UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10
Amendment Number 1
GENERAL FORM FOR REGISTRATION OF SECURITIES
Pursuant to Section 12(b) or (g) of the Securities Exchange Act of 1934
NUTRAFUELS, INC
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
Florida
|
|
46-1482900
|
|
State or Other Jurisdiction of
Incorporation or Organization)
|
|
(I.R.S. Employer
Identification Nos.)
|
|
6601 Lyons Road, Suite L-6, Coconut Creek Fl
|
|
33073
|
|
(Address of principal executive offices)
|
|
(Zip Code)
|
Registrant’s telephone number including area code:
888-509-8901
Securities registered pursuant to Section 12(b) of the Act: None
Securities to be registered pursuant to Section 12(g) of the Act: Common Stock, $.0001 par value per share
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
|
|
|
|
|
|
|
Large accelerated filer
|
|
Accelerated filer
|
|
Non-accelerated filer
(Do not check if a smaller reporting company)
|
|
Smaller reporting company
X
|
1
NUTRAFUELS, INC.
FORM 10
TABLE OF CONTENTS
Page
|
|
|
|
|
|
|
|
|
ITEM 1. BUSINESS
|
4
|
|
ITEM 1A. RISK FACTORS
|
16
|
|
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
24
|
|
ITEM 3. PROPERTIES
|
27
|
|
ITEM 4. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
27
|
|
ITEM 5. DIRECTORS AND EXECUTIVE OFFICERS
|
29
|
|
ITEM 6. EXECUTIVE COMPENSATION
|
31
|
|
ITEM 7. CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS AND DIRECTOR INDEPENDENCE
|
32
|
|
ITEM 8. LEGAL PROCEEDINGS
|
34
|
|
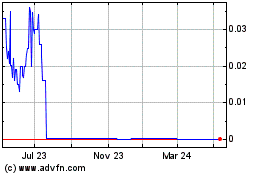
ITEM 9. MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
|
34
|
|
ITEM 10. RECENT SALES OF UNREGISTERED SECURITIES
|
38
|
|
ITEM 11. DESCRIPTION OF SECURITIES
|
48
|
|
ITEM 12. INDEMNIFICATION OF DIRECTORS AND OFFICERS
|
50
|
|
ITEM 13. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
50
|
|
ITEM 14. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
50
|
|
ITEM 15. FINANCIAL STATEMENTS AND EXHIBITS
|
51
|
2
Explanatory / Cautionary Notes
In this Registration Statement, unless otherwise indicated, the terms “Company”, “we”, “us”, and “our” refer to NutraFuels, Inc. We were previously a public reporting company but exited the reporting system, and at that time we were delinquent in meeting our periodic reporting obligations under the federal securities laws.
We are filing this Registration Statement on Form 10 under the Securities Exchange Act of 1934 ("Registration Statement") on a voluntary basis to become a public reporting company a second time in an attempt to provide current public information to the investment community.
Regarding Forward-Looking Statements
Certain of the matters we discuss in this Form 10 Registration Statement may constitute forward-looking statements. You can identify forward-looking statements because they contain words such as “believes”, “expects”, “may”, “will”, “should”, “seeks”, “approximately”, “intends”, “plans”, “estimates”, “anticipates”, or similar expressions which concern our strategy, plans or intentions. All statements we make relating to estimated and projected earnings, margins, costs, expenditures, cash flows, growth rates and financial results are forward-looking statements. In addition, we, through our senior management, from time to time make forward-looking public statements concerning our expected future operations and performance and other developments. All of these forward-looking statements are subject to risks and uncertainties that may change at any time, and, therefore, our actual results may differ materially from those we expected. We derive most of our forward-looking statements from our operating budgets and forecasts, which are based upon many detailed assumptions. While we believe that our assumptions are reasonable, we caution that it is very difficult to predict the impact of known factors, and, of course, it is impossible for us to anticipate all factors that could affect our actual results. There may be other factors not presently known to us or which we currently consider to be immaterial that may cause our actual results to differ materially from the forward-looking statements.
