|
PROSPECTUS SUPPLEMENT
|
Filed Pursuant to Rule
424(b)(5)
|
|
(To Prospectus Dated September 6, 2016)
|
Registration No. 333-207600
|
Anavex
Life Sciences Corp.
Up
to $50,000,000
Common
Stock
We have entered into a Controlled Equity
Offering
SM
Sales Agreement, or Sales Agreement, with Cantor Fitzgerald & Co., or Cantor Fitzgerald, relating
to shares of our common stock,
par value $.001 per share,
offered
by this prospectus supplement and accompanying prospectus. In accordance with the terms of the Sales Agreement, we may offer and
sell shares of our common stock having an aggregate offering price of up to $50,000,000 from time to time through Cantor Fitzgerald,
acting as our sales agent.
Our common stock is currently listed
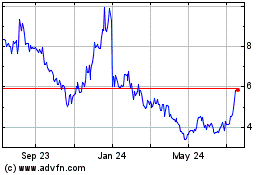
on the Nasdaq Capital Market under the symbol “AVXL”. On July 2, 2018 the last reported sale price
of our common stock was $2.66 per share.
Sales of our common stock, if any, under
this prospectus will be made by any method that is deemed to be an “at the market offering” as defined in Rule 415(a)(4)under
the Securities Act of 1933, as amended, or the Securities Act. Cantor Fitzgerald is not required to sell any specific amount of
securities, but will act as our sales agent using commercially reasonable efforts consistent with its normal trading and sales
practices, on mutually agreed terms between Cantor Fitzgerald and us. There is no arrangement for funds to be received in any escrow,
trust or similar arrangement.
Cantor will be entitled to compensation
at a fixed commission rate of 3.0% of the gross proceeds from the sale of our common stock pursuant to the Sales Agreement. In
connection with the sale of the common stock on our behalf, Cantor Fitzgerald will be deemed to be an “underwriter”
within the meaning of the Securities Act, and the compensation of Cantor Fitzgerald will be deemed to be underwriting commissions
or discounts. We have also agreed to provide indemnification and contribution to Cantor Fitzgerald against certain civil liabilities,
including liabilities under the Securities Act or the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Investing in our securities involves
a high degree of risk. See the section entitled “Risk Factors” on page S-6 of this prospectus supplement and the section
entitled “Risk Factors” beginning on page 9
of the accompanying prospectus, and in the documents we filed
with the Securities and Exchange Commission that are incorporated in this prospectus supplement by reference for certain risks
and uncertainties you should consider.
Neither the Securities and Exchange
Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy
of this prospectus. Any representation to the contrary is a criminal offense.

The date of this prospectus supplement
is July 6, 2018.
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
PROSPECTUS
ABOUT THIS PROSPECTUS SUPPLEMENT
We are providing information to you
about this offering of our common stock in two separate documents that are bound together: (1) this prospectus supplement,
which describes the specific terms of this offering, and (2) the accompanying base prospectus, which provides general information,
some of which may not apply to this offering. This prospectus supplement may also add to, update or change information contained
in the accompanying base prospectus. If information in this prospectus supplement is inconsistent with the accompanying base prospectus,
you should rely on this prospectus supplement. Generally, when we refer to this “prospectus,” we are referring to both
documents combined.
This prospectus supplement, the accompanying
base prospectus and any free-writing prospectus that we prepare or authorize contain and incorporate by reference information that
you should consider when making your investment decision. We have not, and Cantor has not, authorized anyone to provide you with
additional or different information. If anyone provides you with different or inconsistent information, you should not rely on
it. You should not assume that the information contained in this prospectus supplement or the accompanying base prospectus is accurate
as of any date other than the date on the front of those documents or that any information we have incorporated by reference is
accurate as of any date other than the date of the document incorporated by reference. Our business, financial condition, results
of operations and prospects may have changed since those dates.
We are not, and Cantor is not, making
an offer or sale of our common stock in any jurisdiction where such offer or sale is not permitted.
The information in this prospectus supplement
is not complete. You should carefully read this prospectus supplement and the accompanying base prospectus, including the information
incorporated by reference herein and therein, before you invest, as these documents contain information you should consider when
making your investment decision.
None of Anavex Life Sciences Corp.,
Cantor or any of their representatives are making any representation to you regarding the legality of an investment in our common
stock by you under applicable laws. You should consult with your own advisors as to legal, tax, business, financial and related
aspects of an investment in our common stock.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING
STATEMENTS
This prospectus supplement contains
forward-looking statements. All statements other than statements of historical facts contained in this prospectus supplement, including
statements regarding our anticipated future clinical and regulatory milestone events, future financial position, business strategy
and plans and objectives of management for future operations, are forward-looking statements. The words “believe,”
“may,” “estimate,” “continue,” “anticipate,” “intend,” “expect,”
“should,” “forecast,” “could,” “suggest,” “plan,” and similar expressions,
as they relate to us, are intended to identify forward-looking statements. Such forward-looking statements include, without limitation,
statements regarding:
|
|
·
|
our ability to generate any revenue or to continue as a going concern;
|
|
|
·
|
our ability to successfully conduct clinical
and preclinical trials for our product candidates;
|
|
|
·
|
our ability to raise additional capital
on favorable terms;
|
|
|
·
|
our ability to execute our development
plan on time and on budget;
|
|
|
·
|
our products ability to demonstrate efficacy
or an acceptable safety profile;
|
|
|
·
|
our ability to obtain the support of qualified
scientific collaborators;
|
|
|
·
|
our ability, whether alone or with commercial
partners, to successfully commercialize any of our product candidates that may be approved for sale;
|
|
|
·
|
our ability to identify and obtain additional
product candidates;
|
|
|
·
|
intellectual property rights and protections;
|
|
|
·
|
the anticipated start dates, durations
and completion dates of our ongoing and future clinical studies;
|
|
|
·
|
the anticipated designs of our future
clinical studies;
|
|
|
·
|
our anticipated future regulatory submissions
and our ability to receive regulatory approvals to develop and market our product candidates; and
|
|
|
·
|
our anticipated future cash position.
|
We have based these forward-looking
statements largely on our current expectations and projections about future events, including the responses we expect from the
U.S. Food and Drug Administration, or FDA, and other regulatory authorities and financial trends that we believe may affect our
financial condition, results of operations, business strategy, preclinical and clinical trials and financial needs. These forward-looking
statements are subject to a number of risks, uncertainties and assumptions including without limitation the risks described in
“Risk Factors” in “Part I, Item 1A” of or Annual Report on Form 10-K for the year ended September 30, 2017.
These risks are not exhaustive. We operate in a very competitive and rapidly changing environment. New risk factors emerge from
time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors
on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from
those contained in any forward-looking statements. You should not rely upon forward-looking statements as predictions of future
events. We cannot assure you that the events and circumstances reflected in the forward-looking statements will be achieved or
occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by
applicable laws including the securities laws of the United States, we assume no obligation to update or supplement forward-looking
statements.
PROSPECTUS
SUPPLEMENT SUMMARY
This summary highlights information
contained elsewhere in this prospectus supplement and the accompanying base prospectus. It does not contain all of the information
that you should consider before making an investment decision. You should read this entire prospectus supplement, the accompanying
base prospectus and the documents incorporated herein by reference for a more complete understanding of this offering of common
stock. Please read “Risk Factors” in our Annual Report on Form 10-K for the year ended September 30, 2017 for information
regarding risks you should consider before investing in our common stock.
Throughout this prospectus supplement,
when we use the terms “Anavex,” “we,” “us,” “our” or the “Company,”
we are referring either to Anavex Life Sciences Corp. in its individual capacity or to Anavex Life Sciences Corp. and its operating
subsidiaries collectively, as the context requires.
Our Company
Overview
We are a clinical stage biopharmaceutical
company engaged in the development of differentiated therapeutics for the treatment of neurodegenerative and neurodevelopmental
diseases including drug candidates to treat Alzheimer’s disease, other central nervous system (“CNS”) diseases,
pain and various types of cancer. The Company’s lead compound ANAVEX
®
2-73, is being developed to treat Alzheimer’s
disease, Parkinson’s disease and potentially other central nervous system diseases, including rare diseases, such as Rett
syndrome, a severe neurological disorder caused by mutations in the X-linked gene, methyl-CpG-binding protein 2 (MECP2).
In November 2016, a Phase 2a clinical trial,
consisting of PART A and PART B, which lasted a total of 57 weeks, was completed for ANAVEX
®
2-73 in mild-to-moderate
Alzheimer’s patients. This open-label randomized trial met both primary and secondary endpoints, and was designed to assess
the safety and exploratory efficacy of ANAVEX
®
2-73 in 32 patients. ANAVEX
®
2-73 targets sigma-1 and
muscarinic receptors, which have been shown in preclinical studies to reduce stress levels in the brain believed to restore cellular
homeostasis and to reverse the pathological hallmarks observed in Alzheimer’s disease. In October 2017, we presented pharmacokinetic
(PK) and pharmacodynamic (PD) data from the positive Phase 2a study, which established a concentration-effect relationship between
ANAVEX
®
2-73 and study measurements. These measures obtained from all patients who participated in the entire 57
weeks include exploratory cognitive and functional scores as well as biomarker signals of brain activity. Additionally, the study
appears to show that ANAVEX
®
2-73 activity is enhanced by its active metabolite (ANAVEX19-144), which also targets
the sigma-1 receptor and has a half-life approximately twice as long as the parent molecule.
In March 2016, we received approval from the
Ethics Committee in Australia to extend the Phase 2a clinical trial, which had been requested by patients and their caregivers.
The trial extension allows participants who completed the 52-week PART B of the study to roll-over into a new trial and continue
taking ANAVEX
®
2-73 for an additional 104 weeks, providing an opportunity to gather extended safety data. The trial
is independent of our planned larger Phase 2/3 double-blind, placebo-controlled study of ANAVEX
®
2-73 in Alzheimer’s
disease.
In February 2016, we presented positive preclinical data for ANAVEX
®
2-73
in Rett syndrome, a rare neurodevelopmental disease. The study was funded by the International Rett Syndrome Foundation (the Rettsyndrome.org
Foundation). In January 2017, we were awarded a financial grant from the Rettsyndrome.org Foundation of a minimum of $0.6 million
to cover some of the cost of a planned U.S. multicenter Phase 2 clinical trial of ANAVEX
®
2-73 for the treatment
of Rett syndrome. The Phase 2 trial is scheduled to begin following the FDA’s approval of the Company’s investigational
new drug (IND) application and will be a randomized, double blind, placebo-controlled study of ANAVEX
®
2-73 in patients
with Rett syndrome lasting up to 12 weeks. Primary and secondary endpoints include safety as well as Rett syndrome conditions such
as cognitive impairment, motor impairment, behavioral symptoms and seizure activity.
In September 2016, we presented positive preclinical
data for ANAVEX
®
2-73 in Parkinson’s disease, which demonstrated significant improvements on all measures:
behavioral, histopathological, and neuroinflammatory endpoints. The study was funded by the Michael J Fox Foundation. Additional
data was announced in October 2017 from the model for experimental parkinsonism. The data presented indicates that ANAVEX
®
2-73
induces robust neurorestoration in experimental parkinsonism. The encouraging results we have gathered in this model, coupled with
the favorable profile of this compound in the Alzheimer’s disease trial, support the notion that ANAVEX
®
2-73
is a promising clinical candidate drug for Parkinson’s disease. We are also moving forward with a Phase 2 trial with ANAVEX
®
2-73
in Parkinson’s Disease Dementia (PDD), which will study the effect of the compound on both the cognitive and motor impairment
of Parkinson’s disease. The double-blind, randomized, placebo-controlled Phase 2 PDD study has been submitted to regulatory
authorities in Europe, and pending approval, we plan to initiate this clinical trial in the second half of calendar 2018.
Recent Developments
On July 2, 2018, the Human Research
Ethics Committee in Australia approved the initiation of our Phase 2b/3, double-blind, randomized, placebo-controlled 48-week
safety and efficacy trial of ANAVEX®2-73 for the treatment of early Alzheimer’s disease in Australia. This Phase 2b/3
study design incorporates inclusion of genomic precision medicine biomarkers identified in the ANAVEX®2-73 Phase 2a study.
The Phase 2b/3 study, which is expected to enroll approximately 450 patients, randomized 1:1:1 to either two different ANAVEX®2-73
doses or placebo, is scheduled to begin the next month.
