Report of Foreign Issuer (6-k)
June 22 2018 - 4:39PM
Edgar (US Regulatory)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
6-K
REPORT OF
FOREIGN PRIVATE ISSUER
PURSUANT TO RULE
13a-16
OR
15d-16
UNDER THE SECURITIES EXCHANGE ACT OF 1934
For the Month of June 2018
Commission File Number:
001-36697
DBV TECHNOLOGIES S.A.
(Translation of registrant’s name into English)
177-181
avenue Pierre Brossolette
92120 Montrouge France
(Address of principal executive office)
Indicate by check mark whether
the registrant files or will file annual reports under cover of Form
20-F
or Form
40-F:
☒ Form
20-F ☐ Form
40-F
Indicate by check mark if the registrant is submitting the Form
6-K
in paper as permitted by Regulation
S-T
Rule 101(b)(1): ☐
Indicate by check mark if the registrant is submitting the Form
6-K
in paper as permitted by Regulation
S-T
Rule 101(b)(7): ☐
EXHIBIT LIST
|
|
|
|
|
Exhibit
|
|
Description
|
|
|
|
|
99.1
|
|
Press Release dated June 22, 2018.
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned,
thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
|
DBV TECHNOLOGIES S.A.
|
|
|
|
|
|
|
Date: June 22, 2018
|
|
|
|
By:
|
|
/s/ David Schilansky
|
|
|
|
|
|
Name:
|
|
David Schilansky
|
|
|
|
|
|
Title:
|
|
Deputy Chief Executive Officer
|
Exhibit 99.1


DBV Technologies Announces Results of its 2018 Ordinary and Extraordinary General Meeting and the Appointment of Joan
Schmidt as Executive Vice President, General Counsel
Shareholders approve all proposed resolutions
DBV Technologies (Euronext: DBV – ISIN: FR0010417345 – Nasdaq Stock Market: DBVT), a clinical-stage biopharmaceutical company, today held its
Ordinary and Extraordinary General Meeting, which was chaired by Dr. Pierre-Henri Benhamou, Chairman & Chief Executive Officer of DBV Technologies. At the General Meeting, the Company’s shareholders approved all resolutions
submitted by the Board of Directors. These resolutions are posted on the Investors & Media section of the Company’s website,
https://www.dbv-technologies.com/investor-relations/
.
The Company also announced that Joan Schmidt has been appointed as Executive Vice President, General Counsel. She will be responsible for all legal affairs
and compliance at DBV, reporting to the Deputy Chief Executive Officer, David Schilansky. Joan will also serve as a member of the Executive Committee.
“
We are thrilled to welcome Joan as we continue to expand the breadth of talent of our leadership team,
” said
David Schilansky
, Deputy
CEO of DBV Technologies.
“Joan has shown a consistent track record of success, and her legal, business and industry background will be an asset as we advance potential new treatments for food allergies.”
Joan Schmidt joins DBV after three years at Biotronik, a global medical device company, where she was Executive Vice President, Legal & Human
Resources, General Counsel and Secretary. Previously, she held various positions of increasing responsibility at Novo Nordisk, most recently as Corporate Vice President, Legal Affairs. Earlier in her career, she was an associate in the litigation
group of a private law firm in New York, NY. Joan earned a J.D. from Pace University and a B.A. from the University of Connecticut.



Joan Schmidt
said,
“DBV’s mission to develop potentially transformative treatments for
patients with food allergies and other immunological diseases, with its novel Viaskin technology platform, is something I am passionate about. I look forward to building out a team that can support the potential launch of DBV’s first potential
product for the treatment of peanut allergy, if Viaskin Peanut is approved.”
About DBV Technologies
DBV Technologies is developing Viaskin
®
, a proprietary technology platform with broad potential
applications in immunotherapy. Viaskin is based on epicutaneous immunotherapy, or EPIT
®
, DBV’s method of delivering biologically active compounds to the immune system through intact skin.
With this new class of self-administered and
non-invasive
product candidates, the Company is dedicated to safely transforming the care of food allergic patients, for whom there are no approved treatments.
DBV’s food allergies programs include ongoing clinical trials of Viaskin Peanut and Viaskin Milk, and preclinical development of Viaskin Egg. DBV is also pursuing a human
proof-of-concept
clinical study of Viaskin Milk for the treatment of Eosinophilic Esophagitis, and exploring potential applications of its platform in vaccines and other
immune diseases. DBV Technologies has global headquarters in Montrouge, France and New York, NY. The Company’s ordinary shares are traded on segment A of Euronext Paris (Ticker: DBV, ISIN code: FR0010417345), part of the SBF120 index, and the
Company’s ADSs (each representing
one-half
of one ordinary share) are traded on the Nasdaq Global Select Market (Ticker: DBVT).
DBV Investor Relations Contact
Sara Blum Sherman
Senior Director, Investor Relations & Strategy
+1 212-271-0740
sara.sherman@dbv-technologies.com
DBV Media Contact
Raul Damas
Partner, Brunswick Group
+1-212-333-3810
DBV@brunswickgroup.com

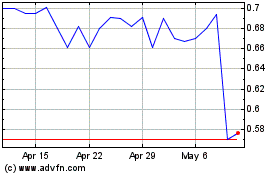
DBV Technologies (NASDAQ:DBVT)
Historical Stock Chart
From Mar 2024 to Apr 2024
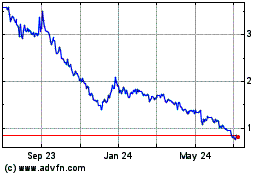
DBV Technologies (NASDAQ:DBVT)
Historical Stock Chart
From Apr 2023 to Apr 2024