PART I
ITEM 1. Business
.
Overview
Osiris Therapeutics, Inc., together with our wholly‑owned subsidiary, Osiris Therapeutics International GmbH (collectively, “we,” “us,” “our,” or the “Company”), researches, develops, manufactures and commercializes regenerative medicine products intended to improve the health and lives of patients and lower overall healthcare costs. We have achieved commercial success with products in wound care, orthopedics, and sports medicine, including the Grafix product line, Stravix, BIO
4
and Cartiform. We continue to advance our research and development (“R&D”) by focusing on innovation in regenerative medicine, including the development of bioengineered stem cell and tissue‑based products.
We began operations in Ohio in 1992 as a Delaware corporation. With the approval of our stockholders we reincorporated as a Maryland corporation in May 2010. Our principal place of business is located at 7015 Albert Einstein Drive, Columbia, MD 21046, and our telephone number is (443) 545‑1800.
Osiris, Osiris and design, Grafix
,
Grafix CORE, Grafix PRIME,
Grafix
XC,
GrafixPL, Stravix, Cartiform, and TruSkin are our trademarks, many of which are federally-registered trademarks in the United States. The trademark registration applications for GrafixPL PRIME and Prestige Lyotechnology are currently pending in the United States.
BIO
4
is a trademark of Howmedica Osteonics Corp., a subsidiary of Stryker Corporation (“Stryker”). Prochymal is a trademark of Mesoblast Limited.
Agreement and Plan of Merger
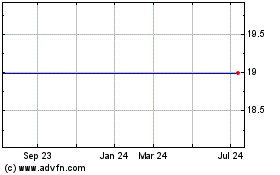
On March 12, 2019, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Smith & Nephew plc, an English public limited liability company (“Parent Holdco”), Smith & Nephew Consolidated, Inc., a Delaware corporation (“Parent”), and Papyrus Acquisition Corp., a Maryland corporation and direct subsidiary of Parent, pursuant to which, and upon the terms and subject to the conditions described therein, Papyrus Acquisition Corp. will commence a cash tender offer (the “Offer”) to acquire all of the outstanding shares of the Company’s common stock at a purchase price of $19.00 per share (the “Offer Price”), in cash, without interest, subject to any required withholding of taxes. The Offer will commence no earlier than five (5) and no later than fifteen (15) business days following the date of the Merger Agreement. The Offer will expire at 12:01 a.m., New York City time, on the 21st business day following the commencement of the Offer (unless the Offer is extended).
The obligation of Papyrus Acquisition Corp. to purchase shares of the Company’s common stock tendered in the Offer is subject to customary closing conditions, including (i) shares of the Company’s common stock having been validly tendered and not validly withdrawn that represent at least a majority of the total number of shares of the Company’s common stock then-outstanding on a fully diluted basis, (ii) the expiration or termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the “HSR Act”), (iii) the absence of injunctions or other legal restraints preventing the consummation of the Offer or the Merger and (iv) certain other conditions set forth in the Merger Agreement. The consummation of the Offer is not subject to any financing condition, and Parent Holdco has guaranteed the performance of the obligations of Parent and Papyrus Acquisition Corp. under the Merger Agreement.
Following the completion of the Offer, subject to the satisfaction or waiver of certain customary conditions set forth in the Merger Agreement, Papyrus Acquisition Corp. will merge with and into the Company, with the Company surviving as a wholly owned subsidiary of Parent, pursuant to the procedure provided for under Section 3-106.1 of the Maryland General Corporation Law, as amended (the “MGCL”), without any stockholder approvals. We refer to this as the “Merger,” and the Offer and the other transactions contemplated by the Merger Agreement as the “Transaction”. The Merger will be effected as promptly as possible following the initial acceptance for payment by Papyrus Acquisition Corp. of shares of the Company’s common stock validly tendered and not validly withdrawn in the Offer. At the
effective time of the Merger (the “Effective Time”), each issued and outstanding share of the Company’s common stock (other than shares owned by Papyrus Acquisition Corp. and any subsidiary of the Company) will be converted into the right to receive the Offer Price, in cash, without interest, subject to any required withholding of taxes. Each unexercised stock option outstanding immediately prior to the Effective Time, whether or not vested, will be canceled at the Effective Time, and the holder of each stock option of the Company will be entitled to receive (i) the excess, if any, of the Offer Price over the exercise price per share of Company common stock subject to the stock option, multiplied by (ii) the number of shares of Company common stock subject to the stock option immediately prior to the Effective Time.
The Merger Agreement also contains a “no shop” provision that, in general, restricts the Company’s ability to solicit third-party acquisition proposals or provide any non-public information to, or engage in discussions or negotiations with, or recommend or otherwise cooperate with respect to proposals from, third parties that have made or that would reasonably be expected to make an acquisition proposal. The no shop provision is subject to a “fiduciary out” provision that allows the Company, under certain circumstances and in compliance with certain obligations, to provide information and participate in discussions and negotiations with respect to any unsolicited third-party acquisition proposal that is reasonably likely to result in a Superior Proposal (as defined in the Merger Agreement) and, subject to compliance with certain obligations, to terminate the Merger Agreement and accept a Superior Proposal upon payment to Parent of the Termination Amount discussed below.
Until the earlier of the termination of the Merger Agreement and the Effective Time, the Company has agreed to operate its business in the ordinary course and has agreed to certain other operating covenants, as set forth more fully in the Merger Agreement. The Merger Agreement includes customary termination provisions for both the Company and Parent and provides that, in connection with the termination of the Merger Agreement under specified circumstances, including termination by the Company to accept a Superior Proposal (as defined in the Merger Agreement), the Company will be required to pay a fee equal to approximately $18.7 million.
Our History
From May 2010 to October 2013, we operated in two business segments, therapeutics and biosurgery. Our therapeutics business focused on developing biologic stem cell drug candidates from a readily available source—adult human bone marrow. Our biosurgery business, now our only segment, focuses on using unique tissue preservation technologies to develop viable human tissue products designed to improve wound closure and surgical outcomes for patients and physicians over standard of care alone.
In October 2013, we sold our therapeutics business, including Prochymal, a stem cell drug for treatment of graft versus host disease, and related assets, to Mesoblast International SARL (“Mesoblast”), a wholly‑owned subsidiary of Mesoblast Limited.
Since 2013, we have focused our resources on our biosurgery business. We have built a substantial direct sales force dedicated exclusively to sell our Grafix and Stravix products. We have also entered into exclusive agreements to market and distribute BIO
4
and Cartiform. We are a fully integrated company, having developed capabilities in R&D, manufacturing, marketing, and sales of our products. We are focused on our long‑term commercial growth through the delivery of differentiated products for use across multiple fields of medicine with clear value propositions to patients, providers, and third‑party payors.
Market Overview
Regenerative medicine focuses on the process of creating living, functional tissues to reconstruct, repair, replace, or supplement tissue or organ function lost due to age, disease, damage, or congenital defects. Osiris has demonstrated more than 25 years of leadership in regenerative medicine and currently markets regenerative medicine products in wound care, orthopedics and sports medicine in the form of skin substitutes, bone grafts, and articular cartilage grafts, respectively.
Wound Care
Our wound care business consists of human placental products used to support natural wound healing processes. Skin substitutes are utilized by qualified healthcare professionals in a variety of medical fields in which repair and regeneration of damaged tissues are critical, including but not limited to wound care centers, podiatric surgery, plastics and reconstructive surgery, vascular surgery, and orthopedic surgery. Skin substitutes represent the largest and fastest growing segment of the wound biologics market, which are part of the larger advanced wound care market. Our Grafix, GrafixPL, and Stravix skin substitutes are human placental tissue‑based products, which are used in conjunction with standard of care for repair and regeneration of damaged tissues when the standard of care alone is not effective. Our products are used primarily for treating chronic wounds of various causes faced by patients in hospital outpatient or physician office settings (i.e., diabetic foot ulcers (“DFUs”), venous leg ulcers (“VLUs”), pressure ulcers, arterial ulcers, severe burns, surgical wounds, and trauma wounds), as well as limb salvage, amputations, and surgical reconstructions in the operating room setting.
Bone Grafts
Bone grafts are used in a variety of orthopedic surgical procedures to fill bone voids to help facilitate repair and regeneration of bones. Bone grafting is one of most frequent types of surgical procedures performed on bones and can be achieved using an autograft (a graft of tissue from one’s own body), or an allograft. Our BIO
4
bone graft is a viable bone matrix allograft primarily used in spine, orthopedics, and trauma surgery to fill bone voids or as part of bone fusion procedures to assist with the natural healing process of bones. Our BIO
4
allograft is an alternative to an autograft, which requires a procedure of harvesting a patient’s own bone (“donor site”) and presents complications and functional restrictions of the donor site commonly known as the donor site morbidity.
Osteochondral Allografts
Osteochondral allografts are used in articular cartilage repair procedures to repair cartilage lesions and, when necessary, underlying bone issues. Osteochondral defects may be the result of trauma or the result of “wear and tear” to the cartilage over time. Patients with osteochondral defects may experience chronic pain, be immobile, have joint instability due to the defect, or have osteoarthritis. The majority of osteochondral grafting procedures are performed in the knee, but these grafts can also be used in other joints. Examples of osteochondral allografts include fresh stored osteochondral allografts, fresh-frozen osteochondral allografts, or our Cartiform
allografts, cryopreserved viable osteochondral allografts. Our Cartiform allografts are cryopreserved viable osteochondral allografts primarily used to treat articular cartilage lesions in knee and ankle joints.
Scientific Background and Our Technology
Over 25 years ago, Osiris was founded on the discovery of mesenchymal stem cells (“MSCs”) in adult human bone marrow. After more than 25 years of R&D, we have gained a deep understanding of cell biology and the importance of MSCs and other biological factors in tissue repair and regeneration. With our knowledge, we have developed Prochymal, as the first approved MSC drug for treatment of graft versus host disease in Canada and New Zealand as well as our current portfolio of regenerative medicine products. As described above, Mesoblast acquired Prochymal in connection with the sale of our therapeutics business.
Our current regenerative medicine products consist of unique placental, bone, and cartilage tissue allografts. To manufacture our products, we rely on a unique proprietary tissue cryopreservation technique, which retains the native tissue components, including mesenchymal stem cells, growth factors and extracellular matrix, and the tissue’s inherent functionalities. Our cryopreservation technology serves as the manufacturing backbone for Grafix and Stravix as wound covers, BIO
4
for bone repair and regeneration, and Cartiform for repair and regeneration of articular cartilage.
Cryopreservation is the conventional method for long‑term storage of living cells and tissues. However, this method requires specialized ultra‑low temperature (below −80 degrees Celsius) equipment for storage and shipment, which creates a barrier to widespread use. In 2017, we announced the development of Prestige
Lyotechnology, a new tissue preservation technology that allows for storage and shipment of living tissue, including our products, at room
temperature. In June 2018, we reported the validation, testing, and upscaling of Prestige
Lyotechnology for manufacturing of our products and eliminated the need to preserve and transport our products at constant ultra-low temperatures. On October 1, 2018, we launched GrafixPL PRIME, our first commercially available product in the lyopreserved formulation.
Products and Pipeline
All of our current commercialized products are marketed as human cells, tissues, and cellular and tissue‑based products (“HCT/Ps”), as defined by the United States Food and Drug Administration (“FDA”), that are regulated solely under Section 361 of the Public Health Service Act (“361 HCT/Ps”), and consequently, do not require pre‑market approval from the FDA. Nevertheless, to support our reimbursement efforts for certain products, we have performed clinical studies to demonstrate the products’ benefits to healthcare providers and third‑party payors as described in greater detail below. Commercialization of future biological drug product candidates in the United States by us, if any, may not qualify as 361 HCT/Ps and therefore may require the submission of Investigational New Drug Applications (“INDs”) and Biologics License Applications (“BLAs”). Commercialization can occur only after successful completion of clinical trials and governmental agency approval.
The table below summarize the status of our products.
361 HCT/Ps
|
|
|
|
|
|
|
Product
|
|
Field of Use
|
|
Status (Market or Discontinued)
|
|
GrafixPL PRIME
|
|
Wounds and Surgical Procedures
|
|
Market
|
|
Grafix
|
|
Wounds and Surgical Procedures
|
|
Market
|
|
Stravix
|
|
Wounds and Surgical Procedures
|
|
Market
|
|
BIO
4
(formerly, OvationOS)
|
|
Bone Repair
|
|
Market
|
|
Cartiform
|
|
Cartilage Repair
|
|
Market
|
|
Menvivo
|
|
Meniscus Repair
|
|
Market, but not actively distributed
|
|
TruSkin
|
|
Wounds
|
|
Market, but not actively distributed
|
|
Ovation
|
|
Surgical Applications
|
|
Discontinued (2014)
|
Products Requiring Pre‑Marketing Approval (IND/BLA)
While we are focused on the development and commercialization of 361 HCT/Ps, we have had early discovery and development programs for biological product candidates, including our cellular drug program. We do not expect to file an IND for, or pursue clinical trials of, any of our biological product candidates in 2019.
Current Products
Our current products span three primary markets: wound care, orthopedics, and sports medicine.
Wound Care
Our
Grafix
products,
initially launched in 2010, include Grafix
PRIME,
Grafix
XC
and Grafix CORE. These products are cryopreserved placental membranes that retain the extracellular matrix, growth factors, and endogenous cells including neonatal epithelial cells (present only in PRIME and XC), MSCs and fibroblasts of the native tissue, all of which are beneficial in supporting natural wound repair. The Grafix PRIME and Grafix XC (a larger size for surgical applications) lines are derived from the amnion, whereas the Grafix CORE line is derived from the chorion. The amnion is the innermost membrane and the chorion is the outermost membrane of the placenta. Our Grafix products are flexible and conforming wound covers designed for direct application to hard‑to‑treat acute and chronic wounds, including but not limited to DFUs, VLUs, and burns.
We previously received an ‘untitled letter’ dated September 26, 2013 (the “Untitled Letter”) from the FDA stating that the Grafix and Ovation products did not meet the definition of a 361 HCT/P. Among the grounds for the FDA’s position were our marketing claims, including wound healing claims for Grafix. We revised all of our marketing claims for Grafix and resolved all issues with the Untitled Letter, which included the discontinuance of Ovation in 2014. Our Grafix products remain on the market as a 361 HCT/P. In April 2016, the FDA performed a routine inspection of the Company, which included a follow‑up on our corrective actions to close out the Untitled Letter. In May 2016, we received a FDA Establishment Inspection Report, which stated that there were no observations, findings, warnings, or untitled letters for either the routine inspection or the Untitled Letter follow‑up.
Our
GrafixPL PRIME is a lyopreserved Grafix product that eliminates the need to preserve and transport a living tissue product at constant ultra-low temperatures. This product was launched for sale on October 1, 2018 as our first commercially available product in the lyopreserved formulation.
Our
Stravix product is a viable cryopreserved human placental tissue, comprised of amniotic and connective layers of umbilical tissue that has been developed as a wound cover or surgical wrap to support soft tissue repair. The product retains native components of the umbilical tissue including the extracellular matrix, growth factors, and endogenous viable cells including epithelial cells, fibroblasts, and MSCs. The product conforms to the site of injury and requires minimal preparation prior to use. The product is thicker and has a stronger tensile strength than our Grafix products. Launched in late 2015, our Stravix product is primarily sold in hospital inpatient settings as a thicker, easy to handle, suturable wound cover for higher risk wounds to prevent amputation (limb salvage) or as a wrap or barrier following tendon or nerve repairs in foot and ankle.
Bone Graft
Our
BIO
4
product is a viable bone matrix containing endogenous bone forming cells including MSCs, osteoprogenitor cells, osteoblasts, and osteoinductive and angiogenic growth factors. The product possesses all four properties required for proper bone repair and regeneration: osteoconductive, osteoinductive, osteogenic, and angiogenic. The product is an alternative to autograft (a graft of tissue from one’s own body, which requires a procedure of harvesting a patient’s own bone (“donor site”) and presents complications and functional restrictions commonly known as the donor site morbidity). Originally marketed as OvationOS and launched in 2014, BIO
4
has been marketed and distributed exclusively by Stryker under the BIO
4
trademark since 2015.
Osteochondral Allograft
Our
Cartiform product, launched in 2012, is a viable osteochondral allograft that contains extracellular matrix, chondrogenic factors and endogenous viable chondrocytes native to the cartilage tissue. The intact architecture of native cartilage is preserved in Cartiform and the graft is intended to treat osteochondral defects. The product can fit to any surface contour. The product has been exclusively available through Arthrex, Inc. (“Arthrex”) since 2014.
Reimbursement
Physicians, hospitals and other healthcare providers use our current products. A significant market for Grafix and GrafixPL PRIME is chronic wounds, which are primarily treated in the hospital outpatient setting or physician’s office. Reimbursement by public and private payors for outpatient treatments typically requires approvals from third‑party payors, which in some cases may be granted after in depth prior authorization or pre-determination review processes. and sometimes independent reviews.
Third‑party payors include, but are not limited to, Medicare, managed care networks, and private insurance plans. To support reimbursement for the use of
Grafix
for chronic wounds, we have published several clinical studies over the past 6 years
. Additional settings of care for the use of Grafix and GrafixPL PRIME are the hospital operating room, as well as government health agencies, including Veteran Affairs Hospitals and Department of Defense Hospitals.
Reimbursement by third‑party payors may be subject to periodic adjustments as a result of legislative, regulatory, and policy changes, as well as budgetary pressures. Even though our customers’ obligations to pay us for our
products are not contingent on whether adequate third‑party reimbursement is available, possible reductions in, or eliminations of, coverage or reimbursement by third‑party payors affects our customers’ ability to purchase our products.
Medicare
Medicare is the largest third‑party payor in the United States. Medicare is a health insurance program primarily for individuals 65 years of age and older and younger individuals with certain disabilities. The Centers for Medicare and Medicaid Services (“CMS”) administers the Medicare program through twelve Medicare Administrative Contractors (“MACs”). A MAC is a multi‑state and private healthcare insurer that has been awarded by CMS a geographic jurisdiction to process Medicare Part A and Part B medical claims for Medicare beneficiaries. Although the overall Medicare reimbursement framework was developed in accordance with the Social Security Act and the applicable CMS regulations, each MAC has the discretion to decide whether to cover our products. In 2017, approximately 33% of Medicare beneficiaries receive Medicare benefits through Medicare Part C, a managed Medicare benefit administered by private health insurers under the brand name of a Medicare Advantage plan. The number of Medicare beneficiaries receiving benefits under Medicare Advantage plans is expected to grow. Part C administrators have discretion to decide product coverage and utilization management policies that can affect coverage for our products by Medicare.
Grafix.
Effective January 2014, CMS issued permanent Healthcare Common Procedure Coding System (“HCPCS”) Q‑codes for Grafix, which assist healthcare providers in facilitating reimbursement in the commercial and Medicare patient populations. In January 2018, CMS added GrafixPL PRIME and GrafixPL
Core
to the existing Grafix HCPCS codes. Since March 2016, all MACs provide coverage for our Grafix products for the treatment of certain chronic wounds, including DFUs. For Medicare reimbursement purposes, CMS has classified our Grafix products as skin substitutes. The majority of Grafix is used in hospital outpatient departments (“HOPDs”). In these settings, Medicare reimburses skin substitutes only in packaged arrangements, meaning Medicare pays the facility for our product and the related application procedure in the form of a single bundled payment. CMS also assigns skin substitutes to either the low cost bundle group or high cost bundle group depending on the product’s average weighted mean unit cost. Grafix has been assigned to the high cost group. The national average high cost bundle reimbursement for each application was $1,427.16 in 2017 and $1,568.32 in 2018. For 2019, the high cost bundle HOPD reimbursement is set at $1,548.96 per application. For private office procedures, reimbursement is based on the average sales price (“ASP”) per square centimeter as published by CMS. As of January 1, 2019, the ASP for Grafix CORE and GrafixPL CORE (HCPCS Q4132) is $135.00 and the ASP for Grafix PRIME and GrafixPL PRIME (HCPCS Q4133) is $135.17. No ASP is published for Grafix XC.
Stravix.
CMS has classified Stravix as a skin substitute, which is also used in HOPD and operating room settings of care. The product has been assigned to the HCPCS Q4133 code as of January 1, 2019. When the product is used for hospital inpatient procedures, reimbursement is bundled into the hospital diagnosis related group (“DRG”) consolidated payment. Reimbursement for hospital outpatient procedures depends on the type of procedures performed and the applicable ambulatory payment classification (“APC”) rate.
BIO
4
and
Cartiform.
Our
BIO
4
and Cartiform products are used in the operating room settings. Like other allografts, they are bundled as part of a hospital’s claim for operating room services under a DRG for inpatient admissions and under an APC for outpatient procedures. Medicare pays a single amount for a patient’s inpatient hospitalization based on the applicable DRG, which depends on the patient’s diagnosis, the surgical procedures involved, and the patient’s age and gender. When the surgery is performed on an outpatient basis, Medicare pays for it based on the APC applicable to the surgical procedure.
Private Third‑Party Payors
Private third‑party payors include, but are not limited to, private health insurance plans and managed care networks. Each private health insurance plan and managed care network has its own coverage and reimbursement policies applicable to our products. Even if a plan covers our products, the reimbursement amount may not cover the entire costs of our product, which could adversely impact the demand for our products.
Intellectual Property
We have intellectual property (patents, patents pending, licenses, know‑how, and trademarks) related to our products, manufacturing processes and other technologies. Our strategy to protect our intellectual property includes generally seeking patent protection for our technology and products primarily in the United States, Canada, and certain countries of the European Union. The intellectual property status of biotechnology generally is highly uncertain and involves complex legal and factual questions. Our success will depend, in part, on whether we can:
|
|
·
|
|
obtain patents to protect our own technologies and products;
|
|
|
·
|
|
protect our trade secrets and know‑how;
|
|
|
·
|
|
obtain licenses or the right to use the technologies of third‑parties, which may be protected by patents;
|
|
|
·
|
|
obtain trademarks to protect our brand names; and
|
|
|
·
|
|
conduct our business without infringing on the intellectual property and proprietary rights of others.
|
Patent Rights
Our success depends, in part, on our ability to obtain patents, maintain trade secret protection, and operate without infringing on the patented rights of third parties. Our policy is to file patent applications to protect technologies and products that we consider important to our business and operations. We have a number of patents issued and applications pending in the United States, Canada, certain countries of the European Union, and a few other foreign jurisdictions. We may need to defend patents from challenges by others from time to time in the future. Certain of our U.S. patents may be challenged by others in a post-grant proceeding under the America Invents Act of 2011. A post‑grant proceeding has become increasingly common in the United States and is costly to defend. Our patented rights may not provide us with a proprietary position or competitive advantages against competitors. Furthermore, even if the outcome is favorable to us, the enforcement of our intellectual property rights can be expensive and time consuming.
Licenses and Related Rights
We sold our culturally expanded mesenchymal stem cell (“ceMSC”) technology (including our stem cell drug Prochymal and other related assets) to Mesoblast, a wholly owned subsidiary of Mesoblast Limited, in October 2013. Pursuant to the purchase agreement with Mesoblast, we retained a royalty free license to all transferred intellectual property, insofar as necessary to continue in our current business. We have agreed not to compete with Mesoblast in the ceMSC business through October 2021, but we retain the rights to develop any other MSC technologies that do not involve culture expansion of cells.
Trade Secrets and Know‑How
We also rely upon trade secrets to protect our proprietary information and technology. A significant amount of our technology, including aspects of the manufacturing processes for our products, is maintained by us as trade secrets. Through our experience with MSC‑based and tissue‑based product development, we have developed expertise and know‑how in this field. To protect our know‑how in manufacturing processes, we enter into confidentiality agreements with our employees, consultants and contractors, manufacturers, outside collaborators, sponsored researchers, advisors and other third parties. These agreements generally provide for protection of confidential information and technology, restrictions on the use of materials and assignment of inventions conceived during the term of the agreement. These agreements may not effectively prevent disclosure of or otherwise protect our confidential information and technology.
Trademarks
Osiris, Osiris and design, Grafix, Grafix CORE, Grafix PRIME, Grafix XC, GrafixPL, Stravix, Cartiform and TruSkin are our trademarks registered in the United States. The trademark registration applications for our GrafixPL
Prime
and Prestige Lyotechnology are currently pending in the United States. We believe that trademark protection is an important part of establishing product and brand recognition. We own a number of registered trademarks and trademark applications in the U.S., Canada and in various other countries throughout the world. The U.S. federal registrations for trademarks may be renewed every 10 years after issuance, provided the mark is still being used in commerce. Trademark registrations in Canada remain in force for 15 years, may be renewed every 15 years after issuance, and are vulnerable to cancellation thereafter if the mark is not being used in commerce. Other countries generally have similar but varying terms and renewal policies with respect to trademarks registered in those countries.
Manufacturing
Our current products are derived from human tissue donated for transplantation. Our Grafix and Stravix allografts are derived from living human donors’ placental tissues, and our BIO
4
and Cartiform products are derived from donated human cadaveric bone and cartilage tissues. We contract with tissue recovery agencies for both placental and cadaveric tissue for our products. These agencies operate on a fee‑for‑service basis. As needed, we intend to enter into contracts with additional tissue recovery agencies to fulfill expected product demand. We have not experienced significant supply issues with tissue recovery agencies, and placental tissue and cadaveric donor bone and cartilage have been generally available to us in sufficient quantities and on acceptable terms. However, there are a limited number of tissue supply agencies, and it requires a long‑lead time to order large quantities of placental and cadaveric tissue. This means that a sudden increase in demand or a supply shortage may prevent us from producing sufficient quantities of our products.
We currently manufacture all of our supply of Grafix and Stravix products at our facility in Columbia, Maryland. Currently, we outsource manufacturing of all of our supply of BIO
4
and Cartiform to Aziyo Biologics, Inc. (“Aziyo”) and Community Tissue Services (“CTS”). A lengthy disruption or shutdown of, or a shortage of supply at, our current manufacturing facility or the manufacturing facilities of Aziyo or CTS, whether due to the occurrence of natural disasters, the need to comply with the requirements of directives from government agencies such as the FDA, the lack of supply of human tissue, or otherwise, could have a material adverse effect on our business, financial condition, and results of operations.
Sales, Marketing and Distribution
Grafix
,
GrafixPL
and
Stravix:
We sell Grafix
,
GrafixPL,
and Stravix through the efforts of our internal direct sales and marketing departments, as well as through a small number of specialty distributors for certain target markets. We focus our marketing efforts for these products in four specific channels: HOPDs, hospital surgical procedures in operating rooms, private physician offices, and Department of Veteran Affairs (“VA”) and Department of Defense (“DOD”) hospitals. For our VA and DOD customers, our products are distributed exclusively through resellers designated as Service‑Disabled, Veteran‑Owned Small Businesses (“SDVOSBs”). SDVOSBs are eligible for set‑asides and other preferences in the federal contracting process. For the VA, SDVOSBs enter into Federal Supply Schedule or Strategic Acquisition Center contracts, and for the DOD, SDVOSBs enter into Distribution and Pricing contracts.
BIO
4
:
In December 2014, we entered into an exclusive agreement with Howmedica Osteonics Corp., also referred to as Stryker Orthopaedics, a subsidiary of Stryker Corporation, for the marketing and distribution of BIO
4
. We are responsible for supply, manufacturing, inventory management, shipments to customers, continued research, and product improvement activities. Stryker is responsible for the sales and marketing of BIO
4
for use in all surgical applications, including spine, trauma, extremity, cranial, and foot and ankle surgery. We collaborate with Stryker on the design and conduct of clinical development programs.
The agreement with Stryker provided for an initial four‑year exclusive term, which commenced in 2015. In February 2019, Stryker extended the exclusive period for an additional four years. We received an exclusivity fee of $3.9 million in February 2019 to extend the initial term. The agreement provides that Stryker has the option to further extend
the term on an exclusive basis for two more years. Pursuant to its terms, Stryker has limited rights to terminate the agreement early. The success of this agreement for us will in part depend upon Stryker’s success in marketing and promoting
BIO
4
.
Cartiform:
In October 2014, we entered into an exclusive agreement with Arthrex for our cartilage product, Cartiform. The agreement provides Arthrex with exclusive commercial distribution rights to Cartiform. We are responsible for manufacturing, continued research, and product improvement activities. We collaborate with Arthrex on the design and conduct of clinical development programs. The agreement provides for an initial eight‑year exclusive term with automatic renewals of additional two‑year periods. Pursuant to the agreement, Arthrex is entitled to certain commission payments on sales of the product.
Information regarding our revenue from sales of the above products is set forth under the heading Results of Operations in Part II, Item 7 of this Form 10‑K and is incorporated by reference in this Item 1.
Competition
In the marketplace, we compete with other companies and organizations that are marketing or developing products competitive with Grafix
,
Stravix, and our other products including those under development. Companies competing with our products include, but are not limited to: Organogenesis Inc., the manufacturer of Apligraf
®
and Dermagraft
®
, MiMedx Group, Inc., the manufacturer of EpiFix
®
, and Integra LifeSciences Corporation, the manufacturer of Integra, all of which compete with Grafix and Stravix. Our BIO
4
product competes with bone tissue products such as Osteocel
®
marketed by NuVasive, Inc. and Trinity
®
marketed by Orthofix International NV. Cartilage allografts which compete with Cartiform include ProChondrix
®
marketed by Stryker and DeNovo
®
marketed by Zimmer Biomet Holdings, Inc. In addition to those listed above, we have other existing and potential competitors developing a variety of products for the same conditions for which we market our products.
Government Regulation
Regulation by governmental authorities in the United States and other countries is a significant factor in the development, manufacture, commercialization, and reimbursement of our products. Our current products, Grafix
,
Stravix, BIO
4
and Cartiform, are HCT/Ps that we believe qualify under 21 CFR Part 1271 to be regulated solely under Section 361 of the Public Health Services Act. Such 361 HCT/Ps are regulated differently from biologics and drugs, and do not require pre‑marketing approval. Some of our product candidates in early development may require pre‑marketing approval of a BLA, referred to as licensure, by the FDA, prior to commercialization.
Criteria for Qualifying as a 361 HCT/P
361 HCT/Ps are human cells, tissues, or cellular and tissue‑based products that are intended for implantation, transplantation, infusion, or transfer into a human recipient and that meet all of the following criteria set forth under 21 CFR Part 1271:
|
|
·
|
|
the product is minimally manipulated;
|
|
|
·
|
|
the product is intended for homologous use only, as reflected in its labeling, advertising, and all other indications of the manufacturer’s objective intent;
|
|
|
·
|
|
the manufacture of the product does not involve the combination with another article except for water, crystalloids, or a sterilizing, preserving, or storage agent, provided that the addition of such articles does not raise new clinical safety concerns with respect to the product; and
|
|
|
·
|
|
the product does not have a systemic effect and is not dependent on the metabolic activity of living cells for its primary function (or if it does, it meets other criteria not applicable to our products, such as being limited to use in first or second degree blood relatives).
|
These criteria form the framework governing our advertising and promotional activities for 361 HCT/Ps. For additional information on FDA’s regulation of 361 HCT/P advertising and promotion, refer to the risk factor entitled, “
Our business is subject to an inherently uncertain and evolving area of regulation.”
under “Risk Factors” in Part I, Item 1A of this Form 10‑K.
We believe all of our current products (Grafix, Stravix, BIO
4
, and Cartiform), as well as our tissue products being developed using our Prestige Lyotechnology
,
meet, or will meet, the FDA’s current interpretations of the criteria for 361 HCT/Ps. For additional information about the FDA regulatory history of our products, which informs our belief that our products qualify as 361 HCT/Ps, refer to the risk factor entitled, “
Should the FDA determine that any of our current products do not meet regulatory requirements that permit qualifying human cells, tissues, and cellular and tissue‑based products to be manufactured, stored, labeled, and distributed without pre‑marketing approval, we may be required to stop manufacturing and distributing such products.”
under “Risk Factors” in Part I, Item 1A of this Form 10‑K.
FDA Regulation of 361 HCT/Ps
The FDA has specific regulations governing HCT/Ps, including some regulations specific to 361 HCT/Ps, which are set forth in 21 CFR Part 1271. All establishments that manufacture 361 HCT/Ps must register and list their HCT/Ps with the FDA’s Center for Biologics Evaluation and Research (“CBER”) within five days after commencing operations. In addition, establishments are required to update their registration annually in December or within 30 days of certain changes and submit changes in HCT/P listing at the time of or within six months of such change.
The regulations in 21 CFR Part 1271 requires us to comply with donor screening, eligibility and testing requirements and federally mandated current Good Tissue Practices (“cGTPs”) regulations to prevent the introduction, transmission, and spread of communicable diseases. The cGTPs govern, as may be applicable, the facilities, controls, and methods used in the manufacture of all HCT/Ps, including processing, storage, recovery, labeling, packaging, and distribution of 361 HCT/Ps. cGTPs require us, and our contract manufacturers, among other things, to maintain a quality program, train personnel, control and monitor environmental conditions as appropriate, control and validate processes, properly store, handle, and test our products and raw materials, maintain our facilities and equipment, keep records, and comply with standards regarding recovery, pre‑distribution, distribution, tracking and labeling of our products, and complaint handling. 21 CFR Part 1271 also mandates compliance with adverse event and cGTP deviation reporting and labeling requirements.
Although we do not currently import any human tissue for our products, we have executed contracts with potential suppliers of placental tissue sourced from Canada, and we are currently in the process of qualifying those suppliers. In the event that we import placental tissue, we will be required to satisfy the regulations on importing 361 HCT/Ps. Those regulations require that the importer of record of HCT/Ps notify the FDA prior to, or at the time of, importation and provide sufficient information for the FDA to make an admissibility decision. In addition, the importer must hold the HCT/P intact and under conditions necessary to prevent transmission of communicable disease until an admissibility decision is made by the FDA.
The FDA conducts periodic inspections of HCT/P manufacturing facilities, and contract manufacturers’ facilities, to assess compliance with cGTP. Such inspections can occur at any time with or without written notice at such frequency as determined by the FDA at its sole discretion. To determine compliance with the applicable provisions, the inspection may include, but is not limited to, an assessment of the establishment’s facilities, equipment, finished and unfinished materials, containers, processes, HCT/Ps, procedures, labeling, records, files, papers, and controls required to be maintained under 21 CFR Part 1271. If the FDA were to find serious non‑compliant manufacturing or processing practices during such an inspection, it could take regulatory actions that could adversely affect our business, results of operations, financial condition, and cash flows. For additional information on these potential enforcement actions, refer to the risk factor entitled, “
Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly, and our failure to comply could result in negative effects on our business.”
under “Risk Factors” in Part I, Item 1A of this Form 10‑K.
