology industry and our competition with larger
companies with greater resources;
|
|
|
·
|
risks related to our reliance on patents and proprietary rights to protect our technology;
|
|
|
·
|
risks related to potential product liability claims;
|
|
|
·
|
risks related to our limited manufacturing experience and reliance on third parties to commercially
manufacture our products, if and when developed;
|
|
|
·
|
risks related to our new products not being accepted by the medical community or consumers;
|
|
|
·
|
risks related to our technologies becoming obsolete;
|
|
|
·
|
risks related to our dependence on third party relationships for research and clinical trials;
|
|
|
·
|
risks related to our license, development, supply and distribution agreement with Adlai Nortye
Biopharma Co. Ltd.;
|
|
|
·
|
risks related to our lack of operating revenues and history of losses;
|
|
|
·
|
uncertainty regarding our ability to obtain third-party reimbursement for the costs of our product;
|
|
|
·
|
risks related to other third-party arrangements;
|
|
|
·
|
risks related to our ability to obtain additional financing to fund future research and development
of our products and to meet ongoing capital requirements;
|
|
|
·
|
risks related to potential increases in the cost of director and officer liability insurance;
|
|
|
·
|
risks related to our dependence on key employees and collaborators;
|
|
|
·
|
risks related to Barbados law, including those relating to the enforcement of judgments obtained
in Canada or the United States;
|
|
|
·
|
risks related to the effect of changes in the law on our corporate structure;
|
|
|
·
|
risks related to expenses in foreign currencies and our exposure to foreign currency exchange rate
fluctuations;
|
|
|
·
|
risks related to data privacy laws;
|
|
|
·
|
risks related to our information technology systems and security breaches;
|
|
|
·
|
risks related to our compliance with the Sarbanes-Oxley Act of 2002, as amended;
|
|
|
·
|
risks related to our status as a foreign private issuer;
|
|
|
·
|
risks related to possible “passive foreign investment company” status;
|
|
|
·
|
risks related to fluctuations in interest rates;
|
|
|
·
|
risks related to information technology systems;
|
|
|
·
|
risks related to business interruptions resulting from pandemics and public health emergencies,
including those related to COVID-19 coronavirus, geopolitical actions, including war and terrorism or natural disasters including
earthquakes, typhoons, floods and fires; and
|
|
|
·
|
risks related to our Securities.
|
This list is not exhaustive of the factors
that may affect any of the Corporation’s forward-looking statements. Some of the important risks and uncertainties that could
affect forward-looking statements are described further under the heading “Risk Factors” in our Annual Report.
If one or more of these risks or uncertainties materializes, or if underlying assumptions prove incorrect, our actual results may
vary materially from those expected, estimated or projected. Forward-looking statements in this document are not a prediction of
future events or circumstances, and those future events or circumstances may not occur. Given these uncertainties, users of the
information included herein, including investors and prospective investors, are cautioned not to place undue reliance on such forward-looking
statements. Investors should consult our quarterly and annual filings with the Canadian and U.S. securities commissions for additional
information on risks and uncertainties relating to forward-looking statements. The Corporation does not undertake any obligation
to publicly update or revise any forward-looking statements other than as required under applicable securities laws.
Prospective investors should carefully
consider the information contained under the heading “Risk Factors” in our Annual Report and all other information
included in or incorporated by reference in this Prospectus before making investment decisions with regard to the Securities.
RISK FACTORS
An investment in the Securities involves
a high degree of risk. Prospective investors should note that there is no market through which the Subscription Receipts, Warrants
or Units may be sold and purchasers may not be able to resell the Subscription Receipts, Warrants or Units purchased under this
Prospectus. This may affect the pricing of these securities in the secondary market, the transparency and availability of trading
prices, the liquidity of the securities, and the extent of issuer regulation.
Prospective investors should consider carefully
the risks described in the documents incorporated by reference in this Prospectus (including in subsequently filed documents incorporated
by reference) and those described in any Prospectus Supplement before purchasing the Securities offered hereby. Discussions of
certain risks affecting the Corporation in connection with its business are provided under the heading “Risk Factors”
in our Annual Report filed with the various securities regulatory authorities, which is incorporated by reference in this Prospectus.
DOCUMENTS INCORPORATED
BY REFERENCE
Information has been incorporated by
reference in this Prospectus from documents filed with securities commissions or similar authorities in Canada. Copies of the
documents incorporated herein by reference may be obtained on request without charge from our Corporate Secretary at 210, 1167
Kensington Crescent N.W., Calgary, Alberta, T2N 1X7 telephone (403) 670-7377, and are available electronically under
the Corporation’s profile on SEDAR (www.sedar.com) and on EDGAR (www.sec.gov/edgar.shtml).