In May 2018, we received approval from
the Human Research Ethics Committee in Australia to further extend the current Phase 2a Alzheimer’s clinical trial of ANAVEX®2-73
for an additional two years. The extension, again requested by existing patients, their caregivers and physicians, will allow patients
who participated in the first 57-week study and continued in the 104-week extension study, to continue taking ANAVEX®2-73 for
an additional two years. As a result, we will have over five years of cumulative safety and tolerability data for ANAVEX®2-73.
The further extension is in addition and independent of the Company’s larger Phase 2b/3 double-blinded, placebo-controlled
study of ANAVEX®2-73 in Alzheimer’s disease discussed above.
Corporate Information
Our principal executive office is located at
51 West 52nd Street, 7th Floor, New York, NY 10019-6163, and our telephone number is 844.689.3939. Our website address is
www.anavex.com
.
No information found on our website is part of this prospectus. Also, this prospectus may include the names of various government
agencies or the trade names of other companies. Unless specifically stated otherwise, the use or display by us of such other parties’
names and trade names in this prospectus is not intended to and does not imply a relationship with, or endorsement or sponsorship
of us by, any of these other parties.
THE OFFERING
|
Common stock offered by us
:
|
Common stock, par value $.001 per share, having an aggregate sales price of up to $50,000,000.
|
|
|
|
|
Common stock to be outstanding after this offering:
|
Up to 63,657,757 shares assuming the sale of 19,083,969 shares of our common stock in this offering at an offering price of $2.62 per share, which was the closing price of our common stock on The Nasdaq Capital Market on June 29, 2018. The actual number of shares issued will vary depending on the sales prices under this offering.
|
|
|
|
|
Plan of Distribution
|
“At the market offering” that may be made from time to time through our sales agent, Cantor Fitzgerald. See “Plan of Distribution.”
|
|
|
|
|
Use of Proceeds
|
We intend to use the net proceeds from this offering, after deducting the sales agent’s commissions and our offering expenses, for general corporate purposes, which may include, among other things, working capital, capital expenditures and funding additional clinical and preclinical development of our pipeline candidates. See “Use of Proceeds” on page S-7.
|
|
|
|
|
Risk Factors
|
You should read the “Risk Factors” section on page S-6 of this prospectus supplement and the other risks identified in the documents incorporated by reference herein before making a decision to purchase common stock in this offering.
|
|
|
|
|
Nasdaq Capital Market symbol
|
“AVXL.”
|
The number of shares of common stock shown above to be outstanding
after this offering is based on 44,573,788 shares of common stock outstanding as of March 31, 2018 and exclude the following:
|
|
·
|
5,867,030 shares of common stock issuable upon the exercise of outstanding
stock options, vested and unvested, with a weighted-average exercise price of $4.00 per share; and
|
|
|
·
|
1,609,309 shares of common stock issuable upon the exercise of outstanding
warrants with a weighted-average exercise price of $2.66 per share.
|
RISK FACTORS
An investment in our common stock
involves a significant degree of risk. Before you invest in our common stock you should carefully consider those risk factors included
in our most recent Annual Report on Form 10-K, our most recent Quarterly Report on Form 10-Q, any subsequently filed Quarterly
Reports on Form 10-Q and any subsequently filed Current Reports on Form 8-K, which are incorporated herein by reference, and those
risk factors that may be included in any applicable prospectus supplement, together with all of the other information included
in this prospectus supplement, the accompanying base prospectus and the documents we incorporate by reference, in evaluating an
investment in our common stock. If any of the risks discussed in the foregoing documents were to occur, our business, financial
condition, results of operations and cash flows could be materially adversely affected. Please read “Cautionary Statement
Regarding Forward-Looking Statements.”
You may experience dilution.
The offering price per share in this offering may exceed
the net tangible book value per share of our common stock outstanding prior to this offering. Assuming that an aggregate of 19,083,969
shares of our common stock are sold at a price of $2.62 per share, the last reported sale price of our common stock on the Nasdaq
Capital Market on June 29, 2018, for aggregate gross proceeds of $50.0 million, and after deducting commissions and estimated offering
expenses payable by us, you would experience immediate dilution of $1.51 per share, representing the difference between our as
adjusted net tangible book value per share as of March 31, 2018, after giving effect to this offering, and the assumed offering
price. The exercise of outstanding stock options and warrants would result in further dilution of your investment. See the section
entitled “Dilution” below for a more detailed illustration of the dilution you would incur if you participate in this
offering. Because the sales of the shares offered hereby will be made directly into the market or in negotiated transactions, the
prices at which we sell these shares will vary and these variations may be significant. Purchasers of the shares we sell, as well
as our existing shareholders, will experience significant dilution if we sell shares at prices significantly below the price at
which they invested.
You may experience future dilution as a result of future
equity offerings.
To raise additional capital, we may in the future offer additional
shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be
the same as the price per share in this offering. We may sell shares or other securities in any other offering at a price per share
that is less than the price per share paid by investors in this offering, and investors purchasing shares or other securities in
the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common
stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price
per share paid by investors in this offering.
The actual number of shares we will issue under the
sales agreement, at any one time or in total, is uncertain.
Subject to certain limitations in the sales agreement with
Cantor, the sales agent in this offering, and compliance with applicable law, we have the discretion to deliver placement notices
to the sales agent at any time throughout the term of the sales agreement. The number of shares that are sold by the sales agent
after delivering a placement notice will fluctuate based on the market price of the common stock during the sales period and limits
we set with the sales agent.
Our management might apply the net proceeds from this
offering in ways with which you do not agree and in ways that may impair the value of your investment.
We currently intend to use the net proceeds from this offering
primarily for working capital and general corporate purposes. Our management has broad discretion as to the use of such proceeds
and you will be relying on the judgment of our management regarding the application of these proceeds. Our management might apply
these proceeds in ways with which you do not agree, or in ways that ultimately do not yield a favorable return. If our management
applies such proceeds in a manner that does not yield a significant return, if any, on our investment of such net proceeds, it
could compromise our ability to pursue our growth strategy and adversely affect the market price of our common stock
USE OF PROCEEDS
We intend to use the net proceeds from this offering, after
deducting the sales agent’s commissions and our offering expenses, for general corporate purposes, which may include, among
other things, working capital, capital expenditures and funding additional clinical and preclinical development of our pipeline
candidates.
DILUTION
If you purchase shares of common stock in this offering,
your ownership interest in the Company will be diluted to the extent of the difference between the public offering price per share
and the as adjusted net tangible book value per share after giving effect to this offering. We calculate net tangible book value
per share by dividing the net tangible book value, which is tangible assets less total liabilities, by the number of outstanding
shares of common stock. Dilution represents the difference between the portion of the amount per share paid by purchasers of shares
in this offering and the as adjusted net tangible book value per share of our common stock immediately after giving effect to this
offering. Our net tangible book value as of March 31, 2018 was approximately $22.5 million, or $0.50 per share.
After giving effect to the sale of common stock pursuant
to this prospectus supplement and accompanying prospectus in the aggregate amount of $50,000,000 at an assumed offering price of
$2.62 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on June 29, 2018, and after deducting
commissions and estimated aggregate offering expenses payable by us, our net tangible book value as of March 31, 2018 would have
been $70.8 million, or $1.11 per share of common stock. This represents an immediate increase in the net tangible book value of
$0.61 per share to our existing stockholders and an immediate dilution in net tangible book value of $1.51 per share to new investors.
The following table illustrates this per share dilution:
|
Assumed public offering price per share
|
|
|
|
|
|
$
|
2.62
|
|
|
Net tangible book value per share as of March 31, 2018
|
|
$
|
0.50
|
|
|
|
|
|
|
Increase per share attributable to new investors
|
|
$
|
0.61
|
|
|
|
|
|
|
As adjusted net tangible book value per share as of March 31, 2018 after giving effect to this offering
|
|
|
|
|
|
$
|
1.11
|
|
|
Dilution per share to new investors purchasing shares in this offering
|
|
|
|
|
|
$
|
1.51
|
|
The table above assumes for illustrative purposes that an
aggregate of 19,083,969 shares of our common stock are sold pursuant to this prospectus supplement and the accompanying prospectus
at a price of $2.62 per share, the last reported sale price of our common stock on the Nasdaq Capital Market on June 29, 2018,
for aggregate gross proceeds of $50.0 million. The shares sold in this offering, if any, will be sold from time to time at various
prices. An increase of $1.00 per share in the price at which the shares are sold from the assumed offering price of $2.62 per share
shown in the table above, assuming all of our common stock in the aggregate amount of $50.0 million is sold at that price, would
result in an adjusted net tangible book value per share after the offering of $1.21 per share and would increase the dilution in
net tangible book value per share to new investors in this offering to $2.41 per share, after deducting commissions and estimated
aggregate offering expenses payable by us. A decrease of $1.00 per share in the price at which the shares are sold from the assumed
offering price of $2.62 per share shown in the table above, assuming all of our common stock in the aggregate amount of $50.0 million
is sold at that price, would result in an adjusted net tangible book value per share after the offering of $0.94 per share and
would decrease the dilution in net tangible book value per share to new investors in this offering to $0.68 per share, after deducting
commissions and estimated aggregate offering expenses payable by us.
The above table and discussion are based on 44,573,788 shares
of common stock outstanding as of March 31, 2018 and exclude the following, all as of March 31, 2018:
|
|
·
|
5,867,030 shares of common stock issuable upon the exercise of outstanding
stock options, vested and unvested, with a weighted-average exercise price of $4.00 per share; and
|
|
|
·
|
1,609,309 shares of common stock issuable upon the exercise of outstanding
warrants with a weighted-average exercise price of $2.66 per share.
|
To the extent that options or warrants outstanding as of
March 31, 2018 have been or are exercised, or other shares are issued, investors purchasing shares in this offering could experience
further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations,
even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is
raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution
to our stockholders.
PLAN OF DISTRIBUTION
We
have entered into a Controlled Equity Offering
SM
Sales Agreement (the “Sales Agreement”) with Cantor
Fitzgerald, pursuant to which we may issue and sell up to $50 million shares of our common stock, $0.01 par value per share, through
Cantor Fitzgerald, acting as sales agent. This summary of the material provisions of the Sales Agreement does not purport to be
a complete statement of its terms and conditions. A copy of the Sales Agreement will be filed as an exhibit to a Current
Report on Form 8-K and will be incorporated by reference into the registration statement of which this prospectus supplement
is a part. See “Where You Can Find More Information” below.
Upon
delivery of a placement notice and subject to the terms and conditions of the Sales Agreement, Cantor Fitzgerald may sell our common
stock by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated
under the Securities Act, including sales made directly on the Nasdaq Capital Market or any other existing trading market for our
common stock. We or Cantor Fitzgerald may suspend the offering of our common stock upon notice and subject to other conditions.
We
will pay Cantor Fitzgerald in cash, upon each sale of our common stock pursuant to the Sales Agreement, a commission in an amount
equal to 3.0% of the aggregate gross proceeds from each sale of our common stock. Because there is no minimum offering amount required
as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not
determinable at this time. We have also agreed to reimburse Cantor Fitzgerald for certain specified expenses, in an aggregate amount
not exceeding $50,000, including the fees and disbursements of its legal counsel. We estimate that the total expenses for
the offering, excluding compensation and reimbursements payable to Cantor Fitzgerald under the terms of the Sales Agreement, will
be approximately $90,000.
Settlement
for sales of common stock will occur on the second business day following the date on which any sales are made, or on another date
that is agreed upon by us and Cantor Fitzgerald in connection with a particular transaction, in return for payment of the net proceeds
to us. Sales of our common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository
Trust Company or by such other means as we and Cantor Fitzgerald may agree upon. There is no arrangement for funds to be received
in an escrow, trust or similar arrangement.
Cantor
Fitzgerald will use its commercially reasonable efforts, consistent with its sales and trading practices, to solicit offers to
purchase shares of our common stock under the terms and subject to the conditions set forth in the Sales Agreement. In connection
with the sale of the common stock on our behalf, Cantor Fitzgerald will be deemed to be an “underwriter” within the
meaning of the Securities Act, and the compensation of Cantor Fitzgerald will be deemed to be underwriting commissions or discounts.
We have agreed to provide indemnification and contribution to Cantor Fitzgerald against certain civil liabilities, including liabilities
under the Securities Act.
The
offering of our common stock pursuant to the Sales Agreement will terminate upon the termination of the Sales Agreement as permitted
therein. We and Cantor Fitzgerald may each terminate the Sales Agreement at any time upon ten days’ prior notice.