FDA Regulatory Approval Process for Biologics
If the FDA determines that any of our current products do not qualify as 361 HCT/Ps, such products would then be regulated as biological products and would require pre‑marketing approval of a BLA, also known as licensure, from the FDA before they could be marketed again in the United States. In addition, certain of our product candidates in early stage development may constitute biological product candidates requiring licensure. For such biological product candidates, the FDA generally requires the following steps prior to their introduction into interstate commerce:
|
|
·
|
|
performance of preclinical (animal and laboratory) tests, in accordance with the FDA’s current Good Laboratory Practice (“cGLP”) regulations and other applicable requirements;
|
|
|
·
|
|
submissions to the FDA of an IND, which must become effective before clinical trials may commence;
|
|
|
·
|
|
approval by an independent institutional review board (“IRB”) of each clinical site before a clinical trial is initiated;
|
|
|
·
|
|
performance of adequate and well‑controlled clinical trials according to FDA’s current Good Clinical Practice (“cGCP”) regulations, and any additional requirements for the protection of human research subjects and their health information, to establish the safety, purity, and potency of the investigational biological product in the intended target population for its intended use;
|
|
|
·
|
|
establishment and validation of a consistent and reproducible manufacturing process intended for commercial use, including the collection of appropriate manufacturing data;
|
|
|
·
|
|
preparation and submission to FDA of a BLA for marketing approval that includes substantial evidence of safety, purity, and potency from results of nonclinical testing and clinical trials;
|
|
|
·
|
|
satisfactory completion of a FDA Advisory Committee review, unless waived by the FDA;
|
|
|
·
|
|
satisfactory completion of a FDA inspection of the manufacturing facility or facilities where the biological product candidate is produced to assess compliance with current Good Manufacturing Practices (“cGMPs”) and to assure that the facilities, methods, and controls are adequate to preserve the biological product candidate’s identity, safety, strength, quality, potency, and purity;
|
|
|
·
|
|
potential FDA inspection of the nonclinical and clinical trial sites that generated the data in support of the BLA; and
|
|
|
·
|
|
FDA review and approval of the BLA before any commercial sale or shipment of the product can begin again.
|
For additional information on the requirements for licensure, refer to the risk factor entitled, “
If the FDA determines that any of our current products are not 361 HCT/Ps, or that any of our future products are not 361 HCT/Ps, we will be required to seek and obtain pre‑marketing regulatory approval.
” under “Risk Factors” in Part I, Item 1A of this Form 10‑K.
Federal Regulation of Clinical Laboratories
Our communicable disease testing is performed by laboratories registered with the FDA to perform donor testing and certified to perform such testing on human specimens in accordance with the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”) and 42 CFR Part 493, or that has met equivalent requirements as determined by CMS. CLIA extends federal oversight to clinical laboratories that examine or conduct testing on materials derived from the human body for the purpose of providing information for the diagnosis, prevention, or treatment of disease or for the assessment of the health of human beings. CLIA requires that these laboratories be certified by the
government, satisfy governmental quality and personnel standards, undergo proficiency testing, be subject to biennial inspections, and remit fees. The sanctions for failure to comply with CLIA include suspension, revocation, or limitation of a laboratory’s CLIA certificate necessary to conduct business, fines, or criminal penalties.
National Organ Transplant Act
Procurement of certain human organs and tissues for transplantation is subject to the restrictions of the National Organ Transplant Act (“NOTA”), which prohibits the transfer of certain human organs, including skin and related tissue, for valuable consideration, but permits reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control, and storage of human tissue and skin. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery, storage and transportation of donated human tissue.
State Tissue Bank Regulations and Related Accreditation
Certain state and local governments regulate our business. As required, we maintain state licensure as a human tissue bank in Maryland, California, Florida, Illinois, Oregon, Delaware and New York. We believe these are the only states in which this specific licensure is required for us. We also received and actively maintain American Association of Tissue Banks (“AATB”) accreditation. In January 2016 the 14th Edition of the AATB standards went into effect, which included specific standards for recovery, screening, testing, labeling, and processing of placental tissue.
Privacy Law
Federal and state laws govern our ability to obtain and, in some cases, to use and disclose data we need to conduct research activities. Through the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), Congress required the Department of Health and Human Services (“HHS”) to issue a series of regulations establishing standards for the electronic transmission of certain health information by healthcare providers, hospitals, health plans, and healthcare clearinghouses, which are known as “Covered Entities”. Among these regulations were standards for the privacy of individually identifiable health information (protected health information or “PHI”). HIPAA applies to us because we are a “Business Associate”, meaning that we are a third‑party who performs functions or services involving PHI for or on behalf of Covered Entities. Congress also enacted the Health Information Technology for Economic and Clinical Health Act (“HITECH”). Among other changes to the laws governing PHI, HITECH strengthened and expanded HIPAA requirements, increased penalties for violations, gave patients new rights to restrict uses and disclosures of their health information, and imposed a number of privacy and security requirements directly on Business Associates. Under HITECH, we must report unauthorized use or disclosure of PHI that meets the definition of a breach to our Covered Entities. We have adopted privacy policies and procedures designed to comply with the applicable requirements set forth in HIPAA and HITECH.
HIPAA and HITECH do not preempt, or override, state privacy laws that provide even more protection for individuals’ health information. These laws’ requirements could further complicate our ability to obtain necessary research data from our research collaborators. In addition, certain state privacy and genetic testing laws may directly regulate our research activities, affecting the manner in which we use and disclose individuals’ health information, potentially increasing our cost of doing business, and exposing us to liability claims. Patients and research collaborators may also have contractual rights that further limit our ability to use and disclose individually identifiable health information. Any claims that we have violated individuals’ privacy rights or breached our contractual obligations, even if we are not found liable, could be expensive and time‑consuming to defend and could result in adverse publicity that could harm our business.
Healthcare Fraud and Abuse Laws
In the United States, we are subject to laws and regulations pertaining to healthcare fraud and abuse, including anti‑kickback laws and physician self‑referral laws that regulate the means by which companies in the healthcare industry may market their products to hospitals and healthcare professionals and may compete by discounting the prices of their products. Our products also are subject to regulation regarding reimbursement, and United States healthcare laws apply when a customer submits a claim for a product that is reimbursed under a federally funded healthcare program.
These laws require that we exercise care in designing our sales and marketing practices, including involving interactions with healthcare professionals. For additional description of these laws and their applicability to us, refer to the risk factor entitled, “
We and our distributor sales representatives must comply with U.S. federal and state fraud and abuse laws, including anti‑kickback and false claims laws and equivalent foreign rules.”
under “Risk Factors” in Part I, Item 1A of this Form 10‑K.
Hazardous Materials
We also are subject to various local, state, and federal laws and regulations relating to safe working conditions, laboratory and manufacturing practices, the distribution of human tissue and tissue products, experimental use of animals and the use and disposal of hazardous or potentially hazardous substances, including chemicals, micro‑organisms, and various radioactive compounds used in connection with our R&D activities. These laws include, but are not limited to, the Occupational Safety and Health Act, the Toxic Test Substances Control Act and the Resource Conservation and Recovery Act. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by state and federal regulations, we cannot assure that accidental contamination or injury to employees and third parties from these materials will not occur. We may not have adequate insurance to cover claims arising from our use and disposal of these hazardous substances.
Foreign Regulation
In the event we decide to distribute our products outside of the United States, we will be required to seek approval for the manufacturing and marketing of each of our products from regulatory authorities in foreign countries prior to the commencement of marketing of the product in those countries. The approval procedure varies among countries, may involve preclinical testing and clinical trials, and the time required may differ from that required by the FDA. For example, although there is now a centralized European Union approval mechanism in place, the mechanism applies only to certain specific medicinal product categories. Each European country also may impose certain of its own procedures and requirements in addition to those requirements set out in the appropriate legislation, many of which could be time‑consuming and expensive.
Employees
As of December 31, 2018, we had 342 full‑time employees and 16 part‑time employees. Of this total, 17 were engaged in R&D and clinical studies, 84 were engaged in manufacturing activities, 165 were engaged in sales and marketing activities, 26 were engaged in reimbursement and market access activities, and 66 were engaged in administration, finance, and facilities. None of our employees are represented by a labor union or covered under a collective bargaining agreement, and we have not experienced any work stoppages.
Available Information
Our reports filed with the SEC can be found on our website at www.osiris.com under the “Investors” heading free of charge. These include our Annual Reports on Form 10‑K, Quarterly Reports on Form 10‑Q, Current Reports on Form 8‑K, and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act. We make these reports available as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC.
The content on any website referred to in this Form 10‑K is not incorporated by reference into this Form 10‑K unless expressly noted.
ITEM 1A. Risk Factors.
We are subject to numerous risks and uncertainties. In addition to the other information contained in this report, you should carefully consider the risks and uncertainties described below. These risks are not the only ones that we may face. Additional risks not presently known to us or that we currently consider immaterial may also impact our business
operations. Our actual results could differ materially from those anticipated in our forward‑looking statements as a result of known and unknown risks including the risks described below or elsewhere in this report.
Risks Related to the Transaction
On March 12, 2019, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Parent Holdco, Parent and Papyrus Acquisition Corp., pursuant to which, and upon the terms and subject to the conditions described therein, Papyrus Acquisition Corp. will commence a cash tender offer (the “Offer”) to acquire all of the outstanding shares of the Company’s common stock at a purchase price of $19.00 per share, in cash, without interest, subject to any required withholding of taxes. Following the completion of the Offer, subject to the satisfaction or waiver of certain customary conditions set forth in the Merger Agreement, Papyrus Acquisition Corp. will merge with and into the Company, with the Company surviving as a wholly owned subsidiary of Parent, pursuant to the procedure provided for under Section 3-106.1 of the MGCL, without any stockholder approvals. We refer to this as the “Merger,” and the Offer and the other transactions contemplated by the Merger Agreement as the “Transaction”.
Failure to complete the Transaction could negatively impact the price of our common stock, as well as our future business and financial results.
The
Merger
Agreement contains a number of conditions that must be satisfied or waived prior to the completion of the Transaction. We cannot assure you that all of the conditions to the Transaction will be so satisfied or waived. If the conditions to the Merger are not satisfied or waived, we may be unable to complete the Transaction.
If the Transaction is not completed, our ongoing business may be adversely affected as follows: (i) we may experience negative reactions from the financial markets, including negative impacts on the market price of our common stock; (ii) some of management's attention will have been directed to the Transaction instead of being directed to our own operations and the pursuit of other opportunities that could have been beneficial to us; (iii) the manner in which customers, suppliers and other third parties perceive us may be negatively impacted, which in turn could affect our ability to compete for business; (iv) we may experience negative reactions from employees; (v) we will have expended time and resources that could otherwise have been spent on our existing business; and (vi) we may be required, in certain circumstances, to pay a termination fee of approximately $18.7 million, as provided in the Merger Agreement.
Additionally, in approving the Merger Agreement, the Board of Directors considered a number of factors and potential benefits, including the fact that the Merger consideration to be received by holders of our common stock represented a 37% premium to the Company’s 90-day volume-weighted average stock price. If the Merger is not completed, neither the Company nor the holders of our common stock will realize this benefit of the Transaction. Moreover, we would also have nevertheless incurred substantial transaction-related fees and costs and the loss of management time and resources.
A significant delay in consummating or a failure to consummate the Transaction could have a material adverse effect on the price of our common stock and our operating results.
Because the Transaction is subject to certain closing conditions, it is possible that the Transaction may not be completed or may not be completed as quickly as expected. If the Transaction is not completed, it could have a material adverse effect on the price of our common stock. In addition, any significant delay in consummating the Transaction could have a material adverse effect on our operating results and adversely affect our relationships with customers and suppliers and would likely lead to a significant diversion of management and employee attention.
Expenses related to the proposed Transaction are significant and will adversely affect our operating results.
We have incurred and expect to continue to incur significant expenses in connection with the proposed Transaction, including legal and investment banking fees. We expect these costs to have an adverse effect on our operating results. If the Transaction is not consummated, we may under certain circumstances be required to pay to Parent a termination fee of approximately $18.7 million. Our financial position and results of operations would be adversely affected if we were required to pay the termination fee to Parent.
We are subject to business uncertainties and contractual restrictions while the Transaction is pending, which could adversely affect our business.
The Merger Agreement requires us to act in the ordinary course of business and restricts us, without the consent of Parent from taking certain specified actions until the proposed Transaction occurs or the Merger Agreement terminates. These restrictions may prevent us from pursuing otherwise attractive business opportunities and making other changes to our business before completion of the Transaction or, if the Transaction is not completed, termination of the Merger Agreement.
Uncertainties associated with the Transaction may cause a loss of management and other key employees and disrupt our business relationships, which could adversely affect our business.
Uncertainty about the effect of the Transaction on our employees, customers and suppliers may have an adverse effect on our business. These uncertainties may impair our ability to attract, retain and motivate key personnel until the Transaction is completed and for a period of time thereafter. Employee retention may be particularly challenging during the pendency of the Transaction. If key employees depart and as we face additional uncertainties relating to the Transaction, our business relationships may be subject to disruption as customers, suppliers and other third parties attempt to negotiate changes in existing business relationships or consider entering into business relationships with parties other than the Company. If key employees depart or if our existing business relationships suffer, our results of operations may be adversely affected. The adverse effects of such disruptions could be further exacerbated by any delay in the completion of the Transaction.
Risks Related to Our Business
Our operating results may fluctuate significantly as a result of a variety of factors, many of which are outside of our control.
The following factors, among others, may negatively affect our operating results:
|
|
·
|
|
failure to obtain reimbursement approvals by, and adequate and timely reimbursements from, third‑party payors, such as Medicare and private health plans, for our products;
|
|
|
·
|
|
removal of our products from the Federal Supply Schedule or change in the prices that government customers will pay for our products;
|
|
|
·
|
|
our ability to attract and retain key personnel;
|
|
|
·
|
|
the announcement or introduction of new or improved products by our competitors;
|
|
|
·
|
|
our ability to obtain the necessary quantities of human tissue to manufacture our products;
|
|
|
·
|
|
our ability to upgrade and develop our systems and infrastructure to accommodate our growth, including adding more manufacturing capacity to enable us to continue to meet market demand;
|
|
|
·
|
|
our ability to manage our relationships with third parties that help us research, develop, manufacture, market, and distribute our products;
|
|
|
·
|
|
the amount and timing of operating costs and capital expenditures relating to the expansion of our business, operations, and infrastructure;
|
|
|
·
|
|
our ability to comply with regulatory requirements related to the marketing, manufacturing and distribution of our products and product candidates, including FDA regulations; and
|
|
|
·
|
|
general economic conditions as well as economic conditions specific to the healthcare industry.
|
We have based our current and future expense levels largely on our investment plans and estimates of future events, although certain of our expense levels are, to a large extent, fixed. We may be unable to adjust spending in a timely manner to compensate for any unexpected revenue shortfall. Accordingly, any significant shortfall in revenue relative to our planned expenditures would have an immediate adverse effect on our business, financial condition and results of operations. Further, as a strategic response to changes in the competitive environment, we may from time to time make certain pricing, service or marketing decisions that could have a material adverse effect on our business, financial condition and results of operations.
We have a history of operating losses and may not achieve or sustain profitability.
We have incurred losses in each year since our inception (except fiscal years 2009, 2010, 2011, 2013, 2017 and 2018), and may incur additional losses in the future. As of December 31, 2018, we had an accumulated deficit of approximately $205.4 million. In earlier years, these losses resulted principally from costs incurred in our R&D programs. In recent years, these losses resulted principally from our growing sales and marketing expenses, primarily due to the expansion of our sales force which was internalized in 2014, and from our growing general and administrative expenses. Our general and administrative expenses included approximately $3.8 million and $9.5 million in 2018 and 2017, respectively, of legal and accounting related to the restatement of our 2015 interim financial statements and the restatement of our 2014 financial statements included in the 2014 Form 10-K/A, as more fully described in the comprehensive Annual Report on Form 10-K for the fiscal years ended December 31, 2015, 2016 and 2017 (the “Restatement”).
We expect to continue to incur significant operating expenses in the foreseeable future as we seek to:
|
|
·
|
|
continue to add sales, operational, compliance, and financial personnel either through additional employees or outsourcing, consistent with expanding our operations and improving our internal control over financial reporting;
|
|
|
·
|
|
expand our manufacturing capacity;
|
|
|
·
|
|
continue to pursue clinical studies for our products to support our reimbursement efforts;
|
|
|
·
|
|
manage regulatory issues and requirements related to the marketing, manufacturing, and distribution of our products and product candidates, including issues related to FDA regulation and third‑party payor reimbursement; and
|
|
|
·
|
|
maintain, expand and protect our intellectual property.
|
The extent of our future operating losses or profits is highly uncertain, and we may not maintain profitability. If we are unable to achieve and then maintain profitability, the market value of our common stock will decline.
We continue to expand our sales and marketing capabilities, and there can be no assurance that these efforts will result in significant increases in sales.
Since 2014, we have been engaged in a major initiative to build and expand our internal sales and marketing capabilities. As a result, we have and are continuing to hire direct sales personnel for certain of our products to allow us to reach new customers. Due to the unique nature of our products, we spend significant time and resources on recruiting, training, retaining, motivating and managing our sales personnel. The increased expenses associated with these selling efforts impact our operating results, and there can be no assurance that we will be successful in significantly increasing sales of our products.
We may have difficulty managing growth in our business, which could have a material adverse effect on our business, financial condition, and results of operations.
As we expand our activities there will be additional demands on our financial, operational and management resources. To manage the growth of our operations and personnel, we must both modify our existing operational and financial systems, procedures and controls and implement new systems, procedures and controls. We must also expand our finance, administrative and operations staff. Management may be unable to hire, train, retain, motivate and manage necessary personnel or to identify, manage and exploit existing and potential strategic relationships and market opportunities. The failure to manage growth effectively could have a material adverse effect on our business, financial condition and results of operations.
Our revenues depend on obtaining coverage and adequate reimbursement from public and private insurers and health systems.
Our success depends on the extent to which reimbursement for the costs of our products will be available from third‑party payors, such as government health administration authorities, private health insurers, health maintenance organizations and pharmacy benefit management companies. A significant number of government and private third‑party payors currently do not provide coverage and reimbursement for our products. If we are not successful in obtaining coverage and adequate reimbursement for our products from more third‑party payors, our ability to sell our products will be adversely affected. Therefore, our ability to grow our revenues is dependent on our ability to meet the requirements for coverage of additional third‑party payors, and to negotiate acceptable reimbursement with such payors once our products have been approved for coverage. Even if we do succeed in obtaining widespread coverage and adequate reimbursement for our products, future changes in coverage and reimbursement policies could have a negative impact on our business, financial condition and results of operations.
Our products may have higher costs than more traditional products, due to the higher cost and complexity associated with their research, development and production, and the complexity associated with their distribution. This higher cost and complexity can make it more difficult to obtain adequate coverage and reimbursement.
Our products may have higher costs or fees associated with them compared with more traditional products, due to the higher cost and complexity associated with their research, development and production, and the complexity associated with their distribution—which requires special handling, storage and shipment procedures and protocols. This, in turn, makes it more difficult for us to obtain approval for coverage and reimbursement from third‑party payors for our products and the procedures in which they are used, particularly if we cannot demonstrate a favorable cost‑benefit relationship. Third‑party payors may also deny coverage because the product has not received approval from the FDA or other government regulators that they believe is necessary, or they believe that the product is experimental, unnecessary or inappropriate.
Even though we are not required to conduct clinical trials in order to market our products in the United States, we may nevertheless be required to conduct one or more clinical studies, and to publish one or more peer reviewed journal articles supporting the products, before we are able to obtain third‑party reimbursement. We may also be required to conduct additional clinical studies that compare the cost effectiveness of our products to other available therapies before third‑party payors will provide reimbursement. Conducting clinical studies is expensive and results in delays in wide scale commercialization and reimbursement. In addition, even if our products otherwise meet the requirements for reimbursement, pricing negotiations with third‑party payors may take months or longer and result in significant delay in obtaining approval for reimbursement.
Coverage and reimbursement policies also sometimes differ depending upon the setting in which the product is to be used. The use of our products in a hospital setting as part of a surgical or other more extensive procedure may have a coverage and reimbursement pathway that differs from a use in an outpatient setting for a more narrowly defined procedure. Thus, for example, the coverage and reimbursement pathway for Grafix—which we expect to be used more often in an outpatient setting—may differ from that for BIO
4
—which we expect to be used more often in an in‑patient hospital setting as part of a surgical procedure. These differences may limit or make coverage and reimbursement more difficult for some products as compared to others, and influence our product development and marketing efforts in ways
that may ultimately prove to be detrimental to our business. Payors’ coverage and reimbursement policies also are subject to change, and the policies in effect at the time a product is marketed may be different from the policies in place when a coverage and reimbursement strategy was developed.
In addition, third‑party payors are increasingly challenging the price and cost‑effectiveness of medical products and services, and many limit coverage and reimbursement for newly approved healthcare products. In particular, third‑party payors may limit the indications for which they will reimburse patients who use our products, or they may not provide reimbursement for our products separately from the procedures in which they are used, to encourage providers to select products based on cost‑effectiveness or for other reasons. Cost‑control initiatives could decrease the price for our products, which would result in lower product revenue to us.
To continue our commercial expansion, we must convince more physicians that our products are appropriate alternatives to traditional methods and products and that our products should be used in their procedures.
While many physicians are using our products, we must continue our efforts to convince other physicians that our products are appropriate alternatives to traditional methods and products. We believe physicians will only adopt our products if they determine, based on experience, clinical data and published peer reviewed journal articles, that the use of our products in a particular procedure is a favorable alternative to conventional methods. Physicians may be slow to change their practices for the following reasons, among others:
|
|
·
|
|
their lack of experience in the field using our products;
|
|
|
·
|
|
lack of evidence supporting additional patient benefits and our products over conventional methods;
|
|
|
·
|
|
perceived liability risks generally associated with the use of new products and procedures;
|
|
|
·
|
|
limited availability of reimbursement from third‑party payors;
|
|
|
·
|
|
the exclusion of our products on the formulary of their affiliated hospital or group purchasing organization (“GPO”), which would preclude their use of our product; and
|
|
|
·
|
|
the time that must be dedicated to training physicians on how to use our products.
|
In addition, hospital acquisition decisions often are affected by physicians’ assessments of products. If physicians do not support adoption of our products or if we are unable to demonstrate favorable long‑term clinical data, hospitals may not use our products, which would significantly reduce our ability to achieve expected revenue.
The potential of our products under development may not be realized.
We are continually evaluating the potential of our current products and products under development. Our products are susceptible to various risks, including undesirable and unintended side effects, inadequate efficacy or other characteristics that may prevent or limit their commercial use, or if required, pre‑marketing approval. We have invested substantial time and resources in developing and commercializing GrafixPL PRIME using our novel Prestige Lyotechnology, a proprietary method to preserve living cells and tissues at room temperatures. Development and commercialization of additional products using Prestige Lyotechnology or new technologies will require additional research, clinical evaluation, significant marketing efforts and substantial additional investment before they can provide us with any revenue. Despite our efforts, any such products may not become commercially successful products for a number of reasons, including:
|
|
·
|
|
we may experience delays in our development programs;
|
|
|
·
|
|
any products that are approved may not be accepted in the marketplace by patients, physicians, or payors;
|
|
|
·
|
|
we may not be able to manufacture any such products in sufficient commercial quantities; and
|
|
|
·
|
|
rapid technological change may make such products obsolete.
|
If the potential of our products is not realized, the value of our products, technology and development programs could be significantly reduced.
Some product development programs are based on novel technologies, which are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of our technology platforms and product candidates creates significant challenges in regards to product development and optimization, processing and manufacturing, government regulation and/or approval, third‑party reimbursement and market acceptance. Therefore, the pathway to development and commercialization of our products may be more complex and lengthy than other products. Additionally, tissue‑ and cell‑based products are subject to donor‑to‑donor variability, which can make standardization more difficult. As a result, the development and commercialization pathway for our products is subject to uncertainty.
We depend on key personnel.
Our current and future success depends to a significant extent on the skills, experience and efforts of our scientific, management, technical and sales personnel. None of our employees is employed for a specified term, and we have experienced significant turnover. Competition for personnel is intense. We may be unable to retain our current personnel or attract or integrate other qualified scientific, management, technical or sales personnel in the future which could harm our business and might significantly delay or prevent the achievement of research, development, sales or other business objectives.
We are in a highly competitive and evolving field and face competition from well‑established tissue product manufacturers as well as new market entrants.
Our business is in a very competitive and evolving field. Competition from other companies and from research and academic institutions is intense and widespread, expected to increase, subject to rapid change and could be significantly affected by new product introductions. The presence of this competition in our market may lead to pricing pressure, which would limit our ability to sell our products at a price that would make us profitable or prevent us from selling our products at all. Our ability to successfully compete will depend on whether we can perfect and protect our intellectual property rights related to our technologies as well as to develop new technologies and new applications for our technologies. Our failure to compete effectively would have a material adverse effect on our business, financial condition and results of operations.
Our products could become obsolete due to rapid technological change.
The technologies underlying our products are subject to rapid and profound technological change. Competition intensifies as technical advances in each field are made and become more widely known. We can give no assurance that others will not develop services, products or processes with significant advantages over the products that we offer or are developing. Any such occurrence could have a material adverse effect on our business, financial condition and results of operations.
Many of our competitors have greater resources or capabilities than we have, or may succeed in developing new or better products more quickly than we do.
In the marketplace, we compete with other companies and organizations that are marketing or developing products competitive with Grafix, Stravix, and our other products and products under development. In many cases, the competing product or candidate is based on bioengineering or other technologies. Companies competing with our products include, but are not limited to: Organogenesis Inc., the manufacturer of Apligraf
®
and Dermagraft
®
, MiMedx Group, Inc., the manufacturer of EpiFix
®
, and Integra LifeSciences Corporation, the manufacturer of Integra, all of
which compete with Grafix and Stravix. BIO
4
competes with bone tissue products such as Osteocel
®
marketed by NuVasive, Inc. and Trinity
®
marketed by Orthofix International NV, while Cartiform competes with cartilage allografts such as ProChondrix
®
marketed by AlloSource and DeNovo
®
marketed by Zimmer Biomet Holdings, Inc. In addition to those listed above, we have other existing and potential competitors developing a variety of products for the same conditions for which we market our products. Many of our current and potential competitors have greater financial and human resources than we have, including more experience in R&D and more established marketing and distribution capabilities.
The biotechnology industry is characterized by rapid technological change, resulting in new product introductions and other technological advancements. Because FDA approval is generally not required for tissue‑based products which are not more than minimally manipulated, competitors might choose to enter this market and produce a substantially similar product, and we may not be able to prevent the marketing and distribution of any such similar products by others. Should others produce a substantially similar product or a new product that renders our current or future products obsolete, we could be subject to increased competition and our potential revenue from distribution of these products may be limited.
Our products are derived from human tissue and therefore have the potential for disease transmission.
Our products consist of human tissue: Grafix is manufactured from human placental tissue; Stravix is manufactured from human placental tissue comprised of amniotic and connective layers of umbilical tissue; BIO
4
is manufactured from cadaveric donor bone; and Cartiform is manufactured from cadaveric donor cartilage.
The utilization of human tissue creates the potential for transmission of communicable disease, including, but not limited to, human immunodeficiency virus, Zika virus, viral hepatitis, syphilis, Creutzfeldt‑Jakob disease (the human form of “mad cow” disease) and other viral, fungal or bacterial pathogens. We, and our suppliers of human adult cadaveric bone, cartilage and placenta tissue are required to comply with federal and state regulations and applicable standards intended to prevent communicable disease transmission. Although we and our suppliers have strict quality controls over the procurement and processing of our tissue:
|
|
·
|
|
we can provide no assurance that these quality controls will be adequate;
|
|
|
·
|
|
we or our suppliers may fail to comply with such regulations and standards;
|
|
|
·
|
|
even with compliance, our products might nevertheless be viewed by the public as being associated with transmission of disease; and
|
|
|
·
|
|
a patient that contracts an infectious disease might assert that the use of our products resulted in disease transmission, even if the patient became infected through another source.
|
Any actual or alleged transmission of communicable disease could result in patient claims, litigation, distraction of management’s attention and potentially increased expenses. Further, any failure in screening, whether by us or other manufacturers of similar products, could adversely affect our reputation, the support we receive from the medical community and overall demand for our products. As a result, such actions or claims, whether or not directed at us, could have a material adverse effect on our reputation with our customers and our ability to distribute our products, which could have a material adverse effect on our business, financial condition and results of operations.
Ethical, legal, and other concerns surrounding the use of human tissue may negatively affect public perception of us or our products, or may result in increased scrutiny of our products and product candidates from a regulatory approval perspective, thereby reducing demand for our products, restricting our ability to market our products or adversely affecting the market price for our common stock.
The commercial success of our products depends in part on general public acceptance of the use of human tissue as a part of the treatment of human diseases and other conditions. The use of human tissue including placental tissue from full‑term normal pregnancies, which is discarded otherwise, has been the subject of debate regarding related
ethical, legal and social issues. We do not use embryonic stem cells or fetal tissue, but the public may fail to differentiate our use of adult tissue, including placental tissue from the use by others of embryonic stem cells or fetal tissue. Ethical concerns have been raised by some about the use of donated human tissue in a for‑profit setting. This could result in a negative perception of our company or our products.
Future adverse events in the field of cellular‑based therapy or changes in public policy could also result in greater governmental regulation of our products and potential regulatory uncertainty or delay relating to any required testing or approval.
Our dependence upon human tissue necessary to produce our products may impact our ability to produce these products on a large scale.
As an accredited and licensed tissue bank, we acquire some of our tissue supply through our own collection efforts. The remaining portion of our tissue supply is obtained through third‑party donor agencies. We and our supplier agencies may not be able to collect sufficient amounts of tissue to meet the demand. Shortages or disruptions in the supply of human tissue can adversely impact our ability to fulfill orders, resulting in decreased sales. For example, in 2016, the FDA issued guidance regarding the Zika virus, which limited our supply of placental tissue for a period of time. Since 2016, we have added additional donor agencies and initiated our own collection efforts. Nevertheless, there can be no assurance that any change in guidance from the FDA or future outbreaks of Zika would not hamper our ability to acquire human placental tissue to meet our manufacturing needs.
The availability of donated tissue could also be adversely impacted by public opinion of the donor process as well as our own reputation in the industry. Moreover, the use of human tissue as a part of the treatment for human disease and medical conditions has increased over recent years and continues to increase, creating greater and continually increasing competition and demand for donated human tissue. Even if we are successful in our efforts to expand our compliment of products, we may not be able to secure quantities of human tissue sufficient to meet the demand.
We may not be able to process our products in sufficient quantities to meet market demand or expand our market for the products.
We currently manufacture all of our supply of Grafix and Stravix products at our facility in Columbia, Maryland. Currently, we outsource manufacturing of all of our supply of BIO
4
and Cartiform to Aziyo and CTS. A lengthy disruption or shutdown of, or a shortage of supply at, our current manufacturing facilities or the manufacturing facilities of Aziyo or CTS, whether due to the occurrence of natural disasters, the need to comply with the requirements of directives from government agencies, such as the FDA, the lack of supply of human tissue, or otherwise, could have a material adverse effect on our business, financial condition and results of operations.
In addition, our product supply chain and manufacturing infrastructure depends on the performance of a number of complex contracts between us on the one hand and our suppliers on the other. If any of our suppliers, contract manufacturers or other service providers cannot or do not perform their contractual obligations, then our production efforts may suffer. If we cannot or do not perform our contractual obligations, then we may be subject to arbitration, mediation or litigation that could have a material adverse effect on us.
Reliance on third parties entails risks to which we would not be subject if we manufactured all of our products and product components ourselves, including:
|
|
·
|
|
reliance on third parties for regulatory compliance and quality assurance;
|
|
|
·
|
|
the possible breach of the manufacturing agreement by the third party; and
|
|
|
·
|
|
the possible termination or nonrenewal of the agreement by the third party, based on its own business priorities, at a time that is costly or inconvenient for us.
|
We use or may use third parties to help us develop, manufacture, market, and/or distribute our products, and our business may be impaired if our third‑party relationships are unsuccessful.
We have arrangements in place with third parties that help us with certain aspects of our business. Each third party supports us in differing capacities, including our R&D, human tissue supply, regulatory compliance, tissue procurement, manufacturing, testing, or marketing and distribution efforts. We are subject to a number of risks associated with our dependence upon our third‑party relationships, including:
|
|
·
|
|
the third parties may not cooperate with us or perform their obligations under our agreements with them;
|
|
|
·
|
|
we cannot control the quality, amount and timing of the third parties’ resources that will be devoted to performing their responsibilities under our agreements with them, and they may choose to pursue alternative technologies in preference to those being developed or commercialized with us;
|
|
|
·
|
|
the third parties may refuse or fail to perform their responsibilities in a timely manner, including breach;
|
|
|
·
|
|
a third party may terminate its agreement with us for reasons outside our control, and in some cases on limited notice;
|
|
|
·
|
|
business combinations and changes in a third party’s business strategy may adversely affect the third party’s willingness or ability to complete its obligations;
|
|
|
·
|
|
loss of significant rights to the other party if we fail to meet our obligations under our agreements;
|
|
|
·
|
|
the ability of a third party to successfully market and promote our products;
|
|
|
·
|
|
withdrawal of support by the third party following development or acquisition by the third party of competing products; and
|
|
|
·
|
|
disagreements with a third party regarding our agreement with such third party or ownership of intellectual property or other proprietary rights.
|
Due to these factors and other possible events, we could suffer delays or experience additional costs in the research, development, supply, manufacture, distribution or sale of our products or we may become involved in litigation or arbitration, which would be time consuming and expensive.
We also rely upon third parties for services and raw materials needed for the manufacture and testing of our products.
In order to produce our products, we require biological media, reagents and other highly specialized materials. This is in addition to the human tissue donations used to manufacture our products. These items must be manufactured and supplied to us in sufficient quantities and in compliance with FDA cGTP regulations. To meet these requirements, we either order from or have entered into supply agreements with firms that manufacture these components to cGTP standards and testing service agreements to perform the necessary quality testing.
We rely on third‑party suppliers, contract manufacturers and service providers and commodity markets to secure raw materials, parts, components and sub‑assembly systems used in our products or to manufacture our products, which expose us to volatility in the prices and availability of these materials. Some of these suppliers or their sub‑suppliers are limited or sole‑source suppliers. Some of these suppliers or their sub‑suppliers are located outside of the United States. A disruption in deliveries from our third‑party suppliers, capacity constraints, production disruptions, price increases, or decreased availability of raw materials or commodities, including as a result of catastrophic events, could have an adverse effect on our ability to meet our commitments to customers or increase our operating costs.
Quality and sourcing issues experienced by third‑party suppliers can also adversely affect the quality of our products and result in liability and reputational harm.
The purchase of components and products from international sources subjects us to extensive U.S. and foreign governmental trade, import, export and customs regulations and laws. If we, our product candidates, or the manufacturing facilities for our product candidates or components, fail to comply with applicable regulatory requirements, a regulatory agency may seize or detain products or refuse to permit the import of products.
Our most significant third‑party arrangement is an exclusive agreement with a subsidiary of Stryker for the distribution of BIO
4
, and our success with this product depends upon the success of this relationship.
We are party to an exclusive service agreement with Stryker for the commercialization of our viable bone matrix allograft under the name BIO
4
. Pursuant to the agreement, Stryker is the exclusive worldwide marketer and distributor of allograft services for BIO
4
for use in surgical applications, including spine, trauma, extremity, cranial and foot and ankle surgery. This agreement is subject to all of the risks and uncertainties applicable to third‑party arrangements generally, including those described above.
The agreement with Stryker provided for an initial four‑year exclusive term, which commenced in 2015. In February 2019, Stryker extended the exclusive period for an additional four years. We received an exclusivity fee of $3.9 million in February 2019 to extend the initial term. The agreement provides that Stryker has the option to further extend the term on an exclusive basis for two more years. Pursuant to its terms, Stryker has limited rights to terminate the agreement early. The success of this agreement for us will in part depend upon Stryker’s success in marketing and promoting
BIO
4
.