We have filed the following documents with
the securities commissions or similar regulatory authorities in certain of the provinces of Canada and such documents are specifically
incorporated by reference in, and form an integral part of, this Prospectus:
|
|
(a)
|
our annual report on Form 20-F (“Annual Report”) dated March 6, 2020, for the
year ended December 31, 2019 (filed in Canada with certain Canadian securities regulatory authorities as our annual information
form for the year ended December 31, 2019);
|
|
|
(c)
|
our audited consolidated financial statements, together with the notes thereto, as at December
31, 2019 and 2018, which comprise the consolidated statements of financial position as at December 31, 2019 and 2018, and the consolidated
statements of loss and comprehensive loss, changes in equity, and cash flows for the years ended December 31, 2019, 2018 and 2017,
together with the independent auditors’ report thereon; and
|
|
|
(e)
|
our unaudited interim consolidated financial statements, together with the notes thereto, as at
March 31, 2020, which comprise the interim consolidated statements of financial position as at March 31, 2020 and December 31,
2019, and the interim consolidated statements of income (loss) and comprehensive income (loss), changes in equity, and cash flows
for the three months ended March 31, 2020 and 2019; and
|
Any documents of the type required by National
Instrument 44-101 - Short Form Prospectus Distributions to be incorporated by reference in a short form prospectus, including
any annual information form, annual report on Form 20-F, comparative annual consolidated financial statements and the auditors’
report thereon, comparative interim consolidated financial statements, management’s discussion and analysis of financial
condition and results of operations, material change report (except a confidential material change report), business acquisition
report and information circular, if filed by us with the securities commissions or similar authorities in Canada after the date
of this Prospectus and prior to the date which is 25 months from the date of this Prospectus, shall be deemed to be incorporated
by reference in this Prospectus.
In addition, to the extent that any document
or information incorporated by reference into this Prospectus is included in any report filed with or furnished to the SEC pursuant
to the United States Securities Exchange Act of 1934, as amended (the “U.S. Exchange Act”), after the date of
this Prospectus, such document or information shall be deemed to be incorporated by reference as an exhibit to the registration
statement of which this Prospectus forms a part (in the case of documents or information deemed furnished on Form 6-K or Form 8-K,
only to the extent specifically stated therein)
Any statement contained in this Prospectus
or in a document incorporated or deemed to be incorporated by reference herein will be deemed to be modified or superseded for
the purposes of this Prospectus to the extent that a statement contained in this Prospectus or in any other subsequently filed
document which also is, or is deemed to be, incorporated by reference into this Prospectus modifies or supersedes that statement.
The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other
information set forth in the document that it modifies or supersedes. The making of a modifying or superseding statement shall
not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation,
an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary
to make a statement not misleading in light of the circumstances in which it was made. Any statement so modified or superseded
shall not be deemed, except as so modified or superseded, to constitute part of this Prospectus.
Upon a new annual information form and
the related audited annual financial statements and management’s discussion and analysis being filed by us with the applicable
securities regulatory authorities during the term of this Prospectus, the previous annual information form, the previous audited
annual financial statements and related management’s discussion and analysis, all unaudited interim financial statements
and related management’s discussion and analysis, material change reports and business acquisition reports filed prior to
the commencement of our financial year in which the new annual information form and the related audited annual financial statements
and management’s discussion and analysis are filed shall be deemed no longer to be incorporated into this Prospectus for
purposes of future offers and sales of Securities hereunder. Upon new interim financial statements and related management’s
discussion and analysis being filed by us with the applicable securities regulatory authorities during the term of this Prospectus,
all interim financial statements and related management’s discussion and analysis filed prior to the new interim consolidated
financial statements and related management’s discussion and analysis shall be deemed no longer to be incorporated into this
Prospectus for purposes of future offers and sales of Securities hereunder. Upon a new information circular relating to an annual
general meeting of holders of Common Shares being filed by us with the applicable securities regulatory authorities during the
term of this Prospectus, the information circular for the preceding annual general meeting of holders of Common Shares shall be
deemed no longer to be incorporated into this Prospectus for purposes of future offers and sales of Securities hereunder.
Any “template version” of any
“marketing materials” (as such terms are defined in National Instrument 41-101) pertaining to a distribution of Securities
will be filed under the Corporation’s profile on SEDAR (www.sedar.com). In the event that such marketing materials are filed
subsequent to the date of filing of the applicable prospectus supplement pertaining to the distribution of the Securities to which
such marketing materials relates and prior to the termination of such distribution, such filed versions of the marketing materials
will be deemed to be incorporated by reference into the Prospectus for purposes of future offers and sales of Securities hereunder.
One or more Prospectus Supplements containing
the specific variable terms for an issue of the Securities and other information in relation to such Securities will be delivered
to purchasers of such Securities together with this Prospectus, except in cases where an exemption from such delivery requirement
has been obtained, and will be deemed to be incorporated by reference into this Prospectus as of the date of the Prospectus Supplement
solely for the purposes of the offering of the Securities covered by any such Prospectus Supplement.
DOCUMENTS FILED AS
PART OF THE REGISTRATION STATEMENT
The following documents have been or will
be filed with the SEC as part of the registration statement of which this Prospectus forms a part: (i) the documents set out
under the heading “Documents Incorporated by Reference”; (ii) the consent of the Corporation’s auditor;
and (iii) the powers of attorney from the directors and certain officers of the Corporation. A copy of the form of warrant
indenture, unit indenture or subscription receipt agreement, as applicable, will be filed by post-effective amendment or by incorporation
by reference to documents filed or furnished with the SEC under the U.S. Exchange Act.