We have agreed to indemnify
Cantor Fitzgerald and specified other persons against certain liabilities relating to or arising out of the Cantor Fitzgerald’s
activities under Sales Agreement and to contribute to payments that Cantor Fitzgerald may be required to make in respect of such
liabilities.
Cantor
Fitzgerald and its affiliates may in the future provide various investment banking, commercial banking and other financial services
for us and our affiliates, for which services they may in the future receive customary fees. To the extent required by Regulation
M, Cantor Fitzgerald will not engage in any market making activities involving our common stock while the offering is ongoing under
this prospectus supplement.
This
prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained by Cantor
Fitzgerald, and Cantor Fitzgerald may distribute this prospectus supplement and the accompanying prospectus electronically.
LEGAL MATTERS
The validity of the securities offered
by this prospectus has been passed upon for us by Snell & Wilmer, L.L.P., Reno, Nevada. Cantor Fitzgerald & Co. is being
represented in connection with this offering by Cooley LLP, New York, New York.
EXPERTS
The financial statements as of September
30, 2017 and 2016 and for each of the three years in the period ended September 30, 2017 and management’s assessment of the
effectiveness of internal control over financial reporting as of September 30, 2017 incorporated by reference in this Prospectus
have been so incorporated in reliance on the reports of BDO USA, LLP, an independent registered public accounting firm, incorporated
herein by reference, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and other reports and other information
with the SEC under the Exchange Act. You may read and copy any reports, statements or other information filed by us at the SEC’s
public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information
on the public reference room. Our filings with the SEC are also available to the public from commercial document retrieval services
and at the SEC’s website at www.sec.gov.
We make available free of charge on our internet website
at www.anavex.com our annual reports on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K and any
amendments to those reports, as soon as reasonably practicable after we electronically file such material with, or furnish it to,
the SEC. Information contained on our website is not incorporated by reference into this prospectus supplement and you should not
consider such information as part of this prospectus supplement.
DOCUMENTS INCORPORATED BY REFERENCE
The SEC allows us to “incorporate
by reference” into this prospectus certain information that we file with the SEC, which means that we can disclose important
information to you by referring you to other documents separately filed by us with the SEC that contain such information. The information
we incorporate by reference is considered to be part of this prospectus and information we later file with the SEC will automatically
update and supersede the information in this prospectus. The following documents filed by us with the SEC pursuant to Section 13(a)
of the Exchange Act and any of our future filings under Sections 13(a), 13(c), 14 or 15 (d) of the Exchange Act, except for
information furnished under Item 2.02 or 7.01 of Current Report on Form 8-K, or exhibits related thereto, made before the termination
of the offering are incorporated by reference herein:
|
|
(1)
|
our Annual Report on Form 10-K for the fiscal year ended September 30, 2017, filed with the SEC
on December 11, 2017;
|
|
|
(2)
|
our Proxy Statement on Schedule 14A filed on March 8, 2018 (excluding those portions that are not
incorporated by reference into our Annual Report on Form 10-K);
|
|
|
(3)
|
our Quarterly Reports on Form 10-Q for the periods ended December 31, 2017 and March 31, 2018,
filed on February 7, 2018 and May 10, 2018, respectively;
|
|
|
(4)
|
our Current Reports on Form 8-K filed on October 6, 2017, March 8, 2018 and April 20, 2018; and
|
|
|
(5)
|
the description of our Common Stock contained in the Registration Statement on Form 8-A (File No.
001-37606) filed with the SEC on October 23, 2005.
|
Any statement contained herein or in
any document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded for the
purposes of this prospectus to the extent that a statement contained herein or in any other subsequently filed document which also
is or is deemed to be incorporated by reference herein modifies or replaces such statement. Any such statement so modified or superseded
shall not be deemed to constitute a part of this prospectus, except as so modified or superseded.
We will provide to each person, including
any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the reports or documents that have been incorporated
by reference in the prospectus contained in the registration statement but not delivered with the prospectus, other than an exhibit
to these filings unless we have specifically incorporated that exhibit by reference into the filing, upon written or oral request
and at no cost to the requester. Requests should be made by writing or telephoning us at the following address:
Anavex Life Sciences Corp.
51 West 52
nd
Street, 7
th
Floor
New York, NY 10019-6163
(844) 689-3939
PROSPECTUS
Anavex
Life Sciences Corp.
$100,000,000
of Shares of Common Stock and Warrants
6,754,609
Shares of Common Stock Offered
by Selling Security Holder
Anavex
Life Sciences Corp., a Nevada corporation (“
us
”, “
we
”, “
our
”, “
Anavex
”
or the “
Company
”) may offer and sell from time to time, in one or more series or issuances and on terms that
Anavex will determine at the time of the offering, shares of our common stock, par value $0.001 per share (“
Common Stock
”)
and warrants (“
Warrants
”) described in this prospectus, up to an aggregate amount of $100,000,000.
This
prospectus also covers the resale by Lincoln Park Capital Fund, LLC (“
Lincoln Park
” or the “
Selling
Security Holder
”), of up to 6,754,609 shares of our Common Stock in one or more transactions in amounts, at prices,
and on terms that will be determined at the time these securities are offered, inclusive of 269,397 shares of Common Stock issued
or issuable to the Selling Security Holder as commitment shares, as described in further detail herein. The shares being offered
for resale by the Selling Security Holder represents approximately 18.9% of the outstanding Common Stock of the Company.
We
will not receive any of the proceeds from the sale of shares of our Common Stock sold by the Selling Security Holder. The Selling
Security Holder may sell the shares of Common Stock described in this prospectus in a number of different ways and at varying
prices. See “Plan of Distribution” for more information about how the Selling Security Holder may sell the shares
of Common Stock being registered pursuant to this prospectus. The Selling Security Holder is an “underwriter” within
the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended (the “
Securities Act
”).
This
prospectus provides you with a general description of the securities offered. We will file prospectus supplements and may provide
other offering material at later dates that will contain specific terms of each offering of securities by us. These supplements
may also add, update or change information contained in this prospectus.
You should carefully read this prospectus and the applicable prospectus supplement before you invest in any of our securities. This
prospectus may not be used to consummate sales of securities unless accompanied by a prospectus supplement.
We
may offer and sell the securities described in this prospectus and any prospectus supplement directly to our stockholders or to
other purchasers or through agents on our behalf or through underwriters or dealers as designated from time to time. If any agents
or underwriters are involved in the sale of any of these securities, the applicable prospectus supplement will provide the names
of the agents or underwriters and any applicable fees, commission or discounts.
Our
Common Stock is currently quoted on the Nasdaq Capital Market under the symbol “AVXL”. On August 30, 2016, the last
reported sale price of our Common Stock was $3.04 per share.
Investing
in our securities involves a high degree of risk. See the section entitled “Risk Factors” on page 9 of this prospectus
and in the documents we filed with the Securities and Exchange Commission that are incorporated in this prospectus by reference
for certain risks and uncertainties you should consider.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
This
prospectus is dated September 6, 2016.
TABLE
OF CONTENTS
i
ABOUT
THIS PROSPECTUS
This
prospectus of Anavex Life Sciences Corp., a Nevada corporation (collectively with all of its subsidiaries, the “Company”,
“Anavex”, or “we”, “us”, or “our”) is a part of a registration statement that
we filed with the Securities and Exchange Commission (“
SEC
”) utilizing a “shelf” registration process.
Under this shelf registration process, we may, from time to time, sell the securities described in this prospectus in one or more
offerings up to a total dollar amount of $100,000,000 as described in this prospectus. In addition, Lincoln Park may, from time
to time, offer and sell up to an aggregate of 6,754,609 shares of our Common Stock in one or more transactions.
The
registration statement containing this prospectus, including the exhibits to the registration statement, provides additional information
about us and the securities offered under this prospectus. The registration statement, including the exhibits and the documents
incorporated herein by reference, can be read on the SEC website or at the SEC offices mentioned under the heading “Prospectus
Summary - Where You Can Find More Information.”
We
may provide a prospectus supplement containing specific information about the amounts, prices and terms of the securities for
a particular offering. The prospectus supplement may add, update or change information in this prospectus. If the information
in the prospectus is inconsistent with a prospectus supplement, you should rely on the information in that prospectus supplement.
You should read both this prospectus and, if applicable, any prospectus supplement. See “Prospectus Summary — Where
You Can Find More Information” for more information.
We
have not authorized any dealer, salesman or other person to give any information or to make any representation other than those
contained or incorporated by reference in this prospectus or any prospectus supplement. You must not rely upon any information
or representation not contained or incorporated by reference in this prospectus or any prospectus supplement. This prospectus
and any prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than
the registered securities to which they relate, nor do this prospectus and any prospectus supplement constitute an offer to sell
or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer
or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus or any prospectus
supplement is accurate on any date subsequent to the date set forth on the front of such document or that any information we have
incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though
this prospectus and any prospectus supplement is delivered or securities are sold on a later date.
1
PROSPECTUS
SUMMARY
The
items in the following summary are described in more detail later in this prospectus. This summary does not contain all of the
information you should consider. Before investing in our securities, you should read the entire prospectus carefully, including
the “Risk Factors” beginning on page 9 and the financial statements incorporated by reference.
Overview
Our
Current Business
We
are a clinical stage biopharmaceutical company engaged in the development of differentiated therapeutics for the treatment of
neurodegenerative and neurodevelopmental diseases including drug candidates to treat Alzheimer’s disease, other central
nervous system (“CNS”) diseases, pain and various types of cancer. The Company’s lead compound ANAVEX 2-73 is
being developed to treat Alzheimer’s disease and potentially other central nervous system diseases, including rare diseases,
such as Rett syndrome.
In
December 2014 a Phase 2a clinical trial was initiated for ANAVEX 2-73, which is being evaluated for the treatment of Alzheimer’s
disease. This randomized trial is designed to assess the safety and exploratory efficacy of ANAVEX 2-73 alone as well as in combination
with donepezil (ANAVEX PLUS) in patients with mild to moderate Alzheimer’s disease. ANAVEX 2-73 targets sigma-1 and muscarinic
receptors, which have been shown in preclinical studies to reduce stress levels in the brain and to reverse the pathological hallmarks
observed in Alzheimer’s disease. ANAVEX 2-73 showed no serious adverse events in a previously performed Phase 1 study. In
pre-clinical studies, ANAVEX 2-73 demonstrated anti-amnesic and neuroprotective properties in various animal models including
the transgenic mouse model Tg2576. In March, 2016, we received approval from the Ethics Committee in Australia to extend the ongoing
Phase 2a clinical trial, which had been requested by patients and their caregivers. The trial extension allows participants who
complete 52 weeks in PART B to roll-over into a new trial and continue taking ANAVEX 2-73 for an additional 104 weeks, providing
an opportunity to gather extended safety data. The trial is independent of the Company’s planned larger Phase 2/3 double-blinded,
placebo-controlled study of ANAVEX 2-73 in Alzheimer’s disease.
We
intend to identify and initiate discussions with potential partners in the next 12 months. Further, we may acquire or develop
new intellectual property and assign, license, or otherwise transfer our intellectual property to further our goals.
Our
Pipeline
Our
pipeline includes one clinical drug candidate and several compounds in different stages of pre-clinical study.
Our
proprietary SIGMACEPTOR™ Discovery Platform produced small molecule drug candidates with unique modes of action, based on
our understanding of sigma receptors. Sigma receptors may be targets for therapeutics to combat many human diseases, including
Alzheimer’s disease. When bound by the appropriate ligands, sigma receptors influence the functioning of multiple biochemical
signals that are involved in the pathogenesis (origin or development) of disease.
Compounds
that have been subjects of our research include the following:
ANAVEX
2-73
ANAVEX
2-73 may offer a disease-modifying approach in Alzheimer’s disease (AD) by using ligands that activate sigma-1 receptors.
In
AD animal models, ANAVEX 2-73 has shown pharmacological, histological and behavioral evidence as a potential neuroprotective,
anti-amnesic, anti-convulsive and anti-depressive therapeutic agent, due to its potent affinity to sigma-1 receptors and moderate
affinities to M1-4 type muscarinic receptors. In addition, ANAVEX 2-73 has shown a potential dual mechanism which may impact both
amyloid and tau pathology. In a transgenic AD animal model Tg2576 ANAVEX 2-73 induced a statistically significant neuroprotective
effect against the development of oxidative stress in the mouse brain, as well as significantly increased the expression of functional
and synaptic plasticity markers that is apparently amyloid-beta independent. It also statistically alleviated the learning and
memory deficits developed over time in the animals, regardless of sex, both in terms of spatial working memory and long-term spatial
reference memory.