Stryker has significantly greater resources than we do, and this agreement is not as core to its business as it is to ours. We rely upon Stryker’s continued performance under this agreement, and any determination by Stryker not to proceed or perform, or any material adverse event that affects Stryker’s ability or desire to perform may have a material adverse effect on our business.
We may also enter into additional third‑party agreements in the future. If we fail to maintain our existing or any future relationships for any reason, we would need to undertake on our own and at our own expense, or find other third parties, to perform the activities we currently anticipate will be performed by third parties. This may substantially increase our cash requirements. We may not have the capability or financial capacity to undertake these activities on our own, or we may not be able to enter third‑party relationships on acceptable terms, or at all. This may limit the programs we can pursue and result in significant delays in the development, sale and manufacture of our products, and may have a material adverse effect on our business.
We distribute products through distribution arrangements that sometimes involve the consignment of inventory to third parties, which results in additional risk and uncertainty as to the viability of consigned inventory, inventory accounting, and tax consequences.
We have historically distributed our products either ourselves or through qualified third‑party distributors. In some situations, we store consigned inventory on site in freezers at end‑use hospital or clinic facilities. We commercialize Grafix and Stravix through the efforts of our own direct distribution and marketing staff, as well as through a network of specialty distributors for certain target markets. BIO
4
is sometimes commercialized through a consignment arrangement, and our agreement with Stryker and the end users includes consignment terms, as does our agreement with Arthrex and the end users for Cartiform.
Inventory management, revenue recognition, and inventory and receivables accounting are complicated by a consignment arrangement. Because our cryopreserved consigned inventory must be stored at −80 degrees Celsius, it is at risk of thawing, resulting in the total loss of that inventory, which risk of loss is borne by us. From the revenue recognition perspective, no revenue is recognized upon the placement of inventory into consignment, as we retain title and maintain the inventory on our balance sheet. For these products, revenue is recognized when we receive appropriate notification that the product has been used in a surgical procedure. The Restatement corrected, among other things,
errors in our prior revenue recognition related to various distributor agreements, including several with consigned inventory. If we are unable to track and maintain proper controls related to consigned inventory, we could experience difficulty in accurately managing and accounting for these consignment arrangements and any related tax implications.
We monitor and verify the condition and status of all consigned inventory on at least an annual basis at our expense. We have increased the controls related to consigned inventory, which has increased our operating expenses, and we will likely incur additional expenses in connection with our future planned improvements in our controls related to consigned inventory. In addition, the FDA’s, The American Association of Tissue Banks’ and other accrediting agencies’ rules, regulations or standards require that we monitor our consigned inventory, and require tracking of human tissue and inventory as it moves through the supply chain.
Moreover, should the FDA or any other regulatory authority determine that we are unable for any reason to continue to distribute consigned inventory, either on account of the viability of that inventory or because of the withdrawal of necessary approvals or other qualifications allowing for the distribution and sale of that inventory, the value of that inventory may have to be completely written off and our balance sheet adjusted accordingly. The complexity of our inventory management, or the application of rules, regulations and standards to our product inventory, or the occurrence of any of these negative events, could have an adverse effect on our business, financial condition and results of operations.
We have no control over whether third parties with whom we contract can comply with applicable regulatory requirements.
Our raw material suppliers, contract manufacturers and distributors, and other third parties that we contract with are subject to many or all of the risks and uncertainties to which we are subject. Similar to us, they are subject to ongoing, periodic, unannounced inspection by the FDA and corresponding state and foreign agencies or their designees to ensure strict compliance with applicable regulations and other governmental regulations and corresponding foreign standards. However, we do not control compliance with these regulations and standards by our suppliers, distributors and other third parties with which we contract. They might not be able to comply with these regulatory requirements. If they fail to comply with applicable regulations, the FDA or other regulatory authorities could issue orders of retention, recall, destruction or cessation of manufacturing, or impose sanctions on us, including fines, injunctions, civil penalties, denial of any required marketing approval, delays, suspension or withdrawal of approvals, license revocation, product seizures or recalls, operating restrictions and criminal prosecutions. Any of these actions could significantly and adversely affect the supply and distribution of our products and could have a material adverse effect on our business, financial condition and results of operations.
In addition to costs incurred in product development and management of the reimbursement processes, we will incur additional operating expenses in connection with the expansion of our business.
We expect to continue to incur significant operating expenses in connection with our planned expansion of our business as we seek to:
|
|
·
|
|
continue to develop, expand and support our distribution network of third‑party distributors and independent sales professionals for the distribution of Grafix, BIO
4
, Cartiform and other products;
|
|
|
·
|
|
continue to expand and support our internal sales force and marketing capabilities, through the hiring of sales and marketing professionals and building an internal sales and marketing organization;
|
|
|
·
|
|
hire or engage additional manufacturing, quality control, quality assurance and management personnel as necessary to expand our manufacturing operations;
|
|
|
·
|
|
expand our manufacturing capacity for our products, all of which must be manufactured in a FDA compliant and validated product manufacturing facility; and
|
|
|
·
|
|
expand and protect our intellectual property portfolio for our products.
|
Our ability to scale up our production capabilities for larger quantities of these products remains to be proven. Our costs in marketing and distributing these products will also increase as production increases.
Our future capital needs are uncertain and we may need to raise funds in the future, and such funds may not be available on acceptable terms or at all.
Continued expansion of our business will be expensive and we may seek funds from public and private stock offerings, borrowings under future credit facilities or other sources. Our capital requirements will depend on many factors, including:
|
|
·
|
|
the revenues generated by sales of our products;
|
|
|
·
|
|
the costs associated with expanding our sales and marketing efforts;
|
|
|
·
|
|
the costs associated with the Restatement and the resolution of related legal proceedings;
|
|
|
·
|
|
the expenses we incur in manufacturing and managing the supply chain for our products;
|
|
|
·
|
|
the costs of developing and commercializing new products or technologies;
|
|
|
·
|
|
the cost of maintaining current products as 361 HCT/Ps or obtaining regulatory approval through the BLA regulatory pathway if any of our products lose their 361 HCT/P status;
|
|
|
·
|
|
the number and timing of any acquisitions and other strategic transactions;
|
|
|
·
|
|
the costs associated with capital expenditures; and
|
|
|
·
|
|
unanticipated general and administrative expenses.
|
As a result of these factors, we may seek to raise capital, and such capital may not be available on favorable terms, or at all. Furthermore, if we issue equity or debt securities to raise capital, our existing stockholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise capital through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our products, potential products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise capital on acceptable terms, we may not be able to develop or enhance our products, execute our business plan, take advantage of future opportunities, or respond to competitive pressure, changes in our supplier relationships, or unanticipated customer requirements. Any of these events could adversely affect our ability to achieve our development and commercialization goals, which could have a material adverse effect on our business, financial condition and results of operations.
If our manufacturing and storage facility is damaged or destroyed, our business and prospects would be negatively affected.
If our manufacturing and storage facility or the equipment in the facility were to be significantly damaged or destroyed, we could suffer a loss of some or all of the stored product, raw and other materials and work in process.
We lease 61,203 square feet of space in Columbia, Maryland that houses essentially all of our operations. Currently, we maintain insurance coverage totaling $21.25 million against damage to our property and equipment, an additional $15.0 million to cover business interruption and extra expenses, including R&D restoration expenses. If we have underestimated our insurance needs, we will not have sufficient insurance to cover losses above and beyond the limits on our policies.
The use of our products in human subjects may expose us to product liability claims, and we may not be able to obtain adequate insurance.
We face an inherent risk of product liability claims and only have limited safety data for our products. We derive the raw materials for our products from human donor sources, the production process is complex and the handling requirements are specific, all of which increase the likelihood of quality failures and subsequent product liability claims. We may not be able to obtain or maintain product liability insurance on acceptable terms with adequate coverage, or at all. If we are unable to obtain insurance, or if claims against us substantially exceed our coverage, then our business could be adversely impacted. Whether or not we are ultimately successful in any product liability litigation, such litigation could consume substantial amounts of our financial and managerial resources and could result in, among other things:
|
|
·
|
|
significant awards against us;
|
|
|
·
|
|
substantial litigation costs;
|
|
|
·
|
|
injury to our reputation; or
|
|
|
·
|
|
adverse regulatory action.
|
Any of these results could have a material adverse effect on our business, financial condition and results of operations.
We may implement a product recall or voluntary market withdrawal, which could significantly increase our costs, damage our reputation and disrupt our business.
The manufacturing and marketing of our tissue products involve an inherent risk that our tissue products or processes do not meet applicable quality standards and requirements. In that event, we may voluntarily implement a recall, report a HCT/P deviation or market withdrawal or may be required to do so by a regulatory authority. A recall or market withdrawal of one of our products would be costly and would divert management resources. A recall, HCT/P deviation or market withdrawal regarding one of our products, or a similar product manufactured by another entity, also could impair sales of our products as a result of confusion concerning the scope of the recall or withdrawal, or as a result of the damage to our reputation for quality and safety.
We and our distributor sales representatives must comply with U.S. federal and state fraud and abuse laws, including anti‑kickback and false claims laws and equivalent foreign rules.
We are exposed to the risk that our employees, independent contractors, principal investigators, consultants, vendors or third‑party distributors may engage in fraudulent or other illegal activity. Misconduct by these parties could include, among other infractions or violations, intentional, reckless and/or negligent conduct or unauthorized activity that violates FDA regulations, manufacturing standards, federal and state healthcare fraud and abuse laws and regulations, laws that require the true, complete and accurate reporting of financial information or data, other commercial or regulatory laws or requirements and equivalent foreign rules. We have policies and procedures intended to prohibit and deter such conduct, including a Code of Ethics for Interactions with Healthcare Professionals, a Code of Conduct, and a Whistleblower Policy. However, it is not always possible to identify and deter misconduct by our employees and third parties. Our precautions to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations.
There are numerous U.S. federal and state laws pertaining to healthcare fraud and abuse, including anti‑kickback and false claims laws. These laws are complex, and even minor irregularities can potentially give rise to
claims that a statute or prohibition has been violated. Our and our distributor’s relationships with physicians, other healthcare professionals and hospitals are subject to scrutiny under these laws. The laws that may affect our ability to operate include:
|
|
·
|
|
the federal Anti‑Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, items or services for which payment may be made, in whole or in part, under federal healthcare programs, such as the Medicare and Medicaid programs. There can be both criminal and civil penalties for violations;
|
|
|
·
|
|
the federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement to induce a false claim payment. There are also criminal penalties, including imprisonment and criminal fines, for making or presenting a false or fictitious or fraudulent claim to the federal government;
|
|
|
·
|
|
HIPAA, which created federal criminal laws that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program including private third‑party payors;
|
|
|
·
|
|
the federal Physician Payment Sunshine Act, which requires manufacturers of drugs, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually (with certain exceptions) to CMS information related to payments or other “transfers of value” made to physicians and teaching hospitals, and requires applicable manufacturers and group purchasing organizations to report annually to CMS ownership and investment interests held by physicians and their immediate family members and payments or other “transfers of value” to such physician owners;
|
|
|
·
|
|
the federal Foreign Corrupt Practices Act and similar anti‑bribery laws in other jurisdictions, which generally prohibit companies and their intermediaries from making improper payments to government officials and/or other persons for the purpose of obtaining or retaining business; and
|
|
|
·
|
|
analogous state and foreign law equivalents of each of the above federal laws, such as:
|
|
|
·
|
|
anti‑kickback and false claims laws which may apply to items or services reimbursed by any third‑party payor, including commercial insurers; or
|
|
|
·
|
|
state laws that require biologic and drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
|
Violations of any of the laws described above or any other governmental regulations are punishable by significant civil, criminal and administrative penalties, damages, fines and exclusion from government‑funded healthcare programs, such as Medicare and Medicaid. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
In the course of conducting our business, we must adequately address quality issues that may arise with our products, as well as defects in third‑party components included in our products. Although we have established internal procedures to minimize risks that may arise from quality issues, we may not be able to eliminate or mitigate occurrences of these issues and associated liabilities. If the quality of our products does not meet the expectations of physicians or patients, then our brand and reputation could suffer and our business could be adversely impacted.
A significant portion of our revenues and accounts receivable come from government accounts.
We have significant sales to the federal government (whether we are selling our products directly to government accounts or through our current distributors). Any disruption of our products on the Federal Supply Schedule or a change in the way the federal government purchases products like ours, or the price it is willing to pay for our products, could materially and adversely affect our business, results of operations and financial condition.
Changes in internal purchasing procedures by the VA may have an adverse effect on our ability to sell our products to VA hospitals and may have a material adverse effect on our sales and results of operations.
Prior to December 2018, the VA required internal pre-authorization by a warranted contracting officer for purchases of certain types of products, including Grafix and Stravix, for greater than $10,000, except for VA owned inventory or a consignment agreement negotiated by a VA contracting officer. On December 1st, 2018, the VA made changes to the pre-authorization requirement that do not require written documentation, but a pre-authorization would still be needed for input of orders into the VA system. The VA also made adjustments to purchase limits. The impact of these recent changes is still unclear, and thus far the changes and new purchase limits varies across all VA facilities. In the past, pre-authorization delays the purchase of our products. In addition, a pre-authorized product may only be used for the patient for whom authorization was granted. If such product is not used for the authorized patient, it may not be used for any other patient and the product must be returned. The impact and implementation of recent changes to the pre-authorization and purchase limit requirements are still unclear and could have an adverse effect on our ability to sell products to one more VA facilities. Furthermore, any other changes in purchasing procedures and policies by the VA could have an adverse effect on our ability to sell our products to VA hospitals.
The ongoing cost‑containment efforts of GPOs and integrated delivery networks (“IDNs”) may have a material adverse effect on our results of operations.
Many customers for our products use GPOs or are members of IDNs in an effort to contain costs. GPOs and IDNs negotiate pricing arrangements with medical supply manufacturers and distributors, which negotiated prices are made available to a GPO’s or IDN’s affiliated hospitals and other members. If we are not one of the providers selected by a GPO or IDN, affiliated hospitals and other members may be less likely to purchase our products, and, if the GPO or IDN has negotiated a strict compliance contract for another manufacturer’s products, we may be precluded from making sales to members of the GPO or IDN for the duration of the contractual arrangement. Our failure to respond to the cost‑containment efforts of GPOs and IDNs may cause us to lose market share to our competitors and could have a material adverse effect on our sales and results of operations.
Significant disruptions of information technology systems or breaches of information security could adversely affect our business.
We rely to a large extent upon information technology systems to operate our business. We collect, store and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property). We also have outsourced significant elements of our operations to third parties, including vital components of our information technology infrastructure. As a result, many third‑party vendors may or could have access to our confidential information. The size and complexity of our information technology and information security systems, and those of our third‑party vendors (and the large amounts of confidential information that is present on them), make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from malicious attacks by third parties. Such attacks are of ever‑increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage and market manipulation) and expertise. Our efforts to prevent service interruptions or security breaches may not be sufficient. Any interruption or breach in our systems could result in the loss of critical or sensitive confidential information or intellectual property, allow third parties to gain material, inside information that they could use to trade our securities, and could result in financial, legal, business, operational and reputational harm to us.
We may expand our business through acquisitions, licenses, investments and other commercial arrangements in other companies or technologies, which contain significant risks.
We periodically evaluate strategic opportunities to acquire companies, divisions, technologies, products and rights through licenses, distribution agreements, investments or outright acquisitions to grow our business. In connection with one or more of those transactions, we may:
|
|
·
|
|
issue additional equity securities that would dilute our stockholders’ value;
|
|
|
·
|
|
use cash that we may need in the future to operate our business;
|
|
|
·
|
|
incur debt that could have terms unfavorable to us or that we might be unable to repay;
|
|
|
·
|
|
structure the transaction in a manner that has unfavorable tax consequences, such as a stock purchase that does not permit a step‑up in the tax basis for the assets acquired;
|
|
|
·
|
|
be unable to realize the anticipated benefits, such as increased revenues, cost savings or synergies from additional sales;
|
|
|
·
|
|
be unable to secure the services of key employees related to the acquisition; and
|
|
|
·
|
|
be unable to succeed in the marketplace with the acquisition.
|
Any of these items could materially and adversely affect our revenues, financial condition and profitability. Business acquisitions also involve the risk of unknown liabilities associated with the acquired business, which could be material. Incurring unknown liabilities or the failure to realize the anticipated benefits of an acquisition could materially and adversely affect our business if we are unable to recover our initial investment. Inability to recover our investment, or any write off of such investment, associated goodwill or assets, could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Regulatory Approval and Other Government Regulations
Should the FDA determine that any of our current products do not meet regulatory requirements that permit qualifying human cells, tissues and cellular and tissue‑based products to be manufactured, stored, labeled, and distributed without pre‑marketing approval, we may be required to stop manufacturing and distributing such products.
The FDA has developed a tiered, risk‑based regulatory framework for human cells, tissues and cellular and tissue‑based products, or so‑called 361 HCT/Ps (meaning that they comply with section 361 of the Public Health Service Act and with 21 CFR Part 1271). The framework includes criteria for facility management, quality assurance, donor selection and manufacture of 361 HCT/Ps. We believe that Grafix
,
Stravix
,
BIO
4
and Cartiform meet the regulatory definition of 361 HCT/P products and as a result do not require the FDA’s pre‑marketing approval. Specifically, we believe all of our current products:
|
|
·
|
|
are minimally manipulated;
|
|
|
·
|
|
are intended for homologous use only, as reflected in our labeling, advertising, and all other indications of our objective intent (
e.g.,
that Grafix be used only as a wound cover, that Stravix be used only as a surgical cover, that BIO
4
be used only for augmentation of bone defects, and that Cartiform be used only as an osteochondral allograft);
|
|
|
·
|
|
are not combined with another article except for water, crystalloids, or a sterilizing, preserving or storage agent in a manner that raises no new clinical safety concerns; and
|
|
|
·
|
|
do not have a systemic effect and are not dependent on the metabolic activity of living cells for their primary function.
|
These criteria form the framework governing our advertising and promotional activities. If we advertise or promote any product in a manner that conveys an intent that it be used for non‑homologous uses, that suggests that the product’s primary function depends on systemic effects or the metabolic activity of living cells, or that indicates that our manufacturing process manipulates the product more than minimally by altering the original relevant characteristics of the tissue relating to its utility for reconstruction, repair, or replacement, we will risk causing our products to no longer qualify as 361 HCT/Ps.
On September 26, 2013, we received the Untitled Letter from the FDA. The agency uses untitled letters to communicate violations that the FDA does not consider of regulatory significance sufficient to lead to an enforcement action. The Untitled Letter stated that Grafix and Ovation did not meet the definition of a 361 HCT/P. Among the grounds for the FDA’s position were our marketing claims, including wound healing claims for Grafix. Specifically, the Untitled Letter indicated that Grafix did not meet the requirements because it is dependent upon the metabolic activity of living cells for its primary function and is not intended for autologous use or allogeneic use in a first or second degree relative. On September 30, 2013, we provided clarifying information to the FDA addressing these concerns. Specifically, we communicated that while Grafix does retain the natural cell population, it is not enriched or expanded in any way; instead, the tissue is preserved so that it closely resembles the source tissue in its native state in accordance with the FDA’s definition of minimal manipulation.
In order to make our marketing claims for Grafix clearer, we committed to the FDA to update our labeling and marketing materials for Grafix to that of a wound cover. By October 2014, we completed all commitments made to the FDA, including the discontinuance of Ovation. In April 2016, the FDA performed a routine inspection of us, which included follow‑up on the actions taken to address the Untitled Letter. In May 2016, we received a FDA Establishment Inspection Report which stated that there were no observations, findings, warnings or untitled letters for either the routine inspection or the Untitled Letter follow‑up.
In March 2017, we completed the development of Prestige
Lyotechnology as an alternative to cryopreservation, which previously had been the only method available for long‑term preservation of living cells and tissues, and which we are using to process our current products. We designed our new technology to preserve living cells within tissues while stored at room temperatures. We have used Prestige
Lyotechnology and completed and launched GrafixPL
Prime
. We intend to use Prestige
Lyotechnology in developing additional placental products. We believe that any additional products based on Prestige
Lyotechnology will comply with the above requirements for 361 HCT/Ps.
We engage in ongoing communication with FDA representatives regarding the applicable regulatory requirements and pathways for our products and product candidates. Determining whether a product complies with these regulatory requirements and pathways is complex and dependent upon numerous factors and subject to varying interpretations and conclusions. In November 2017, the FDA finalized its Guidance Document entitled “Regulatory Considerations for Human Cell, Tissues, and Cellular Tissue‑Based Products: Minimal Manipulation and Homologous Use” and issued a corrected version of this document in December 2017. This document provides the FDA’s current guidance on 361 HCT/Ps. Specifically, it clarifies the FDA’s definitions of minimal manipulation and homologous use. The FDA has given affected companies until November 2020 to determine if they meet the requirements and, if not, to file an IND.
We believe all of our current products (Grafix, GrafixPL, Stravix, BIO
4
and Cartiform), as well as our products being developed using our Prestige
Lyotechnology, meet, or will meet, the FDA’s current interpretations. However, the FDA may not agree with our views on these matters. Should the FDA decide that our current and future products do not meet the regulatory definition of 361 HCT/Ps, we will not be able to produce and distribute these products unless and until we submit a BLA and obtain pre‑marketing approval from the FDA, which would require clinical trials and could take years to obtain, at significant expense. This or any other determination by the FDA that adversely affects our ability to produce or to market any of our products or product candidates would have a material adverse effect on our business, financial condition and results of operations.
Our business is subject to an inherently uncertain and evolving area of regulation.
The regulatory framework that the FDA has developed for 361 HCT/Ps is inherently uncertain and the FDA’s regulation of 361 HCT/Ps is evolving. The FDA may alter or recalibrate its regulatory interpretations and enforcement activities, including in the event a competitor obtains pre‑marketing approval for a product similar to any of our products. Further, the FDA could require that our products, which lack pre‑marketing approval by the FDA, be taken off the market.
In addition, while the FDA’s advertising and promotional labeling regulations do not apply to 361 HCT/Ps, the agency could become more exacting with regard to acceptable advertising and promotional activities for 361 HCT/Ps. Specifically, under FDA regulations, a manufacturer may not promote a 361 HCT/P in a manner that communicates an objective intent of the manufacturer for the HCT/P to be used for non‑homologous uses. In addition, a manufacturer risks undermining its product’s 361 status if it describes its product in a way that suggests that the product does not otherwise meet the criteria for qualifying as a 361 HCT/P, such as by emphasizing the metabolic activity of live cells in the product. Because various government agencies that regulate HCT/Ps, such as the FDA and CMS, employ different terms to describe HCT/Ps and apply different criteria to its decisions, a risk exists that our sales representatives and other employees may use terms applicable to one regulatory regime that are detrimental in another regulatory regime. An example would be that describing an HCT/P as treating a wound for purposes of justifying reimbursement could be interpreted by the FDA as implying that the manufacturer intends the product to be used for non‑homologous wound healing.
If the FDA determines that any of our current products are not 361 HCT/Ps, or that any of our future products are not 361 HCT/Ps, we will be required to seek and obtain pre‑marketing regulatory approval.
If the FDA determines that one or more of our current products do not meet the criteria for 361 HCT/Ps, we will need to pursue pre‑marketing approval applicable to biologics in the United States, which is also referred to as licensure. We are currently considering product candidates that require licensure from the FDA. In the United States, a company must complete rigorous preclinical testing and extensive clinical trials that demonstrate the safety, purity and potency of a biological product in order to apply for licensure to market the product. The steps generally required by the FDA include:
|
|
·
|
|
performance of preclinical (animal and laboratory) tests, in accordance with the FDA’s cGLP regulations and other applicable requirements;
|
|
|
·
|
|
submissions to the FDA of an IND, which must become effective before clinical trials may commence;
|
|
|
·
|
|
approval by an independent IRB of each clinical site before a clinical trial is initiated;
|
|
|
·
|
|
performance of adequate and well‑controlled clinical trials according to the FDA’s cGCP regulations, and any additional requirements for the protection of human research subjects and their health information to establish the safety, purity and potency of the investigational biological product in the intended target population for its intended use;
|
|
|
·
|
|
establishment and validation of a consistent and reproducible manufacturing process intended for commercial use, including the collection of appropriate manufacturing data;
|
|
|
·
|
|
preparation and submission to the FDA of a BLA for marketing approval that includes substantial evidence of safety, purity, and potency from results of nonclinical testing and clinical trials;
|
|
|
·
|
|
satisfactory completion of a FDA inspection of the manufacturing facility or facilities where the biological product candidate is produced to assess compliance with cGMPs and to assure that the facilities, methods and controls are adequate to preserve the biological product candidate’s identity, safety, strength, quality, potency, and purity;
|
|
|
·
|
|
potential FDA inspection of the nonclinical and clinical trial sites that generated the data in support of the BLA; and
|
|
|
·
|
|
FDA review and approval of the BLA before any commercial sale or shipment of the product can begin again.
|
The processes are expensive and can take many years to complete. If we are required to obtain pre‑marketing approval from the FDA for any of our existing or future products, we may not be able to demonstrate the safety, purity and potency of our products to the satisfaction of regulatory authorities. The start of clinical trials can be delayed or take longer than anticipated for many and varied reasons, many of which are out of our control. Safety concerns may emerge that could lengthen the ongoing clinical trials or require additional clinical trials to be conducted. Promising results in early clinical trials may not be replicated in subsequent clinical trials. Regulatory authorities may also require additional testing, and we may be required to demonstrate that our products represent an improved form of treatment over existing therapies, which we may be unable to do without conducting further clinical trials. Moreover, if the FDA grants regulatory approval of a product, the approval may be limited to specific indications or limited with respect to its distribution. Expanded or additional indications for approved products may not be approved, which could limit our revenue opportunities.
Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly and our failure to comply could result in negative effects on our business.
As discussed above, the FDA has specific regulations governing our tissue‑based products, or HCT/Ps. The FDA’s regulation of HCT/Ps includes requirements for registration and listing of products, donor screening and testing, manufacture and distribution, labeling, record keeping and adverse‑reaction reporting, and inspection and enforcement. The FDA has broad regulatory and enforcement powers.
If we fail to comply with the FDA regulations regarding our tissue‑based products, the FDA could take enforcement action, including, without limitation, any of the following sanctions that may be relevant to our current or future business operations, and the manufacture of our products or processing of our tissue could be delayed or terminated:
|
|
·
|
|
untitled letters and warning letters;
|
|
|
·
|
|
orders of retention, recall, destruction and cessation of manufacturing;
|
|
|
·
|
|
product seizures, injunctions and civil penalties;
|
|
|
·
|
|
operating restrictions;
|
|
|
·
|
|
refusing applications for licensure of new products;
|
|
|
·
|
|
suspending current applications for licensure, or revoking or suspending licenses already granted;
|
|
|
·
|
|
refusal to allow the importation of our products or raw materials; and
|
It is likely that the FDA’s regulation of HCT/Ps will continue to evolve in the future. Complying with any such new regulatory requirements may entail significant time delays and expense, which could have a material adverse effect on our business, financial condition and results of operation.
In addition to FDA regulations, we are subject to other laws, rules, regulations, and standards regarding the use of human tissue.
We are registered with the FDA as a tissue bank. In addition, some states have their own tissue banking regulations. We are licensed as a tissue bank in Maryland, California, Florida, Illinois, Oregon, Delaware and New York. If we fail to comply with any of the requirements for licensure as a tissue bank, we will not be able to operate as a tissue bank and collect and store donor tissue. The loss of this licensure could adversely impact the quantity of human tissue available to us and our ability to process our products, which could have a material adverse effect on our business, financial condition and results of operations.
In addition, procurement of certain human organs and tissues for transplantation is subject to the restrictions of NOTA, which prohibits the transfer of certain human organs, including skin and related tissue, for valuable consideration, but permits reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery, storage and transportation of donated human tissue. If we were to be found to have violated NOTA’s prohibition on the sale or transfer of human tissue for valuable consideration, we would potentially be subject to criminal enforcement sanctions, which could materially and adversely affect our business, financial condition and results of operations.
Our business involves the use of hazardous materials that could expose us to environmental and other liability.
We have facilities in Maryland that are subject to various local, state and federal laws and regulations relating to safe working conditions, laboratory and manufacturing practices, and the use and disposal of hazardous or potentially hazardous substances, including chemicals, micro‑organisms and various radioactive compounds used in connection with our R&D and manufacturing activities. These laws include the Occupational Safety and Health Act, the Toxic Test Substances Control Act and the Resource Conservation and Recovery Act. We cannot assure you that accidental contamination or injury to our employees and third parties from hazardous materials will not occur. We do not have insurance to cover claims arising from our use and disposal of these hazardous substances other than limited clean‑up expense coverage for environmental contamination due to an otherwise insured peril, such as fire.
Federal and state laws that protect the privacy and security of personal information may increase our costs and limit our ability to collect and use that information and subject us to liability if we are unable to fully comply with such laws.
Numerous federal and state laws, rules and regulations govern the collection, dissemination, use, security and confidentiality of personal information, including individually identifiable health information. These laws include:
|
|
·
|
|
provisions of HIPAA that limit how covered entities and business associates may use and disclose PHI, provide certain rights to individuals with respect to that information and impose certain security requirements;
|
|
|
·
|
|
HITECH, which strengthens and expands the HIPAA Privacy Rule and Security Rules and imposes data breach notification obligations;
|
|
|
·
|
|
other federal and state laws restricting the use and protecting the privacy and security of personal information, including health information, many of which are not preempted by HIPAA;
|
|
|
·
|
|
federal and state consumer protection laws; and
|
|
|
·
|
|
federal and state laws regulating the conduct of research with human subjects.
|
As part of our business operations, including our medical record keeping, third‑party billing and reimbursement and R&D activities, we collect and maintain PHI in paper and electronic format. Standards related to health information,
whether implemented pursuant to HIPAA, HITECH, state laws, federal or state action or otherwise, could have a significant effect on the manner in which we handle personal information, including healthcare‑related data, and communicate with payors, providers, patients, donors and others, and compliance with these standards could impose significant costs on us or limit our ability to offer services, thereby negatively impacting the business opportunities available to us.
If we are alleged to not comply with existing or new laws, rules and regulations related to personal information we could be subject to litigation and to sanctions that include monetary fines, civil or administrative penalties, civil damage awards or criminal penalties.
We face significant uncertainty in the industry due to government healthcare reform.
There have been and continue to be proposals by the federal government, state governments, regulators and third‑party payors to control healthcare costs, and generally, to reform the healthcare system in the United States. With the Trump Administration and the 115th Congress, there have been certain regulatory and legislative changes to the Patient Protection and Affordable Care Act (the “Affordable Care Act”). For example, the Tax Cuts and Jobs Act enacted on December 22, 2017, eliminated the shared responsibility payment for individuals who fail to maintain minimum essential coverage under section 5000A of the Internal Revenue Code of 1986, commonly referred to as the individual mandate, beginning in 2019. Additional legislative changes to and regulatory changes under the Affordable Care Act remain possible. However, it remains unclear how any new regulations or legislation might affect the prices we may obtain for any of our products. Any reduction in reimbursement from Medicare and other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may harm our business and prevent us from being able to attain and maintain profitability. We also cannot predict what further reform proposals, if any, will be adopted, when they will be adopted, or what impact they may have on us.
Risks Related to Intellectual Property
Given our limited patent position in regard to our products, if we are unable to protect the confidentiality of our proprietary information and know‑how related to these products, our competitive position would be impaired and our business, financial condition, and results of operations could be adversely affected.
Our success depends, in part, on our ability to obtain patents, maintain trade secret protection and operate without infringing on the patented rights of third parties. Our policy is to file patent applications to protect technologies and products that we consider important to our business and operations. We hold an ownership interest in a number of patents issued and applications in the United States, Canada, certain countries of the European Union and a few other foreign countries.
It is possible that we will need to defend patents from challenges by others from time to time. Certain of our U.S. patents may be challenged by others in a post-grant proceeding under the America Invents Act of 2011. A post‑grant proceeding has become increasingly common in the United States and is costly to defend. Our patented rights may not provide us with a proprietary position or competitive advantages against competitors. Furthermore, even if the outcome is favorable to us, the enforcement of our intellectual property rights can be extremely expensive and time consuming.
A significant amount of our technology, including our information regarding the manufacturing process for our products, is patent pending, unpatented or is maintained by us as trade secrets or confidential know‑how. In an effort to protect this proprietary information, we require our employees, consultants, service providers, advisors and other third parties to execute confidentiality agreements upon the commencement of their relationships with us. These agreements require that all confidential information developed by the individual or entity or made known to the individual or entity by us during the individual’s or entity’s relationship with us be kept confidential and not disclosed to third parties without prior written consent by us. These agreements, however, may not provide us with adequate protection against improper use or disclosure of trade secrets or confidential information, and these agreements may be breached. For example, a portion of the manufacturing methodology and know‑how for Grafix is protected by trade secret or through
confidentiality arrangements. A breach of confidentiality could affect our competitive position. Also, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or know‑how.
Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. The disclosure of our trade secrets or know‑how could impair our competitive position and could have a material adverse effect on our business, financial condition and results of operations.
If our patent position does not adequately protect our products, others could compete against us more directly, which would harm our business and have a material adverse effect on our business, financial condition, and results of operations.
Patent law relating to the patentability and scope of claims in the biotechnology field is evolving and our patent rights are subject to this additional uncertainty. The degree of patent protection that will be afforded to our products in the United States and other important commercial markets is uncertain and is dependent upon the scope of protection decided upon by the patent offices, courts and governments in these countries. There is no certainty that our existing patents or others, if obtained, will provide us protection from competition or provide commercial benefit. Others may independently develop similar products or processes to those developed by us, duplicate any of our products or processes or, if patents are issued to us, design around any products and processes covered by our patents. We expect to, when appropriate, file product and process applications with respect to our inventions. However, we may not file any such applications or, if filed, the patents may not be issued. Patents issued to or licensed by us may be infringed by the products or processes of others.
Because of the extensive time required for development, testing and regulatory review of a potential product, it is possible that, before any of our products can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantages of the patent. A portion of our technology, including certain know‑how regarding the production processes for our products, is unpatented and is maintained by us as trade secrets. The lack of patent protection for our products reduces the barrier for entry by others and makes these products susceptible to increased competition, which could be harmful to our business.
If we infringe or are alleged to infringe on intellectual property rights of third parties, it could adversely affect our business, financial condition, and results of operations.
Our research, development and commercialization activities, and the manufacture or distribution of our products, may infringe or be alleged to infringe patents owned by third parties and to which we do not hold licenses or other rights. There may be patent applications that have been filed but not published that, when issued, could be asserted against us. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages. Further, if a patent infringement suit were brought against us, we could be enjoined from certain activities including a stop or delay in research, development, manufacturing or sales activities related to the product or technology that is the subject of the suit.
As a result of patent infringement claims, or in order to avoid potential claims, we may choose or be required to seek a license from the third party. These licenses may not be available on acceptable terms, or at all. Even if we are able to obtain a license, the license would likely obligate us to pay license fees or royalties or both, and the rights granted to us might be nonexclusive, which could result in our competitors gaining access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations, if, as a result of actual or threatened patent infringement claims, we are unable to enter into licenses on acceptable terms.
The cost to us of any patent litigation or other proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Patent litigation and other proceedings may also absorb significant management time. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings
could have a material adverse effect on our ability to compete in the marketplace and, as a result, on our business, financial condition and results of operations.