ADDITIONAL INFORMATION
The Corporation has filed with the SEC
a registration statement on Form F-10 relating to the Securities. This Prospectus, which constitutes a part of the registration
statement, does not contain all of the information contained in the registration statement, certain items of which are contained
in the exhibits to the registration statement as permitted by the rules and regulations of the SEC. See “Documents Filed
as Part of the Registration Statement”. Statements included or incorporated by reference in this Prospectus about the
contents of any contract, agreement or other documents referred to are not necessarily complete, and in each instance you should
refer to the exhibits to the registration statement for a more complete description of the matter involved. The registration statement,
and the items of information omitted from this Prospectus but contained in the registration statement, will be available on EDGAR
(www.sec.gov/edgar.shtml). Each time we sell Securities under the registration statement, we will provide a Prospectus Supplement
that will contain specific information about the terms of that offering. The Prospectus Supplement may also add to, update or change
information contained in this Prospectus.
The Corporation is subject to the information
requirements of the U.S. Exchange Act and applicable Canadian securities legislation and, in accordance therewith, files and
furnishes annual and quarterly financial information and material change reports, business acquisition reports and other material
with the securities commission or similar regulatory authority in each of the provinces of Canada and with the SEC. Under MJDS
adopted by the United States and Canada, documents and other information that the Corporation files with the SEC may be prepared
in accordance with the disclosure requirements of Canada, which are different from those of the United States. As a foreign private
issuer within the meaning of rules made under the U.S. Exchange Act, the Corporation is exempt from the rules under the U.S. Exchange
Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders
are exempt from the reporting and shortswing profit recovery provisions contained in Section 16 of the U.S. Exchange
Act. In addition, the Corporation is not required to publish financial statements as promptly as United States companies.
The Corporation’s
reports and other information filed or furnished with or to the SEC are available from the SEC’s Electronic Document Gathering
and Retrieval System, or EDGAR, at www.sec.gov, as well as from commercial document retrieval services. The Corporation’s
Canadian filings are available on the System for Electronic Document Analysis and Retrieval, or SEDAR, at www.sedar.com.
THE CORPORATION
Oncolytics Biotech Inc. was incorporated
pursuant to the ABCA on April 2, 1998 as 779738 Alberta Ltd. On April 8, 1998, we amended our articles of incorporation (the “Articles”)
and changed our name to Oncolytics Biotech Inc. On July 29, 1999, we amended our Articles by removing the private company restrictions
included therein and subdivided the 2,222,222 Common Shares issued and outstanding into 6,750,000 Common Shares. On February 9,
2007, we amended our Articles to permit shareholder meetings to be held at any place in Alberta or at any other location as determined
by our board of directors. On May 22, 2018, we amended our Articles of Incorporation to effect a consolidation of the Common Shares
on the basis of 9.5 pre-consolidation Common Shares for each one post-consolidation Common Share.
We have two material operating subsidiaries:
Oncolytics Biotech (Barbados) Inc. and Oncolytics Biotech (US) Inc., a Delaware corporation. Oncolytics Biotech (Barbados) Inc.
is incorporated pursuant to the laws of Barbados and is a wholly-owned direct subsidiary of the Corporation. Oncolytics Biotech
(U.S.) Inc. is incorporated pursuant to the laws of Delaware and is a wholly-owned direct subsidiary or Oncolytics Biotech (Barbados)
Inc.
Our head office and principal place of
business is located at 210, 1167 Kensington Crescent N.W., Calgary, Alberta, T2N 1X7. Our registered office is located at 4000,
421 - 7th Avenue S.W., Calgary, Alberta, T2P 4K9.
BUSINESS OF THE CORPORATION
General
Since our inception in April of 1998, Oncolytics
Biotech Inc. has been a development stage company and we have focused our research and development efforts related to pelareorep,
a systemically administered immuno-oncology (“I-O”) viral agent with the potential to treat a variety of cancers.
We have not been profitable since our inception and expect to continue to incur substantial losses as we continue research and
development efforts. We do not expect to generate significant revenues until, if and when, pelareorep becomes commercially viable.
Our potential product for human use, pelareorep,
an unmodified reovirus, is a first in class systemically administered I-O viral agent for the treatment of solid tumors and hematological
malignancies.
Further information regarding the business
of the Corporation is contained in the Annual Report under the heading “Item 4 – Information on the Company”,
which document is incorporated by reference in this Prospectus. See “Documents Incorporated by Reference.”
Recent Developments
On
May 14, 2020 we announced the publication of two abstracts in
connection with the upcoming ASCO Virtual Annual Meeting on May 29-31. The first abstract ("Multiple Myeloma
Abstract"), which has been accepted as an electronic poster, reports on viral replication,
tumor immune responses, and treatment safety in multiple myeloma patients treated with pelareorep in combination
with carfilzomib (Kyprolis®). The second abstract ("Pancreatic Cancer Abstract")
reports on treatment tolerability and efficacy in pancreatic cancer patients treated with pelareorep, in
combination with pembrolizumab (Keytruda®).