2
Based
on the results of pre-clinical testing, we initiated and completed a Phase 1 single ascending
dose (SAD) clinical trial of ANAVEX 2-73 in 2011. In this Phase 1 SAD trial, the maximum
tolerated single dose was defined per protocol as 55–60 mg. This dose is above
the equivalent dose shown to have positive effects in mouse models of AD. There were
no significant changes in laboratory or electrocardiogram (ECG) parameters. ANAVEX 2-73
was well tolerated below the 55–60 mg dose with only mild adverse events in some
subjects. Observed adverse events at doses above the maximum tolerated single dose included
headache and dizziness, which were moderate in severity and reversible. These side effects
are often seen with drugs that target CNS conditions, including AD.
The
ANAVEX 2-73 Phase 1 SAD trial was conducted as a randomized, placebo-controlled study. Healthy male volunteers between the ages
of 18 and 55 received single, ascending oral doses over the course of the trial. Study endpoints included safety and tolerability
together with pharmacokinetic parameters. Pharmacokinetics includes the absorption and distribution of a drug, the rate at which
a drug enters the blood and the duration of its effect, as well as chemical changes of the substance in the body. This study was
conducted in Germany in collaboration with ABX-CRO, a clinical research organization that has conducted several Alzheimer’s
disease studies, and the Technical University of Dresden.
In
December 2014 a Phase 2a clinical trial was initiated for ANAVEX 2-73, which is being evaluated for the treatment of Alzheimer’s
disease. The randomized trial is designed to assess the safety and exploratory efficacy of ANAVEX 2-73 alone as well as in combination
with donepezil (ANAVEX PLUS) in patients with mild to moderate Alzheimer’s disease. ANAVEX 2-73 targets sigma-1 and muscarinic
receptors, which have been shown in preclinical studies to reduce stress levels in the brain and to reverse the pathological hallmarks
observed in Alzheimer’s disease. ANAVEX 2-73 showed no serious adverse events in a previously performed Phase 1 study. In
pre-clinical studies ANAVEX 2-73 demonstrated anti-amnesic and neuroprotective properties in various animal models including the
transgenic mouse model Tg2576.
The
Phase 2a study met both primary and secondary objectives of the study. The 31-week preliminary exploratory safety and efficacy
data from the ongoing Phase 2a study of ANAVEX 2-73 in Alzheimer’s patients demonstrated favorable safety, maximum tolerated
dose, positive dose response, sustained efficacy response through 31 weeks for both cognitive and functional measures, as well
as positive unexpected therapeutic response events. ANAVEX 2-73 continues to demonstrate a favorable adverse event (AE) profile
through 31 weeks in a patient population of elderly Alzheimer’s patients with varying degrees of physical fragility. The
most common side effects across all AE categories tended to be of mild severity grade 1, and were resolved with dose reductions
that were anticipated within the adaptive design of the study protocol. ANAVEX 2-73 data presented is prerequisite information
in order to progress into Phase 2/3 placebo controlled studies.
Recent
preclinical data validates ANAVEX 2-73 as a prospective platform drug for other neurodegenerative diseases beyond Alzheimer’s
as well as neurodevelopmental diseases, more specifically, epilepsy and Rett syndrome. For epilepsy, data demonstrates both significant
and dose related improvement in the reduction of seizures, as well as significant synergy with each of three generations of epilepsy
drugs currently on the market. In Rett syndrome, a rare neurodevelopmental disease indication, administration of ANAVEX 2-73 resulted
in both significant and dose related improvements in an array of behavioural paradigms in the MECP2 HET Rett syndrome disease
model.
ANAVEX
PLUS
ANAVEX
PLUS, a combination of ANAVEX 2-73 with donepezil (Aricept
®
) is
a potential novel combination drug for Alzheimer’s disease. Aricept
®
(donepezil) is now generic. ANAVEX 2-73 showed in combination with donepezil an unexpected and clear synergic effect of memory
improvement by up to 80% in animal models. A patent application was filed in the US for the combination of donepezil and ANAVEX
2-73 and if granted would give patent protection at least until 2033.
In
a humanized calibrated cortical network computer model the unexpected pre-clinical synergy between ANAVEX 2-73 and donepezil was
confirmed and ANAVEX PLUS showed an anticipated ADAS-Cog response of 7 points at 12 weeks and 5.5 points at 26 weeks, which represents
more than 2x the ADAS-Cog of donepezil alone.
ANAVEX
3-71
ANAVEX
3-71, previously named AF710B is a preclinical drug candidate with a novel mechanism of action via sigma-1 receptor activation
and M1 muscarinic allosteric modulation, which has shown to enhance neuroprotection and cognition in Alzheimer’s disease.
ANAVEX 3-71 is a CNS-penetrable mono-therapy that bridges treatment of both cognitive impairments with disease modifications.
It is highly effective in very small doses against the major Alzheimer’s hallmarks in transgenic (3xTg-AD) mice, including
cognitive deficits, amyloid and tau pathologies, and also has beneficial effects on inflammation and mitochondrial
3
dysfunctions.
ANAVEX 3-71 indicates extensive therapeutic advantages in Alzheimer’s and other
protein-aggregation-related diseases given its ability to enhance neuroprotection and
cognition via sigma-1 receptor activation and M1 muscarinic allosteric modulation.
A
recent preclinical study examined the response of ANAVEX 3-71 in aged transgenic animal models, and showed a significant reduction
in rate of cognitive deficit, amyloid beta pathology and inflammation with the administration of ANAVEX 3-71. In April 2016 the
U.S. Food and Drug Administration (“FDA”) granted Orphan Drug Designation (“ODD”) to ANAVEX 3-71 for the
treatment of Frontotemporal dementia (“FTD”).
ANAVEX
1-41
ANAVEX
1-41 is a sigma-1 agonist. Pre-clinical tests revealed significant neuroprotective benefits (i.e., protects nerve cells from degeneration
or death) through the modulation of endoplasmic reticulum, mitochondrial and oxidative stress, which damages and destroys cells
and is believed by some scientists to be a primary cause of AD. In addition, in animal models, ANAVEX 1-41 prevented the expression
of caspase-3, an enzyme that plays a key role in apoptosis (programmed cell death) and loss of cells in the hippocampus, the part
of the brain that regulates learning, emotion and memory. These activities involve both muscarinic and sigma-1 receptor systems
through a novel mechanism of action.
ANAVEX
1037
ANAVEX
1037 is designed for the treatment of prostate cancer. It is a low molecular weight, synthetic compound exhibiting high affinity
for sigma-1 receptors at nanomolar levels and moderate affinity for sigma-2 receptors and sodium channels at micromolar levels.
In advanced pre-clinical studies, this compound revealed antitumor potential with no toxic side effects. It has also been shown
to selectively kill human cancer cells without affecting normal/healthy cells and also to significantly suppress tumor growth
in immune-deficient mice models. Scientific publications describe sigma receptor ligands positively, highlighting the possibility
that these ligands may stop tumor growth and induce selective cell death in various tumor cell lines. Sigma receptors are highly
expressed in different tumor cell types. Binding by appropriate sigma-1 and/or sigma-2 ligands can induce selective apoptosis.
In addition, through tumor cell membrane reorganization and interactions with ion channels, our drug candidates may play an important
role in inhibiting the processes of metastasis (spreading of cancer cells from the original site to other parts of the body),
angiogenesis (the formation of new blood vessels) and tumor cell proliferation.
Our
compounds are in the pre-clinical and clinical testing stages of development, and there is no guarantee that the activity demonstrated
in pre-clinical models will be shown in human testing.
Our
Target Indications
We
have developed compounds with potential application to two broad categories and several specific indications. The two categories
are diseases of the central nervous system, and cancer. Specific indications include:
•
Alzheimer’s disease — In 2015, an estimated 5.3 million Americans were suffering from Alzheimer’s disease.
The Alzheimer’s Association
®
reports that by 2025, 7.1 million
Americans will be afflicted by the disease, a 40 percent increase from currently affected patients. Medications on the market
today treat only the symptoms of AD and do not have the ability to stop its onset or its progression. There is an urgent and unmet
need for both a disease modifying cure for Alzheimer’s disease as well as for better symptomatic treatments.
•
Depression — Depression is a major cause of morbidity worldwide according to the World Health Organization (WHO).
Pharmaceutical treatment for depression is dominated by blockbuster brands, with the leading nine brands accounting for approximately
75% of total sales. However, the dominance of the leading brands is waning, largely due to the effects of patent expiration and
generic competition.
•
Epilepsy — Epilepsy is a common chronic neurological disorder characterized by recurrent unprovoked seizures. These
seizures are transient signs and/or symptoms of abnormal, excessive or synchronous neuronal activity in the brain. According to
the Centers for Disease Control and Prevention, epilepsy affects 2.2 million Americans. Today, epilepsy is often controlled, but
not cured, with medication that is categorized as older traditional anti-epileptic drugs and second generation anti epileptic
drugs. Because epilepsy afflicts sufferers in different ways, there is a need for drugs used in combination with both traditional
anti-epileptic drugs and second generation anti-epileptic drugs. GBI Research estimates that the epilepsy market will increase
to $4.5 billion by 2019.
4
•
Neuropathic Pain — We define neuralgia, or neuropathic pain, as pain that
is not related to activation of pain receptor cells in any part of the body. Neuralgia
is more difficult to treat than some other types of pain because it does not respond
well to normal pain medications. Special medications have become more specific to neuralgia
and typically fall under the category of membrane stabilizing drugs or antidepressants.
•
Malignant Melanoma — Predominantly a skin cancer, malignant melanoma can also occur in melanocytes found in the bowel
and the eye. Malignant melanoma accounts for 75% of all deaths associated with skin cancer. The treatment includes surgical removal
of the tumor, adjuvant treatment, chemo and immunotherapy, or radiation therapy. According to IMS Health the worldwide Malignant
Melanoma market is expected to grow to $4.4 billion by 2022.
•
Prostate Cancer — Specific to men, prostate cancer is a form of cancer that develops in the prostate, a gland in
the male reproductive system. The cancer cells may metastasize from the prostate to other parts of the body, particularly the
bones and lymph nodes. Drug therapeutics for Prostate Cancer are expected to increase to nearly $18.6 billion in 2017 according
to BCC Research.
•
Pancreatic Cancer — Pancreatic cancer is a malignant neoplasm of the pancreas. In the United States approximately
45,000 new cases of pancreatic cancer will be diagnosed this year and approximately 38,000 patients will die as a result of their
cancer. Sales predictions by GlobalData forecast that the market for the pharmaceutical treatment of pancreatic cancer in the
five largest European countries and the United States, will increase to $1.63 billion by 2017.
Corporate
Information
Our
principal executive office is located at 51 West 52
nd
Street, 7
th
Floor, New York, NY 10019-6163, and our telephone number is 844.689.3939. Our website address is
www.anavex.com
.
No information found on our website is part of this prospectus. Also, this prospectus may include the names of various government
agencies or the trade names of other companies. Unless specifically stated otherwise, the use or display by us of such other parties’
names and trade names in this prospectus is not intended to and does not imply a relationship with, or endorsement or sponsorship
of us by, any of these other parties.
The
Offerings
Primary
Offering
We
may offer and sell, from time to time, in one or more offerings, the securities that we describe in this prospectus having a total
initial offering price not exceeding $100,000,000 at prices and on terms to be determined by market conditions at the time of
any offering.
Secondary
Offering
On
October 21, 2015, the Company entered into a purchase agreement (the “
Purchase Agreement
”) with the Selling
Security Holder (such transaction, the “
Financing
”), pursuant to which the Selling Security Holder agreed to
purchase from us up to $50,000,000 of our Common Stock (subject to certain limitations) from time to time over a 36-month period.
In connection with the Financing, the Company also entered into a registration rights agreement with the Selling Security Holder
(the “
RRA
”) whereby the Company agreed to file a registration statement, of which this prospectus is a part,
with the U.S. Securities and Exchange Commission (“
SEC
”) covering the shares of the Company’s Common
Stock that may be issued to the Selling Security Holder under the Purchase Agreement. 6,754,609 shares of Common Stock are being
registered under the registration statement of which this prospectus is a part, in connection with the Company’s obligations
under the Purchase Agreement and the RRA.