We may become involved in lawsuits or administrative proceedings to protect or enforce our patents or the patents of our service providers or licensors, which could be expensive and time consuming.
Litigation may be necessary to enforce patents issued or licensed to us, to protect trade secrets or know‑how, or to determine the scope and validity of proprietary rights. Litigation, post‑grant review, reexamination, opposition or interference proceedings could result in substantial additional costs and diversion of management focus. If we are ultimately unable to protect our technology, trade secrets or know‑how, we may be unable to operate profitably.
Competitors may infringe on our patents or the patents of our service providers or licensors. As a result, we may be required to file infringement claims to protect our proprietary rights. This can be expensive, particularly for a company of our size, and time‑consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours is invalid or is unenforceable, or may refuse to enjoin the other party from using the technology at issue. An adverse determination of any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly. Interference proceedings brought by the United States Patent and Trademark Office may be necessary to determine the priority of inventions with respect to our patent applications or those of our service providers or licensors. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distraction to our management. We may not be able, alone or with our service providers and licensors, to prevent misappropriation of our proprietary rights.
Furthermore, though we would seek protective orders where appropriate, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If investors perceive these results to be negative, the market price for our common stock could be significantly harmed.
The prosecution and enforcement of patents licensed to us by third parties are not within our control, and without these technologies, our products may not be successful and our business would be harmed if the patents were infringed or misappropriated.
We have obtained licenses from third parties for patents and patent application rights, allowing us to use intellectual property rights owned by or licensed to these third parties. We do not control the maintenance, prosecution, enforcement or strategy for many of these patents or patent application rights and as such are dependent in part on the owners of the intellectual property rights to maintain their viability. Their failure to do so could significantly impair our ability to exploit these technologies.
Risks Related to Our Common Stock
Our common stock was delisted from trading on NASDAQ and relisted on August 1, 2018, which we expect to continue to have a material effect on us and our stockholders.
We were delinquent in the filing of our Annual Reports on Form 10‑K for the years ended December 31, 2015 and December 31, 2016, and our Quarterly Reports on Form 10‑Q for the quarters ended March 31, 2016, June 30, 2016, September 30, 2016, March 31, 2017, June 30, 2017 and September 30, 2017. NASDAQ formally delisted our common stock on April 28, 2017 as a result of our failure to timely file our SEC reports. Following the filing of our Comprehensive Form 10-K on March 28, 2018, we applied for relisting to the NASDAQ, which was granted on August 1, 2018. This gap in our listing may adversely affect our common stock share price.
The trading price of the shares of our common stock is highly volatile, and purchasers of our common stock could incur substantial losses.
Our stock price is volatile. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the price they paid for it. The market price for our common stock may be influenced by many factors, including:
|
|
·
|
|
reduced access to a trading market for our common stock as a result of our delisting from NASDAQ;
|
|
|
·
|
|
loss of investor confidence in us due to the Restatement;
|
|
|
·
|
|
the recent changes in our senior management team and departures of other key personnel over the last few years;
|
|
|
·
|
|
the marketing and distribution of new products by our competitors;
|
|
|
·
|
|
regulatory developments in the United States, generally or specific to us and our products;
|
|
|
·
|
|
changes in the structure of healthcare payment systems;
|
|
|
·
|
|
expiration or termination of our significant relationships with third parties;
|
|
|
·
|
|
market conditions in the pharmaceutical and biotechnology sectors and issuance of securities analysts’ reports or recommendations;
|
|
|
·
|
|
sales of substantial amounts of our stock by existing stockholders;
|
|
|
·
|
|
sales of our stock by insiders;
|
|
|
·
|
|
variations in our financial results or those of companies that are perceived to be similar to us;
|
|
|
·
|
|
general economic, industry and market conditions;
|
|
|
·
|
|
announcements by us of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
|
·
|
|
commercial, stockholder class action and derivative, intellectual property or product liability litigation against us; and
|
|
|
·
|
|
the other factors described in this “Risk Factors” section.
|
We do not intend to pay cash dividends.
We currently do not intend to pay cash dividends for the foreseeable future. We currently intend to retain earnings, if any, to finance our operations and growth. As a result, capital appreciation, if any, of our common stock will be an investor’s only source of potential gain from our common stock for the foreseeable future.
Certain provisions of Maryland law and of our charter and bylaws contain provisions that could delay and discourage takeover attempts and any attempts to replace our current directors by stockholders.
Certain provisions of Maryland General Corporation Law (“MGCL”) and of our Maryland charter and Maryland bylaws contain provisions that may make it more difficult to or prevent a third party from acquiring control of us or changing our Board and management. These include, but are not limited to, the following:
|
|
·
|
|
authorization of the board of directors to issue shares of preferred stock generally without stockholder approval;
|
|
|
·
|
|
requirements that special meetings of stockholders may only be called by stockholders, upon request of stockholders holding at least 20% of the capital stock issued and outstanding; and
|
|
|
·
|
|
requirements that our stockholders comply with advance notice procedures in order to nominate candidates for election to our Board or to place stockholders’ proposals on the agenda for consideration at stockholder meetings.
|
Maryland law also prohibits “business combinations” between us and an interested stockholder or an affiliate of an interested stockholder for five years after the most recent date on which the interested stockholder becomes an interested stockholder. These business combinations include a merger, consolidation, share exchange or, in certain circumstances specified in the statute, an asset transfer or issuance or reclassification of equity securities. Maryland law defines an interested stockholder as any person who beneficially owns 10% or more of the voting power of the corporation’s stock, or an affiliate or associate of the corporation who, at any time within the two‑year period prior to the date in question, was the beneficial owner of 10% or more of the voting power of the corporation’s then‑outstanding voting stock. A person is not an interested stockholder if the board of directors of the corporation approved in advance the transaction by which the person otherwise would have become an interested stockholder. However, such approval may be conditional.
After the five‑year prohibition, any business combination between the corporation and an interested stockholder or an affiliate of an interested stockholder generally must be recommended by the board of directors and approved by the affirmative vote of at least 80% of the votes entitled to be cast by holders of the then‑outstanding shares of voting stock, and two‑thirds of the votes entitled to be cast by holders of the voting stock other than stock held by the interested stockholder with whom or with whose affiliate the business combination is to be effected or stock held by an affiliate or associate of the interested stockholder. These super‑majority vote requirements do not apply if the holders of the common stock receive a minimum price, as defined under Maryland law, for their stock in the form of cash or other consideration in the same form as previously paid by the interested stockholder for its stock.
The statute permits various exemptions from its provisions, including business combinations that are approved or exempted by the board of directors before the time that the interested stockholder becomes an interested stockholder. Our Board has not exempted us from the business combination statute. Consequently, unless the Board adopts an exemption from this statute in the future, the statute will be applicable and may affect business combinations between us and other persons. The statute may discourage others from trying to acquire control of us or increase the difficulty of consummating any such acquisition.
Subtitle 8 of Title 3 of the MGCL (“Subtitle 8”) permits a Maryland corporation with a class of equity securities registered under the Exchange Act, and with at least three independent directors to elect to be subject to any or all of five provisions:
|
|
·
|
|
a two‑thirds vote requirement to remove a director;
|
|
|
·
|
|
a requirement that the number of directors be fixed only by the vote of the directors;
|
|
|
·
|
|
a requirement that a vacancy on the board be filled only by the remaining directors and for the remainder of the full term of the directorship in which the vacancy occurred; and
|
|
|
·
|
|
a majority requirement for the calling of a special meeting of stockholders.
|
An eligible Maryland corporation like us can elect into this statute by provision in its charter or bylaws or by a resolution of its board of directors, without stockholder approval. Furthermore, we can elect to be subject to the above provisions regardless of any contrary provisions in the charter or bylaws. Pursuant to Subtitle 8, we have elected to provide that vacancies on our Board may be filled only by the remaining directors and for the remainder of the full term of the class of directors in which the vacancy occurred.
Concentration of ownership of our common stock among our existing executive officers, directors, and principal stockholders may prevent others from influencing significant corporate decisions, and provisions in our charter allowing for a stockholder vote by consent in lieu of a meeting may make it easier for stockholders holding a majority of our common stock to take action.
Our executive officers, directors and beneficial owners of 5% or more of our common stock and their affiliates, in aggregate, beneficially own approximately 52.1% of our outstanding common stock as of February 28, 2018. Included among this 52.1%, Peter Friedli, the Chairman of the Board, and certain entities with which he is affiliated, beneficially own approximately 42.9% of our outstanding common stock as of February 28, 2019. These persons, acting together, will be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors and any merger or other significant corporate transactions. The interests of this group of stockholders may not coincide with our interests or the interests of other stockholders.
Moreover, as permitted by the MGCL, our charter provides that the holders of common stock entitled to vote generally in the election of directors may take action or consent to any action by delivering a consent in writing or by electronic transmission of the stockholders entitled to cast not less than the minimum number of votes (which is generally either a majority of votes cast or a majority of votes entitled to be cast) that would be necessary to authorize or take the action at a stockholders meeting if the corporation gives notice of the action not later than ten (10) days after the effective date of the action to each holder of the class of common stock and to each stockholder who, if the action had been taken at a meeting, would have been entitled to notice of the meeting.
Accordingly, these persons acting together, and Mr. Friedli specifically, currently has, and will continue to have, a significant influence over the outcome of all corporate actions requiring stockholder approval, including any actions that may be taken by stockholder consent in lieu of a meeting.
Risks Related to the Restatement of Our Financial Statements and Failure to File SEC Reports
We have restated our prior financial statements, which may lead to additional risks and uncertainties, including loss of investor confidence and negative impacts on our stock price.
As a result of the Restatement, we have become subject to a number of additional costs and risks, including costs for accounting and legal fees in connection with or related to the Restatement and the remediation of our material weaknesses in internal control over financial reporting. In addition, the attention of our management team has been diverted by these efforts. The Restatement and related matters could impair our reputation or could cause our counterparties to lose confidence in us. Each of these occurrences could have a material adverse effect on our business, financial condition, results of operations and stock price.
Our management has identified material weaknesses in the Company’s internal control over financial reporting which could, if not remediated, result in additional material misstatements in our consolidated financial statements. We may be unable to develop, implement and maintain appropriate controls in future periods.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, and the Sarbanes‑Oxley Act of 2002 and SEC rules require that our management report annually on the
effectiveness of the Company’s internal control over financial reporting. Among other things, our management must conduct an assessment of the Company’s internal control over financial reporting to allow management to report on, and our independent registered public accounting firm to audit, the effectiveness of the Company’s internal control over financial reporting, as required by Section 404 of the Sarbanes‑Oxley Act. As disclosed in Part II, Item 9A, “Controls and Procedures” of this Form 10‑K, our management, with the participation of our current Chief Executive Officer and our Chief Financial Officer, has determined that we had material weaknesses in the Company’s internal control over financial reporting as of December 31, 2018.
A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim consolidated financial statements will not be prevented or detected on a timely basis. We are actively engaged in developing and implementing a remediation plan designed to address such material weaknesses. However, additional material weaknesses in the Company’s internal control over financial reporting may be identified in the future. Any failure to implement or maintain required new or improved controls, or any difficulties we encounter in their implementation, could result in additional material weaknesses, or could result in material misstatements in our consolidated financial statements. These misstatements could result in a further restatement of our consolidated financial statements, cause us to fail to meet our reporting obligations, reduce our ability to obtain financing or cause investors to lose confidence in our reported financial information, leading to a decline in our stock price.
Although we are working to remedy the ineffectiveness of the Company’s internal control over financial reporting, there can be no assurance as to when the remediation plan will be fully developed and implemented. Until our remediation plan is fully implemented, our management will continue to devote significant time, attention and financial resources to these efforts. If we do not complete our remediation in a timely fashion, or at all, or if our remediation plan is inadequate, there will continue to be an increased risk that we will be unable to timely file future periodic reports with the SEC and that our future consolidated financial statements could contain errors that will be undetected. Further and continued determinations that there are material weaknesses in the effectiveness of the Company’s internal control over financial reporting could also reduce our ability to obtain financing or could increase the cost of any financing we obtain and require additional expenditures of both money and our management’s time to comply with applicable requirements. For more information relating to the Company’s internal control over financial reporting, the material weaknesses that existed as of December 31, 2018 and the remediation activities undertaken by us, see Part II, Item 9A, “Controls and Procedures” of this Form 10‑K.
Our failure to prepare and timely file our periodic reports with the SEC limits our access to the public markets to raise debt or equity capital.
We did not file our Annual Reports on Form 10‑K for the years ended December 31, 2015 and December 31, 2016 and our Quarterly Reports on Form 10‑Q for the quarters ended March 31, 2016, June 30, 2016, September 30, 2016, March 31, 2017, June 30, 2017 and September 30, 2017 as required by the SEC. Because we did not comply with our reporting requirements with the SEC, we are limited in our ability to access the public markets to raise debt or equity capital. Our limited ability to access the public markets could prevent us from pursuing transactions or implementing business strategies that we might otherwise believe are beneficial to our business. Even if we maintain compliance with our SEC reporting obligations prospectively, until one year from the date we regain and maintain status as a current filer, we will be ineligible to use shorter and less costly filing forms, such as Form S‑3, to register our securities for sale. We may use Form S‑1 to register a sale of our stock to raise capital or complete acquisitions, but doing so would likely take longer than using a shorter and less costly form, increase transaction costs and adversely impact our ability to raise capital or complete acquisitions of other companies in a timely manner.
ITEM 1B. Unresolved Staff Comments.
Not Applicable.
ITEM 2. Properties.
Our corporate headquarters are located in Columbia, Maryland, where we lease 61,203 square feet of laboratory, production, warehouse and office space, currently at a rent and associated expenses of approximately $1.2 million per annum. Our lease expires in October 2023, and includes options to extend the term for two additional five‑year periods.
ITEM 3. Legal Proceedings.
From time to time in the ordinary course of business, we are subject to claims, asserted or unasserted, or named as a party to lawsuits, arbitrations or investigations. Litigation, in general, and intellectual property and securities litigation in particular, can be expensive and disruptive to normal business operations. Moreover, the results of legal proceedings cannot be predicted with any certainty and in the case of more complex legal proceedings, such as intellectual property and securities litigation, the results are difficult to predict. Except as described below, we are not aware of any asserted or unasserted legal proceedings or claims that we believe would have a material adverse effect on our business, financial condition or results of operations.
Securities Class Action.
On November 23, 2015, a putative class action lawsuit was filed in the United States District Court for the District of Maryland by a single plaintiff, individually and on behalf of other persons similarly situated, against the Company and three current or former executive officers of the Company. An amended complaint clarifying plaintiff's claims was filed on April 6, 2018. The action, captioned Kiran Kumar Nallagonda v. Osiris Therapeutics, Inc. et al., Case 1:15-cv-03562 (the “Nallagonda Action”), alleged, among other things, that the defendants made materially false or misleading statements and material omissions in the Company’s filings with the SEC in violation of the federal securities laws. The complaint sought certification as a class action, unspecified damages and reimbursement of attorneys’ fees. On March 21, 2016, the court entered an order appointing Dr. Raffy Mirzayan as lead plaintiff and the firm of Hagens Berman Sobol Shapiro LLP as lead counsel. On March 11, 2018, we entered into a memorandum of understanding to settle the Nallagonda Action. Subsequently, on June 5, 2018, the parties executed a Stipulation and Settlement Agreement in which the Company agreed in principle to pay $18.5 million in cash to create a settlement fund for the benefit of class members. On June 12, 2018, the lead plaintiff filed an Unopposed Motion for Preliminary Approval of the parties’ settlement. On September 4, 2018, the Court entered an order preliminarily approving the settlement and scheduling a hearing for February 4, 2019 to determine whether the proposed settlement is fair, reasonable and adequate and whether the case should therefore be dismissed with prejudice. On October 3, 2018, the Company deposited the $18.5 million settlement payment into an escrow account, pending final Court approval of the settlement. On November 11, 2018, the lead plaintiff filed a request for an award of attorneys’ fees and expenses from the settlement fund. At a February 4, 2019 hearing, the Court found the settlement fair, reasonable and adequate and therefore dismissed the case with prejudice in its order of that date.
The Company had a $5.0 million executive and corporate securities liability insurance policy in place at the time of the allegations. The Company received the remaining $4.8 million of unused policy coverage for the shareholder settlement of the Nallagonda Action on October 9, 2018.
Shareholder Derivative Actions.
On March 2, 2016, a shareholder derivative complaint was filed in the Circuit Court for Howard County in the State of Maryland (Case No. 13C16106811) by a single plaintiff, derivatively and on behalf of the Company, against certain current and former directors and certain former executive officers (the “Connelley Action”). This action, captioned Kevin Connelley v. Lode Debrabandere et al., alleges that each of the individual directors and officers named as defendants (i) violated their fiduciary duties to the Company’s shareholders; (ii) abused their ability to control and influence the Company; (iii) engaged in gross mismanagement of the assets and business of the Company; and (iv) was unjustly enriched at the expense of, and to the detriment of, the Company. The alleged claims generally relate to the matters that are the subject of the Nallagonda Action. The plaintiff seeks, among other things, unspecified monetary damages, reimbursement of attorneys’ fees and shareholder votes on amendments to the Company’s Articles of
Incorporation and Bylaws with respect to various corporate governance policies. On June 2, 2016, the Court entered an order that, subject to certain qualifications, stayed the action until 30 days after the entry of an order either: (1) denying all motions to dismiss in the Nallagonda Action, or (2) finally dismissing the Nallagonda Action with prejudice. On March 12, 2018, the plaintiff filed an amended complaint clarifying his claims. On or about July 31, 2018, the parties advised the Court that the Company and the plaintiffs in various derivative actions, including the plaintiff in the Connelley Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below).
On February 9, 2017, a shareholder derivative complaint was filed in the United States District Court for the District of Maryland (Case No. 1:17-cv-00381-JKB) by a single plaintiff, derivatively and on behalf of the Company, against certain current and former directors (the “Recupero Action”). This action, captioned Recupero v. Friedli et al., alleges, among other things, that each of the individual directors named as defendants (i) violated their fiduciary duties to the Company’s shareholders, including that such violations constituted constructive fraud; (ii) engaged in gross mismanagement of the assets and business of the Company; and (iii) was unjustly enriched at the expense of, and to the detriment of, the Company. The plaintiff seeks, among other things, unspecified monetary damages, reimbursement of attorneys’, accountants’ and experts’ fees, and that the Company take all necessary actions to improve and comply with corporate governance, internal procedures and existing laws. On March 28, 2017, the Court entered an order that stays the action until: (1) the Nallagonda Action is dismissed with prejudice and all appeals relating thereto have been exhausted; (2) all motions to dismiss the Nallagonda Action are denied; or (3) either party provides 30 days’ notice that they no longer consent to a stay. On April 5, 2018, the plaintiff filed an amended complaint clarifying her claims. On July 31, 2018, the parties notified the Court that the Company and the plaintiffs in certain derivative actions, including the plaintiff in the Recupero Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below). On August 1, 2018, the Court stayed all further proceedings in the Recupero Action pending consideration of the proposed settlement by the Court in the Salley Action. On February 21, 2019, the parties filed a Stipulation of Dismissal with Prejudice, which is awaiting Court approval.
On May 11, 2017, a shareholder derivative complaint was filed in the Circuit Court for Howard County in the State of Maryland, (Case No. 13C17111441) by a single plaintiff, derivatively on behalf of the Company, against certain former executive officers and certain current and former directors (the “Lee Action”). This action, captioned Brian Lee v. Peter Friedli, et. al., alleges that each of the individual defendants violated their fiduciary duties by allegedly failing to adopt and implement adequate accounting and financial reporting systems and for allegedly causing the Company to make false and misleading statements regarding its financial condition. The alleged claims generally relate to the matters that are the subject of the Nallagonda Action and seek substantially similar relief. On September 5, 2017, the defendants moved to either stay or dismiss the plaintiffs’ complaint. That motion was subsequently withdrawn. On February 14, 2018, the plaintiff filed an amended derivative complaint. No defendant has responded to that pleading. On July 31, 2018, the parties advised the Court that the Company and the plaintiffs in various derivative actions, including the plaintiff in the Lee Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below). The Court subsequently denied the parties’ joint request for a stay of proceedings. Thereafter, on August 15, 2018, the parties filed a Joint Stipulation of Dismissal Without Prejudice and the Lee Action was closed.
On December 21, 2017, a shareholder derivative action was filed in the United States District Court for the District of Maryland (Case No. 1:17-cv-03777) by a single plaintiff, derivatively and on behalf of the Company, against certain former executive officers and certain current and former directors (the “Salley Action”) . This action, captioned Todd Salley v. Lode Debrabandere, et. al., alleges that each of the individual defendants violated their fiduciary duties by failing to maintain adequate internal controls and by causing the Company to make false and misleading statements regarding the Company’s financial condition. The alleged claims generally relate to the matters that are the subject of the Nallagonda Action and seek substantially similar relief. No defendant has responded to the complaint. On July 31, 2018, the parties notified the Court that the Company and the plaintiffs in certain derivative actions, including the plaintiff in the Salley Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below). On August 1, 2018, the Court stayed all further proceedings in the Salley Action except for those related to the proposed settlement.
On October 24, 2018, the parties to the derivative actions entered into a settlement agreement to resolve those cases. The parties’ agreement called for the Company to adopt certain governance changes. Plaintiffs also sought Court approval of an award of attorneys’ fees and expenses in the amount of $0.9 million. We accrued $0.9 million as our
estimated settlement cost in the consolidated balance sheets at December 31, 2018. On November 1, 2018, the United States District Court for the District of Maryland entered an order preliminarily approving the settlement agreement. Immediately after a final settlement hearing held on February 1, 2019, the District Court approved the settlement and dismissed the Salley Action.
Government Investigations
On November 2, 2017, the Company announced the resolution of an investigation by the SEC into the Company’s historical accounting practices. The Company agreed to settle with the SEC, without admitting or denying the allegations of the SEC, by consenting to the entry of a final judgment, subject to court approval, that permanently restrains and enjoins the Company from violating certain provisions of the federal securities laws. As part of the settlement, the Company paid a civil penalty in the amount of $1.5 million. On November 7, 2017, this settlement was approved by the United States District Court for the District of Maryland, through entry of a final judgment, resolving as to the Company the matters alleged by the SEC in the civil complaint against the Company. The SEC civil case is continuing against four former Company officers.
The Company previously announced that a criminal investigation was being conducted by the U.S. Attorney’s Office for the Southern District of New York (“SDNY”) relating to matters that were also being investigated by the SEC. The SDNY investigation resulted in a former chief financial officer of the Company entering into a guilty plea with the government. As previously disclosed, based on communications the Company has had with the SDNY since that time and given that sentencing of the former company officer has now occurred, the Company believes that, subject to any newly discovered information, the SDNY has concluded the criminal investigation with respect to Company‑related matters.
The Company believes that both the previously disclosed SEC and SDNY investigations are concluded with respect to the Company.
ITEM 4. Mine Safety Disclosures.
Not Applicable.
PART II
ITEM 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
During the years ended December 31, 2015 and December 31, 2016, our common stock traded on NASDAQ under the symbol “OSIR.” As a result of the Restatement and our failure to file SEC reports, we were not in compliance with NASDAQ’s requirements for continued listing and, as a result, our common stock was suspended from trading on NASDAQ on March 14, 2017 and delisted from NASDAQ on April 28, 2017.
Following the filing of our Comprehensive Form 10-K on March 28, 2018, we applied for relisting to the NASDAQ, which was granted on August 1, 2018.
Stockholders
As of March 1, 2019, there were approximately 121 stockholders of record of our common stock.
We Do Not Pay Dividends
We did not declare or pay any cash dividends on our common stock during 2017 or 2018 and do not plan to pay cash dividends in the foreseeable future.
Unregistered Sales of Securities and Use of Proceeds
None.
Issuer Purchase of Equity Securities
There were no repurchases by us of our securities during 2018 or 2017 other than in connection with exercise of stock options.
Securities Authorized for Issuance under Equity Compensation Plans
In April 2006, we adopted our 2006 Omnibus Plan. We amended and restated this plan in 2008 and 2010, and further amended it in 2012 and 2014, in each case to, among other things, increase the number of shares available for grant (the “Amended and Restated 2006 Omnibus Plan”). As a result of the 2014 amendment, the Amended and Restated 2006 Omnibus Plan has a termination date of May 6, 2024. The Amended and Restated 2006 Omnibus Plan was approved by our stockholders and authorizes the issuance of various forms of stock‑based awards, including incentive and non‑qualified stock options, stock purchase rights, stock appreciation rights, restricted and unrestricted stock awards and performance units. A total of 3,000,000 shares of our common stock were reserved for issuance under the Amended and Restated 2006 Omnibus Plan. As of December 31, 2018:
|
|
|
|
|
|
|
|
|
|
|
Number of Securities
|
|
Weighted‑Average
|
|
Number of Securities
|
|
|
|
to be Issued Upon
|
|
Exercise Price of
|
|
Remaining Available
|
|
|
|
Exercise of
|
|
Outstanding Options,
|
|
for Future Issuance
|
|
|
|
Outstanding Options,
|
|
Warrants and Rights
|
|
Under Equity
|
|
Plan Category
|
|
Warrants and Rights
|
|
($/Sh)
|
|
Compensation Plans
|
|
Equity compensation plans approved by security holders
|
|
599,251
|
|
11.46
|
|
1,454,334
|
|
Equity compensation plans not approved by security holders
|
|
—
|
|
—
|
|
—
|
ITEM 6. Selected Financial Data
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) is intended to help the reader understand our results of operations, financial condition and our current business environment. This MD&A is provided as a supplement to—and should be read in conjunction with—our consolidated financial statements and the accompanying notes thereto included in Part II, Item 8 of this Form 10‑K.
This MD&A is organized into the following sections:
|
|
·
|
|
Restatement and Related Actions
—a brief overview of the Restatement and related legal proceedings; and the related costs and expenses.
|
|
|
·
|
|
Overview of Osiris
—a general description of our business, products and history.
|
|
|
·
|
|
Critical Accounting Policies
—a discussion of accounting policies that require critical judgments and estimates.
|
|
|
·
|
|
Recent and Pending Accounting Pronouncements
—a discussion of recent and pending accounting policies under review by us that require critical judgments and estimates.
|
|
|
·
|
|
Results of Operations
—an analysis of our consolidated results of operations for the two years presented in our consolidated financial statements.
|
|
|
·
|
|
Liquidity and Capital Resources
—an analysis of our liquidity and financial positions; cash flows; off‑balance sheet arrangements and aggregate contractual obligations.
|
Restatement and Related Actions
Expenses Incurred
In 2018 and 2017, we recorded $3.8 million and $9.5 million, respectively, of expenses for professional fees related to the Restatement and the related legal proceedings. These expenses are included in “General and administrative expenses” in the accompanying consolidated statements of comprehensive income. These expenses, all of which were expensed as incurred, include costs of (i) outside accounting professionals we engaged to assist with the Restatement and recommend improvements to our internal control over financial reporting; (ii) our current independent registered public accounting firm related to its audits of our 2015 and 2016 consolidated financial statements incurred, (iii) reimbursement of expenses of certain current and former officers and directors related to legal proceedings; and (iv) our outside legal counsel related to these matters.
Legal Proceedings
As previously disclosed, we believe that both the previously disclosed SEC and SDNY investigations are now concluded with respect to the Company. In November 2017, we paid a civil penalty in the amount of $1.5 million as part of the SEC settlement, which we recorded as an expense in the fourth quarter of 2015. The SEC civil case is continuing against four of our former officers. We are currently advancing expenses incurred by three of these former officers. As the SEC civil case against these three former officers is continuing, the amount of expenses that we may be required to advance for these three former officers in 2019 could be significant.
As discussed above, on March 11, 2018, we entered into a memorandum of understanding to settle the Nallagonda Action. Subsequently, on June 5, 2018, the parties executed a Stipulation and Settlement Agreement in which the Company agreed in principle to pay $18.5 million in cash to create a settlement fund for the benefit of class members, which was recorded as an expense in the fourth quarter of 2015. On October 3, 2018, the Company deposited the $18.5 million settlement payment into an escrow account, pending final Court approval of the settlement. On November 11, 2018, the lead plaintiff filed a request for an award of attorneys’ fees and expenses from the settlement fund. At a February 4, 2019 hearing, the Court found the settlement fair, reasonable and adequate and therefore dismissed the case with prejudice in its order of that date, which released the monies from escrow to the plaintiffs. Of the $18.5 million settlement payment, we were reimbursed approximately $4.8 million from our insurance carrier in October 2018.
On October 24, 2018, the parties to the derivative actions entered into a settlement agreement to resolve those cases. The parties’ agreement called for the Company to adopt certain governance changes. Plaintiffs also sought Court approval of an award of attorneys’ fees and expenses in the amount of $0.9 million. We accrued $0.9 million as our estimated settlement cost in the consolidated balance sheets at December 31, 2018. On November 1, 2018, the United States District Court for the District of Maryland entered an order preliminarily approving the settlement agreement. Immediately after a final settlement hearing was held on February 1, 2019, with the District Court approving the settlement and dismissed the Salley Action.
Future Costs
We expect to continue to incur significant expenses in 2019 associated with the advancement of expenses to the three former officers in connection with the SEC’s civil case against those individuals. We will recognize these expenses as services are received. Other costs related to the Restatement that may be incurred in 2019 include incremental expenses for remediation activities related to improving our internal control over financial reporting.
Insurance Coverage
We maintained $5.0 million of director’s and officer’s liability insurance coverage during the years ended December 31, 2018 and 2017. Approximately $0.2 million had been paid to us as of December 31, 2017 for reimbursable expenses incurred by us and the remaining $4.8 million was paid to us in October 2018 in connection with the settlement of the securities class action described above.
Remediation
Our management, with the oversight of the Audit Committee, has undertaken a plan to remediate our material weaknesses. Our remediation efforts are intended to address the identified material weaknesses as well as to strengthen our overall financial control environment. See Part II, Item 9A, “Controls and Procedures” of this Form 10‑K for more information about the material weaknesses and our remediation activities.
Overview of Osiris
Osiris Therapeutics, Inc. researches, develops, manufactures, markets, and sells regenerative medicine products intended to improve the health and lives of patients and lower overall healthcare costs. We continue to advance our R&D by focusing on innovation in regenerative medicine, including the development of bioengineered stem cell and tissue‑based products. We have achieved commercial success with products in orthopedics, sports medicine, and wound care, including the Grafix product line, Stravix, BIO
4
and Cartiform.
We are a fully integrated company, having developed capabilities in research and development, manufacturing, marketing, and sales of our products. We are focused on the long‑term commercial growth of the Company through the delivery of differentiated products for use across multiple fields of medicine with clear value propositions to patients, healthcare providers, and third‑party payors.
Our History
We began operations in Ohio in 1992 as a Delaware corporation. With the approval of our stockholders, we reincorporated as a Maryland corporation in May 2010.
From May 2010 to October 2013, we operated as two business segments, therapeutics and biosurgery. Our therapeutics business focused on developing biologic stem cell drug candidates from a readily available source—adult bone marrow. Our biosurgery business, now our only segment, focuses on using unique tissue preservation technologies to develop viable human tissue products designed to improve wound closure and surgical outcomes for patients and physicians over standard of care alone.
In October 2013, we sold our therapeutics business, including Prochymal, a stem cell drug for treatment of graft versus host disease, and related assets, to Mesoblast. The agreement with Mesoblast provided for the receipt of $50 million in initial consideration and up to an additional $50 million in payments upon Mesoblast achieving certain clinical and regulatory milestones.
Since 2013, we have focused our resources on our biosurgery business and have built a substantial direct sales force dedicated exclusively to sell our Grafix and Stravix products. As a result, sales of our Grafix products increased significantly. However, the contribution of our Grafix products to our operating income has been limited due to the heavy investment in sales and marketing. We operate in a competitive and challenging business environment and recognize that our execution of our product development and market penetration strategy will face a number of challenges. As a result, we may experience variability in our overall results, and in our quarter to quarter revenue.
In September 2013, we received the Untitled Letter from the FDA stating that Grafix and Ovation did not meet the definition of a
361
HCT/P. Among the grounds for the FDA’s position were our marketing claims, including wound healing claims for Grafix. We revised all of our marketing claims for Grafix and resolved all issues with the Untitled Letter, which included the discontinuance of Ovation in 2014. Our Grafix products remain on the market as a 361
HCT/P. Production of Ovation was discontinued in the third quarter of 2014 and we sold or shipped to consignees all of our remaining Ovation inventory by the end of October 2014.
In the fourth quarter of 2014, we executed marketing and distribution agreements with Stryker and Arthrex for the distribution of BIO
4
and Cartiform, respectively. Sales under these exclusive distribution arrangements commenced in 2015.
The agreement with Stryker provided for an initial four‑year exclusive term, which commenced in 2015. In February 2019, Stryker extended the exclusive period for an additional four years. We received an exclusivity fee of $3.9 million in February 2019 to extend the initial term. The agreement provides that Stryker has the option to further extend the term on an exclusive basis for two more years. Pursuant to its terms, Stryker has limited rights to terminate the agreement early. The success of this agreement for us will in part depend upon Stryker’s success in marketing and promoting
BIO
4
.
Current Business Environment
Our ability to generate revenue and achieve and sustain profitability is primarily affected by our ability to obtain coverage and adequate reimbursement from public and private insurers and health systems, and our ability to compete in a highly competitive and evolving field.
A significant number of government and private third-party payors currently do not provide coverage and reimbursement for our products and those third-payors that do provide coverage and reimbursement are increasingly challenging the price and cost-effectiveness of medical products and are increasingly limiting coverage and reimbursement for medical products. Our products may have higher costs or fees associated with them compared with more traditional wound dressings, due to the higher cost and complexity associated with their research, development and production, and the complexity associated with their distribution—which requires special handling, storage and shipment procedures and protocols. This, in turn, makes it more difficult for us to obtain approval for coverage and reimbursement from third‑party payors for our products and the procedures in which they are used, particularly if we cannot demonstrate a favorable cost‑benefit relationship. Third‑party payors may also deny coverage because the product has not received approval from the FDA or other government regulators that they believe is necessary, or they believe that the product is experimental, unnecessary or inappropriate.
Even though we are not required to conduct clinical trials in order to market our products in the United States, we may nevertheless be required to conduct one or more clinical studies, and to publish one or more peer reviewed journal articles supporting the products, before we are able to obtain third‑party reimbursement.
Our business is also subject to a very competitive and changing field. Competition from other companies and from research and academic institutions is intense and widespread and has continued to increase, which could lead to pricing pressure, thereby limiting our ability to sell our products at a price that would make our business profitable or prevent us from selling our products at all. Our ability to compete in today’s climate depends both on our ability to develop new technologies and new applications for our technologies and on our ability to expand our market access and sales capabilities. We are currently engaged in an initiative to build and expand our internal sales and marketing capabilities in order to allow us to reach new markets and compete in an ever changing industry. These initiatives have and will continue to result in an increase in our operating expenses, including our sales and marketing expenses.
Our business requires a significant amount of sales, marketing, administrative and compliance support and infrastructure. We believe that we will need to expand our product offerings in order to provide our sales and marketing personnel with additional products to sell to our customers and in order to gain economies of scale with our infrastructure. Over the last several years, we have had to conserve cash due to the legal proceedings related to the Restatement and other challenges we faced. As a result, we slowed our spending on research and development in 2017.