The
Multiple Myeloma Abstract highlighted the following:
|
|
·
|
Reported selective infection of cancerous cells with pelareorep,
with associated CD8+ and natural killer (NK) cell recruitment, PD-L1 upregulation, activated caspase-3 expression, and increased
pelareorep protein production within myeloma cells.
|
|
|
·
|
Two patients achieved unconfirmed partial responses, two patients
had stable disease, and two patients had progressive disease. All patients had advanced and challenging to treat carfilzomib-refractory
disease.
|
|
|
·
|
Of the two responding patients, one patient developed a fever
and grade 4 thrombocytopenia that resolved, and the other patient developed a cytokine storm, associated with rapid tumor lysis.
|
|
|
·
|
Data supports the potential of future studies investigating pelareorep,
carfilzomib, and immune checkpoint inhibitor combination therapy in multiple myeloma patients.
|
|
|
·
|
Additional data to be presented at the poster session on May
29, 2020.
|
The
Pancreatic Cancer Abstract highlighted the following:
|
|
·
|
Preliminary data indicate that the combination of pelareorep
and pembrolizumab resulted in tumor-specific replication, a high degree of T cell repertoire turnover, and the creation of new
T cell clones in the peripheral blood during treatment.
|
|
|
·
|
The treatment was found to be well tolerated, with most treatment-related
adverse events being grade 1 or 2.
|
|
|
·
|
One patient achieved a partial response and three achieved stable
disease, with an overall disease control rate of 30% in evaluable patients.
|
|
|
·
|
The study will not proceed to stage 2 in unselected patients,
however further evaluation of the anti-tumor activity of pelareorep and anti-PD-1 therapy is now planned in biomarker defined pancreatic
patients in a subsequent study.
|
|
|
·
|
Detailed translational and biomarker data from this study will
be published later in the year.
|
On May 26, 2020, we announced the publication
of an electronic-poster (“ePoster”) with clinical data from the Corporation's AWARE-1 window-of-opportunity
breast cancer study. The data demonstrates a pelareorep-induced adaptive immune response in the tumor microenvironment (“TME”)
and the potential of a predictive biomarker (T cell clonality) to identify patients with breast cancer most likely to respond to
pelareorep. The ePoster was published on May 23, 2020 and presented as part of the European Society for Medical Oncology Breast
Cancer Virtual Meeting.
Key data and conclusions
from this ePoster include:
|
|
·
|
Intravenous systemic administration resulted in tumor cell-specific pelareorep replication.
|
|
|
·
|
All patients treated with pelareorep demonstrated an increase in CD8+ T cells as confirmed in tumor
biopsies (range of 1.6-fold to 11.2-fold increase).
|
|
|
·
|
All patients treated with pelareorep experienced an increase in the number of PD-L1 positive cells
in their tumors in as early as three weeks after beginning treatment (range of 1.3-fold to 11.0-fold increase).
|
|
|
·
|
Four out of six evaluated patients exhibited an increase in tumor cellularity and tumor-infiltrating
lymphocytes (“CelTIL”), which is associated with favorable clinical response, the study's primary endpoint.
|
|
|
·
|
Peripheral T cell clonality correlated with changes in the TME and CelTIL, highlighting its potential
as a compelling biomarker of pelareorep response in breast cancer.
|
The results show intravenous delivery of
pelareorep reaches the primary breast cancer tumor target. Importantly, the large increase in CD8+ T cells is prognostic
of positive treatment outcomes, and the increased expression of PD-L1 suggests a treatment synergy when combining pelareorep and
anti-PD-L1 checkpoint inhibitors, such as Tecentriq®. The correlation between T cell clonality and tumor inflammation continues
to show promise across multiple indications and increases our ability to identify patients likely to respond to treatment.
On May 29, 2020, we announced the publication
of an ePoster with clinical proof-of-concept data from the Corporation's phase 1b study in carfilzomib-refractory multiple myeloma
patients treated with pelareorep in combination with carfilzomib (Kyprolis®). Data presented in this ePoster demonstrates that
the pelareorep-carfilzomib combination treatment results in selective replication of pelareorep in cancer cells and beneficial
induction of an inflamed tumor environment associated with clinical responses. The ePoster was published and presented as part
of the American Society of Clinical Oncology Virtual Annual Meeting.