After
the registration statement, of which this prospectus is a part, is declared effective, the Company may, from time to time and
at its sole discretion, direct the Selling Security Holder to purchase up to 50,000 shares of our Common Stock on any such business
day, provided that in no event shall the Selling Security Holder purchase more than $2,000,000 worth of our Common Stock on any
single business day, plus an additional “accelerated amount” under certain circumstances. Except as described in this
prospectus, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing
and amount of any sales of our Common Stock to the Selling Security Holder. The purchase price of the up to 50,000 shares that
may be sold to the Selling Security Holder under the Purchase Agreement on any business day will be based on the market price
of our Common Stock immediately preceding the time of sale as computed under the Purchase Agreement without any fixed discount.
The purchase price per share will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, forward or
reverse stock split, or other similar transaction occurring during the business days used to compute such price. We may at any
time in our sole discretion terminate the Purchase Agreement without fee, penalty or cost upon one business day’s notice.
5
The
Purchase Agreement contains customary representations, warranties, covenants, closing
conditions and indemnification and termination provisions by, among and for the benefit
of the parties. The Selling Security Holder has covenanted not to cause or engage in
any manner whatsoever, any direct or indirect short selling or hedging of the Company’s
Common Stock.
In
consideration for entering into the Purchase Agreement, the Company issued to the Selling Security Holder, 179,598 shares of Common
Stock as a commitment fee and shall issue up to 89,799 shares pro rata, which commitment fee shares are also being registered
hereunder, when and if, the Selling Security Holder purchases at the Company’s discretion the $50,000,000 aggregate commitment.
The Purchase Agreement may be terminated by the Company at any time at its discretion without any cost to the Company.
This
prospectus provides you with a general description of the securities we and the Selling Security Holder may offer. Each time we
offer a type or series of securities under this prospectus, we will provide a prospectus supplement that will describe the specific
amounts, prices and other important terms of the securities.
General
The
prospectus supplement also may add, update or change information contained in this prospectus or in documents we have incorporated
by reference into this prospectus. However, no prospectus supplement will fundamentally change the terms that are set forth in
this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
Where
You Can Find More Information
We
are subject to the information requirements of the Securities Exchange Act of 1934 (the “
Exchange
Act
”).
Accordingly, we file annual, quarterly and current reports, proxy statements as may be required and other information with the
SEC and filed a registration statement on Form S-3 under the Securities Act relating to the securities offered by this prospectus.
This prospectus, which forms part of the registration statement, does not contain all of the information included in the registration
statement. For further information, you should refer to the registration statement and its exhibits.
You
may read and copy the registration statement and any document we file with the SEC at the SEC’s Public Reference Room at
100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of
the Public Reference Room. You can also review our filings by accessing the website maintained by the SEC at
http://www.sec.gov
.
The site contains reports, proxy and information statements and other information regarding issuers that file electronically with
the SEC. In addition to the foregoing, we maintain a website at
http://www.anavex.com
. Our website content is made available
for informational purposes only. It should neither be relied upon for investment purposes nor is it incorporated by reference
into this prospectus. We make available at
http://www.anavex.com/investors/share-data/
copies of our Annual Reports on
Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K and any amendments to such document as soon as practicable
after we electronically file such material with or furnish such documents to the SEC.
6
SELECTED
FINANCIAL DATA
The
following selected financial data should be read in conjunction with our financial statements and the related notes contained
in Item 8 of Part II of our Annual Report on Form 10-K for the fiscal year ended September 30, 2015 and our interim consolidated
financial statements and the related notes contained in Item 1 of Part I of our Quarterly Report on Form 10-Q for the quarter
ended June 30, 2016, which are incorporated by reference into this prospectus, except that share and per share information for
the years ended September 30, 2015 and 2014 and for the nine months ended June 30, 2016 and 2015 have been revised to reflect
the reverse stock split of our issued and outstanding shares of Common Stock effective on October 7, 2015, at a ratio of 1 to
4. The selected data in this section is not intended to replace such financial statements, except that share and per share information
has been revised for the periods presented to reflect the reverse stock split at a ratio of 1 to 4.
We
have derived the statements of operations data for each of the years ended September 30, 2015 and 2014 and the balance sheet data
as of September 30, 2015 and 2014 from the audited consolidated financial statements contained in Item 8 of Part II of our Annual
Report on Form 10-K for the year ended September 30, 2015. The consolidated statement of operations data set forth below for the
nine months ended June 30, 2016 and 2015 and the consolidated balance sheet data as of June 30, 2016 has been derived from our
consolidated financial statements included in Item 1 of Part I of our Quarterly Report on Form 10-Q for the quarter ended June
30, 2016, which is incorporated by reference into this prospectus.
The
historical financial information set forth below may not be indicative of our future performance and should be read together with
“Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical consolidated
financial statements and notes to those statements included in Item 7 of Part II and Item 8 of Part II, respectively, of our Annual
Report on Form 10-K for the year ended September 30, 2015, “Management’s Discussion and Analysis of Financial Condition
and Results of Operations” and our historical financial statements and notes to those statements included in Item 2 of Part
I and Item 1 of Part I, respectively, of our Quarterly Report on Form 10-Q for the quarter ended June 30, 2016, and updates thereto
reflected in subsequent filings with the SEC, and all other annual, quarterly and other reports that we file with the SEC after
the date of this prospectus and that also are incorporated herein by reference.
|
|
|
Nine
months ended June 30,
|
|
|
|
|
|
|
|
Research
and development expenses
|
|
$
|
2,915,432
|
|
|
$
|
1,525,233
|
|
|
General
and administrative expenses
|
|
|
6,090,835
|
|
|
|
1,616,744
|
|
|
Net
loss
|
|
|
(8,225,666
|
)
|
|
|
(6,751,821
|
)
|
|
Net
loss per share, basic and diluted
|
|
|
(0.24
|
)
|
|
|
(0.44
|
)
|
|
Weighted
average number of common shares, basic and diluted
|
|
|
34,961,838
|
|
|
|
15,438,000
|
|
|
|
|
Year
ended September 30,
|
|
|
|
|
|
|
|
Research
and development expenses
|
|
$
|
2,271,736
|
|
|
$
|
732,395
|
|
|
|
|
|
|
|
|
|
|
|
|
General
and administrative expenses
|
|
|
4,836,978
|
|
|
|
2,236,580
|
|
|
Net
loss
|
|
|
(12,108,130
|
)
|
|
|
(9,968,353
|
)
|
|
Net
loss per share
|
|
|
|
|
|
|
|
|
|
Basic
|
|
|
(0.65
|
)
|
|
|
(1.02
|
)
|
|
Diluted
|
|
|
(0.65
|
)
|
|
|
(1.02
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
average number of common shares, basic and diluted
|
|
|
18,584,820
|
|
|
|
9,804,539
|
|
7
Selected
Balance Sheet Data
|
|
|
At
June 30, 2016
|
|
At
September 30,
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$9,727,040
|
|
$15,290,976
|
|
$7,262,138
|
|
Working
Capital
|
|
7,670,925
|
|
12,808,083
|
|
5,910,106
|
|
Total
Assets
|
|
9,843,973
|
|
15,469,913
|
|
7,353,502
|
|
Long-term
debt
|
|
345
|
|
332
|
|
263,727
|
|
Total
Stockholders’ Equity
|
|
|
7,671,086
|
|
|
12,809,003
|
|
|
192,626
|
|
|
|
|
|
|
|
|
|
|
|
8
R
ISK
FACTORS
An
investment in our securities which may be offered hereby is subject to numerous risks, including the risks described under the
caption “Risk Factors” in our Annual Report on Form 10-K for the year ended September 30, 2015, which is incorporated
by reference herein. You should carefully consider these risks, along with the information provided elsewhere in this prospectus
and the documents we incorporate by reference in this prospectus before investing in our securities. You could lose all or part
of your investment in the securities. You should consider these matters in conjunction with the other information included or
incorporated by reference in this prospectus. The risks and uncertainties described in this prospectus, any applicable prospectus
supplement and the documents incorporated by reference herein are not the only ones facing us. Additional risks and uncertainties
that we do not presently know about or that we currently believe are not material may also adversely affect our business. Our
business, results of operations or financial condition could be seriously harmed, and the trading price of our Common Stock may
decline due to any of these or other risks.
This
prospectus contains statements that constitute forward-looking statements within the meaning of the Private Securities Litigation
Reform Act of 1995. These statements appear in a number of places in this prospectus and include statements regarding the intent,
belief or current expectations of our management, directors or officers primarily with respect to our future operating performance.
Prospective purchasers of our securities are cautioned that these forward-looking statements are not guarantees of future performance
and involve risks and uncertainties. Actual results may differ materially from those in the forward-looking statements due to
various factors. The accompanying information contained in this prospectus, including the information set forth below, identifies
important factors that could cause these differences. See “Forward-Looking Statements” below.
Risks
Relating to the Purchase Agreement with the Selling Security Holder
The
sale or issuance of our Common Stock to the Selling Security Holder may cause dilution and the sale of the shares of Common Stock
acquired by the Selling Security Holder, or the perception that such sales may occur, could cause the price of our Common Stock
to fall.
On
October 21, 2015, we entered into the Purchase Agreement with the Selling Security Holder, pursuant to which the Selling Security
Holder has committed to purchase up to $50,000,000 of shares of our Common Stock. Concurrently with the execution of the Purchase
Agreement on October 21, 2015, we issued 179,598 shares of our Common Stock to the Selling Security Holder as a fee for its commitment
to purchase additional shares of our Common Stock under the Purchase Agreement. We have not issued additional shares of Common
Stock to the Selling Security Holder since the execution of the Purchase Agreement. Additional purchase shares that may be sold
pursuant to the Purchase Agreement may be sold by us to Lincoln Park at our discretion from time to time for a 36-month period,
commencing after the SEC declares the registration statement that this prospectus forms a part effective.
The
purchase price for the shares that we may sell to the Selling Security Holder under the Purchase Agreement will fluctuate based
on the price of our Common Stock. Depending on market liquidity at the time, sales of such shares may cause the trading price
of our Common Stock to fall.
We
generally have the right to control the timing and amount of any sales of our shares to the Selling Security Holder. Sales of
our Common Stock, if any, to the Selling Security Holder will depend upon market conditions and other factors to be determined
by us. Therefore, the Selling Security Holder may ultimately purchase all, some or none of the shares of our Common Stock that
may be sold pursuant to the Purchase Agreement and, after it has acquired shares, the Selling Security Holder may sell all, some
or none of those shares. Sales to the Selling Security Holder by us could result in substantial dilution to the interests of other
holders of our Common Stock. Additionally, the sale of a substantial number of shares of our Common Stock to the Selling Security
Holder, or the anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in
the future at a time and at a price that we might otherwise wish to effect sales.
We
may not be able to access sufficient funds under the Purchase Agreement with Lincoln Park when needed.
Our
ability to sell shares to Lincoln Park and obtain funds under the Purchase Agreement is limited by the terms and conditions in
the Purchase Agreement, including restrictions on when we may sell shares to Lincoln Park, restrictions on the amounts we may
sell to Lincoln Park at any one time, and a limitation on our ability to sell shares to Lincoln Park to the extent that it would
cause Lincoln Park to beneficially own more than 9.99% of our outstanding Common Stock. Therefore, we might not have access to
the full amount available to us under the Purchase Agreement. In addition, any amounts we sell under the Purchase
9
Agreement
may not satisfy all of our funding needs, even if we are able and choose to sell all $50,000,000 of our Common Stock under the
Purchase Agreement.
We
elected to enter into the Purchase Agreement with Lincoln Park as we expect that amount of capital over the next three years will
be required for us to fully implement our business, operating and development plans. The extent we rely on Lincoln Park as a source
of funding will depend on a number of factors including, the prevailing market price of our Common Stock and the extent to which
we are able to secure working capital from other sources. If obtaining sufficient funding from Lincoln Park were to prove unavailable
or prohibitively dilutive, we will need to secure another source of funding in order to satisfy our working capital needs. Even
if we sell all $50,000,000 of shares of our Common Stock under the Purchase Agreement, we may still need additional capital to
fully implement our business, operating and development plans. Should the financing we require to sustain our working capital
needs be unavailable or prohibitively expensive when we require it, the consequences could be a material adverse effect on our
business, operating results, financial condition and prospects.