Our increased research and development expense in 2018 was primarily related to testing and clinical trials of our lyopreservation technology. We will need to maintain or increase our spending on R&D to develop new products or implement innovations.
If we are unable to grow our sales at a faster rate than our growth in operating expenses, then our profitability will decline. Additionally, if we experience a decline in sales in any fiscal period or over time, we may not be able to offset any such decline through a reduction in expenses quickly enough, or at all. There can be no assurance that we will be able to continue to grow revenues or maintain profitability.
Critical Accounting Policies
The preparation of consolidated financial statements and related disclosures in conformity with generally accepted accounting principles in the United States (“GAAP”) requires management to make judgments, assumptions and estimates that affect the amounts reported. Due to the inherent uncertainty involved in making those assumptions, actual results could differ from those estimates. We believe that the most significant estimates that affect our financial statements are those that relate to the fair value of our available for sale investments, the amounts of our deferred tax assets and liabilities and related valuation allowance, the net realizable value of inventory and related allowance for obsolescence, the ultimate realizability of our accounts receivable and estimates of collectability, the reserve for sales returns, and the fair value of our share‑based awards. Note 2, “Summary of Significant Accounting Policies,” to our consolidated financial statements included in Part II, Item 8 of this Form 10‑K describes the significant accounting policies and methods used in the preparation of our consolidated financial statements.
The policies and estimates discussed in this section are considered critical because they had or could have a material impact on our consolidated financial statements and because they require significant judgments, assumptions or estimates. We base our estimates on historical experience and other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates and such differences could be material. We have reviewed these critical accounting policies and related disclosures with the Audit Committee.
Revenue Recognition
The Company began accounting for revenue in accordance with Topic 606 on January 1, 2018, using the modified retrospective method. Under the new revenue standard for arrangements that are determined to be within the scope of Topic 606, the Company recognizes revenue following the five-step model: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, once the contract is determined to be within the scope of Topic 606, the Company assesses the goods or services promised within each contract and determines the performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
Accordingly, the Company recognizes revenue when title to the product, ownership and risk of loss transfer to the customer, which is either the date of receipt by the customer or when we receive appropriate notification that the product has been used or implanted in a patient. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to in exchange for transferring the goods. The nature of the Company’s contracts gives rise to certain types of variable consideration, including rebates and other discounts. The Company includes estimated amounts of variable consideration in the transaction price to the extent that it is probable there will not be a significant reversal of revenue. Estimates are based on historical or anticipated performance and represent our best judgment at the time. Any estimates are evaluated on a quarterly basis until the uncertainty is resolved.
The Company provides a variety of products and services to its customers. Most of the Company’s contracts consist of a single, distinct performance obligation or promise to transfer goods to a customer. For contracts that include multiple performance obligations, the Company allocates the total transaction price to each performance obligation using our best estimate of the standalone selling price of each identified performance obligation.
The Company’s incremental costs of obtaining a contract would generally have less than a one-year duration; therefore, the Company applies the practical expedient available and expenses costs to obtain a contract when incurred.
We record deferred revenue when we receive payments prior to the time we recognize revenue.
Trade Accounts Receivable
Accounts receivable are reported at their net realizable value. We consider receivables outstanding longer than the time specified in the respective customer’s contract to be past due. In making the determination of the appropriate allowance for doubtful accounts, management considers prior experience with customers, analysis of accounts receivable aging reports, changes in customer payment patterns, and historical write‑offs.
Inventory Valuation
Inventory consists of raw materials, products in process, finished goods available for sale and products held by customers under consignment sales arrangements. We determine our inventory values using the first‑in, first‑out method. Inventory is valued at the lower of cost or net realizable value and excludes units that we anticipate distributing for clinical evaluation.
Fair Value Accounting
We disclose the fair value of our financial assets in our consolidated financial statements included in Part II, Item 8 of this Form 10‑K. Fair value is defined as the price at which an asset could be exchanged or a liability transferred (an exit price) in an orderly transaction between knowledgeable, willing parties in the principal or most advantageous market for the asset or liability. Where available, fair value is based on observable market prices or parameters or derived from such prices or parameters. Where observable prices or inputs are not available, valuation models are applied.
Income Taxes
Our income taxes are accounted for under the asset and liability method. Deferred income tax assets and liabilities are recognized for the future tax consequences attributable to differences between the carrying amounts of existing assets and liabilities in the consolidated financial statements and their respective tax basis. Deferred income tax assets and liabilities are measured using enacted tax rates expected to be applied to taxable income in the years in which those temporary differences
are expected to be recovered or settled.
Valuation allowances are recorded if it is more likely than not that some portion of the deferred income tax assets will not be realized. In evaluating the need for a valuation allowance, we take into account various factors, including the expected level of future taxable income and available tax planning strategies. Any changes in judgment about the valuation allowance are recorded through income tax provision (benefit) and are based on changes in facts and circumstances regarding realizability of deferred tax assets.
We must presume that an income tax position taken in a tax return will be examined by the relevant tax authority and determine whether it is more likely than not that the tax position will be sustained upon examination based upon the technical merits of the position. An income tax position that meets the more-likely-than-not recognition threshold is measured to determine the amount of benefit to recognize in the financial statements. We establish a liability for unrecognized income tax benefits for income tax positions for which it is more likely than not that a tax position will not be sustained upon examination by the respective taxing authority to the extent such tax positions reduce our income tax liability. We recognize interest and penalties related to unrecognized income tax benefits in the income tax provision (benefit) in the consolidated statements of comprehensive income.
Recent and Pending Accounting Pronouncements
See Note 2 to our consolidated financial statements included in Part II, Item 8 of this Form 10‑K for a full description of recent accounting pronouncements and our expectation of their impact on our consolidated financial statements.
Results of Operations
The following tables setting forth selected financial data for the years ended December 31, 2018 and 2017 have been prepared in accordance with GAAP:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31,
|
|
(in thousands)
|
|
2018
|
|
2017
|
|
Revenue
|
|
$
|
142,824
|
|
$
|
118,514
|
|
Cost of revenue
|
|
|
38,139
|
|
|
32,681
|
|
Gross profit
|
|
|
104,685
|
|
|
85,833
|
|
Operating expenses:
|
|
|
|
|
|
|
|
Research and development
|
|
|
6,764
|
|
|
4,138
|
|
Sales and marketing
|
|
|
67,542
|
|
|
61,545
|
|
General and administrative
|
|
|
19,626
|
|
|
22,139
|
|
Shareholder litigation expense
|
|
|
900
|
|
|
—
|
|
Total operating expenses
|
|
|
94,832
|
|
|
87,822
|
|
Income (loss) from continuing operations
|
|
|
9,853
|
|
|
(1,989)
|
|
Other income (expense), net
|
|
|
675
|
|
|
(645)
|
|
Income (loss) before income taxes from continuing operations
|
|
|
10,528
|
|
|
(2,634)
|
|
Income tax benefit
|
|
|
26,005
|
|
|
1,398
|
|
Net income (loss) from continuing operations
|
|
|
36,533
|
|
|
(1,236)
|
|
Mesoblast income from discontinued operations, net of tax(1)
|
|
|
368
|
|
|
10,021
|
|
Net income
|
|
$
|
36,901
|
|
$
|
8,785
|
|
|
(1)
|
|
In October 2013, we sold our therapeutics business to Mesoblast. The agreement with Mesoblast provided for the receipt of $50 million in initial consideration and up to an additional $50 million in payments upon Mesoblast achieving certain clinical and regulatory milestones. In addition, we are entitled to earn single to low double‑digit percentage cash royalties on future sales by Mesoblast of Prochymal and other products utilizing the acquired technology. Our former therapeutics business generated royalty revenue and related expenses in 2014. See Note 4 to the Consolidated Financial Statements in Part II, Item 8 of this Form 10‑K.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2018
|
|
2017
|
|
|
|
|
Product
|
|
Percent of
|
|
Product
|
|
Percent of
|
|
|
Product (in thousands)
|
|
Revenue
|
|
Total
|
|
Revenue
|
|
Total
|
|
|
Grafix/Stravix
|
|
$
|
105,287
|
|
73.7
|
%
|
$
|
85,344
|
|
72.0
|
%
|
|
BIO
4
|
|
|
27,685
|
|
19.4
|
%
|
|
24,166
|
|
20.4
|
%
|
|
Cartiform
|
|
|
9,845
|
|
6.9
|
%
|
|
8,988
|
|
7.6
|
%
|
|
Other
|
|
|
7
|
|
—
|
%
|
|
16
|
|
—
|
%
|
|
Total
|
|
$
|
142,824
|
|
100.0
|
%
|
$
|
118,514
|
|
100.0
|
%
|
Results of Operations for the year ended December 31, 2018 compared to the year ended December 31, 2017
Revenue
Revenue was $142.8 million for the year ended December 31, 2018, which increased $24.3 million, or 20.5%, compared with revenue of $118.5 million in 2017.
The increase in revenue was due to the following:
Grafix/Stravix revenue increased $19.9 million, or 23.4%, primarily due to increased demand from improved market awareness and acceptance as we increased selling efforts in the operating room and surgical settings as well as hospital outpatient wound care centers. In October 2018, we launched our GrafixPL PRIME product which contributed to higher sales in the fourth quarter of 2018. In addition, we received a one-time settlement payment of $1.3 million from a former distributor that was accounted for on a cash basis, as collection was not reasonably assured, to settle amounts owed to us from previous years, primarily 2015 and 2016. While we are experiencing some pressure to reduce prices, the pricing of our Grafix
/
Stravix products did not significantly impact sales growth during the year ended December 31, 2018.
BIO
4
revenue increased $3.5 million, or 14.6%, primarily due to increased demand through our distribution arrangement with Stryker. Pricing of our BIO
4
products did not significantly impact sales growth during the year ended December 31, 2018.
Cartiform revenue increased $0.9 million, or 9.5%, primarily due to increased sales of larger units through our distribution arrangement with Arthrex. Pricing of our Cartiform products did not significantly impact sales growth during the year ended December 31, 2018.
Gross Profit and Gross Margin
Gross profit is affected by a number of factors including product mix, cost of labor and raw materials, unit volumes, pricing, competition, new products, new customer programs, and capacity utilization. In the case of new products and new customer programs, profitability may lag revenue growth due to product start‑up costs, lower manufacturing volumes in the start‑up phase, operational inefficiencies, and under‑absorbed overhead. Gross margin can improve over time if manufacturing volumes increase and if our utilization rates and overhead absorption improves. As a result of these and other factors, our gross margin may vary from period to period.
Gross profit was $104.7 million for the year ended December 31, 2018, which increased $18.9 million, or 22.0%,
compared with gross profit of $85.8 million in 2017. Gross margin improved to 73.3% for the year ended December 31, 2018, compared with 72.4% in 2017. This increase was primarily due to higher revenues, and the collection of the $1.3 million settlement from a former distributor that was accounted for on a cash basis which did not have any cost of revenue as the cost of revenue was recognized in the periods the product was shipped. Gross margin improved as a result of lower cost of sales associated with our GrafixPL
Prime
product, primarily resulting from lower shipping and packaging costs.
Research and Development Expenses
Our R&D expenses were $6.8 million in 2018, or 4.7% of revenue, compared with $4.1 million, or 3.5% of revenue, in 2017. This increase of $2.7 million was primarily related to higher costs associated with the development, testing and clinical trials of our lyopreservation technology that allows for ambient storage of viable tissues.
Sales and Marketing Expenses
Sales and marketing expenses totaled $67.5 million, or 47.3% of sales, during the year ended December 31, 2018, compared with $61.5 million, or 51.9 % of sales for the year ended December 31, 2017. The increase in sales and marketing expense of $6.0 million was primarily due to higher labor costs, including commissions due to higher sales
and a larger sales force. The reduction in sales and marketing expense as a percentage of revenue was due, in part, to lower labor and labor related costs as a percentage of revenue of 2.3%, and the effect of the additional $1.3 million in revenue from the one-time settlement with a former distributor, which reduced the ratio by 0.9%.
General and Administrative Expenses
General and administrative expenses totaled $19.6 million, or 13.7% of sales, during the year ended December 31, 2018, compared with $22.1 million, or 18.7% of sales, for the year ended December 31, 2017. This decrease of $2.5 million, as well as the decrease as a percentage of revenue, was primarily due to lower accounting and legal fees as a result of completing the filing of our restated historical financial statements.
Shareholder Litigation Expense
As discussed above, we settled a securities class action and multiple shareholder derivative actions. On March 11, 2018, we entered into a memorandum of understanding to settle the securities class action lawsuit. By the terms of the memorandum, the Company agreed in principle to a total payment of $18.5 million in cash. We made this payment to settle the class action lawsuit in October 2018. In addition, we received approximately $4.8 million in insurance proceeds related to this settlement.
During the third quarter of 2018 we recorded a liability of $0.9 million related to the settlement of shareholder derivative actions. We expect to make this payment to settle the derivative action in March 2019.
In November 2017, we paid a civil penalty in the amount of $1.5 million as part of the SEC settlement with regard to the SEC investigation previously discussed, all of which was recorded as an expense in the fourth quarter of 2015.
Other Income (Expense), Net
Other income (expense), net in 2018 was $0.7 million, an increase of $1.3 million compared to other income (expense), net of $(0.6) million in 2017. The increase is primarily due to a loss recognized in 2017 on the sale of Mesoblast Limited common stock.
Income Taxes
Income tax benefit from continuing operations for 2018 was $26.0 million primarily due to the partial release of our valuation allowance and the recognition of the Orphan Drug tax credit that was reported as an uncertain tax position as of December 31, 2017. During 2018, the Company performed a detailed study to recalculate the value of the Orphan Drug tax credit based on available documentation and support. This resulted in a supportable tax credit reported in our deferred tax asset of $45.0 million, net of a reserve of $3.0 million. As the Company is in a net cumulative income position for the past three years, the Company expects to realize some of its deferred tax assets, mainly consisting of the general business credits, which includes the Orphan Drug tax credit, and net operating loss carryforwards. The income tax benefit from continuing operations for 2017 was $1.4 million primarily due to the release of the valuation allowance on the AMT carryforwards, offset by state tax expense where the Company did not have state credit or net operating losses available. See Note 10, “
Income Taxes
”, in Item 8. Financial Statements and Supplementary Data, for additional information.
Since realization of deferred tax assets is dependent upon future earnings, a partial valuation allowance has been recorded on the net deferred tax assets as of December 31, 2018 and a full valuation allowance has been recorded as of December 31, 2017. We have general business credits of approximately $45.6 million that may be utilized to reduce our federal tax.
Mesoblast Income (Loss) from Discontinued Operations
As noted above, in October 2013, we sold our therapeutics business to Mesoblast. The agreement with Mesoblast provided for the receipt of $50 million in initial consideration and up to an additional $50 million in payments upon Mesoblast achieving certain clinical and regulatory milestones. In addition, we are entitled to earn royalties on future sales by Mesoblast of Prochymal and other products utilizing the acquired technology. We received all of the initial consideration by April 2014, consisting of $35 million in cash and $15 million of Mesoblast Limited common stock which we classified as a trading security. The Mesoblast Limited common stock was subject to a holding period which was ultimately extended to May 2015. During this holding period, Mesoblast Limited provided us limited protection against a decline in the market value of the stock. In May 2015, concurrent with the expiration of the holding period, Mesoblast paid us $6.2 million representing the decline in the market value of the stock through that date. Later in 2015, we sold all of the Mesoblast Limited common stock for $6.8 million, resulting in a realized loss of $2.6 million, which was recorded in other income (expense), net in our consolidated statement of comprehensive income loss for the year ended December 31, 2015.
As noted above, in connection with the sale of our therapeutics business to Mesoblast, we are entitled to receive contingent consideration as a result of Mesoblast achieving certain milestones. In July 2017, we received contingent consideration of $10.0 million in Mesoblast Limited common stock and $350 thousand in cash as a milestone payment. We recorded $10.0 million, net of $0.3 million in tax, in discontinued operations in 2017. All of the Mesoblast Limited common stock was sold in 2017, resulting in a loss of approximately $1.9 million, which was reported in other income (expense), net in our consolidated statement of comprehensive income for the year ended December 31, 2017.
Liquidity and Capital Resources
Liquidity
At December 31, 2018, we had $16.4 million in cash, $10.0 million in certificates of deposit and $8.3 million in investments available for sale. As a result of the Restatement and related legal proceedings, in October 2018, we deposited an $18.5 million settlement payment into an escrow account, pending final court approval of the settlement. In addition, in October 2018, we received $4.8 million in insurance proceeds related to the securities class action lawsuit.
We have not had any outstanding debt at any time since 2008. We anticipate our cash on hand, certificate of deposits, investments and cash flows from operations will be sufficient to finance our operations for the next twelve months.
Cash Flows
The following table sets forth a summary of our cash flows for each of our two most recently completed fiscal years:
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
(in thousands)
|
|
2018
|
|
2017
|
|
Net cash provided by (used in) operating activities
|
$
|
7,304
|
$
|
(5,223)
|
|
Net cash provided by investing activities
|
$
|
5,975
|
$
|
5,343
|
|
Net cash provided by financing activities
|
$
|
7
|
$
|
128
|
Operating Activities
Net cash provided by operating activities was $7.3 million during 2018, and was primarily due to our income before income taxes from continuing operations of $10.5 million, collections on our accounts receivable, net of $3.6 million, and an increase in accounts payable, accrued expenses and other liabilities of $3.8 million. In connection with the securities class action lawsuit, in October 2018 we deposited an $18.5 million settlement payment into an escrow
account, pending final court approval of the settlement. In addition, in October 2018, we received $4.8 million in insurance proceeds related to the securities class action lawsuit.
Net cash used in operating activities was $5.2 million during 2017, and was primarily due to our loss from continuing operations of $1.2 million, plus a realized loss on investment of approximately $2.0 million, and changes in operating assets and liabilities, which included an increase in accounts receivable, net, of $2.8 million, an increase in prepaid and other current assets of $1.6 million, due in part to an increase in income tax receivable, and a net decrease in accounts payable, accrued expenses and other liabilities of $1.1 million, and payment of an accrued civil penalty of $1.5 million to the SEC in 2017.
Investing Activities
Net cash provided by investing activities was $6.0 million during 2018,
and primarily reflects net proceeds from the sale of our investments of $16.7 million partially offset by purchases of certificates of deposit totaling $10.0 million and other investments of $0.5 million. We sold investments in order to pay the settlement of the securities class action lawsuit.
Net cash provided by investing activities was $5.3 million during 2017, and was primarily due to the net proceeds from the sale of Mesoblast Limited common stock of approximately $7.9 million, partially offset by $2.0 million in purchases of property and equipment made during the year.
Financing Activities
There were immaterial financing activities during the year ended December 31, 2018.
Net cash provided by financing activities was $0.1 million during 2017, and reflects the proceeds from the exercise of options to purchase our common stock.
Off‑Balance Sheet Arrangements
We had no off‑balance sheet financing arrangements as of December 31, 2018 or 2017.
Contractual Obligations
Our most significant contractual obligations include operating leases and agreement with contract research organization (“CROs”). Operating leases consist of our lease for 61,203 square feet of laboratory, production, warehouse and office space in Columbia, Maryland, which lease expires in 2023.
The contractual obligations table does not include obligations relating to contract research organizations (“CROs”). CROs perform many of the tasks required under our clinical studies, and we utilize their expertise to ensure objectivity of the clinical results. We pay fees directly to the CROs for their professional services, which may be payable upon specified study milestones or as they provide services, depending on the structure of the contract. We are also responsible for reimbursing the CROs for certain pass through expenses they incur in administering the study. The timing of our payments to the CROs is dependent upon the progress of the various studies, which is highly variable dependent upon the speed with which the CROs are able to enroll patients and testing sites. As such, we are unable to specifically predict the timing of future payments to CROs in connection with a specific clinical study.
ITEM 7A. Quantitative and Qualitative Disclosures about Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
ITEM 8. Financial Statements and Supplementary Data
See our consolidated financial statements beginning on page F‑1
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
ITEM 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow for timely decisions regarding required disclosure.
Our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, has carried out an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a‑15(e) and 15d‑15(e) under the Exchange Act) as of December 31, 2018. Based upon that evaluation, and in light of the material weaknesses in the operation of our internal controls over financial reporting disclosed below, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2018, our disclosure controls and procedures were not effective.
Management’s Annual Report on Internal Control over Financial Reporting
Our management, under the supervision of our Chief Executive Officer and Chief Financial Officer, is responsible for the preparation, integrity and fair presentation of information in our financial statements, including estimates and judgments. The financial statements presented in this Annual Report on Form 10‑K have been prepared in accordance with GAAP. Our management believes the financial statements and other financial information included in this Annual Report on Form 10‑K fairly present, in all material respects, our financial condition, results of operations, cash flows and notes thereto as of and for the periods presented in this Annual Report on Form 10‑K.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting and for the assessment of the effectiveness of internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a‑15(f) and 15d‑15(f) under the Exchange Act as a process designed by, or supervised by, our principal executive and principal financial officers, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
Our internal control over financial reporting is supported by written policies and procedures that
|
|
·
|
|
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect our transactions and dispositions of our assets;
|
|
|
·
|
|
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and
|
|
|
·
|
|
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
|
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of the effectiveness of such controls to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In connection with the preparation of our annual financial statements, our management, under the supervision of our Chief Executive Officer and Chief Financial Officer, has undertaken an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2018 based on the criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO Framework”). Management’s assessment included an evaluation of the design and operational effectiveness of our internal control over financial reporting. Based on this assessment, and in light of the material weaknesses in the operation of our internal control over financial reporting disclosed below, management has concluded that our internal control over financial reporting was not effective as of December 31, 2018.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
We identified material weaknesses in internal control over financial reporting in the 2017 Form 10-K that was filed with
the Securities and Exchange Commission on March 28, 2018
. The Audit Committee, the Board and management initiated extensive remediation efforts, as discussed below in "Remediation Activities in Response to Material Weaknesses," to begin to remediate the material weaknesses in control over financial reporting that existed at December 31, 2017. These material weaknesses cannot be considered remediated until the applicable remedial controls operate for a sufficient period of time and management is able to conclude, through testing, that these controls are operating effectively consistent with the monitoring component of the COSO Framework. As of December 31, 2018, we identified the following control deficiencies that we believe constituted individually, and in the aggregate, material weaknesses in the control activity components of the COSO Framework, which include some of the material weaknesses previously disclosed in our 2017 Form 10-K:
|
|
·
|
|
Revenue Recognition.
We did not maintain controls, including relevant application controls, that were operating effectively over the timing and manner of revenue recognition and failed to properly and timely assess the five-step model in assessing revenue recognition: (i) identifying the contract(s) with a customer; (ii) identifying the performance obligation(s) in the contract; (iii) determining the transaction price; (iv) allocating the transaction price to the performance obligations in the contract; and (v) recognizing revenue when (or as) we satisfy a performance obligation.
|
|
|
·
|
|
Inventory.
We did not maintain effective processes and controls,
including relevant application controls,
over the computation of costs, quantities and reserves for recording inventory.
|
|
|
·
|
|
Reserves, Estimates, and Valuation Accounts.
We did not maintain effective processes and controls,
including relevant application controls,
over reserves, estimates, and valuation accounts, including the collectability of accounts receivable and determining our bad debt reserve, rebates reserve, returns reserve, income tax valuation allowance and uncertain tax positions.
|
|
|
·
|
|
Purchasing and Disbursements.
We did not maintain effective controls over the purchasing of goods and services, including the processing and payment of vendor invoices, the classification of various expenses, capitalization of assets and the processing of employee payroll and other compensation arrangements.
|
Changes in Internal Control over Financial Reporting
With the exception of the remediation efforts described below, there have been no changes in our internal control over financial reporting that occurred in the annual period covered by this report that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting. The Audit Committee, the Board and current management are committed to remediating the material weaknesses in our internal control environment. As a result, we have initiated the remediation efforts outlined below, but these efforts are ongoing and cannot be considered
remediated until the applicable remedial controls operate for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively.
Remediation Activities in Response to Material Weaknesses
Our management, with the oversight of the Audit Committee, has undertaken a plan to remediate the material weaknesses identified above. We have engaged a new third-party consultant to assist us with our remediation efforts, which are intended to address the identified material weaknesses. The following provides a summary of these remediation efforts:
|
|
·
|
|
We have implemented new and enhanced controls for proper revenue recognition, although there was not an adequate amount of time during 2018 when the controls were operating in order for us to test those controls. These controls include:
|
|
|
o
|
|
the timely assessment of the revenue recognition five step model:
(i) identifying the contract(s) with a customer; (ii) identifying the performance obligation(s) in the contract; (iii) determining the transaction price; (iv) allocating the transaction price to the performance obligations in the contract; and (v) recognizing revenue when (or as) we satisfy a performance obligation
;
|
|
|
o
|
|
review and approval of sale and distribution contracts by both our legal and finance departments;
|
|
|
o
|
|
monthly reconciliation of billing with revenue;
|
|
|
o
|
|
review, approval and documentation of customer credits issued for returns, refunds, price adjustments and similar commercial accommodations; and
|
|
|
o
|
|
implementing a company-wide budgeting and forecasting process.
|
|
|
·
|
|
We have implemented new and enhanced controls over inventory,
although there was not an adequate amount of time during 2018 when the controls were operating in order for us to test those controls.
These controls include:
|
|
|
o
|
|
procedures to monitor and analyze consignment inventories, such as periodic field counts held at customer sites;
|
|
|
o
|
|
enhancements to ensure that if a finished good has been determined to be damaged, obsolete or otherwise not fit for sale, it is reserved for or written off on a timely basis;
|
|
|
o
|
|
additional review of the inventory reserve analysis, including the involvement of both finance and operational executives;
|
|
|
o
|
|
additional analysis and review of inventory on hand at the product line level to assess excess and obsolete inventory based on the current inventory on hand in relation to the demand forecast and related reserve;
|
|
|
o
|
|
hiring of accounting personnel with relevant cost accounting experience whose daily job responsibility is to monitor, analyze and develop processes to properly value our inventory levels; and
|
|
|
o
|
|
preparing standard inventory cost valuation reserves, purchase price and manufacturing inventory variance adjustments, and obsolescence reserves.
|
|
|
·
|
|
We have implemented new and enhanced controls for developing and evaluating the appropriateness of reserves, estimates, and valuation accounts,
although there was not an adequate amount of time during 2018 where the controls were operating in order for us to test those controls.
These controls include:
|
|
|
o
|
|
new procedures to assess the collectability of trade accounts receivable and determine bad debt reserve, which will be overseen by our credit and collections manager;
|
|
|
o
|
|
new procedures to evidence review and approval of collectability of accounts receivable and determining an allowance for doubtful accounts;
|
|
|
o
|
|
new procedures to evidence review and approval of the allowance of returns;
|
|
|
o
|
|
engaging a third-party tax expert to assist in estimating deferred income tax assets, deferred income tax liabilities, uncertain tax positions, and valuation allowances;
|
|
|
o
|
|
new procedures to evidence review and approval of accruals; and
|
|
|
o
|
|
new procedures to evidence review and approval of "other-than-temporary" impairment of investments.
|
|
|
·
|
|
We have enhanced, or are in the process of enhancing, certain controls for purchasing and disbursements that continued to be ineffective as of December 31, 2018 including:
|
|
|
o
|
|
approving and validating vendor invoices received by verifying purchase authorization and the receipt of the goods or services; and
|
|
|
o
|
|
reviewing the classification of costs, expenses and capital purchases.
|
Management has implemented and is monitoring the effectiveness of these and other processes, procedures and controls and will make any further changes deemed appropriate. Management believes its planned remedial efforts will remediate the identified material weaknesses. As we continue to evaluate and work to improve our internal control over financial reporting, management may determine to take additional measures to address control deficiencies or determine to modify the remediation plan described above.
Remediation of Previously Reported Material Weaknesses Related to
processing of Director’s Expense Reports
:
During the year ended December 31, 2018, we implemented new and enhanced controls relating to expense reimbursement for our directors, including the adoption of a formal director expense reimbursement policy to address a material weakness previously identified in our control environment related to
processing of Director’s expense reports.
We believe that these remediation efforts have improved our internal control over financial reporting, as well as our disclosure controls and procedures, and that the previously identified material weakness related to our processing of Director’s expense reports has been remediated as of December 31, 2018.
Remediation of Previously Reported Material Weaknesses Related to
Information Technology
:
During the year ended December 31, 2018, we implemented the following remediation actions to address a material weakness previously identified in our control environment related to
information technology
:
|
|
·
|
|
requiring management approval for all levels of access to financial systems prior to such access being granted to new employees or transfers;
|
|
|
·
|
|
requiring appropriate periodic review of all system access to ensure granted access remains appropriate;
|
|
|
·
|
|
implementation of a new termination policy requiring that all access held by terminated employees is terminated in a timely manner;
|
|
|
·
|
|
clearly segregating duties and information access among employees, including limiting administrative rights and “privileged access” rights to financial systems to certain information technology management members;
|
|
|
·
|
|
requiring that all new programs and changes to existing programs be formally approved by appropriate levels of management, and that all programs and changes be fully developed and tested in a test environment prior to migration into the production environment; and
|
|
|
·
|
|
reviewing, on an annual basis, internal controls existing at third party/vendor service organizations to evaluate whether information processed by those parties is complete and accurate and can be relied upon.
|
We believe that these remediation efforts have improved our internal control over financial reporting, as well as our disclosure controls and procedures, and that the previously identified material weakness related to information technology has been remediated as of December 31, 2018.
Our independent registered public accounting firm is engaged to express an opinion on our internal control over financial reporting as of December 31, 2018, which opinion is included in its report in Part II, Item 8 of this Form 10‑K under the caption “Report of Independent Registered Public Accounting Firm—Internal Control Over Financial Reporting.”
ITEM 9B. Other Information.
None.
PART III
We are a smaller reporting company as defined by Rule 12b-2 of the Exchange Act and are not required to provide certain information required in Part III.
ITEM 10. Directors, Executive Officers and Corporate Governance.
Board of Directors
Our Charter and Bylaws provide that our business is to be managed by or under the direction of our Board. Our Board is currently comprised of four directors, all of whom have terms expiring at the next annual meeting. In each case, the directors remain in office, notwithstanding the expiration of the otherwise applicable term, until their respective successors have been duly elected and qualified, or until their earlier death, resignation, retirement or removal.
The current members of our Board, along with the positions and ages of such members, are as follows:
|
|
|
|
|
|
|
|
|
|
|
Committee Membership
|
|
Name
|
Age
|
Positions
|
Audit
|
Compensation
|
Nominating
|
|
Peter Friedli
|
65
|
Chairman of the Board
|
|
|
|
|
Thomas J. Knapp
|
66
|
Director
|
X
|
|
X
|
|
Willi Miesch(1)
|
55
|
Chairman, Nominating Committee and Compensation Committee
|
X
|
X
|
X
|
|
Charles A. Reinhart, III(2)
|
58
|
Chairman, Audit Committee
|
X
|
X
|
|
(1)
Mr. Miesch was appointed to the Board in February 2018.
(2)
Mr. Reinhart was appointed to the Board in September 2018.
Set forth below are descriptions of the backgrounds and principal occupations of each of our current directors, and the period during which each director has served as a member of the Board.
Peter Friedli
, age 65, is a co‑founder of the Company and, except for the period between February and June 2004, has been a director since January 1996. Since 2012, Mr. Friedli has served as the President and sole owner of Peter Friedli & Co., Inc. Since 1986, Mr. Friedli has served as a principal of Friedli Corporate Finance, Inc., a leading Swiss venture capital firm, which has made significant investments in the biotechnology industry and has been the primary source of financing for the Company. Since 1997, Mr. Friedli has served as the President of New Venturetec Ltd., a Swiss publicly traded investment company. Mr. Friedli has worked in the field of international management consulting for service and industrial companies in Europe and the United States throughout his career and serves as a director for certain private companies. Mr. Friedli brings to our Board his extensive experience as an independent investment manager in venture capital, specialization in investments domiciled in the United States in the areas of biotechnology and technology, service on the boards of other companies and organizations, and a longtime commitment to the Company. Mr. Friedli is the largest single shareholder of the Company.
Thomas J. Knapp
, age 66, was appointed to the Board in February 2017. Mr. Knapp is currently a consultant for SELLAS Life Sciences Group, Inc. (NASDAQ:SLS), which completed its merger with Galena Biopharma, Inc. (“Galena”) (NASDAQ:GALE) in December 2017. From June 2015 to December 2017, Mr. Knapp served as Interim General Counsel and Corporate Secretary of Galena. Previously, from February 2010 to July 2015, he was Executive Vice President, Chief Legal Officer and Corporate Secretary of Sucampo Pharmaceuticals, Inc. (NASDAQ:SCMP). His other experience includes serving as Vice President, General Counsel and Corporate Secretary at NorthWestern Corporation and in senior in‑house attorney positions at The Boeing Company and The Burlington Northern & Santa Fe Railway Company. Mr. Knapp has over 25 years of corporate and law firm experience with regulated industries, which experience includes federal and state regulatory matters, federal government affairs, risk management, compliance, litigation, complex commercial transactions and corporate governance. Mr. Knapp brings to our Board his vast legal experience working with companies in regulated industries as well as his knowledge regarding the pharmaceutical industry.
Willi Miesch
, age 55, was appointed to the Board in February 2018. Since 1998, Mr. Miesch has served as Chief Executive Officer of Medartis AG, a medical technology company. Prior to working at Medartis AG, he held executive roles at Institut Straumann AG Switzerland and USA and Stratec Medical. Mr. Miesch has extensive entrepreneurial experience developing healthcare companies and marketing medical devices. Mr. Miesch has a Superior Technician degree from ABB Engineering School Baden and completed postgraduate studies in market‑oriented Business Management at the University of Central Switzerland. Mr. Miesch brings to our Board his leadership experience and marketing expertise.
Charles A. Reinhart, III
, age 58, was appointed to the Board in September 2018. Mr. Reinhart is currently the Chief Financial Officer at Pacira Pharmaceuticals, Inc. He joined Pacira in 2016. Prior to that, Mr. Reinhart was the Chief Financial Officer at Covis Pharmaceuticals, Inc., a specialty pharmaceutical company, from September 2014 to October 2015. From September 2011 to August 2014, he served as Executive Vice President and Chief Financial Officer of Archimedes Pharma Ltd., a specialty pharmaceutical company. Mr. Reinhart also served as Senior Vice President and Chief Financial Officer of PharmAthene, Inc., a biodefense company engaged in the development of next-generation medical countermeasures against biological and chemical threats, from 2009 to 2011. In addition, Mr. Reinhart has previously held senior financial roles at Millennium Pharmaceuticals, Inc., Cephalon, Inc. and several early-stage life sciences companies. Mr. Reinhart earned his Bachelor of Science degree from Lehigh University and his MBA from the Wharton School of the University of Pennsylvania. He is also a Certified Public Accountant.
Executive Officers
The following table sets forth certain information concerning our current executive officers.
|
|
|
|
|
|
|
Name
|
|
Age
|
Positions
|
Employment
Date
|
|
Samson Tom. Ph.D., MBA
|
46
|
President and Chief Executive Officer
|
November 2018
|
|
Joel Rogers
|
57
|
Chief Financial Officer
|
October 2017
|
|
Jason Keefer
|
47
|
Chief Commercial Officer
|
February 2014
|
|
Alla Danilkovitch, Ph.D.
|
55
|
Chief Scientific Officer
|
April 2003
|
Set forth below are descriptions of the backgrounds of each of our current executive officers.