The ePoster, Oncolytic
virus Pelareorep [P] plus Carfilzomib & Dexamethasone [Kd] phase 1 trial in Carfilzomib-refractory patients (NCI9603): responses
with cytokine storm was co-authored by Dr. Douglas W. Sborov MD, MS, Assistant Professor, Division of Hematology and Hematologic
Malignancies, University of Utah – Huntsman Cancer Institute, and Craig Hofmeister, M.D., MPH, Associate Professor, Department
of Hematology and Medical Oncology Emory University School of Medicine, as well as several other colleagues at institutions across
the United States. Key data and conclusions from six patients in the study are presented in this ePoster and include:
|
|
·
|
Pelareorep targets and selectively replicates in multiple myeloma tumor cells.
|
|
|
·
|
Pelareorep, when combined with carfilzomib, activates a profound inflammatory response accompanied
by a 50% overall response rate and 83% clinical benefit rate.
|
|
|
·
|
Three partial responses, one minimal response, one stable disease, and one progressive disease were
achieved among patients with advanced and difficult-to-treat carfilzomib-refractory disease.
|
|
|
·
|
Significant and rapid T cell activation led to a single incidence of cytokine storm associated with
tumor response after treatment with pelareorep and carfilzomib.
|
CONSOLIDATED CAPITALIZATION
Since March 31, 2020, the Corporation has
issued:
|
|
(a)
|
an aggregate of 2,449,658 Common Shares pursuant to an equity distribution agreement dated October
24, 2018 with Canaccord Genuity LLC at prices ranging from US$1.34 to US$2.34 per Common Share, with the weighted average price
being US$1.73 per Common Share;
|
|
|
(b)
|
an aggregate of 213,181 Common Shares upon the exercise of Common Share purchase warrants at a
price of US$0.90 per Common Share;
|
|
|
(c)
|
an
aggregate of 7,324 Common Shares upon the exercise of outstanding stock options granted
pursuant to its amended and restated stock option plan at a price of $2.41 per Common
Share; and
|
|
|
(d)
|
an
aggregate of 4,734 Common Shares upon the vesting of restricted share awards granted
pursuant to its amended and restated incentive share award plan.
|
Except as set forth above, there has been
no material change in the share and loan capital of the Corporation on a consolidated basis since March 31, 2020.
USE OF PROCEEDS
The use of proceeds from the issue and
sale of specific Securities pursuant to this Prospectus will be described in the Prospectus Supplement relating to the issuance
and sale of such Securities.
DESCRIPTION OF SHARE
CAPITAL
Authorized Capital
Our authorized capital consists of an unlimited
number of Common Shares. The following is a summary of the provisions attached to our Common Shares.
Common Shares
The holders of our Common Shares are entitled
to one vote per share at meetings of shareholders, to receive such dividends as declared by the Board and to receive our remaining
property and assets upon dissolution or wind up. Our Common Shares are not subject to any future call or assessment and there are
no pre-emptive, conversion or redemption rights attached to such shares.
As at the date hereof, we have 40,492,010
Common Shares issued and outstanding. After giving effect to the exercise of all outstanding options to acquire Common Shares
and all outstanding share awards granted under the Corporation’s Incentive Share Award Plan, we would have 42,938,570 Common
Shares issued and outstanding.
Common Share Purchase
Warrants
As of the date hereof, we have 16,443,500
Common Share purchase warrants (the “2017 Warrants”) issued and outstanding. Each 9.5 2017 Warrants entitles
the holder to purchase one Common Share until June 1, 2022, at an exercise price of $9.025. The 2017 Warrants are subject to acceleration
if the volume weighted average price of the Common Shares equals or exceeds $2.50 for a period of 15 consecutive trading dates.
As of the date hereof, we have 265,757
Common Share purchase warrants (the “2019 Warrants”) issued and outstanding. Each 2019 Warrant entitles the
holder to purchase one common share at an exercise price of US$0.90 until August 16, 2024.
In addition, as of the date hereof, the
Corporation has outstanding a Common Share purchase warrant (the “Adlai Warrant”) exercisable by the holder
thereof until November 14, 2020 to purchase such number of Common Shares as is calculated by dividing US$6,000,000 by the Exercise
Price.
For purposes of the Adlai Warrant, the
term “Exercise Price” means an amount equal to 120% of the volume weighted average trading price of the Common
Shares on the Nasdaq Capital Market (or, if the Common Shares are not then listed on the Nasdaq Capital Market, on the Toronto
Stock Exchange) for the five trading days immediately preceding the exercise date.
The Adlai Warrant is subject to a right
to call by the Corporation upon the date of the enrollment of the fiftieth (50th) patient in a Phase 3 Study related
to pelareorep.
DESCRIPTION OF SUBSCRIPTION
RECEIPTS
The following description of the terms
of Subscription Receipts sets forth certain general terms and provisions of Subscription Receipts in respect of which a Prospectus
Supplement may be filed. The particular terms and provisions of Subscription Receipts offered by any Prospectus Supplement, and
the extent to which the general terms and provisions described below may apply thereto, will be described in the Prospectus Supplement
filed in respect of such Subscription Receipts.
Subscription Receipts may be offered separately
or in combination with one or more other Securities. The Subscription Receipts will be issued under a subscription receipt agreement
(the ”Subscription Receipt Agreement”). A copy of the Subscription Receipt Agreement will be filed by us
with the applicable securities regulatory authorities after it has been entered into by us and will be available electronically
under the Corporation’s profile on SEDAR (www.sedar.com) and, if applicable, we will file with the SEC via EDGAR (www.sec.gov/edgar.shtml)
as exhibits to the registration statement of which this Prospectus is a part, or will incorporate by reference from a Report of
Foreign Private Issuer on Form 6-K that we file with the SEC, any Subscription Agreement describing the terms and conditions of
such Subscription Receipts that we are offering before the issuance of such Subscription Receipts.