10
DOCUMENTS
INCORPORATED BY REFERENCE
The
SEC allows us to “incorporate by reference” into this prospectus certain information that we file with the SEC, which
means that we can disclose important information to you by referring you to other documents separately filed by us with the SEC
that contain such information. The information we incorporate by reference is considered to be part of this prospectus and information
we later file with the SEC will automatically update and supersede the information in this prospectus. The following documents
filed by us with the SEC pursuant to Section 13(a) of the Exchange Act and any of our future filings under Sections 13(a), 13(c),
14 or 15 (d) of the Exchange Act, except for information furnished under Item 2.02 or 7.01 of Current Report on Form 8-K, or exhibits
related thereto, made before the termination of the offering are incorporated by reference herein:
(1)
our Annual Report on Form 10-K for the fiscal year ended September 30, 2015, filed with the SEC on December 29, 2015;
(2)
our Quarterly Reports on Form 10-Q for the fiscal periods ended: (i) December 31, 2015, as filed with the SEC on February
8, 2016; (ii) March 31, 2016, as filed with the SEC on May 11, 2016; and (iii) June 30, 2016, as filed with the SEC on August
11, 2016;
(3)
our Current Reports on Form 8-K, as filed with the SEC on September 28, 2015, October 6, 2015, October 7, 2015, October
26, 2015, May 6, 2016, May 11, 2016, June 23, 2016, July 7, 2016 and July 22, 2016;
(4)
all other reports filed pursuant to Section 13(a) or 15(d) of the Exchange Act and all proxy or information statements
filed pursuant to Section 14 of the Exchange Act since the end of the fiscal year covered by the Annual Report referenced in (1)
above; and
(5)
The description of our Common Stock contained in the Registration Statement on Form 8-A filed with the SEC on December
6, 2005.
In
addition, all documents subsequently filed by us pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act before the
date our offering is terminated or complete are deemed to be incorporated by reference into, and to be a part of, this prospectus.
We
will provide to each person, including any beneficial owner, to whom a prospectus is delivered, a copy of any or all of the reports
or documents that have been incorporated by reference in the prospectus contained in the registration statement but not delivered
with the prospectus, other than an exhibit to these filings unless we have specifically incorporated that exhibit by reference
into the filing, upon written or oral request and at no cost to the requester. Requests should be made by writing or telephoning
us at the following address:
Anavex
Life Sciences Corp.
51 West 52
nd
Street, 7
th
Floor
New York, NY 10019-6163
(844) 689-3939
11
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus contains forward-looking statements within the meaning of Section 27A of the Securities Act regarding our business,
financial condition, results of operations and prospects. Words such as “expects”, “anticipates”, “intends”,
“plans”, “believes”, “seeks”, “estimates” and similar expressions or variations
of such words are intended to identify forward-looking statements. However, these are not the exclusive means of identifying forward-looking
statements. Although forward-looking statements contained in this prospectus reflect our good faith judgment, such statements
can only be based on facts and factors currently known to us. Consequently, forward-looking statements are inherently subject
to risks and uncertainties, and actual outcomes may differ materially from the results and outcomes discussed in the forward-looking
statements. Further information about the risks and uncertainties that may impact us are described or incorporated by reference
in “Risk Factors” beginning on page 9. You should read that section carefully. You should not place undue reliance
on forward-looking statements, which speak only as of the date of this prospectus. We undertake no obligation to update publicly
any forward-looking statements in order to reflect any event or circumstance occurring after the date of this prospectus or currently
unknown facts or conditions or the occurrence of unanticipated events.
12
USE
OF PROCEEDS
Unless
otherwise specified in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities
described in this prospectus in connection with the primary offering for general corporate and operations purposes and to fund
our anticipated growth. The applicable prospectus supplement will provide more details on the use of proceeds of any specific
offering. We retain broad discretion in determining how we allocate the net proceeds received in connection with the primary offering.
However, we expect that such cash will be used to further our business plan of advancing human clinical trials of AVAVEX 2-73
and for general corporate and administrative purposes.
We
will not receive any proceeds from the sale of shares of our Common Stock by the Selling Security Holder named in such prospectus
supplement.
13
PLAN
OF DISTRIBUTION
Primary
Offering
We
may sell the securities described in this prospectus on a continuous or delayed basis directly to purchasers, through underwriters,
broker-dealers or agents that may receive compensation in the form of discounts, concessions or commissions from us or the purchasers
of the securities, in “at the market offerings” within the meaning of Rule 415(a)(4) of the Securities Act, to or
through a market maker or into an existing trading market, on an exchange, or otherwise or through a combination of any such methods
of sale. Discounts, concessions or commissions as to any particular underwriter, broker-dealer or agent may be in excess of those
customary in the types of transactions involved.
The
securities may be sold from time to time in one or more transactions at fixed prices, which may be changed from time to time,
at prevailing market prices at the time of sale, at varying prices determined at the time of sale or at negotiated prices. These
sales may be effected in transactions, which may involve crosses or block transactions:
•
on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of
sale;
•
in the over-the-counter market;
•
in transactions otherwise than on these exchanges or services or in the over-the-counter market; or
•
through the writing of options, whether the options are listed on an options exchange or otherwise.
Each
time that we use this prospectus to sell our securities, we shall also provide a prospectus supplement. For each series of securities,
the applicable prospectus supplement will set forth the terms of the offering including:
•
the public offering price;
•
the name or names of any underwriters, dealers or agents;
•
the purchase price of the securities;
•
the proceeds from the sale of the securities to us;
•
any underwriting discounts, agency fees, or other compensation payable to underwriters or agents;
•
any discounts or concessions allowed or reallowed or repaid to dealers; and
•
the securities exchanges on which the securities will be listed, if any.
If
we use underwriters in the sale of securities, the securities will be acquired by the underwriters for their own account. The
underwriters may then resell the securities in one or more transactions at a fixed public offering price or at varying prices
determined at the time of sale or thereafter. The securities may be either offered to the public through underwriting syndicates
represented by managing underwriters, or directly by underwriters. The obligations of the underwriters to purchase the securities
will be subject to certain conditions. The underwriters will be obligated to purchase all the securities offered if they purchase
any securities. The public offering price and any discounts or concessions allowed or re-allowed or paid to dealers may be changed
from time to time.
If
we use dealers in the sale of securities, we will sell securities to such dealers as principals. The dealers may then resell the
securities to the public at varying prices to be determined by such dealers at the time of resale. We may solicit offers to purchase
the securities directly, and we may sell the securities directly to institutional or other investors, who may be deemed underwriters
within the meaning of the Securities Act with respect to any resales of those securities. The terms of these sales will be described
in the applicable prospectus supplement. If we use agents in the sale of securities, unless otherwise indicated in the prospectus
supplement, they will use their reasonable best efforts to solicit purchases for the period of their appointment. Unless otherwise
indicated in a prospectus supplement, if we sell directly, no underwriters, dealers or agents would be involved. We will not make
an offer of securities in any jurisdiction that does not permit such an offer.
We
may grant underwriters who participate in the distribution of securities an option to purchase additional securities to cover
overallotments, if any, in connection with the distribution. Any underwriter may engage in overallotment, stabilizing
14
transactions,
short covering transactions and penalty bids in accordance with SEC orders, rules and regulations and applicable law. To the extent
permitted by applicable law and SEC orders, rules and regulations, an overallotment involves sales in excess of the offering size,
which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing
bids do not exceed a specified maximum. To the extent permitted by applicable law and SEC orders, rules and regulations, short
covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short
positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold
by the dealer is purchased in a covering transaction to cover short positions. Those activities may cause the price of the Common
Stock to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any
underwriters who are qualified market makers on the NASDAQ Stock Market may engage in passive market making transactions in our
Common Stock on the NASDAQ Stock Market in accordance with Rule 103 of Regulation M, during the business day prior to the pricing
of the offering, before the commencement of offers or sales of the Common Stock. Passive market makers must comply with applicable
volume and price limitations and must be identified as passive market makers. In general a passive market maker must display its
bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive
market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are
exceeded. The anti-manipulation rules under the Exchange Act may apply to sales of shares in the market. Furthermore, Regulation
M may restrict the ability of any person engaged in the distribution of the shares to engage in market-making activities for the
particular securities being distributed for a period of up to five business days before the distribution. The restrictions may
affect the marketability of the shares and the ability of any person or entity to engage in market-making activities for the shares.
Underwriters,
dealers and agents that participate in any distribution of securities may be deemed to be underwriters as defined in the Securities
Act. Any discounts, commissions or profit they receive when they resell the securities may be treated as underwriting discounts
and commissions under the Securities Act. Only underwriters named in the prospectus supplement are underwriters of the securities
offered in the prospectus supplement. We may have agreements with underwriters, dealers and agents to indemnify them against certain
civil liabilities, including certain liabilities under the Securities Act, or to contribute with respect to payments that they
may be required to make.
We
may authorize underwriters, dealers or agents to solicit offers from certain institutions whereby the institution contractually
agrees to purchase the securities from us on a future date at a specific price. This type of contract may be made only with institutions
that we specifically approve. Such institutions could include banks, insurance companies, pension funds, investment companies
and educational and charitable institutions. The underwriters, dealers or agents will not be responsible for the validity or performance
of these contracts.
Each
series of securities will be a new issue of securities. Our Common Stock is quoted on the Nasdaq Capital Market. Unless otherwise
specified in the applicable prospectus supplement, our securities (other than our Common Stock) will not be listed on any exchange.
It has not presently been established whether the underwriters, if any, of the securities will make a market in the securities.
If the underwriters make a market in the securities, such market making may be discontinued at any time without notice.
Agents,
dealers and underwriters may be entitled to indemnification by us against certain civil liabilities, including liabilities under
the Securities Act, or to contribution with respect to payments which the agents, dealers or underwriters may be required to make
in respect thereof. Agents, dealers or underwriters may be customers of, engage in transactions with, or perform services for
us and our subsidiaries in the ordinary course of business.
Secondary
Offering
The
Common Stock offered by this prospectus is being offered by the Selling Security Holder. The Common Stock may be sold or distributed
from time to time by the selling stockholder directly to one or more purchasers or through brokers, dealers, or underwriters who
may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at
negotiated prices, or at fixed prices, which may be changed. The sale of the Common Stock offered by this prospectus could be
affected in one or more of the following methods:
•
ordinary brokers’ transactions;
•
transactions involving cross or block trades;
15
•
through brokers, dealers, or underwriters who may act solely as agents;
•
“at the market” into an existing market for the Common Stock;
•
in other ways not involving market makers or established business markets, including direct sales to purchasers or sales
effected through agents;
•
in privately negotiated transactions; or
•
any combination of the foregoing.
In
order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed
brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for
sale in the state or an exemption from the state’s registration or qualification requirement is available and complied with.
The
Selling Security Holder is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
The
Selling Security Holder has informed us that it intends to use an unaffiliated broker-dealer to effectuate all sales, if any,
of the Common Stock that it may purchase from us pursuant to the Purchase Agreement. Such sales will be made at prices and at
terms then prevailing or at prices related to the then current market price. Each such unaffiliated broker-dealer will be an underwriter
within the meaning of Section 2(a)(11) of the Securities Act. The Selling Security Holder has informed us that each such broker-dealer
will receive commissions from the Selling Security Holder that will not exceed customary brokerage commissions. In compliance
with the guidelines of the Financial Industry Regulatory Authority, Inc., or FINRA, the maximum consideration or discount to be
received by any FINRA member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered
pursuant to this prospectus.
Brokers,
dealers, underwriters or agents participating in the distribution of the shares as agents may receive compensation in the form
of commissions, discounts, or concessions from the selling stockholder and/or purchasers of the Common Stock for whom the broker-dealers
may act as agent. The compensation paid to a particular broker-dealer may be less than or in excess of customary commissions.
Neither we nor the Selling Security Holder can presently estimate the amount of compensation that any agent will receive. We know
of no existing arrangements between the Selling Security Holder or any other stockholder, broker, dealer, underwriter or agent
relating to the sale or distribution of the shares offered by this prospectus. At the time a particular offer of shares is made,
a prospectus supplement, if required, will be distributed that will set forth the names of any agents, underwriters or dealers
and any compensation from the selling stockholder, and any other required information.
We
will pay the expenses incident to the registration, offering, and sale of the shares to the Selling Security Holder. We have agreed
to indemnify and certain other persons against certain liabilities in connection with the offering of shares of Common Stock offered
hereby, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute amounts required
to be paid in respect of such liabilities. Lincoln Park has agreed to indemnify us against liabilities under the Securities Act
that may arise from certain written information furnished to us by specifically for use in this prospectus or, if such indemnity
is unavailable, to contribute amounts required to be paid in respect of such liabilities.
Lincoln
Park has represented to us that at no time prior to the Purchase Agreement has or its agents, representatives or affiliates engaged
in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined in Rule 200 of Regulation
SHO of the Exchange Act) of our Common Stock or any hedging transaction, which establishes a net short position with respect to
our Common Stock. Lincoln Park has agreed that during the term of the Purchase Agreement, it, its agents, representatives or affiliates
will not enter into or effect, directly or indirectly, any of the foregoing transactions.