Samson Tom, Ph.D., MBA
, age 46, rejoined Osiris in November 2018 as President and Chief Executive Officer. Previously, Dr. Tom served as Vice President, Research & Development for Surgical Orthobiologics at Bioventus, LLC from 2016 to 2018. Prior to Bioventus, Dr. Tom served as Group Director, Biosurgery Research & Development at Ethicon, Inc. (a Johnson & Johnson company), where he was employed from 2011 to 2016. Dr. Tom worked at Osiris from 2003 to 2011 in several leadership positions spanning multiple functional areas including Quality, Operations, Clinical Development, and Product Development, culminating in a Senior Director role during his tenure. Preceding Osiris, Dr. Tom held scientific positions with increasing responsibility at Vion Pharmaceuticals, Inc., Prevalere Life Sciences, Inc., and the Bristol-Myers Squibb Company. Dr. Tom earned a Bachelor of Arts degree in Biology and Chemistry from Cornell University, a Master of Science degree and a Doctor of Philosophy degree in Biochemistry from the University of Rochester School of Medicine and Dentistry, and a Master of Business Administration degree in General Management from the Johns Hopkins University Carey Business School. He has attended executive education programs at the Harvard Business School, the Vlerick Business School, and the Raymond A. Mason School of Business at the College of William & Mary. He is a certified Project Management Professional (PMP®), Risk Management Professional (PMI-RMP®), and Strategic Project Leader (SPL®).
Joel Rogers
, age 57, was appointed Chief Financial Officer, effective July 10, 2018. Mr. Rogers joined the Company in October 2017 as Corporate Controller. Previously, Mr. Rogers served as Corporate Controller at ARC Group Worldwide, Inc. (NASDAQ: ARCW) from August 2014 to October 2017. Prior to ARC Group Worldwide, Inc., Mr. Rogers served in accounting management roles at DigitalGlobe, Inc. (NYSE: DGI), Allos Therapeutics, Inc. (NASDAQ: ALTH), and Flex LTD. (NASDAQ: FLEX). Mr. Rogers began his career as an auditor at Deloitte & Touche LLP. He holds a Bachelor of Arts (Accounting) from the University of Washington as well as a Master of Business Administration from Cornell University. He is a Certified Public Accountant (Colorado).
Jason Keefer
, age 47, has served as Chief Commercial Officer since November 2018. Mr. Keefer joined the Company in 2014, was named Vice President, Marketing in August 2016 and previously served as our Interim President and Chief Executive Officer from June 2017 to July 2017 and from February 2018 to November 2018. Mr. Keefer assumed the additional responsibilities to lead Market Access in October 2017. Prior to joining the Company, Mr. Keefer served as Zone Sales Director, Central United States, for Shire Plc, where he was employed from 2011 to 2014, and he worked at Pfizer Inc. from 1998 to 2011. He has 20 years of experience in the pharmaceutical and medical device industries and has held roles in sales, training, senior sales management and marketing. Mr. Keefer earned his Bachelor of Arts degree from Colgate University.
Alla Danilkovitch, Ph.D.
, age 55, has served as our Chief Scientific Officer since April 2015. Dr. Danilkovitch joined the Company in 2003 and previously served as our Vice President of Research & Development and, prior to that, as our Head of Discovery. Dr. Danilkovitch has over 25 years of broad biomedical research experience including stem cell biology, immunology and cancer research. Prior to joining the Company in April 2003, Dr. Danilkovitch conducted research at the National Cancer Institute of National Institutes of Health, the Max‑Plank Institute of Biochemistry in Munich and at Moscow State University. Dr. Danilkovitch earned a Doctor of Philosophy degree in cell biology and a Master of Science degree in cellular immunology and microbiology from Moscow State University.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires that our executive officers and directors, and persons who own more than 10% of a registered class of our equity securities, file reports of ownership and changes in ownership on Forms 3, 4 and 5 with the SEC and the NYSE. Executive officers, directors and greater than 10% shareholders are required by the SEC to furnish us with copies of all Forms 3, 4 and 5 that they file.
Based on our review of the copies of such forms, and/or on written representations from the reporting persons that they were not required to file a Form 5 for the fiscal year, we believe that these filing requirements were satisfied by the reporting persons during the years ended December 31, 2018.
Codes of Conduct and Ethics
We have adopted a Corporate Code of Conduct that applies to all of our employees, including our Chief Executive Officer and Chief Financial Officer, and every member of our Board. We have also adopted a Code of Business Conduct and Ethics for Members of the Board of Directors and Executive Officers. Copies of these documents are publicly available on the Investors Relations—Corporate Governance section of our website at
http://investor.osiris.com/corporate‑governance.
Disclosure regarding any amendments to, or waivers from, provisions of our Corporate Code of Conduct or our Code of Business Conduct and Ethics for Members of the Board of Directors and Executive Officers that apply to our directors and principal executive and financial officers will be posted on our website at
http://investor.osiris.com/corporate‑governance
.
Audit Committee
We have an Audit Committee consisting of the following directors, all of whom were determined to be independent under the independence standards promulgated by the SEC and by the NASDAQ rules, as such standards apply specifically to members of audit committees: Charles A. Reinhart, III (Chairman), Thomas J. Knapp and Willi Miesch. The Board has determined that Mr. Reinhart is an “audit committee financial expert,” as defined by the rules and regulations of the SEC. The current charter for the Audit Committee is posted on the Company's website at http://investor.osiris.com/corporate-governance. Pursuant to its charter, the Audit Committee's general responsibilities include:
|
|
·
|
|
Overseeing financial and compliance functions as assigned by the Board;
|
|
|
·
|
|
Reviewing areas of potential significant financial risk to the Company;
|
|
|
·
|
|
Monitoring the independence and performance of the independent registered public accounting firm; and
|
|
|
·
|
|
Providing an avenue of communication among the independent auditors, management, our internal audit function and the Board.
|
ITEM 11. Executive Compensation.
Summary Compensation Table
The following table shows the total compensation paid or accrued during fiscal 2018 and 2017 to our named executive officers.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option
|
|
All Other
|
|
|
|
|
|
|
|
Salary
|
|
Bonus
|
|
Awards
|
|
Compensation
|
|
Total
|
|
Name and Principal Position
|
|
Year
|
|
($)
|
|
($)(1)
|
|
($)(2)
|
|
($)(3)
|
|
($)
|
|
Current Executive Officers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Samson Tom, Ph.D., MBA (4)
|
|
2018
|
|
24,000
|
|
—
|
|
—
|
|
—
|
|
24,000
|
|
President and CEO
|
|
2017
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
Joel Rogers(5)
|
|
2018
|
|
222,885
|
|
20,000
|
|
—
|
|
4,115
|
|
247,000
|
|
Chief Financial Officer
|
|
2017
|
|
—
|
|
—
|
|
—
|
|
—
|
|
—
|
|
Jason Keefer(6)
|
|
2018
|
|
230,192
|
|
70,000
|
|
138,079
|
|
57,431
|
|
495,702
|
|
Chief Commercial Officer
|
|
2017
|
|
210,735
|
|
—
|
|
71,197
|
|
132,647
|
|
414,579
|
|
Alla Danilkovitch, Ph.D.
|
|
2018
|
|
250,962
|
|
—
|
|
—
|
|
4,825
|
|
255,787
|
|
Chief Scientific Officer
|
|
2017
|
|
243,349
|
|
—
|
|
221,689
|
|
4,855
|
|
469,893
|
|
Former Executive Officers
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
Linda Palczuk(7)
|
|
2018
|
|
51,923
|
|
—
|
|
—
|
|
1,041
|
|
52,964
|
|
Former President and CEO
|
|
2017
|
|
216,811
|
|
—
|
|
109,317
|
|
40,712
|
|
366,840
|
(1)
Unless otherwise noted, these amounts represents bonus payments that were paid in 2018 on account of named executive officer performance in 2017.
(2)
These amounts reflect the non‑cash aggregate grant date fair value of these awards computed in accordance with FASB ASC Topic 718 “Stock Compensation,” which includes the effect of estimated forfeitures. The assumptions and methodologies used to calculate the amounts reported are discussed in Note 8 (Share‑Based Compensation and Employee Benefit Plans) to the Company’s financial statements included in Part II, Item 8 of this Form 10‑K. Under GAAP, compensation expense with respect to stock awards and option awards granted to our employees is generally recognized over the vesting periods applicable to the awards.
(3)
Unless otherwise noted, all other compensation represents matching contributions under our 401(k) plan.
(4)
Dr. Tom was appointed as President and Chief Executive Officer, effective as of November 26, 2018.
(5)
Mr. Rogers was appointed as Chief Financial Officer, effective as of July 10, 2018. He previously served as Corporate Controller. Mr. Rogers bonus was awarded for his performance as Corporate Controller. As Mr. Rogers was not a named executive officer prior to 2018, his summary compensation information for 2017 is omitted.
(6)
Mr. Keefer was appointed as Chief Commercial Officer, effective as of November 26, 2018. He previously served as Interim President and Chief Executive Officer from June 21, 2017 to July 10, 2017 and was reappointed for the same positions, effective as of February 9, 2018, serving through his appointment to Chief Commercial Officer. Mr. Keefer’s bonus represents an estimated bonus, subject to Compensation Committee approval, to be paid in 2019 on account of performance in 2018. All other compensation represents commissions earned in 2018 of $53,206 and 2017 of $128,118 and matching contributions under our 401(k) plan in 2018 of $4,225 and 2017 of $4,529.
(7)
Ms. Palczuk’s all other compensation for 2017 is comprised of a $25,000 lump sum payment made under the Separation and Release Agreement, between the Company and Ms. Palczuk, dated February 9, 2018, matching
contributions under our 401(k) plan and reimbursement for rent she paid for an apartment. Ms. Palczuk was appointed as President and Chief Executive Officer, effective as of July 10, 2017 and resigned from the position, effective as of February 9, 2018.
Outstanding Equity Awards at Fiscal Year‑End
The following table provides information on the outstanding equity awards held by our 2018 named executive officers as of December 31, 2018:
|
|
|
|
|
|
|
|
|
|
|
|
|
Option Awards
|
|
|
|
Number of Securities
|
|
|
|
|
|
|
|
Underlying Unexercised
|
|
Option
|
|
Option
|
|
|
|
Options (#)
|
|
Exercise
|
|
Expiration
|
|
Name
|
|
Exercisable
|
|
Unexercisable
|
|
Price ($)
|
|
Date(1)
|
|
Jason Keefer
|
|
4,000
|
|
12,000
|
|
6.80
|
|
7/19/2027
|
|
|
|
—
|
|
20,000
|
|
10.53
|
|
6/26/2028
|
|
Alla Danilkovitch, Ph.D.
|
|
8,000
|
|
—
|
|
7.74
|
|
3/12/2020
|
|
|
|
5,000
|
|
—
|
|
6.46
|
|
5/27/2020
|
|
|
|
9,000
|
|
—
|
|
7.13
|
|
2/14/2021
|
|
|
|
6,000
|
|
—
|
|
5.08
|
|
3/23/2022
|
|
|
|
10,000
|
|
—
|
|
7.73
|
|
2/12/2023
|
|
|
|
20,000
|
|
—
|
|
14.00
|
|
5/6/2024
|
|
|
|
18,750
|
|
6,250
|
|
18.40
|
|
3/6/2025
|
|
|
|
12,500
|
|
37,500
|
|
6.80
|
|
7/19/2027
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
Options expire on the tenth anniversary of the award date and vest in equal installments on the first, second, third and fourth anniversary of the award date. Upon termination of employment, vested options remain exercisable for up to 90‑days if the former employee left in good standing.
Employment Contracts, Termination of Employment and Change in Control Arrangements
Except in the case of Ms. Palczuk, none of our named executive officers during fiscal 2018 were employed for a specified term, and each named executive’s officer’s employment with us was subject to termination at any time by either party for any reason, with or without cause.
Ms. Palczuk
On June 24, 2017, we entered into an employment agreement with Ms. Palczuk. Ms. Palczuk resigned as President and Chief Executive Officer effective as of February 9, 2018. On February 9, 2018, we entered into a Separation and Release Agreement with Ms. Palczuk, that provides for, among other terms, (i) a separation date of February 9, 2018, (ii) a lump sum payment to Ms. Palczuk of $25,000, (iii) up to $4,800 to reimburse Ms. Palczuk for costs associated with the termination of her apartment and furniture rentals, (iv) provision of health benefits through the end of February 2018, (v) mutual non‑disparagement, (vi) compliance with certain post‑employment restrictions in Ms. Palczuk’s employment agreement, including with respect to confidentiality, inventions and patents, non‑competition and non‑solicitation, and (vii) a release of claims by Ms. Palczuk.
Director Compensation
Each director who is not an employee is eligible to receive compensation from us for his or her services as a member of our Board or any of its standing committees. Director compensation is determined by the Compensation Committee, subject to approval by the entire Board. In determining compensation for directors, the Compensation Committee’s decision is generally guided by three goals: compensation should fairly pay the directors for work required of directors of a company of our size and scope; and the structure of the compensation should be simple, transparent and easy for stockholders to understand. As a result, our non-employe director compensation consists of an annual Board
retainer and an annual Chairman of the Board retainer. All directors are reimbursed for their out-of-pocket expenses incurred in attending meetings.
The following table summarizes compensation earned by our non‑employee directors for their service on our Board during the fiscal year ended December 31, 2018.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock
|
|
Option
|
|
All Other
|
|
|
|
Name
|
|
Fees ($)(1)
|
|
Awards ($)
|
|
Awards ($)
|
|
Compensation ($)
|
|
Total ($)
|
|
Peter Friedli(2)
|
|
400,000
|
|
—
|
|
—
|
|
—
|
|
400,000
|
|
Thomas J. Knapp
|
|
60,000
|
|
—
|
|
—
|
|
—
|
|
60,000
|
|
Willi Miesch
|
|
60,000
|
|
—
|
|
—
|
|
—
|
|
60,000
|
|
Charles A. Reinhart, III
|
|
30,000
|
|
—
|
|
—
|
|
—
|
|
30,000
|
|
Uwe Sommer*
|
|
41,800
|
|
—
|
|
—
|
|
—
|
|
41,800
|
|
David L. White*
|
|
41,100
|
|
—
|
|
—
|
|
—
|
|
41,100
|
|
Thomas M. Brandt, Jr.*
|
|
29,167
|
|
—
|
|
—
|
|
—
|
|
29,167
|
(1)
Annual fee for non‑employee directors other than the Chairman is set at $60,000. Fees for directors who only served for a portion of 2018 have been pro‑rated.
(2)
The Compensation Committee determined to increase the Chairman of the Board retainer from $150,000 to $400,000 in 2018. In making this determination, the Compensation Committee considered the amount of work required of the Chairman over the last several years as a result of the significant turnover in the Company’s senior management. In making this determination, the Compensation Committee considered the Chairman’s time commitment and responsibilities more akin to an executive chairman role. In recognition of Mr. Friedli’s continued work in this role, the Committee determined to increase the Chairman retainer from $400,000 to $500,000 for 2019.
*
Indicates individuals who were no longer directors as of December 31, 2018.
ITEM 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of February 28, 2019 for (a) each of our current directors and the named executive officers, (b) all of our current directors and named executive officers as a group, and (c) each stockholder that it knows to be the beneficial owner of more than 5% of our common stock. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. We deem shares of common stock that may be acquired by an individual or group within 60 days of February 28, 2019 pursuant to the exercise of options to be outstanding for the purpose of computing the percentage ownership of such individual or group, but those shares are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them based on information provided to us by these stockholders. Percentage of ownership is based on 34,526,636 shares of common stock outstanding on February 28, 2019.
|
|
|
|
|
|
|
|
|
|
Amount and
|
|
|
|
|
|
|
Nature of
|
|
|
|
|
|
|
Beneficial
|
|
Percent of
|
|
|
Name and Address of Beneficial Owners
|
|
Ownership
|
|
Class(2)
|
|
|
Named Executive Officers and Directors(1)
|
|
|
|
|
|
|
Alla Danilkovitch
|
|
104,875
|
(3)
|
|
|
|
Peter Friedli
|
|
14,810,455
|
(4)
|
42.9
|
%
|
|
Jason Keefer
|
|
4,000
|
|
|
|
|
Thomas J. Knapp
|
|
—
|
|
|
|
|
Willi Miesch
|
|
—
|
|
|
|
|
Charles A. Reinhart, III
|
|
—
|
|
|
|
|
Joel Rogers
|
|
—
|
|
|
|
|
Samson Tom
|
|
—
|
|
|
|
|
All directors and named executive officers as a group (8 persons)
|
|
14,919,330
|
|
43.2
|
%
|
|
Other 5% Stockholders
|
|
9,814,181
|
|
28.4
|
%
|
|
New Venturetec AG
|
|
4,103,301
|
|
11.9
|
%
|
|
c/o Osiris Therapeutics, Inc.
|
|
|
|
|
|
|
7015 Albert Einstein Drive
|
|
|
|
|
|
|
Columbia, Maryland 21046
|
|
|
|
|
|
|
Thomas Schmidheiny
|
|
3,053,267
|
(5)
|
8.8
|
%
|
|
Zurcherstrasse 156
|
|
|
|
|
|
|
8645 Jona Switzerland
|
|
|
|
|
|
|
BIH SA
|
|
2,658,113
|
|
7.7
|
%
|
|
3 Fauboug de’Hopital
|
|
|
|
|
|
|
2000 Neuchatel, Switzerland
|
|
|
|
|
|
(1)
Mailing address for all directors and officers is c/o Osiris Therapeutics, Inc., 7015 Albert Einstein Drive, Columbia, Maryland 21046.
(2)
Percentage not provided if less than 1%.
(3)
Consists of 9,375 shares owned by Dr. Danilkovitch and 95,500 shares issuable upon exercise of options. Dr. Danilkovitch separately has 37,500 options that have not yet vested.
(4)
Consists of 10,204,404 shares owned directly by Mr. Friedli, 4,103,301 shares held by New Venturetec, AG, 500,000 shares held by Mr. Friedli’s minor daughter and 2,750 shares held by his spouse.
Mr. Friedli has sole voting power with respect to 14,810,455 shares, sole dispositive power with respect to 10,204,404 shares, and shared dispositive power with respect to 4,606,051 shares. Mr. Friedli serves as President of New Venturetec AG. Mr. Friedli has disclaimed beneficial ownership in the shares held by his daughter and spouse, beyond the extent of his pecuniary interest therein.
(5)
Consists of 395,154 shares owned directly by Mr. Schmidheiny and 2,658,113 shares owned by BIH SA. Mr. Schmidheiny is the Chairman and controlling shareholder of BIH SA.
Information regarding securities authorized for issuance under equity compensation plans is set forth under the heading Securities Authorized for Issuance under Equity Compensation Plans in Part II, Item 5 of this Form 10‑K and is incorporated by reference in this Item 12.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence.
The Audit Committee reviews and approves all related person transactions, as defined by SEC rules. The Audit Committee may approve only those related party transactions that are in, or are not inconsistent with, the best interests of us and our stockholders, as the Audit Committee determines in good faith. In making such a determination, the Audit
Committee is required to consider all of the relevant facts and circumstances relating to the transaction including, but not limited to, the following:
•
the benefits to the Company;
•
if the transaction involves a director, a member of the director’s immediate family or entity affiliated with the director, the impact on the director’s independence;
•
the availability of other sources for comparable products or services;
•
the terms of the transaction; and
•
the terms available to unrelated third parties.
Our Board has determined that the following members of the Board qualify as “independent” under the definition promulgated by NASDAQ: Thomas J. Knapp, Willi Miesch, and Charles A. Reinhart, III. Furthermore, our Board has determined that none of the current members of the Audit Committee, the Compensation Committee or the Nominating Committee of the Board has any material relationship with us (directly or as a partner, stockholder or officer of an organization that has a relationship with us), and therefore, each committee is “independent” within the meaning of the NASDAQ standards and the rules and regulations of the SEC.
ITEM 14. Principal Accounting Fees and Services.
Pursuant to the rules of the SEC, the following table sets forth the amount of audit fees, audit‑related fees, tax fees, and all other fees billed for services by Ernst & Young for the audit of our financial statements and the effectiveness of internal control over financial reporting for the year ended December 31, 2018.
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year Ended
|
|
Fiscal Year Ended
|
|
|
(in thousands)
|
|
12/31/2018
|
|
12/31/2017
|
|
|
Audit Fees(1)
|
|
$
|
1,315
|
|
$
|
1,312
|
|
|
Audit‑Related Fees(2)
|
|
|
—
|
|
|
—
|
|
|
Tax Fees(3)
|
|
|
52
|
|
|
—
|
|
|
All Other Fees(4)
|
|
|
—
|
|
|
—
|
|
|
Total
|
|
$
|
1,367
|
|
$
|
1,312
|
|
(1)
Audit fees are fees billed to the Company for professional services performed for the audit of financial statements included in the Form 10‑K, for review of quarterly unaudited financial information included as a footnote in the annual financial statements, and for the audit of our internal control over financial reporting.
(2)
Audit‑related fees consisted principally of fees billed to the Company for assurance and related services performed that are reasonably related to the performance of the audit or review of our financial statements, including due diligence related to mergers and acquisitions, audits of employee benefit and compensation plans, audits of carve‑out entities, and other agreed upon procedures to meet various statutory and regulatory requirements.
(3)
Tax fees are fees billed to the Company for professional services performed with respect to U.S. federal, state and local tax planning, advice and compliance, assistance with tax audits and appeals and the preparation of original and amended tax returns for the Company.
(4)
All other fees are fees for any permissible services performed that do not meet the above category descriptions, however, we generally do not engage our independent registered public accountants for “other” services.
Under our pre‑approval policy, concurrent with the appointment of the independent registered public accounting firm, the Audit Committee specifically pre‑approves the recurring audit and audit‑related services and
estimated fees. All the work performed for the Company by Ernst & Young for the audit of our financial statements for the years ended December 31, 2018 and 2017 and the related fees, were pre‑approved by the Audit Committee.
The Audit Committee has considered whether the provision of audit and non‑audit services by Ernst & Young to the Company for fiscal years 2018 and 2017 is compatible with maintaining the auditor’s independence. We have been advised by Ernst & Young that neither the firm, nor any member of the firm, has any financial interest, direct or indirect, in any capacity in the Company.
PART IV
ITEM 15. Exhibits and Financial Statement Schedules.
(A) The following documents are filed as part of this report:
(1) The financial statements are included in Part II, Item 8 of this Form 10‑K
(2) Financial Statement Schedules
Schedules have been omitted since the required information is not significant or is included in the Notes to Consolidated Financial Statements.
(3) Exhibits:
|
Exhibit
Number
|
|
Description of Exhibit
|
|
3.1
|
|
Articles of Restatement of the Registrant as filed with the State Department of Assessments and Taxation of Maryland on June 4, 2010 (Incorporated herein by reference to Exhibit 3.1 to the Quarterly Report on Form 10‑Q filed by the Registrant with the SEC on August 6, 2010
).
|
|
3.2
|
|
Articles of Amendment of the Registrant as filed with the State Department of Assessments and Taxation of Maryland on June 10, 2015 (Incorporated herein by reference to Exhibit 3.2 to the Annual Report on Form 10-K filed by the Registrant on March 28, 2018).
|
|
3.3
|
|
Articles of Merger between Osiris Therapeutics, Inc., a Delaware corporation, and Osiris Maryland, Inc., a Maryland corporation, as survivor, changing the name of “Osiris Maryland, Inc.” to “Osiris Therapeutics, Inc.” as filed with the State Department of Assessments and Taxation of Maryland on May 27, 2010, and effective May 31, 2010 (Incorporated herein by reference to Exhibit 3.2 to the Current Report on Form 8‑K filed by the Registrant with the SEC on June 2, 2010).
|
|
3.4
|
|
Articles of Amendment of the Registrant as filed with the State Department of Assessments and Taxation of Maryland on June 28, 2018 (Incorporated herein by reference to Exhibit 3.4 to the Registration Statement on Form 8-A filed by the Registrant with the SEC on July 27, 2018).
|
|
3.5
|
|
Amended and Restated Bylaws of the Registrant (Incorporated herein by reference to Exhibit 3.5 to the Registration Statement on Form 8‑A filed by the Registrant with the SEC on July 27, 2018).
|
|
4.1
|
|
Form of Common Stock Certificate (Incorporated herein by reference to corresponding Exhibit to the Registrant’s Registration Statement on Form S 1, which was declared effective by the SEC on August 3, 2006).
|
|
10.1.1
|
|
Amended and Restated 2006 Omnibus Plan, effective as of May 27, 2010 (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8‑K filed by the Registrant with the SEC on June 2, 2010).
|
|
10.1.2
|
|
First Amendment to Amended & Restated 2006 Omnibus Plan, effective as of June 11, 2012 (Incorporated herein by reference to Exhibit 4.4 to the Registration Statement on Form S‑8 filed by the Registrant with the SEC on November 9, 2012).
|
|
10.1.3
|
|
Second Amendment to Amended & Restated 2006 Omnibus Plan, effective as of May 6, 2014 (Incorporated herein by reference to Exhibit 4.5 to the Registration Statement on Form S‑8 filed by the Registrant with the SEC on October 3, 2014).
|
|
10.1.4
|
|
Form of
Stock Option Award Agreement under the 2006 Omnibus Plan (filed herewith)
.
|
|
10.2
|
|
Separation and Release Agreement, dated February 9, 2018, between Linda Palczuk and the R
egistrant
(Incorporated by reference to Exhibit 10.9 to the Annual Report on Form 10-K filed by the Registrant on March 28, 2018).
|
|
10.3
|
|
Agreement of Lease by and between the Registrant and Columbia Gateway S‑28, L.L.C., dated June 6, 2006 (Incorporated herein by reference to corresponding Exhibit to the Registrant’s Registration Statement on Form S 1, which was declared effective by the SEC on August 3, 2006).
|
|
10.4
|
|
Third Amendment to Agreement of Lease by and between the Registrant and Columbia Gateway S‑28, L.L.C., dated September 30, 2014 (Incorporated herein by reference to Exhibit 10.7 to the Annual Report on Form 10‑K/A filed by the Registrant with the SEC on March 27, 2017).
|
|
10.5
|
|
Form of Indemnification and Advancement of Expenses Letter Agreement (Incorporated herein by reference to Exhibit 10.1 to the Current Report on Form 8 K filed by the Registrant with the SEC on July 2, 2018).
|
|
10.6
|
|
Exclusive Service Agreement by and between the Registrant and Howmedica Osteonics Corp., also referred to as Stryker Orthopaedics, dated as of December 19, 2014 (Incorporated herein by reference to Exhibit 10.9 to the Annual Report on Form 10-K/A filed by the Registrant with the SEC on March 27, 2017).
|
|
16.1
|
|
Letter from BDO USA, LLP, dated December 17, 2015 (Incorporated by reference to Exhibit 1.1 to the Current Report on Form 8‑K filed by the Registrant with the SEC on December 17, 2015).
|
|
21
|
|
List of Subsidiaries (filed herewith).
|
|
23.1.1
|
|
Consent of Ernst & Young LLP (filed herewith).
|
|
31.1.1
|
|
Certification of Chief Executive Officer pursuant to Rule 13a‑14(a) or Rule 15d‑14(a) (filed herewith).
|
|
31.2.1
|
|
Certification of Chief Financial Officer pursuant to Rule 13a‑14(a) or Rule 15d‑14(a) (filed herewith).
|
|
32.1.1
|
|
Section 1350 Certification of Chief Executive Officer and Chief Financial Officer (filed herewith).
|
|
Exhibit
Number
|
|
Description of Exhibit
|
|
101
|
|
The following materials from the Registrant’s Annual Report on Form 10‑K for the year ended December 31, 2018, formatted in Extensible Business Reporting Language (XBRL), include: (i) the Statements of Income, (ii) the Balance Sheets, (iii) the Statements of Cash Flows, and (iv) related notes (filed herewith).
|
ITEM 16. Form 10‑K Summary
.
None.
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
OSIRIS THERAPEUTICS, INC.
|
|
|
|
|
|
March 15, 2019
|
|
By:
|
/s/ SAMSON TOM
|
|
|
|
|
Samson Tom
|
|
|
|
|
President and Chief Executive Officer
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
|
|
|
|
|
|
/s/ SAMSON TOM
|
|
President and Chief Executive Officer
|
|
March 15, 2019
|
|
Samson Tom
|
|
(principal executive officer)
|
|
|
|
|
|
|
|
|
|
/s/ JOEL ROGERS
|
|
Chief Financial Officer (principal financial officer
|
|
March 15, 2019
|
|
Joel Rogers
|
|
and principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ PETER FRIEDLI
|
|
Director
|
|
March 15, 2019
|
|
Peter Friedli
|
|
|
|
|
|
|
|
|
|
|
|
/s/ THOMAS J. KNAPP
|
|
Director
|
|
March 15, 2019
|
|
Thomas J. Knapp
|
|
|
|
|
|
|
|
|
|
|
|
/s/ WILLI MIESCH
|
|
Director
|
|
March 15, 2019
|
|
Willi Miesch
|
|
|
|
|
|
|
|
|
|
|
|
/s/ CHARLES A. REINHART
|
|
Director
|
|
March 15, 2019
|
|
Charles A. Reinhart
|
|
|
|
|
OSIRIS THERAPEUTICS, INC.
INDEX
TO
CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Osiris Therapeutics, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Osiris Therapeutics, Inc.’s internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, because of the effect of the material weaknesses described below on the achievement of the objectives of the control criteria, Osiris Therapeutics, Inc. (the Company) has not maintained effective internal control over financial reporting as of December 31, 2018, based on the COSO criteria.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. As more fully described in Management’s Annual Report on Internal Control over Financial Reporting included in Item 9A, management has identified material weaknesses in control activities related to revenue recognition; inventory; reserves, estimates and valuation accounts; and purchasing and disbursements.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of Osiris Therapeutics, Inc. as of December 31, 2018 and 2017, and the related consolidated statements of comprehensive income, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2018, and the related notes. These material weaknesses were considered in determining the nature, timing and extent of audit tests applied in our audit of the 2018 consolidated financial statements, and this report does not affect our report dated March 15, 2019, which expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting included in Item 9A Controls and Procedures. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that
transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Baltimore, Maryland
March 15, 2019
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Osiris Therapeutics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Osiris Therapeutics, Inc. (the Company) as of December 31, 2018 and 2017, and the related consolidated statements of comprehensive income, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2018, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company’s internal control over financial reporting as of December 31, 2018, based on criteria established in Internal Control‑Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework), and our report dated March 15, 2019 expressed an adverse opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2017.