Pursuant to the Subscription Receipt Agreement,
original purchasers of Subscription Receipts will have a contractual right of rescission against the Corporation, following the
issuance of the underlying Common Share or other securities to such purchasers upon the surrender or deemed surrender of the Subscription
Receipts, to receive the amount paid for the Subscription Receipts in the event that this Prospectus or a Prospectus Supplement,
and any amendment thereto, contains a misrepresentation or is not delivered to such purchaser, provided such remedy for rescission
is exercised within 180 days from the closing date of the offering of Subscription Receipts.
The description of general terms and provisions
of Subscription Receipts described in any Prospectus Supplement will include, where applicable:
|
|
·
|
the number of Subscription Receipts offered;
|
|
|
·
|
the price at which the Subscription Receipts will be offered;
|
|
|
·
|
if other than Canadian dollars, the currency or currency unit in which the Subscription Receipts
are denominated;
|
|
|
·
|
the procedures for the exchange of the Subscription Receipts into Common Shares or other securities;
|
|
|
·
|
the number of Common Shares or other securities that may be obtained upon exercise of each Subscription
Receipt;
|
|
|
·
|
the designation and terms of any other Securities with which the Subscription Receipts will be
offered, if any, and the number of Subscription Receipts that will be offered with each Security;
|
|
|
·
|
the terms applicable to the gross proceeds from the sale of the Subscription Receipts plus any
interest earned thereon;
|
|
|
·
|
the material Canadian tax consequences of owning such Subscription Receipts; and
|
|
|
·
|
any other material terms, conditions and rights (or limitations on such rights) of the Subscription
Receipts.
|
We reserve the right to set forth in a
Prospectus Supplement specific terms of the Subscription Receipts that are not within the options and parameters set forth in this
Prospectus. In addition, to the extent that any particular terms of the Subscription Receipts described in a Prospectus Supplement
differ from any of the terms described in this Prospectus, the description of such terms set forth in this Prospectus shall be
deemed to have been superseded by the description of such differing terms set forth in such Prospectus Supplement.
DESCRIPTION OF WARRANTS
The following description of the terms
of Warrants sets forth certain general terms and provisions of Warrants in respect of which a Prospectus Supplement may be filed.
The particular terms and provisions of Warrants offered by any Prospectus Supplement, and the extent to which the general terms
and provisions described below may apply thereto, will be described in the Prospectus Supplement filed in respect of such Warrants.
Warrants may be offered separately or in combination with one or more other Securities. If applicable, we will file with the SEC
as exhibits to the registration statement of which this Prospectus is a part, or will incorporate by reference from a current report
on Form 6-K that we file with the SEC, any warrant indenture or form of warrant describing the terms and conditions of such Warrants
that we are offering before the issuance of such Warrants.
The description of general terms and provisions
of Warrants described in any Prospectus Supplement will include, where applicable:
|
|
·
|
the designation and aggregate number of Warrants offered;
|
|
|
·
|
the price at which the Warrants will be offered;
|
|
|
·
|
if other than Canadian dollars, the currency or currency unit in which the Warrants are denominated;
|
|
|
·
|
the designation and terms of the Common Shares that may be acquired upon exercise of the Warrants;
|
|
|
·
|
the date on which the right to exercise the Warrants will commence and the date on which the right
will expire;
|
|
|
·
|
the number of Common Shares that may be purchased upon exercise of each Warrant and the price at
which and currency or currencies in which that amount of securities may be purchased upon exercise of each Warrant;
|
|
|
·
|
the designation and terms of any Securities with which the Warrants will be offered, if any, and
the number of the Warrants that will be offered with each Security;
|
|
|
·
|
the date or dates, if any, on or after which the Warrants and the related Securities will be transferable
separately;
|
|
|
·
|
the minimum or maximum amount, if any, of Warrants that may be exercised at any one time;
|
|
|
·
|
whether the Warrants will be subject to redemption or call, and, if so, the terms of such redemption
or call provisions; and
|
|
|
·
|
any other material terms, conditions and rights (or limitations on such rights) of the Warrants.
|
We reserve the right to set forth in a
Prospectus Supplement specific terms of the Warrants that are not within the options and parameters set forth in this Prospectus.
In addition, to the extent that any particular terms of the Warrants described in a Prospectus Supplement differ from any of the
terms described in this Prospectus, the description of such terms set forth in this Prospectus shall be deemed to have been superseded
by the description of such differing terms set forth in such Prospectus Supplement.