We
have advised the Selling Security Holder that it is required to comply with Regulation M promulgated under the Exchange Act. With
certain exceptions, Regulation M precludes the selling stockholder, any affiliated purchasers, and any broker-dealer or other
person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase
any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any
bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All
of the foregoing may affect the marketability of the securities offered by this prospectus.
Our
Common Stock is quoted on the Nasdaq Capital Market under the symbol “AVXL.”
When
we refer to “Selling Security Holder” in this prospectus, we mean the entity listed in the table below.
16
SELLING
SECURITY HOLDER
An
aggregate of up to 6,754,609 shares of Common Stock and up to 269,397 shares of Common Stock may be offered for sale and sold
from time to time pursuant to this prospectus by the Selling Security Holder. Except as may be set forth in any accompanying prospectus
supplement, we will pay all of the expenses in connection with the registration and the sale of the shares, other than selling
commissions and the fees and expenses of counsel and other advisors to the Selling Security Holder. We will not receive any proceeds
from the sale of shares by the Selling Security Holder.
On
October 21, 2015, the Company entered into the Purchase Agreement with the Selling Security Holder. In connection with the Financing,
the Company also entered into the RRA whereby the Company agreed to file a registration statement with the SEC covering the shares
of the Company’s Common Stock that may be issued to the Selling Security Holder under the Purchase Agreement. The shares
being registered hereunder are being registered pursuant to the terms of the RRA.
After
the SEC has declared effective this registration statement related to the Financing, the Company has the right, in its sole discretion
over a 36-month period, to sell to the Selling Security Holder up to an aggregate commitment of $50,000,000 of shares of Common
Stock. The Company controls the timing and amount of any future sales, if any, of shares of Common Stock to the Selling Security
Holder.
The
Purchase Agreement contains customary representations, warranties, covenants, closing conditions and indemnification and termination
provisions by, among and for the benefit of the parties. The Selling Security Holder has covenanted not to cause or engage in
any manner whatsoever, any direct or indirect short selling or hedging of the Company’s Common Stock.
In
consideration for entering into the Purchase Agreement, the Company issued to the Selling Security Holder, 179,598 shares of Common
Stock as a commitment fee and shall issue up to 89,799 shares pro rata, when and if the Selling Security Holder purchases at the
Company’s discretion the $50,000,000 aggregate commitment. For example, if we elect, at our sole discretion, to require
the Selling Security Holder to purchase $100,000 of our stock then we would issue 180 shares of the pro rata commitment fee which
is the product of $100,000 (the amount we have elected to sell) divided by $50,000,000 (the amount we can sell the Selling Security
Holder under the Purchase Agreement multiplied by 89,799 (the total number of pro rata commitment shares). The pro rata commitment
shares will only be issued pursuant to this formula as and when we elect at our discretion to sell stock to the Selling Security
Holder. The Selling Security Holder may not assign or transfer its rights and obligations under the Purchase Agreement. The Purchase
Agreement may be terminated by the Company at any time at its discretion without any cost to the Company.
Pursuant
to the Registration Rights Agreement, dated as of October 21, 2015, among the Selling Security Holder and the Company, the Selling
Security Holder has the right to request that the Company include their shares on the registration statement of which this prospectus
forms a part under the Securities Act. Under the Registration Rights Agreement, the Selling Security Holder has registration rights
with respect to the shares of Common Stock set forth in the table below.
As
of August 30, 2016, the Selling Security Holder beneficially owned, in the aggregate, 179,598 shares of Common Stock, or 0.50%
of our outstanding Common Stock. We cannot provide an estimate as to the number of shares of Common Stock that will be held by
the Selling Security Holder upon consummation of any offering or offerings covered by this prospectus because the Selling Security
Holder may offer some, all or none of their shares of Common Stock in any such offering or offerings. The Selling Security Holder
does not and has not within the past three years had, any position, office or material relationship with us or any of our predecessors
or affiliates.
|
|
|
Shares
Beneficially Owned Before this Offering
|
|
Percentage
of Outstanding Shares Beneficially Owned Before this Offering
|
|
No.
of Shares to be Registered in this Offering
|
|
Percentage
of Outstanding Shares Beneficially Owned After this Offering
|
|
Lincoln
Park Capital Fund, LLC
(1)
|
|
179,598
|
(2)
|
|
0.50
|
%
|
|
6,754,609
|
(4)
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
17
The
Lincoln Park Transaction
General
On
October 21, 2015, we entered into the Purchase Agreement and the Registration Rights Agreement with Lincoln Park. Pursuant to
the terms of the Purchase Agreement, Lincoln Park has agreed to purchase from us up to $50,000,000 of shares of our Common Stock
(subject to certain limitations). Pursuant to the terms of the Registration Rights Agreement, we have filed with the SEC the registration
statement that includes this prospectus to register for resale under the Securities Act the shares that have been or may be issued
to Lincoln Park under the Purchase Agreement.
After
the SEC has declared the registration statement effective, we may, from time to time over a 36-month period and at our sole discretion,
but no more frequently than every other business day, direct Lincoln Park to purchase 50,000 shares of our Common Stock on any
such business day, which amounts may be increased under certain circumstances, provided that in no event shall Lincoln Park purchase
more than $2,000,000 worth of our Common Stock on any single business day, plus an additional “accelerated amount”
under certain circumstances, at a purchase price per share based on the market price of our Common Stock immediately preceding
the time of sale as computed under the Purchase Agreement without any fixed discount. The amount of shares of our Common Stock
that we direct Lincoln Park to purchase depends on the closing price of our Common Stock.
The
Company has previously entered into transactions with Lincoln Park. On July 5, 2013, the Company entered into a purchase agreement
with Lincoln Park (the “
2013 Purchase Agreement
”) to purchase $10,000,000 shares, pursuant to which 2013 Purchase
Agreement the Company could sell and issue to Lincoln Park, and Lincoln Park was obligated to purchase, up to $10,000,000 in value
of its shares of Common Stock from time to time over a 25 month period. In connection therewith, the Company also entered into
a registration rights agreement with Lincoln Park whereby the Company agreed to file a registration statement with the Commission
covering the shares of the Company’s Common Stock that may be issued to Lincoln Park under the 2013 Purchase Agreement.
Under
the 2013 Purchase Agreement, the Company determined, at its own discretion, the timing and amount of its sales of Common Stock,
subject to certain conditions and limitations. The purchase price of the shares that were sold to Lincoln Park under the 2013
Purchase Agreement was based on the market price of the Company’s shares of Common Stock immediately preceding the time
of sale without any fixed discount, provided that in no event could such shares be sold to Lincoln Park when the closing sale
price was less than $0.50 per share ($2.00 on a post reverse split basis).
Pursuant
to the 2013 Purchase Agreement, Lincoln Park initially purchased 62,500 shares of the Company’s Common Stock for $100,000.
In consideration for entering into the 2013 Purchase Agreement, the Company issued to Lincoln Park 85,465 shares of Common Stock
as a commitment fee and issued up to 33,353 shares pro rata, when Lincoln Park purchased the remaining $10,000,000 aggregate commitment.
The sale of shares under the 2013 Purchase Agreement did not seem to have any discernable effect on the market price of the Company’s
stock. During the 2-week period subsequent to the effective date of the registration statement on Form S-1 relating to the 2013
Lincoln Park transaction, there was a decrease in the market price of the Company’s Common Stock. During this period, the Company
issued 75,489 shares of Common Stock to Lincoln Park, which represented 0.81% of the Company’s issued and outstanding shares as of
the effective date.
Purchase
of Shares Under the Purchase Agreement
Under
the Purchase Agreement, on any business day selected by us, we may direct Lincoln Park to purchase 50,000 shares of our Common
Stock on any such business day. On any day that the closing sale price of our Common Stock is not below $7.00 the purchase amount
may be increased, at our sole discretion, to up to 75,000 shares of our Common Stock per purchase; on any day that the closing
sale price of our Common Stock is not below $9.00 the purchase amount may be increased, at our
18
sole
discretion, to up to 100,000 shares of our Common Stock per purchase; and on any day that the closing sale price of our Common
Stock is not below $11.00 the purchase amount may be increased, at our sole discretion, to up to 125,000 shares of our Common
Stock per purchase and on any day that the closing sale price of our Common Stock is not below $13.00 the purchase amount may
be increased, at our sole discretion, to up to 150,000 shares of our Common Stock per purchase. Such purchases are hereinafter
referred to as “Regular Purchases”. In no event shall Lincoln Park purchase more than $2,000,000 worth of our Common
Stock pursuant to a Regular Purchase on any single business day. The purchase price per share for each such Regular Purchase will
be equal to the lower of:
•
the lowest sale price for our Common Stock on the purchase date of such shares; or
•
the arithmetic average of the three lowest closing sale prices for our Common Stock during the 10 consecutive business
days ending on the business day immediately preceding the purchase date of such shares.
In
addition to Regular Purchases described above, we may also direct Lincoln Park, on any business day on which we have properly
submitted a Regular Purchase notice, to purchase an additional amount of our Common Stock, which we refer to as an Accelerated
Purchase, not to exceed the lesser of:
•
30% of the aggregate shares of our Common Stock traded during normal trading hours on the purchase date; and
•
200% of the number of purchase shares purchased pursuant to the corresponding Regular Purchase.
The
purchase price per share for each such Accelerated Purchase will be equal to the lower of:
•
96% of the volume weighted average price during (i) the entire trading day on the purchase date, if the volume of shares
of our Common Stock traded on the purchase date has not exceeded a volume maximum calculated in accordance with the Purchase Agreement,
or (ii) the portion of the trading day of the purchase date (calculated starting at the beginning of normal trading hours) until
such time at which the volume of shares of our Common Stock traded has exceeded such volume maximum; or
•
the closing sale price of our Common Stock on the purchase date.
In
the case of both Regular Purchases and Accelerated Purchases, the purchase price per share will be equitably adjusted for any
reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction occurring during
the business days used to compute the purchase price.
The
Purchase Agreement limits our sales of shares of Common Stock to Lincoln Park to the maximum number of shares of our Common Stock
that we may issue without breaching our obligations under applicable rules of the NASDAQ Stock Market (approximately 6,754,609
shares, or 19.99% of our total outstanding Common Stock upon entering into the Purchase Agreement) or obtaining stockholder approval
under such rules.
Other
than as set forth above, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will control
the timing and amount of any sales of our Common Stock to Lincoln Park.
Events
of Default
Events
of default under the Purchase Agreement include the following:
•
the effectiveness of the registration statement of which this prospectus forms a part lapses for any reason (including,
without limitation, the issuance of a stop order), or any required prospectus supplement and accompanying prospectus are unavailable
for the resale by Lincoln Park of our Common Stock offered hereby, and such lapse or unavailability continues for a period of
10 consecutive business days or for more than an aggregate of 30 business days in any 365-day period;
•
suspension by our principal market of our Common Stock from trading for a period of three consecutive business days;
•
the delisting of the Common Stock from the OTCQB operated by the OTC Markets Group, Inc., provided, however, that the Common
Stock is not immediately thereafter trading on the New York Stock Exchange, The NASDAQ Capital Market, The NASDAQ Global Market,
The NASDAQ Global Select Market, the NYSE MKT, the NYSE
19
Arca,
the OTC Bulletin Board or the OTCQX operated by the OTC Markets Group, Inc. (or nationally recognized successor to any of the
foregoing). If at any time after the Commencement Date, the Exchange Cap (as defined in the Purchase Agreement) is reached unless
and until stockholder approval is obtained pursuant to Section 2(e) of the Purchase Agreement. The Exchange Cap shall be deemed
to be reached at such time if, upon submission of a Regular Purchase Notice or Accelerated Purchase Notice under this Agreement,
the issuance of such shares of Common Stock would exceed that number of shares of Common Stock which the Company may issue under
this Agreement without breaching the Company’s obligations under the rules or regulations of the Principal Market (as defined
in the Purchase Agreement);
•
the transfer agent’s failure for three business days to issue to Lincoln Park shares of our Common Stock which Lincoln
Park is entitled to receive under the Purchase Agreement;
•
any breach of the representations or warranties or covenants contained in the Purchase Agreement or any related agreement
which has or which could have a material adverse effect on us subject to a cure period of five business days; and
•
any voluntary or involuntary participation or threatened participation in insolvency or bankruptcy proceedings by or against
us; and
•
if at any time we are not eligible to transfer our Common Stock electronically or a material adverse change in our business,
financial condition, operations or prospects has occurred.