Baltimore, Maryland
March 15, 2019
OSIRIS THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(amounts in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2018
|
|
2017
|
|
|
Assets
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
16,367
|
|
$
|
3,081
|
|
|
Certificates of deposit
|
|
|
10,044
|
|
|
—
|
|
|
Short-term investments
|
|
|
8,276
|
|
|
24,807
|
|
|
Trade receivables, net
|
|
|
22,469
|
|
|
26,053
|
|
|
Inventory, net
|
|
|
9,614
|
|
|
11,278
|
|
|
Insurance receivable
|
|
|
—
|
|
|
4,788
|
|
|
Prepaid expenses and other current assets
|
|
|
3,477
|
|
|
2,920
|
|
|
Total current assets
|
|
|
70,247
|
|
|
72,927
|
|
|
Property and equipment, net
|
|
|
2,882
|
|
|
3,587
|
|
|
Deferred income taxes, net
|
|
|
25,205
|
|
|
—
|
|
|
Other assets
|
|
|
3,168
|
|
|
1,608
|
|
|
Total assets
|
|
$
|
101,502
|
|
$
|
78,122
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Equity
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
4,144
|
|
$
|
5,269
|
|
|
Accrued liabilities
|
|
|
13,820
|
|
|
9,399
|
|
|
Accrued shareholder litigation
|
|
|
900
|
|
|
18,500
|
|
|
Other current liabilities
|
|
|
1,728
|
|
|
1,934
|
|
|
Total current liabilities
|
|
|
20,592
|
|
|
35,102
|
|
|
Other long-term liabilities
|
|
|
2,382
|
|
|
1,626
|
|
|
Total liabilities
|
|
|
22,974
|
|
|
36,728
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value, 5,000 shares authorized, none issued and outstanding at December 31, 2018, and 20,000 shares authorized, none issued and outstanding at December 31, 2017
|
|
|
—
|
|
|
—
|
|
|
Common stock, $0.001 par value, 72,000 shares authorized, 34,527 shares issued and outstanding at December 31, 2018, and 90,000 shares authorized, 34,526 shares issued and outstanding at December 31, 2017
|
|
|
35
|
|
|
35
|
|
|
Additional paid-in-capital
|
|
|
284,189
|
|
|
283,905
|
|
|
Accumulated other comprehensive loss
|
|
|
(259)
|
|
|
(208)
|
|
|
Accumulated deficit
|
|
|
(205,437)
|
|
|
(242,338)
|
|
|
Total equity
|
|
|
78,528
|
|
|
41,394
|
|
|
Total liabilities and equity
|
|
$
|
101,502
|
|
$
|
78,122
|
|
See accompanying notes to consolidated financial statements
OSIRIS THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(amounts in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31,
|
|
|
|
2018
|
|
2017
|
|
Revenue
|
|
$
|
142,824
|
|
$
|
118,514
|
|
Cost of revenue
|
|
|
38,139
|
|
|
32,681
|
|
Gross profit
|
|
|
104,685
|
|
|
85,833
|
|
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
Research and development
|
|
|
6,764
|
|
|
4,138
|
|
Sales and marketing
|
|
|
67,542
|
|
|
61,545
|
|
General and administrative
|
|
|
19,626
|
|
|
22,139
|
|
Shareholder litigation expense
|
|
|
900
|
|
|
—
|
|
Total operating expenses
|
|
|
94,832
|
|
|
87,822
|
|
Income (loss) from continuing operations
|
|
|
9,853
|
|
|
(1,989)
|
|
Other income (expense), net
|
|
|
675
|
|
|
(645)
|
|
Income (loss) before income taxes from continuing operations
|
|
|
10,528
|
|
|
(2,634)
|
|
Income tax benefit
|
|
|
26,005
|
|
|
1,398
|
|
Net income (loss) from continuing operations
|
|
|
36,533
|
|
|
(1,236)
|
|
Discontinued operations, net of tax
|
|
|
368
|
|
|
10,021
|
|
Net income
|
|
|
36,901
|
|
|
8,785
|
|
Other comprehensive income:
|
|
|
|
|
|
|
|
Unrealized loss on investments
|
|
|
(51)
|
|
|
(119)
|
|
Comprehensive income
|
|
$
|
36,850
|
|
$
|
8,666
|
|
Net income per share from continuing operations:
|
|
|
|
|
|
|
|
Basic
|
|
$
|
1.06
|
|
$
|
(0.04)
|
|
Diluted
|
|
$
|
1.06
|
|
$
|
(0.04)
|
|
Net income per share from discontinued operations:
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.01
|
|
$
|
0.29
|
|
Diluted
|
|
$
|
0.01
|
|
$
|
0.29
|
|
Net income per share:
|
|
|
|
|
|
|
|
Basic
|
|
$
|
1.07
|
|
$
|
0.25
|
|
Diluted
|
|
$
|
1.07
|
|
$
|
0.25
|
|
Weighted average common shares outstanding:
|
|
|
|
|
|
|
|
Basic
|
|
|
34,526
|
|
|
34,524
|
|
Diluted
|
|
|
34,573
|
|
|
34,525
|
See accompanying notes to consolidated financial statements
OSIRIS THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(amounts in thousands, except for share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
Other
|
|
|
|
|
|
|
|
|
|
Common Stock
|
|
Paid-in
|
|
Comprehensive
|
|
Accumulated
|
|
Total
|
|
|
|
Shares
|
|
Amount
|
|
Capital
|
|
(Loss) Income
|
|
Deficit
|
|
Equity
|
|
Balance at January 1, 2017
|
|
34,500,886
|
|
$
|
35
|
|
$
|
283,678
|
|
$
|
(89)
|
|
$
|
(251,123)
|
|
$
|
32,501
|
|
Stock issued upon exercise of stock options, net of shares acquired
|
|
25,000
|
|
|
—
|
|
|
128
|
|
|
—
|
|
|
—
|
|
|
128
|
|
Stock based compensation
|
|
—
|
|
|
—
|
|
|
99
|
|
|
—
|
|
|
—
|
|
|
99
|
|
Unrealized loss on available for sale investments
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(119)
|
|
|
—
|
|
|
(119)
|
|
Net income
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
8,785
|
|
|
8,785
|
|
Balance at December 31, 2017
|
|
34,525,886
|
|
|
35
|
|
|
283,905
|
|
|
(208)
|
|
|
(242,338)
|
|
|
41,394
|
|
Stock issued upon exercise of stock options, net of shares acquired
|
|
750
|
|
|
—
|
|
|
7
|
|
|
—
|
|
|
—
|
|
|
7
|
|
Stock based compensation
|
|
—
|
|
|
—
|
|
|
277
|
|
|
—
|
|
|
—
|
|
|
277
|
|
Unrealized loss on available for sale investments
|
|
—
|
|
|
—
|
|
|
—
|
|
|
(51)
|
|
|
—
|
|
|
(51)
|
|
Net income
|
|
—
|
|
|
—
|
|
|
—
|
|
|
—
|
|
|
36,901
|
|
|
36,901
|
|
Balance at December 31, 2018
|
|
34,526,636
|
|
$
|
35
|
|
$
|
284,189
|
|
$
|
(259)
|
|
$
|
(205,437)
|
|
$
|
78,528
|
See accompanying notes to consolidated financial statements
OSIRIS THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(amounts in thousands)
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
2018
|
|
2017
|
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
|
|
|
|
|
|
|
Net income
|
|
$
|
36,901
|
|
$
|
8,785
|
|
Adjustments to reconcile net income to net cash provided by (used in) operating activities:
|
|
|
|
|
|
|
|
Deferred income taxes
|
|
|
(25,205)
|
|
|
—
|
|
Receipt of Mesoblast common stock
|
|
|
—
|
|
|
(10,000)
|
|
Provision for excess and obsolete inventory
|
|
|
1,529
|
|
|
525
|
|
Realized loss on investments
|
|
|
219
|
|
|
2,028
|
|
Loss on disposal of fixed assets
|
|
|
74
|
|
|
123
|
|
Depreciation
|
|
|
873
|
|
|
688
|
|
Stock-based compensation expense
|
|
|
277
|
|
|
99
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
Settlement of shareholder and regulatory actions
|
|
|
(18,500)
|
|
|
(1,500)
|
|
Receipt of insurance proceeds regarding the settlement of shareholder actions
|
|
|
4,788
|
|
|
—
|
|
Accounts receivables, net
|
|
|
3,584
|
|
|
(2,805)
|
|
Inventory, net
|
|
|
135
|
|
|
(437)
|
|
Prepaid expenses and other assets
|
|
|
(2,117)
|
|
|
(1,641)
|
|
Accounts payable, accrued expenses, and other liabilities
|
|
|
3,846
|
|
|
(1,088)
|
|
Accrued shareholder litigation
|
|
|
900
|
|
|
—
|
|
Net cash provided by (used in) operating activities
|
|
|
7,304
|
|
|
(5,223)
|
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
|
|
|
|
|
|
|
Purchases of property and equipment
|
|
|
(242)
|
|
|
(2,008)
|
|
Proceeds from sale of investments
|
|
|
16,721
|
|
|
32,857
|
|
Purchases of certificates of deposit
|
|
|
(10,000)
|
|
|
—
|
|
Purchases of investments
|
|
|
(504)
|
|
|
(25,506)
|
|
Net cash provided by investing activities
|
|
|
5,975
|
|
|
5,343
|
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
|
|
|
|
|
|
|
Proceeds from the exercise of options to purchase common stock
|
|
|
7
|
|
|
128
|
|
Net cash provided by financing activities
|
|
|
7
|
|
|
128
|
|
NET INCREASE IN CASH AND CASH EQUIVALENTS
|
|
|
13,286
|
|
|
248
|
|
Cash and cash equivalents at beginning of period
|
|
|
3,081
|
|
|
2,833
|
|
Cash and cash equivalents at end of period
|
|
$
|
16,367
|
|
$
|
3,081
|
|
|
|
|
|
|
|
|
|
Cash (refunded) paid during the period for:
|
|
|
|
|
|
|
|
Income taxes (refunded) paid
|
|
$
|
(318)
|
|
$
|
134
|
See accompanying notes to consolidated financial statements
OSIRIS THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Business
Osiris Therapeutics, Inc. (“we”, “us”, “our”, “Osiris”, or the “Company”) researches, develops, manufactures and commercializes regenerative medicine products intended to improve the health and lives of patients and lower overall healthcare costs. We are headquartered in Columbia, Maryland. We continue to advance our research and development (“R&D”) by focusing on innovation in regenerative medicine, including the development of bioengineered stem cell and tissue‑based products. We have achieved commercial success with products in orthopedics, sports medicine and wound care.
We operate in one segment and are focused on using unique tissue preservation technologies to develop viable human tissue products designed to improve wound closure and surgical outcomes for patients and physicians over standard of care alone. We launched Grafix in 2010, Ovation in 2011 and discontinued it in 2014, Cartiform in 2012, BIO
4
(previously branded as OvationOS) in 2014 and Stravix in 2015. Sales of these products have increased with the expansion of our direct sales force and distribution network and the broadening of our reimbursement coverage. Our direct sales force focuses exclusively on Grafix and Stravix sales. We entered into two exclusive distribution agreements in the fourth quarter of 2014, with a subsidiary of Stryker Corporation (“Stryker”) for the marketing and distribution of BIO
4
, and with Arthrex, Inc. (“Arthrex”) for the marketing and distribution of Cartiform. The first sales under these agreements began in 2015.
We are a fully integrated company, having developed capabilities in research and development, manufacturing, marketing and sales of our products. We are focused on the long‑term commercial growth of the Company through the delivery of differentiated products for use across multiple fields of medicine with clear value propositions to patients, providers and third‑party payors.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly‑owned subsidiary, Osiris Therapeutics International GmbH, which was formed in 2016. All intercompany transactions have been eliminated in consolidation. Osiris Therapeutics International GmbH does not have any operations.
Use of Estimates
We make certain estimates and assumptions in preparing our consolidated financial statements in accordance with generally accepted accounting principles in the United States (“GAAP”). These estimates and assumptions affect the reported amount of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statement and the reported amounts of revenue and expenses for the period presented. Actual results may differ from these estimates.
Cash and Cash Equivalents
Cash and cash equivalents includes all financial instruments purchased with an initial maturity of three months or less when purchased, which includes certificates of deposit that can be redeemed on demand and have maturities of less than three months, when purchased. As of December 31, 2018, approximately $5.0 million of the Company’s cash and cash equivalents were certificates of deposits with one financial institution.
Certificates of Deposit
The Company considers certificates of deposit that can be redeemed on demand and have maturities of less than three months, when purchased, to be cash equivalents, and therefore, excludes them from the Certificates of Deposit line in the accompanying consolidated balance sheets. Certificates of deposit with an initial maturity date exceeding three months and which can be redeemed upon demand are included in this classification. The Company invested approximately $10.0 million in certificates of deposit during 2018 with an initial maturity date exceeding three months and has classified them accordingly in the accompanying consolidated balance sheets as of December 31, 2018.
Investments
All of the Company’s investments are classified as “available‑for‑sale” and are carried at fair value, with the exception of the Mesoblast Limited common stock, which was classified as a trading security (see Note 3). Securities which have been classified as Level 1 are measured using quoted market prices in active markets for identical assets or liabilities while those which have been classified as Level 2 are measured using quoted prices for identical assets and liabilities in markets that are not active (see Note 9). The Company’s policy is to classify all investments with contractual maturities within one year as current. Investment income is recognized when earned and reported net of investment expenses. Net unrealized holding gains or losses on non-equity securities are excluded from earnings and are reported as “Accumulated other comprehensive income (loss)” in the accompanying consolidated balance sheets and consolidated statements of comprehensive income (loss) until realized, unless the losses are deemed to be other‑than‑temporary. Realized gains or losses, including any provision for other‑than‑temporary declines in value, are included in “Other income (expense), net” in the accompanying consolidated statements of comprehensive income.
If a debt security is in an unrealized loss position and the Company has the intent to sell the debt security, or it is more likely than not that the Company will have to sell the debt security before recovery of its amortized cost basis, the decline in value is deemed to be other‑than‑temporary and is recorded to other‑than‑temporary impairment losses recognized in income in the consolidated statements of comprehensive income. For impaired debt securities that the Company does not intend to sell or it is more likely than not that the Company will not have to sell such securities, but the Company expects that it will not fully recover the amortized cost basis, the credit component of the other‑than‑temporary impairment is recognized in other‑than‑temporary impairment losses recognized in income in the consolidated statements of comprehensive income and the non‑credit component of the other‑than‑temporary impairment is recognized in other comprehensive loss.
The credit component of an other‑than‑temporary impairment is determined by comparing the net present value of projected future cash flows with the amortized cost basis of the debt security. The net present value is calculated by discounting the best estimate of projected future cash flows at the effective interest rate implicit in the debt security at the date of acquisition. Cash flow estimates are driven by assumptions regarding probability of default, including changes in credit ratings, and estimates regarding timing and amount of recoveries associated with a default. Furthermore, unrealized losses entirely caused by non‑credit related factors related to debt securities for which the Company expects to fully recover the amortized cost basis continue to be recognized in accumulated other comprehensive loss.
As of December 31, 2018 and 2017, there were no unrealized losses that the Company believed to be other‑than‑temporary.
Trade Receivables, net
Trade receivables, net are reported net of an allowance for doubtful accounts. We consider receivables outstanding longer than the time specified in the respective customer’s contract to be past due. In making the determination of the appropriate allowance for doubtful accounts, management considers prior experience with customers, analysis of accounts receivable aging reports, changes in customer payment patterns, and historical write‑offs.
Inventory, net
Inventory, net consists of raw materials, products in process, finished goods available for sale and products held by customers under consignment sales arrangements. We determine our inventory values using the first‑in, first‑out method. The Company periodically reviews its quantities of inventories on hand and compares these amounts to the expected usage of each particular product. The Company records as a charge to Cost of revenue any amounts required to reduce the carrying value of inventories to net realizable value. Inventory excludes units that we anticipate distributing for clinical evaluation.
Property and Equipment, net
Property and equipment, net are valued at cost less accumulated depreciation. Depreciation is recognized on a straight‑line basis over the estimated useful lives of the related assets and is allocated between cost of sales and selling, general and administrative expenses. Maintenance and repairs, which do not improve or extend the life of the respective assets, are charged to expense as incurred. Assets recorded under capital leases are included in property and equipment and are amortized using the straight‑line method over the shorter of the estimated useful lives of the assets or the lease term and is recorded in depreciation expense in the consolidated statements of comprehensive income.
We review long‑lived assets, which consist solely of property and equipment, for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or group of assets may not be fully recoverable based on the criteria for accounting for the impairment or disposal of long‑lived assets under Accounting Standard Code (“ASC”) Topic 360,
Property, Plant and Equipment
. These events or changes in circumstances may include a significant deterioration of operating results, changes in business plans, or changes in anticipated future cash flows. If an impairment indicator is present, we evaluate recoverability by a comparison of the carrying amount of the assets group to future undiscounted net cash flows expected to be generated by the assets group. Assets are grouped at the lowest level for which there is identifiable cash flows that are largely independent of the cash flows generated by other asset groups. If the total of the expected undiscounted future cash flows is less than the carrying amount of the asset, an impairment loss is recognized for the difference between the fair value and carrying value of assets within the group. Fair value is generally determined by estimates of discounted cash flows. The discount rate used in any estimate of discounted cash flows would be the rate required for a similar investment of like risk. There were no impairment losses recognized during the years ended December 31, 2018 or 2017.
Accrued liabilities
As of December 31, 2018 and 2017, accrued liabilities consisted of:
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
(in $000's)
|
|
2018
|
|
2017
|
|
Payroll and related
|
|
$
|
3,098
|
|
$
|
1,980
|
|
Commissions
|
|
|
5,968
|
|
|
5,651
|
|
Accounting and audit fees
|
|
|
1,859
|
|
|
905
|
|
Lease liabilities
|
|
|
321
|
|
|
120
|
|
Other
|
|
|
2,574
|
|
|
743
|
|
Total
|
|
$
|
13,820
|
|
$
|
9,399
|
Stockholders’ Equity
On June 26, 2018, the Company amended its charter, which was approved by the Company’s stockholders, which decreased the total number of authorized shares of common stock from ninety million (90,000,000) shares to seventy-two million (72,000,000) shares and the total number of authorized shares of preferred stock from twenty million (20,000,000) shares to five million (5,000,000) shares.
.
Revenue Recognition
The Company began accounting for revenue in accordance with Topic 606 on January 1, 2018, using the modified retrospective method. Under the new revenue standard for arrangements that are determined to be within the scope of Topic 606, the Company recognizes revenue following the five-step model: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the customer. At contract inception, once the contract is determined to be within the scope of Topic 606, the Company assesses the goods or services promised within each contract and determines the performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
Accordingly, the Company recognizes revenue when title to the product, ownership and risk of loss transfer to the customer, which is either the date of receipt by the customer or when we receive appropriate notification that the product has been used or implanted in a patient, which is consistent with revenue recognition under ASC 605, which was applicable for the year ended December 31, 2017. The amount of revenue recognized reflects the consideration to which the Company expects to be entitled to in exchange for transferring the goods. The nature of the Company’s contracts gives rise to certain types of variable consideration, including rebates and other discounts. The Company includes estimated amounts of variable consideration in the transaction price to the extent that it is probable there will not be a significant reversal of revenue. Estimates are based on historical or anticipated performance and represent our best judgment at the time. Any estimates are evaluated on a quarterly basis until the uncertainty is resolved.
The Company provides a variety of products and services to its customers. Most of the Company’s contracts consist of a single, distinct performance obligation or promise to transfer goods to a customer. For contracts that include multiple performance obligations, the Company allocates the total transaction price to each performance obligation using our best estimate of the standalone selling price of each identified performance obligation.
The Company’s incremental costs of obtaining a contract would generally have less than a one-year duration; therefore, the Company applies the practical expedient available and expenses costs to obtain a contract when incurred.
In December 2014, we entered into exclusive distribution agreements with a subsidiary of Stryker Corporation for the marketing and distribution of BIO
4
, previously branded by the Company as OvationOS, our viable bone matrix allograft. Pursuant to the agreement, Stryker has been granted worldwide distribution rights to the product and any improvements, for all surgical applications. We are responsible for supply, manufacturing, inventory management, shipments to customers, continued research and product improvement activities. We maintain all inventory and collection risk and we control the product. We recognize revenue as the principal in the arrangement, for the amounts charged to end‑use customers for the BIO
4
product. Commissions and administrative support fees paid to Stryker are accounted for as selling expenses, as Stryker is acting as an outside sales and marketing agent for the Company. We received an up‑front payment of $5.0 million from Stryker in 2015, related to its initial exclusivity, and are entitled to additional fees upon any exercise by Stryker of its right to extend the initial term, whether on an exclusive or non‑exclusive basis. The exclusivity fee is recognized as a reduction of commission expense over the four‑year term of the agreement. The exclusivity fee was fully recognized during fiscal year 2018 with no remaining balance at December 31, 2018. At December 31, 2017, we had a remaining balance of $1.4 million related to the exclusivity payment of which $1.25 million was classified as other current liabilities in the consolidated balance sheets at the end of the year.
In February 2019, Stryker extended the exclusive period for an additional four years. We received an exclusivity fee of $3.9 million in February 2019 to extend the initial term, which will be recognized as a reduction of commissions expense over the exclusivity period.
In October 2014, we entered into an exclusive marketing and sales representative agreement for our cartilage product, Cartiform, with Arthrex. The agreement provides Arthrex with exclusive commercial distribution rights to
Cartiform beginning in 2015. We are responsible for manufacturing, continued research and product improvement activities. Pursuant to the agreement, Arthrex is entitled to a certain commission on Cartiform sales. We maintain all inventory and collection risk. We recognize revenue as the principal in the agreement, for the amounts charged to end‑use customers for the Cartiform product. We account for the commission payments to Arthrex as sales and marketing expense, as Arthrex is acting as outside sales and marketing agent for the Company.
The following table presents our revenues disaggregated by product line:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2018
|
|
2017
|
|
|
(in $000’s)
|
|
|
|
|
|
|
|
|
Grafix/Stravix
|
|
$
|
105,287
|
|
$
|
85,344
|
|
|
BIO4
|
|
|
27,685
|
|
|
24,166
|
|
|
Cartiform
|
|
|
9,845
|
|
|
8,988
|
|
|
Other
|
|
|
7
|
|
|
16
|
|
|
Total
|
|
$
|
142,824
|
|
$
|
118,514
|
|
Research and Development Costs (“R&D”)
R&D expenditures are charged to expense as incurred. R&D expenses include the costs of certain personnel, preclinical studies, clinical trials, regulatory affairs, biostatistical data analysis, and manufacturing costs for development of drug materials for use in clinical trials. We accrue R&D expenses for activity as incurred during the fiscal year and prior to receiving invoices from clinical sites and third party clinical research organizations.
Share‑Based Compensation
We account for share‑based employee compensation under the fair value recognition and measurement provisions in accordance with applicable accounting standards, which require all share‑based payments to employees, including grants of stock options, to be measured based on the grant date fair value of the awards, with the resulting expense generally recognized on an accelerated basis over the period during which the employee is required to perform service in exchange for the award.
Income Taxes
We use the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the period that such tax rate changes are enacted. The measurement of a deferred tax asset is reduced, if necessary, by a valuation allowance if it is more likely than not that some portion or all of the deferred tax asset will not be realized.
We use a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more‑likely‑than‑not to be sustained upon examination by taxing authorities. We recognize interest and penalties accrued on any unrecognized tax exposures as a component of income tax expense.
On December 22, 2017, the Tax Cuts and Jobs Act of 2017 (the “Tax Act”) was enacted into law and the new legislation contains certain key tax provisions that affected the Company, including a reduction of the corporate income tax rate to 21% effective January 1, 2018, the repeal of Alternative Minimum Tax (“AMT”) (and changes to the utilization of AMT credit carryforwards that exist at December 31, 2017), among others. We recognized the effect of the tax law changes in the period of enactment, such as re‑measuring our U.S. deferred tax assets and liabilities as well as reassessing the net realizability of our deferred tax assets and liabilities. See Note 10 for additional information.
Concentration of Risk
We maintain cash with high credit quality financial institutions and do not believe we are exposed to a significant credit risk, although such balances exceed federally insured limits. We also invest excess cash in certificates of deposit or investment‑grade securities with average maturities of approximately two years.
Accounting Pronouncements Adopted
In August 2016, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-15, “Statement of Cash Flows Topic 203.” This ASU addresses eight specific cash flow issues and clarifies their presentation and classification in the statement of cash flows. The Company adopted this ASU on January 1, 2018 and concluded that the adoption of this ASU did not have a material impact on our condensed consolidated financial statements. As such, no retrospective adjustment was recorded.
On December 22, 2017, the SEC issued Staff Accounting Bulletin No. 118 (“SAB 118”) to address the application of GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the newly-enacted U.S. Tax Cuts and Jobs Act (the “Act”). SAB 118 allows registrants to include a provisional amount to account for the implications of the Act where a reasonable estimate can be made and requires the completion of the accounting no later than one year from the date of enactment of the Act or December 22, 2018. We finalized our accounting for the effects of the Act in 2018 with no significant changes to provisional amounts recorded in 2017.
Accounting Pronouncements Not Yet Adopted
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842).” This ASU requires, among other things, a lessee to recognize assets and liabilities associated with the rights and obligations attributable to most leases but also recognize expenses similar to current lease accounting. This ASU also requires certain qualitative and quantitative disclosures designed to assess the amount, timing and uncertainty of cash flows arising from leases, along with additional key information about leasing arrangements. Originally, entities were required to adopt Leases (Topic 842) using a modified retrospective transition approach for all leases existing at, or entered into after, the date of initial application. However, in July 2018, the FASB issued ASU 2018-11, “Leases (Topic 842): Targeted Improvements,” which now allows entities the option of recognizing the cumulative effect of applying the new standard as an adjustment to the opening balance of retained earnings in the year of adoption while continuing to present all prior periods under previous lease accounting guidance. In July 2018, the FASB also issued ASU 2018-10, “Codification Improvements to Topic 842, Leases,” which clarifies how to apply certain aspects of ASU 2016-02. ASU 2016-02, ASU 2018-10 and ASU 2018-11 (now commonly referred to as ASC Topic 842 (ASC 842)) is effective for the Company’s fiscal year beginning January 1, 2019. The Company plans to elect the transition option provided under the ASU, which will not require adjustments to comparative periods nor require modified disclosures in those comparative periods. Upon adoption, the Company expects to elect the transition package of practical expedients permitted within the new standard, which among other things, allows the carryforward of the historical lease classification. Based on its anticipated election of practical expedients, the Company anticipates recognizing right of use assets and a lease liability of approximately $6.2 million related to its leases on its consolidated balance sheet as of January 1, 2019. The Company will apply this ASU and its related updates on a modified retrospective basis as of January 1, 2019. The impact on the consolidated statement of comprehensive income and the consolidated statement of cash flows is not expected to be material.
In June 2016, the FASB issued ASU 2016‑13, “
Financial Instruments—Credit Losses Topic 326”
. The guidance eliminates the probable initial recognition threshold that was previously required prior to recognizing a credit loss on financial instruments. The credit loss estimate can now reflect an entity’s current estimate of all future expected credit losses. Under the previous guidance, an entity only considered past events and current conditions. The guidance is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years. Early adoption is permitted for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. The adoption of certain amendments of this guidance must be applied on a modified retrospective basis and the adoption of the remaining amendments must be applied on a prospective basis. The Company is currently evaluating the impact of this guidance although we do not believe the impact of adoption will be material.
In February 2018, the FASB issued ASU 2018-02, “Income Statement - Reporting Comprehensive Income (Topic 220), Reclassification of Certain Tax Effects from Accumulated Other Comprehensive Income.” This ASU gives entities the option to reclassify the disproportionate income tax effects caused by the Act from accumulated other comprehensive income to retained earnings. The update also requires new disclosures, some of which are applicable for all entities. The guidance in ASU 2018-02 is effective for annual reporting periods beginning after December 15, 2018, with early adoption permitted. The Company is currently evaluating the impact of this ASU on its consolidated financial statements and the timing of adoption although we do not believe the impact of adoption will be material.
In August 2018, the FASB issued ASU 2018-13, “Fair Value Measurement (Topic 820), Disclosure Framework – Changes to the Disclosure Requirements for Fair Value Measurement.” This ASU eliminates, adds, and modifies certain disclosure requirements for fair value measurements. Entities will no longer be required to disclose the amount of and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, but public companies will be required to disclose the range and weighted average used to develop significant unobservable inputs for Level 3 fair value measurements. The guidance in ASU 2018-13 is effective for annual reporting periods beginning after December 15, 2019, with early adoption permitted. The Company is currently evaluating the impact of this ASU on its consolidated financial statements and the timing of adoption although we do not believe the impact of adoption will be material
.
In August 2018, the FASB issued ASU 2018-15, “Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract.” This ASU requires implementation costs incurred by customers in cloud computing arrangements to be deferred over the non-cancellable term of the cloud computing arrangements plus any optional renewal periods (1) that are reasonably certain to be exercised by the customer or (2) for which exercise of the renewal option is controlled by the cloud service provider. The guidance in ASU 2018-15 is effective for annual reporting periods beginning after December 15, 2019, with early adoption permitted. The Company is currently evaluating the impact of this ASU on its consolidated financial statements and the timing of adoption although we do not believe the impact of adoption will be material.
3. Investments
Our investments consisted of the following as of December 31, 2018 and 2017:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2018
|
|
|
|
|
|
|
Gross
|
|
Gross
|
|
|
|
|
|
|
Amortized
|
|
unrealized
|
|
unrealized
|
|
|
Estimated
|
|
(in $000's)
|
|
cost
|
|
gains
|
|
losses
|
|
|
fair Value
|
|
U.S. government agencies
|
|
$
|
2,117
|
|
$
|
—
|
|
$
|
(56)
|
|
$
|
2,061
|
|
Obligations of government sponsored enterprises(1)
|
|
|
892
|
|
|
—
|
|
|
(24)
|
|
|
868
|
|
Corporate debt securities
|
|
|
4,541
|
|
|
—
|
|
|
(153)
|
|
|
4,388
|
|
Foreign government bonds
|
|
|
984
|
|
|
—
|
|
|
(25)
|
|
|
959
|
|
Total (2)
|
|
$
|
8,534
|
|
$
|
—
|
|
$
|
(258)
|
|
$
|
8,276
|
|
|
(1)
|
|
Includes investments in notes issued by the Federal Home Loan Bank and the Federal Farm Credit Bank.
|
|
|
(2)
|
|
During the year ended December 31, 2018, investments were sold or matured which resulted in net proceeds of $16.7 million and realized losses of $0.2 million. Investments were sold in order to provide the funds necessary in cash and cash equivalents for the payment of the settlement of the securities class action lawsuit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017
|
|
|
|
|
|
|
Gross
|
|
Gross
|
|
|
|
|
|
|
Amortized
|
|
unrealized
|
|
unrealized
|
|
|
Estimated
|
|
(in $000's)
|
|
cost
|
|
gains
|
|
losses
|
|
|
fair Value
|
|
U.S. government agencies
|
|
$
|
6,077
|
|
$
|
—
|
|
$
|
(73)
|
|
$
|
6,004
|
|
Obligations of government sponsored enterprises(1)
|
|
|
3,737
|
|
|
—
|
|
|
(31)
|
|
|
3,706
|
|
Corporate debt securities
|
|
|
12,479
|
|
|
21
|
|
|
(66)
|
|
|
12,434
|
|
Foreign government bonds
|
|
|
2,689
|
|
|
—
|
|
|
(26)
|
|
|
2,663
|
|
Total
|
|
$
|
24,982
|
|
$
|
21
|
|
$
|
(196)
|
|
$
|
24,807
|
|
|
(1)
|
|
Includes investments in notes issued by the Federal Home Loan Bank and the Federal Farm Credit Bank.
|
Unrealized losses on all fixed maturity investments in a continuous loss position for more than twelve consecutive months were $0.3 million and $0.2 million as of December 31, 2018 and 2017, respectively. Unrealized losses on all fixed maturity investments in a continuous loss position for less than twelve consecutive months were not material as of December 31, 2018 and 2017. As of December 31, 2018 and 2017, there were no material unrealized losses that the Company believed to be other-than-temporary. The following table summarizes maturities of our investments available‑for‑sale as of December 31, 2018:
|
|
|
|
|
|
|
|
|
|
|
December 31, 2018
|
|
(in $000’s)
|
|
Cost
|
|
Fair Value
|
|
Maturities:
|
|
|
|
|
|
|
|
Within 1 year
|
|
$
|
1,366
|
|
$
|
1,323
|
|
After 1 year through 5 years
|
|
|
7,168
|
|
|
6,953
|
|
Total investments available for sale
|
|
$
|
8,534
|
|
$
|
8,276
|
Realized losses, net of investment income were $0.2 million and $2.0 million, for the years ended December 31, 2018 and 2017, respectively, and have been included as a component of “Other income (expense) net,” in the accompanying consolidated statements of comprehensive income. Realized losses for the year ended December 31, 2017 includes losses on sale of Mesoblast Limited common stock of $1.9 million, which was classified as a trading security (see Note 4).
4. Discontinued Operations
In October 2013, we sold our therapeutics business, including Prochymal, a stem cell drug for treatment of graft versus host disease, and related assets, to Mesoblast International SARL (“Mesoblast”), a wholly‑owned subsidiary of Mesoblast Limited. The agreement with Mesoblast provided for the receipt of $50 million in initial consideration, which was received in 2013 and 2014, and up to an additional $50 million, plus royalties as described below, in contingent consideration. The additional $50.0 million of contingent consideration is based on Mesoblast achieving certain clinical and regulatory milestones
as follows:
|
|
|
|
|
|
Milestone
|
|
Amount
(in $000's)
|
|
First marketing authorization received in the U.S.
|
|
$
|
20,000
|
|
First marketing authorization received from France, Germany, or European Union
|
|
|
10,000
|
|
Receipt of final data for the Crohn's trial or first marketing approval for Crohn's
|
|
|
10,000
|
|
Completion of the enrollment of the Phase 3 Crohn's Trial or Mesoblast's election to discontinue the trial
|
|
|
10,000
|
|
Total contingent consideration
|
|
$
|
50,000
|
In 2017, we received contingent consideration of $10.0 million, for the completion of the Phase 3 Crohn’s trial, in Mesoblast Limited common stock (classified as a trading security when received) and $350 thousand in cash. We recorded income of $10.0 million, net of $0.3 million in income tax expense, in discontinued operations in the 2017 consolidated statement of comprehensive income. All of the Mesoblast Limited common stock was sold in 2017,
resulting in a loss of approximately $1.9 million, which was reported in other income (expense), net in our consolidated statement of comprehensive income for the year ended December 31, 2017.
The first three milestones listed in the table above totaling $40.0 million have not been met or earned yet and the Company has not recorded any income or asset related to these specific milestones in any period.
In addition, as part of the contingent consideration, we are entitled to earn royalties ranging from 4% to up to 10% on future sales by Mesoblast of specified products utilizing the acquired technology. The royalties are payable annually by Mesoblast to Osiris in cash. Royalties can be earned for 10 years beginning with the first commercial sale of specified products by Mesoblast.
In 2018, we received cash payments from Mesoblast of approximately $0.4 million for royalties from Mesoblast. In 2017, we received cash payments from Mesoblast of approximately $0.2 million for royalties from Mesoblast. Income from royalties is included in discontinued operations in the consolidated statements of comprehensive income.
5. Inventory, net
Inventory, net consists of the following:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
|
|
|
2018
|
|
2017
|
|
|
(in $000's)
|
|
|
|
|
|
|
|
|
Raw materials and supplies
|
|
$
|
1,049
|
|
$
|
1,330
|
|
|
Work-in-process
|
|
|
5,337
|
|
|
5,605
|
|
|
Finished goods
|
|
|
6,764
|
|
|
6,350
|
|
|
|
|
|
13,150
|
|
|
13,285
|
|
|
Reserve for excess and obsolete inventory
|
|
|
(3,536)
|
|
|
(2,007)
|
|
|
Inventory, net
|
|
$
|
9,614
|
|
$
|
11,278
|
|
Work‑in‑process inventory is largely product that is in quarantine pending completion of our quality assurance procedures. Finished goods inventory includes gross consigned inventory of $1.7 million and $1.
5
million as of December 31, 2018 and 2017, respectively. The reserves for excess and obsolete inventory includes $0.5 million and $0.6 million for consigned finished goods inventory as of December 31, 2018 and 2017, respectively.
6. Property and Equipment, net
Property and equipment, net consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciable Life
|
|
Year Ended December 31,
|
|
|
(in $000's)
|
|
(in years)
|
|
2018
|
|
2017
|
|
|
Laboratory and manufacturing equipment
|
|
3-7
|
|
$
|
2,792
|
|
$
|
3,083
|
|
|
Computer hardware, furniture and fixtures
|
|
3-7
|
|
|
921
|
|
|
1,134
|
|
|
Leasehold improvements
|
|
(A)
|
|
|
6,359
|
|
|
6,344
|
|
|
|
|
|
|
|
10,072
|
|
|
10,561
|
|
|
Accumulated depreciation and amortization
|
|
|
|
|
(7,190)
|
|
|
(6,974)
|
|
|
Property and equipment, net
|
|
|
|
$
|
2,882
|
|
$
|
3,587
|
|
|
|
(A)
|
|
Shorter of economic life or lease term.
|
Depreciation expense was $0.9 million and $0.
7
million for the years ended December 31, 2018 and 2017, respectively.
7. Share‑Based Compensation and Employee Benefit Plans
In April 2006, we adopted our 2006 Omnibus Plan. We amended and restated this plan in 2008, 2010, 2012 and 2014, in each case to, among other things, increase the number of shares available for grant (the “Amended and Restated 2006 Omnibus Plan”). As a result of the 2014 amendment, the Amended and Restated 2006 Omnibus Plan has a termination date of May 2024. The plan authorizes the issuance of various forms of stock‑based awards, including incentive and non‑qualified stock options, stock purchase rights, stock appreciation rights and restricted and unrestricted stock awards. A total of 3,000,000 shares of our common stock have been reserved for issuance under the Amended and Restated 2006 Omnibus Plan. At December 31, 2018, there were 1,454,334 shares available for future awards under the Amended and Restated 2006 Omnibus Plan.
A summary of stock option activity for the years ended December 31, 2018 and 2017 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
Weighted Average
|
|
Aggregate
|
|
|
|
Number of
|
|
Average
|
|
Remaining
|
|
Intrinsic
|
|
|
|
Options
|
|
Exercise
|
|
Contractual
|
|
Value(1)
|
|
|
|
(in 000’s)
|
Price
|
|
Life (in years)
|
|
(in $000’s)
|
|
Outstanding at December 31, 2016
|
|
840
|
|
|
12.33
|
|
|
|
|
|
|
Granted
|
|
109
|
|
|
6.77
|
|
|
|
|
|
|
Exercised
|
|
(25)
|
|
|
5.14
|
|
|
|
|
|
|
Forfeited or cancelled
|
|
(258)
|
|
|
13.13
|
|
|
|
|
|
|
Expired
|
|
(43)
|
|
|
7.83
|
|
|
|
|
|
|
Outstanding at December 31, 2017
|
|
623
|
|
|
11.63
|
|
|
|
|
|
|
Granted
|
|
110
|
|
|
9.18
|
|
|
|
|
|
|
Exercised
|
|
(1)
|
|
|
8.90
|
|
|
|
|
|
|
Forfeited or cancelled
|
|
(127)
|
|
|
10.03
|
|
|
|
|
|
|
Expired
|
|
(6)
|
|
|
17.27
|
|
|
|
|
|
|
Outstanding at December 31, 2018
|
|
599
|
|
$
|
11.46
|
|
5.6
|
|
$
|
1,760
|
|
Vested at December 31, 2018
|
|
477
|
|
$
|
12.03
|
|
4.9
|
|
$
|
1,181
|
|
Vested and expected to vest at December 31, 2018
|
|
520
|
|
$
|
11.96
|
|
5.5
|
|
$
|
1,334
|
|
|
(1)
|
|
The aggregate intrinsic value is based upon the difference between the Company’s closing stock price at the date of the Consolidated Balance Sheet and the exercise price of the stock option for in‑the‑money stock options. The intrinsic value of outstanding stock options fluctuates based upon the trading value of the Company’s Common stock.
|
The weighted‑average grant date fair value of stock options granted during the years ended December 31, 2018, and 2017 was $6.03
and
$4.42, respectively. The total fair value of shares vested during the years ended December 31, 2018 and 2017 was $0.7 million and $1.0 million, respectively.