DESCRIPTION OF UNITS
We may issue Units comprised of one or
more of the other Securities described in this Prospectus in any combination. Each Unit will be issued so that the holder of the
Unit is also the holder of each Security included in the Unit. Thus, the holder of a Unit will have the rights and obligations
of a holder of each included Security. The unit agreement, if any, under which a Unit is issued may provide that the Securities
comprising the Unit may not be held or transferred separately, at any time or at any time before a specified date. If applicable,
we will file with the SEC as exhibits to the registration statement of which this Prospectus is a part, or will incorporate by
reference from a current report on Form 6-K that we file with the SEC, any unit agreement describing the terms and conditions of
such Units that we are offering before the issuance of such Units.
The particular terms and provisions of
Units offered by any Prospectus Supplement, and the extent to which the general terms and provisions described below may apply
to them, will be described in the Prospectus Supplement filed in respect of such Units.
The particular terms of each issue of Units
will be described in the related Prospectus Supplement. This description will include, where applicable:
|
|
·
|
the designation and aggregate number of Units offered;
|
|
|
·
|
the price at which the Units will be offered;
|
|
|
·
|
if other than Canadian dollars, the currency or currency unit in which the Units are denominated;
|
|
|
·
|
the terms of the Units and of the Securities comprising the Units, including whether and under
what circumstances those securities may be held or transferred separately;
|
|
|
·
|
any provisions for the issuance, payment, settlement, transfer or exchange of the Units or of the
Securities comprising the Units; and
|
|
|
·
|
any other material terms, conditions and rights (or limitations on such rights) of the Units.
|
We reserve the right to set forth in a
Prospectus Supplement specific terms of the Units that are not within the options and parameters set forth in this Prospectus.
In addition, to the extent that any particular terms of the Units described in a Prospectus Supplement differ from any of the terms
described in this Prospectus, the description of such terms set forth in this Prospectus shall be deemed to have been superseded
by the description of such differing terms set forth in such Prospectus Supplement with respect to such Units.
PLAN OF DISTRIBUTION
We may sell the Securities to or through
one or more underwriters or dealers purchasing as principals and we may also sell the Securities to one or more purchasers directly,
through applicable statutory exemptions, or through one or more agents designated from time to time. The Securities may be sold
from time to time in one or more transactions at fixed prices or not at fixed prices, such as market prices prevailing at the time
of sale, prices related to such prevailing market prices or prices to be negotiated with purchasers, which prices may vary as between
purchasers and during the period of distribution of the Securities. The Prospectus Supplement relating to a particular offering
and sale of Securities will identify each underwriter, dealer or agent engaged in connection with the offering and sale of such
Securities, as well as the method of distribution and the terms of the offering and sale of such Securities, including the initial
offering price (in the event the offering is a fixed price distribution), the manner of determining the offering price(s) (in the
event the offering is not a fixed price distribution), the net proceeds to us and, to the extent applicable, any fees, discounts
or any other compensation payable to underwriters, dealers or agents and any other material terms. Only underwriters so named in
the Prospectus Supplement are deemed to be underwriters in connection with the Securities offered and sold thereby.
If the underwriters purchase Securities
from us as principal, the Securities will be acquired by the underwriters for their own account and may be resold from time to
time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined
at the time of sale, at market prices prevailing at the time of sale or at prices related to such prevailing market prices. The
obligations of the underwriters to purchase such Securities as principal will be subject to certain conditions precedent, and the
underwriters will be obligated to purchase all the Securities offered and sold by the Prospectus Supplement if any of such Securities
are purchased. Any public offering price and any discounts or concessions allowed or re-allowed or paid to underwriters, dealers
or agents may be changed from time to time.
The Securities may be sold from time
to time in one or more transactions at a fixed price or prices which may be changed or at market prices prevailing at the time
of sale, at prices related to such prevailing market prices or at negotiated prices. The prices at which the Securities may be
offered may vary as between purchasers and during the period of distribution. If, in connection with the offering of Securities
at a fixed price or prices, the underwriters have made a bona fide effort to sell all of the Securities at the initial
offering price fixed in the applicable Prospectus Supplement, the public offering price may be decreased and thereafter further
changed, from time to time, to an amount not greater than the initial public offering price fixed in such Prospectus Supplement,
in which case the compensation realized by the underwriters will be decreased by the amount that the aggregate price paid by purchasers
for the Securities is less than the gross proceeds paid to us by the underwriters. Any such reduction to the public offering price
will not affect the net proceeds received by the Corporation.
The Securities may also be sold directly
by us, pursuant to applicable statutory exemptions, at such prices and upon such terms as agreed to by us and the purchaser or
through one or more agents designated by us from time to time. Any agent involved in the offering and sale of the Securities in
respect of which this Prospectus is delivered will be named, and any commissions payable by us to such agent will be set forth,
in the Prospectus Supplement. Unless otherwise indicated in the Prospectus Supplement, any agent would be acting on a best efforts
basis for the period of its appointment.
We may agree to pay the underwriters a
commission for various services relating to the issue and sale of any Securities offered hereby. Any such commission will be paid
out of our general funds. Underwriters, dealers and agents who participate in the distribution of the Securities may be entitled
under agreements to be entered into with us to indemnification by us against certain liabilities under securities legislation,
or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof.
