Atossa Genetics to Host Conference Call to Announce Preliminary Results from Male Phase 1 Study of Topical Endoxifen Thursday...
September 12 2018 - 8:00AM

Atossa Genetics Inc. (NASDAQ:
ATOS), a
clinical-stage biopharmaceutical company developing novel
therapeutics and delivery methods for breast cancer and other
breast conditions, will host a conference call on September 13,
2018 at 10 am EDT to discuss preliminary results from its Phase 1
dose-escalation study of its proprietary topical Endoxifen in male
subjects.
The Phase 1 study was a double-blind, placebo-controlled, repeat
dose study of 24 healthy male subjects. Atossa assessed
safety, tolerability and the pharmacokinetics of proprietary
formulations of topical Endoxifen at varying dose levels over 28
days. The study was conducted on behalf of Atossa by CPR Pharma
Services Pty Ltd., Thebarton, SA, Australia.
Due to expected high call attendance, participants are asked to
preregister for the call through the following link:
http://dpregister.com/10124008. Please note that registered
participants will receive their dial in number upon registration
and will dial directly into the call without delay. Those without
internet access or who are unable to pre-register may dial in by
calling: 1-844-824-3830 (domestic), 1-412-317-5140 (international)
and Canada Toll Free: 1-855-669-9657. Callers should ask to be
joined into the Atossa Genetics call.
The conference call will also be available through a live
webcast at https://services.choruscall.com/links/atos180913.html
which is also available at www.atossagenetics.com on the Company’s
IR events page at http://ir.atossagenetics.com/ir-calendar.
Management will answer pre-submitted questions gathered prior to
the conference call in the Question and Answer period of the call.
Interested parties may submit questions for management’s
consideration prior to the call by submitting them in writing to
Atossa Genetics’ Investor Relations at scottg@coreir.com.
A replay of the call will be available approximately one hour
after the end of the call through October 13, 2018. The replay can
be accessed via Atossa's website or by dialing 877-344-7529
(domestic) or 412-317-0088 (international) or Canada Toll Free at
855-669-9658. The replay access code is 10124008.
About Atossa Genetics
Atossa Genetics Inc., is a clinical-stage biopharmaceutical
company developing novel therapeutics and delivery methods to treat
breast cancer and other breast conditions. For more information,
please
visit www.atossagenetics.com.
Forward-Looking Statements
Forward-looking statements in this press release, which Atossa
undertakes no obligation to update, are subject to risks and
uncertainties that may cause actual results to differ materially
from the anticipated or estimated future results, including the
risks and uncertainties associated with any variation between
preliminary and final clinical results, actions and inactions by
the FDA, the outcome or timing of regulatory approvals needed by
Atossa including those needed to commence studies, lower than
anticipated rate of patient enrollment, estimated market size of
drugs under development, the safety and efficacy of Atossa's
products and services, performance of clinical research
organizations and investigators, obstacles resulting from
proprietary rights held by others with respect to fulvestrant, such
as patent rights, potential market sizes for Atossa’s drugs under
development and other risks detailed from time to time in Atossa's
filings with the Securities and Exchange Commission, including
without limitation its periodic reports on Form 10-K and 10-Q, each
as amended and supplemented from time to time. Atossa
Genetics Company Contact:
Atossa Genetics Inc. Kyle Guse CFO and General Counsel Office:
866 893-4927 kyle.guse@atossagenetics.com
Investor Relations Contact:
Scott Gordon CoreIR 377 Oak Street Concourse 2 Garden City, NY
11530 Office: 516 222-2560 scottg@CoreIR.com
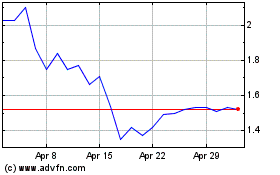
Atossa Therapeutics (NASDAQ:ATOS)
Historical Stock Chart
From Mar 2024 to Apr 2024
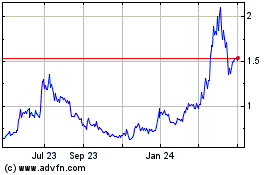
Atossa Therapeutics (NASDAQ:ATOS)
Historical Stock Chart
From Apr 2023 to Apr 2024