Options outstanding at December 31, 2018 are summarized below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding
|
|
Options Exercisable
|
|
|
|
|
|
Weighted Average
|
|
Weighted
|
|
|
|
Weighted
|
|
|
|
Number
|
|
Remaining
|
|
Average
|
|
Number
|
|
Average
|
|
|
|
Outstanding
|
|
Contractual
|
|
Exercise
|
|
Outstanding
|
|
Exercise
|
|
Range of Exercise Prices
|
|
(in 000’s)
|
Life (in years)
|
|
Price
|
|
(in 000’s)
|
Price
|
|
$4.01-$7.00
|
|
119
|
|
6.4
|
|
$
|
6.25
|
|
70
|
|
$
|
5.86
|
|
$7.25-$12.75
|
|
196
|
|
5.4
|
|
|
8.99
|
|
136
|
|
|
8.82
|
|
$12.76-$16.00
|
|
160
|
|
5.5
|
|
|
14.12
|
|
159
|
|
|
14.10
|
|
$16.01-$18.40
|
|
124
|
|
5.5
|
|
|
16.94
|
|
112
|
|
|
16.84
|
|
|
|
599
|
|
5.6
|
|
|
11.46
|
|
477
|
|
|
12.03
|
We estimated the fair value of stock options using the Black‑Scholes option‑pricing model. Fair value is amortized on an accelerated basis over the requisite service periods of the awards. We generally issue stock option
awards that vest over four years and have a ten-year contractual life. The fair value of stock options granted during each of the years was estimated using the following weighted‑average assumptions:
|
|
|
|
|
|
|
|
|
|
Year ended
|
|
|
|
|
December 31,
|
|
|
|
|
2018
|
|
2017
|
|
|
Interest rate
|
|
2.7
|
%
|
2.0
|
%
|
|
Dividend yield
|
|
0.0
|
%
|
0.0
|
%
|
|
Term (in years)
|
|
6.2
|
|
6.2
|
|
|
Volatility
|
|
71.0
|
%
|
71.9
|
%
|
Interest Rate
—The risk‑free interest rate was determined using the yield available for zero‑coupon United States government issues with a remaining term approximating the expected life of the options.
Dividend
—We do not expect to pay a dividend in the foreseeable future, and as such, the expected dividend yield is zero.
Term
—Since the Company has limited option exercise history, it has generally elected to estimate the expected life of an award based upon the SEC‑approved “simplified method” noted under the provisions of Staff Accounting Bulletin No. 107 with the continued use of this method extended under the provisions of Staff Accounting Bulletin No. 110.
Volatility
—Expected volatility is based on the historical volatility of the Company’s common stock, which we believe will be indicative of future experience.
The table below reflects the total share‑based compensation expense (benefit) recognized in our consolidated statements of comprehensive income for the years ended December 31, 2018 and 2017. Share‑based compensation benefit is the result of the cancellation of option awards prior to vesting for which compensation expense had previously been recorded.
|
|
|
|
|
|
|
|
|
|
|
Year ended
|
|
|
|
December 31,
|
|
(in $000’s)
|
|
2018
|
|
2017
|
|
Cost of product revenue
|
|
$
|
19
|
|
$
|
51
|
|
Sales and marketing
|
|
|
178
|
|
|
(17)
|
|
Research and development
|
|
|
94
|
|
|
123
|
|
General and administrative
|
|
|
(14)
|
|
|
(58)
|
|
Total
|
|
$
|
277
|
|
$
|
99
|
As of December 31, 2018, there was $0.1 million of total unrecognized compensation expense related to non‑vested stock options, which is expected to be recognized over a weighted‑average period of 1.5 years. We estimate expected forfeitures and recognize expense only for those option grants expected to vest. Our estimate of expected forfeitures may be adjusted throughout the requisite service period based on the extent to which actual forfeitures differ, or are likely to differ, from our previous estimates. At the end of the service period, compensation cost will have been recognized only for those awards for which the employee has provided the requisite service.
401(k) Plan
We sponsor a 401(k) plan. We match employee contributions based upon the amount of the employees’ contributions, subject to certain limitations and vesting requirements, and we may make a discretionary contribution. Company matching contributions to the 401(k) plan totaled $0.
5
million and $0.4 million for the years ended December 31, 2018 and 2017, respectively. There were no discretionary contributions made in 2018 or 2017.
8
. Net Income (Loss) Per Share from Continuing Operations
Basic net earnings per share (“EPS”) is calculated by dividing net income by the weighted average number of common shares outstanding for the applicable period. Diluted net EPS is computed based on the weighted average number of common shares outstanding increased by the number of additional shares that would have been outstanding had the potentially dilutive common shares been issued and reduced by the number of shares the Company could have repurchased with the proceeds from the issuance of the potentially dilutive shares.
|
|
|
|
|
|
|
|
|
|
|
Year ended
|
|
|
|
December 31,
|
|
|
|
2018
|
|
2017
|
|
Computation of net income (loss) per share from continuing operations
|
|
|
|
|
|
|
|
Net income (loss) from continuing operations
|
|
$
|
36,533
|
|
$
|
(1,236)
|
|
Shares used in computation:
|
|
|
|
|
|
|
|
Weighted-average shares outstanding
|
|
|
34,526
|
|
|
34,524
|
|
Basic earnings (loss) per share from continuing operations
|
|
$
|
1.06
|
|
$
|
(0.04)
|
|
|
|
|
|
|
|
|
|
Diluted earnings (loss) per share:
|
|
|
|
|
|
|
|
Net income (loss) from continuing operations
|
|
$
|
36,533
|
|
$
|
(1,236)
|
|
Shares used in computation:
|
|
|
|
|
|
|
|
Weighted-average shares outstanding
|
|
|
34,526
|
|
|
34,524
|
|
Weighted-average share equivalents from stock options
|
|
|
47
|
|
|
—
|
|
Weighted-average shares and share equivalents outstanding
|
|
|
34,573
|
|
|
34,524
|
|
Diluted earnings (loss) per share from continuing operations
|
|
$
|
1.06
|
|
$
|
(0.04)
|
Dilutive earnings per share for the years ended December 31, 2018 and 2017 excludes 363,219 and 654,564 of our outstanding options as of that date, respectively, as their impact on our net income per share is anti-dilutive.
Included in the calculation of diluted net income per share from discontinued operations are
46,849
and
1,012
potentially dilutive securities for the year ended December 31, 2018 and 2017, respectively.
9. Financial Instruments and Fair Value
Fair value is defined as the price at which an asset could be exchanged or a liability transferred (an exit price) in an orderly transaction between knowledgeable, willing parties in the principal or most advantageous market for the asset or liability. Where available, fair value is based on observable market prices or parameters or derived from such prices or parameters. Where observable prices or inputs are not available, valuation models are applied.
Financial assets recorded at fair value in the accompanying financial statements are categorized based upon the level of judgment associated with the inputs used to measure their fair value. The levels are directly related to the amount of subjectivity associated with the inputs to fair valuation of these assets and liabilities, and are as follows:
|
|
|
|
Level 1
|
Inputs are unadjusted, quoted prices in active markets for identical assets at the reporting date. Active markets are those in which transactions for the asset or liability occur in sufficient frequency and volume to provide pricing information on an ongoing basis.
|
|
Level 2
|
Inputs are other than quoted prices included in Level 1, which are either directly or indirectly observable for the asset or liability through correlation with market data at the reporting date and for the duration of the instrument’s anticipated life.
|
|
Level 3
|
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities and which reflect management’s best estimate of what market participants would use in pricing the asset or liability at the reporting date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
|
When quoted prices in active markets for identical assets are available, we use these quoted market prices to determine the fair value of financial assets and classify these assets as Level 1. In other cases where a quoted market price for identical assets in an active market is either not available or not observable, we obtain the fair value from a third‑party vendor that uses pricing models, such as matrix pricing, to determine fair value. These financial assets would then be classified as Level 2. In the event quoted market prices were not available, we would determine fair value using broker quotes or an internal analysis of each investment’s financial statements and cash flow projections. In these instances, financial assets would be classified based upon the lowest level of input that is significant to the valuation. Thus, financial assets might be classified in Level 3 even though there could be some significant inputs that may be readily available.
Assets and liabilities measured at fair value on a recurring basis are summarized below as of December 31, 2018 and 2017:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2018
|
|
(in $000's)
|
|
Level I
|
|
Level II
|
|
Level III
|
|
Total
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investments: Available for Sale Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government agencies
|
|
$
|
—
|
|
$
|
2,061
|
|
$
|
—
|
|
$
|
2,061
|
|
Obligations of government sponsored enterprises
|
|
|
—
|
|
|
868
|
|
|
—
|
|
|
868
|
|
Corporate debt securities
|
|
|
—
|
|
|
4,388
|
|
|
—
|
|
|
4,388
|
|
Foreign government bonds
|
|
|
—
|
|
|
959
|
|
|
—
|
|
|
959
|
|
Total investments
|
|
$
|
—
|
|
$
|
8,276
|
|
$
|
—
|
|
$
|
8,276
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2017
|
|
(in $000's)
|
|
Level I
|
|
Level II
|
|
Level III
|
|
Total
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Investments: Available for Sale Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government agencies
|
|
$
|
—
|
|
$
|
6,004
|
|
$
|
—
|
|
$
|
6,004
|
|
Obligations of government sponsored enterprises
|
|
|
—
|
|
|
3,706
|
|
|
—
|
|
|
3,706
|
|
Corporate debt securities
|
|
|
—
|
|
|
12,434
|
|
|
—
|
|
|
12,434
|
|
Foreign government bonds
|
|
|
—
|
|
|
2,663
|
|
|
—
|
|
|
2,663
|
|
Total investments
|
|
$
|
—
|
|
$
|
24,807
|
|
$
|
—
|
|
$
|
24,807
|
For the years ended December 31, 2018 and 2017 the Company did not transfer any assets between fair value levels.
The carrying value of financial instruments, including trade receivables, accounts payable, and accrued liabilities approximate fair value because of the short‑term maturity of these items. The estimated fair values may not represent actual values of the financial instruments that could be realized as of the balance sheet date or that will be realized in the future.
All of the Company’s investments at each year end are classified as “available‑for‑sale” and are carried at fair value.
10. Income Taxes
The benefit and expense for income taxes consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended December 31,
|
|
(in $000's)
|
|
2018
|
|
2017
|
|
|
Current:
|
|
|
|
|
|
|
|
|
Federal
|
|
$
|
(1,041)
|
|
$
|
(1,526)
|
|
|
State
|
|
|
208
|
|
|
128
|
|
|
Total current benefit
|
|
|
(833)
|
|
|
(1,398)
|
|
|
Deferred:
|
|
|
|
|
|
|
|
|
Federal
|
|
|
(23,936)
|
|
|
—
|
|
|
State
|
|
|
(1,236)
|
|
|
—
|
|
|
Total deferred benefit
|
|
|
(25,172)
|
|
|
—
|
|
|
Income tax benefit
|
|
$
|
(26,005)
|
|
$
|
(1,398)
|
|
A reconciliation of the federal statutory rate to the effective income tax rate is as follows:
|
|
|
|
|
|
|
|
|
|
Year ended December 31,
|
|
|
|
2018
|
|
2017
|
|
|
Federal income tax rate
|
|
21.0
|
%
|
35.0
|
%
|
|
State income taxes
|
|
6.1
|
%
|
0.9
|
%
|
|
Nondeductible expenses
|
|
3.3
|
%
|
(16.2)
|
%
|
|
Stock-based compensation
|
|
0.4
|
%
|
(2.7)
|
%
|
|
Valuation allowance
|
|
182.9
|
%
|
220.2
|
%
|
|
Credits
|
|
(460.0)
|
%
|
2.1
|
%
|
|
Change in tax rate
|
|
(1.2)
|
%
|
(184.8)
|
%
|
|
Other
|
|
0.6
|
%
|
(1.4)
|
%
|
|
Effective tax rate
|
|
(246.9)
|
%
|
53.1
|
%
|
The components of our net deferred tax assets and liabilities at December 31 are as follows:
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
(in $000’s)
|
|
2018
|
|
2017
|
|
Deferred tax assets arising from:
|
|
|
|
|
|
|
|
Tax credits and net operating losses
|
|
$
|
48,777
|
|
$
|
528
|
|
Inventory adjustments
|
|
|
1,142
|
|
|
2,114
|
|
Accrued expenses and reserves
|
|
|
3,320
|
|
|
7,449
|
|
Property and equipment
|
|
|
463
|
|
|
488
|
|
Stock compensation
|
|
|
87
|
|
|
64
|
|
Deferred rent
|
|
|
508
|
|
|
265
|
|
Deferred revenue
|
|
|
—
|
|
|
316
|
|
Other
|
|
|
252
|
|
|
197
|
|
Deferred tax assets
|
|
|
54,549
|
|
|
11,421
|
|
Less: valuation allowance
|
|
|
(29,344)
|
|
|
(10,212)
|
|
Net deferred tax assets
|
|
|
25,205
|
|
|
1,209
|
|
Deferred tax liabilities arising from:
|
|
|
|
|
|
|
|
Insurance receivable
|
|
|
—
|
|
|
(1,209)
|
|
Net deferred tax asset (liability)
|
|
$
|
25,205
|
|
$
|
—
|
As of the date of the filing of the 2017 Form 10-K, we were in the process of obtaining documentation and support for carryforward orphan drug credits. We did not include the orphan drug credit (“the Credits”) in our deferred tax assets as of December 31, 2017 due to the lack of support and instead the potential for $71.3 million in credits was disclosed. During 2018, we performed a detailed study to recalculate the value of the Credits based on available
documentation and support, which resulted in a deferred tax asset of $45.0 million, net of a reserve of $3.0 million as of December 31, 2018. The net impact of all of the changes to the orphan drug credit resulted in an additional $11.4 million of federal and state net operating losses and $1.0 million of additional alternative minimum tax credit carryforward.
We recognized a tax benefit of $26.0 million for the year ended December 31, 2018. This benefit primarily relates to the partial release of a valuation allowance and the recognition of certain general business credits. As of December 31, 2018 and 2017, our net income tax receivable was $2.8 million and $2.0 million, respectively, which were recorded in prepaid expenses and other current assets in the consolidated balance sheets. In addition, the Company has an AMT credit carryforward that is now refundable under the Tax Act of $1.3 million and $1.5 million that is recorded in other assets on the consolidated balance sheets as of December 31, 2018 and 2017, respectively.
As of December 31, 2018, we had federal operating loss carryforwards of $14.1 million and credits of $45.6 million expiring beginning in 2022 and state operating loss carryforwards and credits of $228 thousand, net of federal benefit, expiring beginning in 2019.
As of December 31, 2017, we maintained a full valuation allowance on our net deferred tax assets of $10.2 million, reducing the carrying value of these assets on our balance sheet to zero. As of December 31, 2018, we had gross deferred tax assets of $54.5 million comprised of $45.6 million and $3.1 million related to general business credits and net operating losses, respectively. The remaining $5.8 million relates to various timing differences that represent amounts that we are able to use to reduce future taxable income.
We assess the need for a valuation allowance on an ongoing basis. This assessment process can be highly judgmental and requires us to consider all available positive and negative evidence when evaluating if it is more likely than not we will be able to realize the benefit of the deferred tax assets in the future. Such evidence consists of reversing temporary differences, and projected future income. Realization of our deferred tax assets is dependent upon our ability to generate sufficient taxable income in future periods. Although we believe it is more likely than not that future taxable income will be sufficient to allow us to recover some of the value of our deferred tax assets, realization is not assured and future events could cause us to change our judgment. In the event that actual results differ from our estimates, or we adjust these estimates in the future periods, further adjustments to our valuation allowance may be recorded, which could materially impact our financial position and net income (loss) in the period of the adjustment.
In light of the fact that the majority of our general business credits expire in 2026-2029, a key consideration in our analysis was the projection of future taxable income and the ability to utilize these credits. In performing our analysis, we projected future taxable income and the expected usage of our general business credits. The result of this analysis is that $29.2 million of general business credits are expected to expire unused, which represents the majority of the $29.3 million valuation allowance that remains as of December 31, 2018.
Utilization of the net operating losses and credit carryforwards may be subject to an annual limitation due to the ownership change limitations provided by the Internal Revenue Code of 1986, as amended, and similar state provisions. We have not performed a detailed analysis to determine whether an ownership change under Section 382 of the Internal Revenue Code occurred. The effect of an ownership change would be the imposition of an annual limitation on the use of credits attributable to periods before the change and could result in a reduction in the total credits available.
We file income tax returns in the United States and various state and local jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment to apply. We had tax net operating losses and credit carryforwards that are subject to examination for a number of years beyond the year in which they are generated for tax purposes. Since a portion of these carryforwards may be utilized in the future, many of these attribute carryforwards remain subject to examination. We remain subject to examinations by these jurisdictions for years 2006-2018.
Our policy is to recognize interest and penalties related to income tax matters in income tax expense. Interest and penalties related to uncertain tax positions of $41 thousand and $34 thousand were recognized during the years ended December 31, 2018 and 2017, respectively related to state tax nexus issues. As of December 31, 2018 and 2017, accrued interest and penalties were $232 thousand and $176 thousand, respectively, and are recorded as other long term
liabilities on the consolidated balance sheets. As of December 31, 2018, our total unrecognized tax benefits would favorably affect our effective tax rate if recognized.
The beginning balance of unrecognized tax benefits mainly consists of the Credits in the amount of $71.3 million. As a result of the study performed, the Credits have a reduced net value of $45.0 million and have moved out of uncertain tax positions and is reported as a deferred tax asset. A reserve of $6.2 million remains in uncertain tax positions related to Credits. The aggregate change in the balance of unrecognized tax benefits, and which excludes interest and penalties is as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31,
|
|
|
|
2018
|
|
2017
|
|
Balance, beginning of year
|
|
$
|
71,754
|
|
$
|
71,665
|
|
Increase in current year position
|
|
|
8
|
|
|
89
|
|
Increase in prior year position
|
|
|
11
|
|
|
—
|
|
Decrease in prior year position
|
|
|
(65,093)
|
|
|
—
|
|
Settlements of prior year position
|
|
|
(22)
|
|
|
—
|
|
Balance, end of year
|
|
$
|
6,658
|
|
$
|
71,754
|
11. Commitments and Contingencies
Leases
We lease facilities and equipment under various non‑cancellable operating lease agreements expiring through 2023. Rent and associated expense was $1.2 million and $1.4 million for the years ended December 31, 2018 and 2017, respectively.
At December 31, 2018, the Company’s minimum obligations under non‑cancellable operating leases are as follows:
|
|
|
|
|
|
(in $000's)
|
|
|
|
|
2019
|
|
$
|
1,270
|
|
2020
|
|
|
1,282
|
|
2021
|
|
|
1,292
|
|
2022
|
|
|
1,312
|
|
2023
|
|
|
1,117
|
|
Thereafter
|
|
|
—
|
|
Total
|
|
$
|
6,273
|
Legal
The Company is party to outstanding legal proceedings, investigations and claims as described below. The Company cannot predict with any certainty the final outcome of any legal proceedings, investigations (including any settlement discussions with the government seeking to resolve such investigations) or claims made against it as described in the paragraphs below, and there can be no assurance that the ultimate resolution of any such matter will not have a material adverse impact on the Company’s consolidated financial position, results of operations, or cash flows.
The Company records accruals for certain outstanding legal proceedings, investigations or claims when it is probable that a liability will be incurred and the amount of the loss can be reasonably estimated. The Company evaluates, on a quarterly basis, developments in legal proceedings, investigations and claims that could affect the amount of any accrual, as well as any developments that would make a loss contingency both probable and reasonably estimable. When a loss contingency is not both probable and reasonably estimable, the Company does not accrue the loss. However, if the loss (or an additional loss in excess of the accrual) is at least a reasonable possibility and material, then
the Company discloses a reasonable estimate of the possible loss or range of loss, if such reasonable estimate can be made. If the Company cannot make a reasonable estimate of the possible loss, or range of loss, then that is disclosed.
The assessments of whether a loss is probable or a reasonable possibility, and whether the loss or range of loss is reasonably estimable, often involve a series of complex judgments about future events. Among the factors that the Company considers in this assessment are the nature of existing legal proceedings, investigations and claims, the asserted or possible damages or loss contingency (if reasonably estimable), the progress of the matter, existing law and precedent, the opinions or views of legal counsel and other advisers, the involvement of the U.S. Government and its agencies in such proceedings, the Company’s experience in similar matters and the experience of other companies, the facts available to the Company at the time of assessment, and how the Company intends to respond, or has responded, to the proceeding, investigation or claim. The Company’s assessment of these factors may change over time as individual proceedings, investigations or claims progress. For matters where the Company is not currently able to reasonably estimate the range of reasonably possible loss, the factors that have contributed to this determination include the following: (i) the damages sought are indeterminate, or an investigation has not manifested itself in a filed civil or criminal complaint, (ii) the matters are in the early stages, (iii) the matters involve novel or unsettled legal theories or a large or uncertain number of actual or potential cases or parties, and/or (iv) discussions with the government or other parties in matters that may be expected ultimately to be resolved through negotiation and settlement have not reached the point where the Company believes a reasonable estimate of loss, or range of loss, can be made. In such instances, the Company believes that there is considerable uncertainty regarding the timing or ultimate resolution of such matters, including a possible eventual loss, fine, penalty or business impact, if any.
In addition, the Company does not accrue for estimated legal fees and other directly related costs as they are expensed as incurred.
In addition to the matters described in the paragraphs below, in the normal course of its business, the Company is involved in various lawsuits from time to time and may be subject to certain other contingencies, none of which we believe to be material.
Based on our analysis and assessment as described above, including consultation with our legal counsel, management believes there are no matters that are probable or reasonably possible that require accrual or disclosure, except for the matters described below.
Securities Class Action
On November 23, 2015, a putative class action lawsuit was filed in the United States District Court for the District of Maryland by a single plaintiff, individually and on behalf of other persons similarly situated, against the Company and three current or former executive officers of the Company. An amended complaint clarifying plaintiff's claims was filed on April 6, 2018. The action, captioned Kiran Kumar Nallagonda v. Osiris Therapeutics, Inc. et al., Case 1:15-cv-03562 (the “Nallagonda Action”), alleged, among other things, that the defendants made materially false or misleading statements and material omissions in the Company’s filings with the SEC in violation of the federal securities laws. The complaint sought certification as a class action, unspecified damages and reimbursement of attorneys’ fees. On March 21, 2016, the court entered an order appointing Dr. Raffy Mirzayan as lead plaintiff and the firm of Hagens Berman Sobol Shapiro LLP as lead counsel. On March 11, 2018, we entered into a memorandum of understanding to settle the Nallagonda Action. Subsequently, on June 5, 2018, the parties executed a Stipulation and Settlement Agreement in which the Company agreed in principle to pay $18.5 million in cash to create a settlement fund for the benefit of class members. On June 12, 2018, the lead plaintiff filed an Unopposed Motion for Preliminary Approval of the parties’ settlement. On September 4, 2018, the Court entered an order preliminarily approving the settlement and scheduling a hearing for February 4, 2019 to determine whether the proposed settlement is fair, reasonable and adequate and whether the case should therefore be dismissed with prejudice. On October 3, 2018, the Company deposited the $18.5 million settlement payment into an escrow account, pending final Court approval of the settlement. On November 11, 2018, the lead plaintiff filed a request for an award of attorneys’ fees and expenses from the settlement fund. At a February 4, 2019 hearing, the Court found the settlement fair, reasonable and adequate and therefore dismissed the case with prejudice in its order of that date.
The Company had a $5.0 million executive and corporate securities liability insurance policy in place at the time of the allegations. The Company received the remaining $4.8 million of unused policy coverage for the shareholder settlement of the Nallagonda Action on October 9, 2018 which was recorded as Insurance receivable in the consolidated balance sheets as of December 31, 2017.
Shareholder Derivative Actions
On March 2, 2016, a shareholder derivative complaint was filed in the Circuit Court for Howard County in the State of Maryland (Case No. 13C16106811) by a single plaintiff, derivatively and on behalf of the Company, against certain current and former directors and certain former executive officers (the “Connelley Action”). This action, captioned Kevin Connelley v. Lode Debrabandere et al., alleges that each of the individual directors and officers named as defendants (i) violated their fiduciary duties to the Company’s shareholders; (ii) abused their ability to control and influence the Company; (iii) engaged in gross mismanagement of the assets and business of the Company; and (iv) was unjustly enriched at the expense of, and to the detriment of, the Company. The alleged claims generally relate to the matters that are the subject of the Nallagonda Action. The plaintiff seeks, among other things, unspecified monetary damages, reimbursement of attorneys’ fees and shareholder votes on amendments to the Company’s Articles of Incorporation and Bylaws with respect to various corporate governance policies. On June 2, 2016, the Court entered an order that, subject to certain qualifications, stayed the action until 30 days after the entry of an order either: (1) denying all motions to dismiss in the Nallagonda Action, or (2) finally dismissing the Nallagonda Action with prejudice. On March 12, 2018, the plaintiff filed an amended complaint clarifying his claims. On or about July 31, 2018, the parties advised the Court that the Company and the plaintiffs in various derivative actions, including the plaintiff in the Connelley Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below).
On February 9, 2017, a shareholder derivative complaint was filed in the United States District Court for the District of Maryland (Case No. 1:17-cv-00381-JKB) by a single plaintiff, derivatively and on behalf of the Company, against certain current and former directors (the “Recupero Action”). This action, captioned Recupero v. Friedli et al., alleges, among other things, that each of the individual directors named as defendants (i) violated their fiduciary duties to the Company’s shareholders, including that such violations constituted constructive fraud; (ii) engaged in gross mismanagement of the assets and business of the Company; and (iii) was unjustly enriched at the expense of, and to the detriment of, the Company. The plaintiff seeks, among other things, unspecified monetary damages, reimbursement of attorneys’, accountants’ and experts’ fees, and that the Company take all necessary actions to improve and comply with corporate governance, internal procedures and existing laws. On March 28, 2017, the Court entered an order that stays the action until: (1) the Nallagonda Action is dismissed with prejudice and all appeals relating thereto have been exhausted; (2) all motions to dismiss the Nallagonda Action are denied; or (3) either party provides 30 days’ notice that they no longer consent to a stay. On April 5, 2018, the plaintiff filed an amended complaint clarifying her claims. On July 31, 2018, the parties notified the Court that the Company and the plaintiffs in certain derivative actions, including the plaintiff in the Recupero Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below). On August 1, 2018, the Court stayed all further proceedings in the Recupero Action pending consideration of the proposed settlement by the Court in the Salley Action. On February 21, 2019, the parties filed a Stipulation of Dismissal with Prejudice, which is awaiting Court approval.
On May 11, 2017, a shareholder derivative complaint was filed in the Circuit Court for Howard County in the State of Maryland, (Case No. 13C17111441) by a single plaintiff, derivatively on behalf of the Company, against certain former executive officers and certain current and former directors (the “Lee Action”). This action, captioned Brian Lee v. Peter Friedli, et. al., alleges that each of the individual defendants violated their fiduciary duties by allegedly failing to adopt and implement adequate accounting and financial reporting systems and for allegedly causing the Company to make false and misleading statements regarding its financial condition. The alleged claims generally relate to the matters that are the subject of the Nallagonda Action and seek substantially similar relief. On September 5, 2017, the defendants moved to either stay or dismiss the plaintiffs’ complaint. That motion was subsequently withdrawn. On February 14, 2018, the plaintiff filed an amended derivative complaint. No defendant has responded to that pleading. On July 31, 2018, the parties advised the Court that the Company and the plaintiffs in various derivative actions, including the plaintiff in the Lee Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below). The Court subsequently denied the parties’ joint request for a stay of proceedings. Thereafter, on August 15, 2018, the parties filed a Joint Stipulation of Dismissal Without Prejudice and the Lee Action was closed.
On December 21, 2017, a shareholder derivative action was filed in the United States District Court for the District of Maryland (Case No. 1:17-cv-03777) by a single plaintiff, derivatively and on behalf of the Company, against certain former executive officers and certain current and former directors (the “Salley Action”). This action, captioned Todd Salley v. Lode Debrabandere, et. al., alleges that each of the individual defendants violated their fiduciary duties by failing to maintain adequate internal controls and by causing the Company to make false and misleading statements regarding the Company’s financial condition. The alleged claims generally relate to the matters that are the subject of the Nallagonda Action and seek substantially similar relief. No defendant has responded to the complaint. On July 31, 2018, the parties notified the Court that the Company and the plaintiffs in certain derivative actions, including the plaintiff in the Salley Action, had entered into a Memorandum of Understanding to resolve those derivative actions (see below). On August 1, 2018, the Court stayed all further proceedings in the Salley Action except for those related to the proposed settlement.
On October 24, 2018, the parties to the derivative actions entered into a settlement agreement to resolve those cases. The parties’ agreement called for the Company to adopt certain governance changes. Plaintiffs also sought Court approval of an award of attorneys’ fees and expenses in the amount of $0.9 million. We accrued $0.9 million as our estimated settlement cost in the consolidated balance sheets at December 31, 2018. On November 1, 2018, the United States District Court for the District of Maryland entered an order preliminarily approving the settlement agreement. Immediately after a final settlement hearing held on February 1, 2019, the District Court approved the settlement and dismissed the Salley Action.
Government Investigations
On November 2, 2017, the Company announced the resolution of an investigation by the SEC into the Company’s historical accounting practices. The Company agreed to settle with the SEC, without admitting or denying the allegations of the SEC, by consenting to the entry of a final judgment, subject to court approval, that permanently restrains and enjoins the Company from violating certain provisions of the federal securities laws. As part of the settlement, the Company paid a civil penalty in the amount of $1.5 million on November 9, 2017. This $1.5 million settlement was expensed by the Company in the fourth quarter of 2015. On November 7, 2017, this settlement was approved by the United States District Court for the District of Maryland, through entry of a final judgment, resolving as to the Company the matters alleged by the SEC in the civil complaint against the Company. The SEC civil case is continuing against four former Company officers.
The Company previously announced that a criminal investigation was being conducted by the U.S. Attorney’s Office for the Southern District of New York (“SDNY”) relating to matters that were also being investigated by the SEC. The SDNY investigation resulted in a former chief financial officer of the Company entering into a guilty plea with the government. As previously disclosed, based on communications the Company has had with the SDNY since that time and given that sentencing of the former company officer has now occurred, the Company believes that, subject to any newly discovered information, the SDNY has concluded the criminal investigation with respect to Company‑related matters.
The Company believes that both the previously disclosed SEC and SDNY investigations are concluded with respect to the Company.
12. Significant Distributors
We have historically provided credit in the normal course of business to contract counterparties and to the distributors of our products. Trade receivables in the accompanying consolidated balance sheets consist primarily of amounts due from distributors and end‑user customers of our products. The concentration of the Company’s business with a relatively small number of distributors may expose it to a material adverse effect if one or more of these large distributors were to experience financial difficulty or were to cease being a distributor for non‑financial related issues. The Company’s revenue concentrations through distributors of 10% or greater are as follows:
|
|
|
|
|
|
|
|
|
|
Percentage of Revenue
|
|
Distributor
|
|
2018
|
|
2017
|
|
|
A
|
|
19
|
%
|
21
|
%
|
The Company’s accounts receivable concentrations of 10% or greater are as follows:
|
|
|
|
|
|
|
|
|
|
Percentage of Receivables
|
|
|
|
|
December 31,
|
|
|
Distributor
|
|
2018
|
|
2017
|
|
|
A
|
|
24
|
%
|
24
|
%
|
13. Subsequent Events
On March 12, 2019, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Smith & Nephew plc, an English public limited liability company (“Parent Holdco”), Smith & Nephew Consolidated, Inc., a Delaware corporation (“Parent”), and Papyrus Acquisition Corp., a Maryland corporation and direct subsidiary of Parent, pursuant to which, and upon the terms and subject to the conditions described therein, Papyrus Acquisition Corp. will commence a cash tender offer (the “Offer”), to acquire all of the outstanding shares of the Company’s common stock at a purchase price of $19.00 per share (the “Offer Price”), in cash, without interest, subject to any required withholding of taxes. The Offer will commence no earlier than five (5) and no later than fifteen (15) business days following the date of the Merger Agreement.
The Offer will expire at 12:01 a.m., New York City time,
on the 21st business day following the commencement of the Offer
(unless the Offer is extended).
The obligation of Papyrus Acquisition Corp. to purchase shares of the Company’s common stock tendered in the Offer is subject to customary closing conditions, including (i) shares of the Company’s common stock having been validly tendered and not validly withdrawn that represent at least a majority of the total number of shares of the Company’s common stock then-outstanding on a fully diluted basis, (ii) the expiration or termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976, as amended (the “HSR Act”), (iii) the absence of injunctions or other legal restraints preventing the consummation of the Offer or the Merger and (iv) certain other conditions set forth in the Merger Agreement. The consummation of the Offer is not subject to any financing condition
, and Parent Holdco has guaranteed the performance of the obligations of Parent and Papyrus Acquisition Corp. under the Merger Agreement
.
Following the completion of the Offer, subject to the satisfaction or waiver of certain customary conditions set forth in the Merger Agreement, Papyrus Acquisition Corp. will merge with and into the Company, with the Company surviving as a wholly owned subsidiary of Parent (the “Merger”), pursuant to the procedure provided for under Section 3-106.1 of the Maryland General Corporation Law, as amended (the “MGCL”), without any stockholder approvals. The Merger will be effected as promptly as possible following the initial acceptance for payment by Papyrus Acquisition Corp. of shares of the Company’s common stock validly tendered and not validly withdrawn in the Offer. At the effective time of the Merger (the “Effective Time”), each issued and outstanding share of the Company’s common stock (other than shares owned by Papyrus Acquisition Corp. and any subsidiary of the Company will be converted into the right to receive the Offer Price, in cash, without interest, subject to any required withholding of taxes.
Each unexercised stock option outstanding immediately prior to the Effective Time, whether or not vested, will be canceled at the Effective Time and the holder of each stock option of the Company will be entitled to receive (i) the excess, if any, of
the Offer Price over the exercise price per share of Company common stock subject to the stock option, multiplied by (ii) the number of shares of Company common stock subject to the stock option immediately prior to the Effective Time.
Until the earlier of the termination of the Merger Agreement and the Effective Time, the Company has agreed to operate its business in the ordinary course and has agreed to certain other operating covenants, as set forth more fully in the Merger Agreement. The Merger Agreement includes customary termination provisions for both the Company and Parent and provides that, in connect
ion with the termination of the Merger Agreement under specified circumstances, including termination by the Company to accept a Superior Proposal (as defined in the Merger Agreement), the Company will be required to pay a fee of approximately $18.7 million.
Osiris Therapeutics, Inc. (NASDAQ:OSIR)
Historical Stock Chart
From Mar 2024 to Apr 2024
Osiris Therapeutics, Inc. (NASDAQ:OSIR)
Historical Stock Chart
From Apr 2023 to Apr 2024