Report of Foreign Issuer (6-k)
January 03 2020 - 6:06AM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
Under the Securities Exchange Act of 1934
For the month of January 2020
Commission File Number 001-38716
GAMIDA CELL LTD.
(Translation of registrant’s name into
English)
5 Nahum Heftsadie Street
Givaat Shaul, Jerusalem 91340 Israel
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will
file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the
Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the
Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7): ☐
On January 2, 2020, Gamida Cell Ltd. (the “Company”)
issued a press release, a copy of which is furnished as Exhibit 99.1 to this Form 6-K, announcing the completion of patient
enrollment in the Company’s ongoing Phase 3 clinical study of omidubicel. Omidubicel, the Company’s lead clinical
program, is an advanced cell therapy under development as a potential life-saving bone marrow transplant solution for patients
with hematologic malignancies.
The international, multi-center,
randomized Phase 3 study is designed to evaluate the safety and efficacy of omidubicel compared to standard umbilical cord blood
in patients with high-risk hematologic malignancies who need a bone marrow transplant and do not have an available matched donor.
The primary endpoint is time to neutrophil engraftment. The study includes approximately 120 patients aged 12-65 with acute lymphoblastic
leukemia, acute myelogenous leukemia, chronic myelogenous leukemia, myelodysplastic syndrome or lymphoma. The study is taking
place at over 50 clinical centers in the U.S., Latin America, Europe and Asia. Topline data from the study are expected in the
first half of 2020.
This report on Form 6-K is hereby
incorporated by reference into the Company’s Registration Statement on Form F-3 (File No. 333-234701).
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act
of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
GAMIDA CELL LTD.
|
|
January 2, 2020
|
|
|
|
By:
|
|
|
|
|
|
|
|
|
|
Shai Lankry
|
|
|
|
|
|
|
|
Chief Financial Officer
|
Gamida Cell (NASDAQ:GMDA)
Historical Stock Chart
From Mar 2024 to Apr 2024
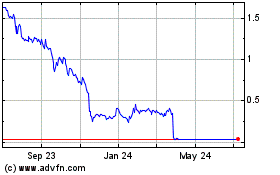
Gamida Cell (NASDAQ:GMDA)
Historical Stock Chart
From Apr 2023 to Apr 2024