Lincoln
Park does not have the right to terminate the Purchase Agreement upon any of the events of default set forth above. During an
event of default, all of which are outside of Lincoln Park’s control, shares of our Common Stock cannot be sold by us or
purchased by Lincoln Park under the Purchase Agreement.
Our
Termination Rights
We
have the unconditional right, at any time, for any reason and without any payment or liability to us, to give notice to Lincoln
Park to terminate the Purchase Agreement. In the event of bankruptcy proceedings by or against us, the Purchase Agreement will
automatically terminate without action of any party.
No
Short-Selling or Hedging by Lincoln Park
Lincoln
Park has agreed that neither it nor any of its affiliates shall engage in any direct or indirect short-selling or hedging of our
Common Stock during any time prior to the termination of the Purchase Agreement.
Effect
of Performance of the Purchase Agreement on Our Stockholders
All
the shares of our Common Stock registered in this offering which may be sold by us to Lincoln Park under the Purchase Agreement
are expected to be freely tradable. It is anticipated that shares registered in this offering will be sold over a period of up
to 36 months commencing on the date that the registration statement including this prospectus becomes effective. The sale by Lincoln
Park of a significant amount of shares registered in this offering at any given time could cause the market price of our Common
Stock to decline and to be highly volatile. Lincoln Park may ultimately purchase all, some or none of the shares of Common Stock
registered in this offering that Lincoln Park has not previously purchased. Lincoln Park may sell all, some or none of the shares
it has purchased or will purchase under the Purchase Agreement. Therefore, sales to Lincoln Park by us under the Purchase Agreement
may result in substantial dilution to the interests of other holders of our Common Stock. In addition, if we sell a substantial
number of shares to Lincoln Park under the Purchase Agreement, or if investors expect that we will do so, the actual sales of
shares or the mere existence of our arrangement with Lincoln Park may make it more difficult for us to sell equity or equity-related
securities in the future at a time and at a price that we might otherwise wish to effect such sales. However, we have the right
to control the timing and amount of any sales of our shares to Lincoln Park and the Purchase Agreement may be terminated by us
at any time at our discretion without any cost to us.
20
Pursuant
to the terms of the Purchase Agreement, we have the right, but not the obligation, to direct Lincoln Park to purchase up to $50,000,000
of our Common Stock, exclusive of the 179,598 shares issued to Lincoln Park as a commitment fee. Depending on the price per share
at which we sell our Common Stock to Lincoln Park, we may be authorized to issue and sell to Lincoln Park under the Purchase Agreement
more shares of our Common Stock than are offered under this prospectus. If we choose to do so, we must first register for resale
under the Securities Act any such additional shares, which could cause additional substantial dilution to our stockholders. The
number of shares ultimately offered for resale by Lincoln Park under this prospectus is dependent upon the number of shares we
direct Lincoln Park to purchase under the Purchase Agreement.
The
following table sets forth the amount of gross proceeds we would receive from Lincoln Park from our sale of shares to Lincoln
Park under the Purchase Agreement registered in this offering at varying purchase prices:
Assumed
Average
Purchase Price
Per Share
|
|
Number
of Registered
Shares to be Issued
if Full Purchase
(1)(2)
|
|
Percentage
of
Outstanding Shares
After Giving Effect to
the Issuance to
Lincoln Park
(3)(5)
|
|
Proceeds
from the Sale
of Shares to Lincoln
Park Under the
$50,000,000
Purchase Agreement
|
|
$1.00
|
|
|
6,485,212
|
|
15.34%
|
|
|
$6,485,212
|
|
|
|
|
|
|
|
|
|
|
|
$2.00
|
|
|
6,485,212
|
|
15.34%
|
|
|
$12,970,424
|
|
|
|
|
|
|
|
|
|
|
|
$3.04
(4)
|
|
|
6,485,212
|
|
15.34%
|
|
|
$19,715,044
|
|
|
|
|
|
|
|
|
|
|
|
$5.00
|
|
|
6,485,212
|
|
15.34%
|
|
|
$32,426,060
|
|
|
|
|
|
|
|
|
|
|
|
$10.00
|
|
|
5,000,000
|
|
12.25%
|
|
|
$50,000,000
|
21
DESCRIPTION
OF OUR CAPITAL STOCK
Common
Stock
We
are authorized to issue 100,000,000 shares of Common Stock with a par value of $0.001. As at August 30, 2016 we had 35,710,862
common shares outstanding. Upon liquidation, dissolution or winding up of the corporation, the holders of Common Stock are entitled
to share ratably in all net assets available for distribution to stockholders after payment to creditors. The Common Stock is
not convertible or redeemable and has no preemptive, subscription or conversion rights. There are no conversion, redemption, sinking
fund or similar provisions regarding the Common Stock. Each outstanding share of Common Stock is entitled to one vote on all matters
submitted to a vote of stockholders. There are no cumulative voting rights.
Each
stockholder is entitled to receive the dividends as may be declared by our board of directors out of funds legally available for
dividends and, in the event of liquidation, to share pro rata in any distribution of our assets after payment of liabilities.
Our board of directors is not obligated to declare a dividend. Any future dividends will be subject to the discretion of our board
of directors and will depend upon, among other things, future earnings, the operating and financial condition of our Company,
its capital requirements, general business conditions and other pertinent factors. It is not anticipated that dividends will be
paid in the foreseeable future.
On
October 7, 2015, the Company effected a reverse stock split at a ratio of 1 for 4, whereby every 4 shares of Common Stock became
1 share of Common Stock. The authorized shares of Common Stock of the Company were therefore proportionally reduced from 400,000,000
to 100,000,000.
Our
Common Stock is quoted on the Nasdaq Capital Market under the trading symbol “AVXL”. On August 30, 2016, the last
reported sale price of our Common Stock was $3.04 per share.
Nevada
Anti-Takeover Law and Charter and Bylaws Provisions
Nevada
Revised Statutes sections 78.378 to 78.3793 provide state regulation over the acquisition of a controlling interest in certain
Nevada corporations unless the articles of incorporation or bylaws of the corporation provide that the provisions of these sections
do not apply. The statute creates a number of restrictions on the ability of a person or entity to acquire control of a Nevada
company by setting down certain rules of conduct and voting restrictions in any acquisition attempt, among other things. The statute
is limited to corporations that are organized in the state of Nevada and that have 200 or more shareholders, at least 100 of whom
are shareholders of record and residents of the State of Nevada; and do business in the State of Nevada directly or through an
affiliated corporation. Because of these conditions, the statute does not apply to our Company.
There
are no provisions in our articles of incorporation or our bylaws that would delay, defer or prevent a change in control of our
Company.
DESCRIPTION OF WARRANTS
We may issue Warrants from time to time
in one or more series for the purchase of our Common Stock. Warrants may be issued independently or together with any shares of
Common Stock or offered by any prospectus supplement and may be attached to or separate from Common Stock. Each series of Warrants
may be issued under a separate warrant agreement to be entered into between us and a warrant agent, or any other bank or trust
company specified in the related prospectus supplement relating to the particular issue of Warrants. A warrant agent may act as
our agent in connection with the Warrants and would not assume any obligation or relationship of agency or trust for or with any
holders of Warrants or beneficial owners of Warrants. The specific terms of any series of Warrants will be described in the applicable
prospectus supplement relating to that series of Warrants along with any general provisions applicable to that series of warrants.
The following is a
general description of the Warrants we may issue. The applicable prospectus supplement will describe the specific terms of any
issuance of Warrants. The terms of any Warrants we offer may differ from the terms described in this prospectus. As a result,
we will describe in the prospectus supplement the specific terms of the particular series of Warrants offered by that prospectus
supplement. Accordingly, for a description of the terms of a particular series of Warrants, you should carefully read this prospectus,
the applicable prospectus supplement, and the applicable warrant agreement, which will be filed as an exhibit to the registration
statement of which this prospectus forms a part.
Terms
.
If Warrants are offered
by us, the prospectus supplement will describe the terms of the Warrants, including the following if applicable to the particular
offering:
|
|
•
|
|
the title of the Warrants;
|
|
|
•
|
|
the total number of Warrants;
|
|
|
•
|
|
the number of shares of Common Stock purchasable upon exercise of
the Warrants to purchase Common Stock and the price at which such shares of Common Stock may be purchased upon exercise;
|
|
|
•
|
|
the date on and after which the Warrants and the related Common Stock
will be separately transferable;
|
|
|
•
|
|
if applicable, the date on which the right to exercise the Warrants
will commence and the date on which this right will expire;
|
|
|
•
|
|
if applicable, the minimum or maximum amount of the Warrants which
may be exercised at any one time;
|
|
|
•
|
|
a discussion of federal income tax, accounting and other special considerations,
procedures and limitations relating to the Warrants; and
|
|
|
•
|
|
any other terms of the Warrants including terms, procedures and limitations
relating to the exchange and exercise of the Warrants.
|
Warrants may be exchanged for new warrants
of different denominations, may be presented for registration of transfer, and may be exercised at our principal executive office,
the warrant agent or any other office indicated in the prospectus supplement.
Before the exercise of their Warrants,
holders of Warrants will not have any of the rights of holders of shares of Common Stock, including the right to receive payments
of dividends, if any, on the shares of Common Stock or to exercise any applicable right to vote.
Exercise of Warrants.
Each Warrant
will entitle the holder to purchase a number of shares of Common Stock at an exercise price as will in each case be described in, or calculable from, the prospectus supplement relating to those Warrants. Warrants may be exercised at the times set forth
in the prospectus supplement relating to the Warrants. After the close of business on the expiration date (or any later date to
which the expiration date may be extended by us), unexercised Warrants will become void. Subject to any restrictions and additional
requirements that may be set forth in the prospectus supplement relating thereto, Warrants may be exercised by delivery to the
warrant agent, or at our principal executive office or any other office indicated in the prospectus supplement, of the certificate
evidencing the Warrants properly completed and duly executed, and of payment as provided in the prospectus supplement of the amount
required to purchase the shares of Common Stock purchasable upon such exercise. The exercise price will be the price applicable
on the date of payment in full, as set forth in the prospectus supplement relating to the Warrants. Upon receipt of the payment,
and the certificate representing the Warrants to be exercised properly completed and duly executed at our principal executive
office, the warrant agent or any other office indicated in the prospectus supplement, we will, as soon as practicable, issue and
deliver the shares of Common Stock purchasable upon such exercise. If fewer than all of the Warrants represented by that certificate
are exercised, a new certificate will be issued for the remaining amount of Warrants.
The description in the applicable prospectus
supplement and other offering material of any Warrants we offer will not necessarily be complete and will be qualified in its
entirety by reference to the applicable warrant agreement, which will be filed with the SEC if we offer Warrants. For more information
on how you can obtain copies of the applicable warrant agreement if we offer Warrants, see “Where You Can Find More Information”.
We urge you to read the applicable warrant agreement and the applicable prospectus supplement in their entirety.
22
LEGAL
MATTERS
The
validity of the securities offered by this prospectus has been passed upon for us by Snell & Wilmer, L.L.P., Reno, Nevada.
If legal matters in connection with offerings made pursuant to this prospectus are passed upon by counsel for the underwriters,
dealers or agents, if any, such counsel will be named in the prospectus supplement relating to such offering.
EXPERTS
The
financial statements as of September 30, 2015 and 2014 and for each of the two years in the period ended September 30, 2015 incorporated
by reference in this prospectus have been so incorporated in reliance on the report of BDO USA, LLP, an independent registered
public accounting firm, incorporated herein by reference, given on the authority of said firm as experts in auditing and accounting.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION
Insofar
as indemnification for liabilities arising under the Securities Act, as amended, may be permitted to directors, officers, and
controlling persons of the registrant pursuant to the Company’s constituent documents, or otherwise, the registrant has
been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and
is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by
the registrant of expenses incurred or paid by a director, officer, or controlling person in the successful defense of any action,
suit, or proceeding) is asserted by such director, officer, or controlling person connected with the securities being registered,
we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will
be governed by the final adjudication of such issue.
23
Anavex
Life Sciences Corp.
Up
to $50,000,000
Common
Stock
PROSPECTUS
SUPPLEMENT

July 6, 2018
Anavex Life Sciences (NASDAQ:AVXL)
Historical Stock Chart
From Mar 2024 to Apr 2024
Anavex Life Sciences (NASDAQ:AVXL)
Historical Stock Chart
From Apr 2023 to Apr 2024