Any offering of Subscription Receipts,
Warrants or Units will be a new issue of securities with no established trading market. Unless otherwise specified in the applicable
Prospectus Supplement, the Subscription Receipts, Warrants or Units will not be listed on any securities exchange. Certain dealers
may make a market in these Securities, but will not be obligated to do so and may discontinue any market making at any time without
notice. No assurance can be given that any dealer will make a market in these Securities or as to the liquidity of the trading
market, if any, for these Securities. See “Risk Factors”.
Unless otherwise specified in a Prospectus
Supplement, in connection with any offering of the Securities, the underwriters or agents may over-allot or effect transactions
which stabilize or maintain the market price of the Securities offered at a higher level than that which might exist in the open
market. Such transactions, if commenced, may be interrupted or discontinued at any time.
PRIOR SALES
Information regarding prior sales of Securities
will be provided as required in a Prospectus Supplement with respect to the issuance of Securities pursuant to such Prospectus
Supplement.
TRADING PRICE AND
VOLUME
Information regarding trading price and
volume of the Securities will be provided as required for all of the Corporation’s issued and outstanding Securities that
are listed on any securities exchange, as applicable, in each Prospectus Supplement.
CERTAIN INCOME TAX
CONSIDERATIONS
The applicable Prospectus Supplement may
describe certain Canadian federal income tax consequences which may be applicable to a purchaser of Securities offered thereunder,
and may also include a discussion of certain United States federal income tax consequences to the extent applicable.
LEGAL MATTERS AND
INTEREST OF EXPERTS
Unless otherwise specified in the Prospectus
Supplement relating to an offering and sale of Securities, certain legal matters relating to such offering and sale of Securities
will be passed upon on behalf of the Corporation by McCarthy Tétrault LLP with respect to matters of Canadian law and Dorsey
& Whitney LLP, with respect to matters of U.S. law. In addition, certain legal matters in connection with an offering and sale
of Securities will be passed upon for any underwriters, dealers or agents by counsel to be designated at the time of such offering
and sale by such underwriters, dealers or agents with respect to matters of Canadian and, if applicable, United States or other
foreign law. As at the date hereof, the partners and associates of McCarthy Tétrault LLP, as a group, own less than 1% of
the outstanding securities of the Corporation.
AUDITORS, TRANSFER
AGENT AND REGISTRAR
The auditors of the Corporation are Ernst
& Young LLP, Chartered Accountants, Calgary City Centre, 2200, 215 – 2nd Street S.W., Calgary, Alberta, T2P
1M4. Ernst & Young LLP is independent of the Corporation in accordance with the Rules of Professional Conduct as outlined by
the Chartered Professional Accountants of Alberta. Ernst & Young LLP is registered with the U.S. Public Corporation Accounting
Oversight Board.
The transfer agent and registrar for the
Common Shares is AST Trust Company (Canada) at its principal offices located in Montreal, Quebec.
ENFORCEABILITY OF
CIVIL LIABILITIES AGAINST NON-U.S. PERSONS
The Corporation is a corporation existing
under the Business Corporations Act (Alberta). Most of the Corporation’s directors and officers, and some or all of
the experts named in this Prospectus, are residents of Canada or otherwise reside outside the United States, and all or a substantial
portion of their assets, and substantially all of the Corporation’s assets, are located outside the United States. The Corporation
has appointed an agent for service of process in the United States, but it may be difficult for holders of Securities who reside
in the United States to effect service within the United States upon those directors, officers and experts who are not residents
of the United States. It may also be difficult for holders of Securities who reside in the United States to realize in the United
States upon judgments of courts of the United States predicated upon the Corporation’s civil liability and the civil liability
of its directors, officers and experts under the United States federal securities laws.
The Corporation filed with the SEC, concurrently
with its registration statement on Form F-10 of which this Prospectus is a part, an appointment of agent for service of process
on Form F-X. Under the Form F-X, the Corporation appointed DL Services Inc. as its agent for service of process
in the United States in connection with any investigation or administrative proceeding conducted by the SEC, and any civil suit
or action brought against or involving the Corporation in a United States court arising out of or related to or concerning the
offering of the Securities under this Prospectus.
AGENT FOR SERVICE
OF PROCESS
Messrs. Wayne Pisano and Leonard Kruimer
and Drs. William G. Rice and Bernd R. Seizinger are directors are directors of the Corporation who reside outside of Canada. Messrs.
Pisano and Kruimer and Drs. Rice and Seizinger have appointed the Corporation, at its principal place of business, as agent for
service of process. Purchasers are advised that it may not be possible for investors to enforce judgments obtained in Canada against
any person that resides outside of Canada, even if the party has appointed an agent for service of process.
Oncolytics Biotech (NASDAQ:ONCY)
Historical Stock Chart
From Mar 2024 to Apr 2024
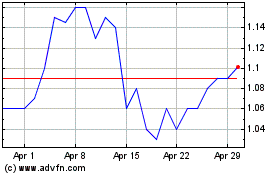
Oncolytics Biotech (NASDAQ:ONCY)
Historical Stock Chart
From Apr 2023 to Apr 2024