All forward-looking statements and projections attributable to us or persons acting on our behalf apply only as of the date of this Registration Statement and are expressly qualified in their entirety by the cautionary statements included in this Registration Statement. We undertake no obligation to publicly update or revise any written or oral forward-looking statements, made by us or on our behalf including any of the projections presented herein, to reflect events or circumstances after the date made or to reflect the occurrence of unanticipated events.
Emerging Growth Company
We are an emerging growth company under the JOBS Act. We shall continue to be deemed an emerging growth company until the earliest of:
·
The last day of our fiscal year during which we have had total annual gross revenues of $1,000,000,000 (as such amount is indexed for inflation every five (5) years by the Securities and Exchange Commission to reflect the change in the Consumer Price Index for All Urban Consumers published by the Bureau of Labor Statistics, setting the threshold to the nearest 1,000,000) or more;
·
The last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective IPO registration statement;
·
The date on which we have, during the previous three (3)-year period, issued more than $1,000,000,000 in non-convertible debt; or
·
The date on which we are deemed to be a ‘large accelerated filer’, as defined in section 240.12b-2 of title 17, Code of Federal Regulations, or any successor thereto.
3
As an emerging growth company, we are exempt from Section 404(b) of Sarbanes Oxley. Section 404(a) requires issuers to publish information in their annual reports concerning the scope and adequacy of the internal control structure and procedures for financial reporting. This statement shall also assess the effectiveness of such internal controls and procedures. Section 404(b) requires that the registered accounting firm shall, in the same report, attest to and report on the assessment and the effectiveness, of the internal control structure and procedures for financial reporting.
As an emerging growth company, we are also exempt from Section 14A (a) and (b) of the Securities Exchange Act of 1934 which requires the shareholder approval of executive compensation and golden parachutes. These exemptions are also available to us as a Smaller Reporting Company.
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2) of the JOBS Act, that allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies, until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
ITEM 1.
BUSINESS
Form and Year of Organization
NutraFuels, Inc, a Florida corporation (“us”, “we” or “our”) was formed as a limited liability company in the state of Florida on April 1, 2010, to engage in the development and distribution of
CBD (Cannabidiol)
oil products and nutritional and dietary oral spray products. On December 3, 2012, we converted from a Limited Liability Company to a Florida Corporation.
The address of our principal executive office and contact information are below:
NutraFuels, Inc.
6601 Lyons Road, L6
Coconut Creek
Florida 33073
Tel: 888-509-8901
Fax: 754-227-5970
Website: www.nutrafuels.com
Bankruptcy, Receivership or Similar Proceedings
We have not been involved in a bankruptcy receivership or similar proceeding. Additionally, we have not been involved in a reclassification, merger, consolidation, or purchase or sale of a significant amount of assets not in the ordinary course of business.
Organization
We were formed as a limited liability company in the state of Florida on April 1, 2010, to engage in the development and distribution of nutritional and dietary oral spray products. On December 3, 2012, we converted from a limited liability company to a Florida Corporation.
Our principal executive office is located at 6601 Lyons Road L 6, Coconut Creek, Florida 33073, and our telephone number is 888-509-8901. Our website is located at www.nutrafuels.com. Information contained in, or accessible through, our website does not constitute part of this Form 10 Registration Statement.
4
During the years ended December 31, 2015, December 31, 2016, and nine (9) months ended September 30, 2017, we received $1,191,700, $936,000, and
$1,379,500 respectively from the sale of our securities.
For the years ended December 31, 2015, December 31, 2016 and nine months ended September 30, 2017, our revenues were $134,006, $225,293 and $1,029,727, respectively from the sale of our products. For the years ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, we incurred net losses of $2,174,541, $2,098,771 and $23,202,212.
Our Business
Since our inception in April of 2010, we have engaged in the development, manufacturing, and distribution of nutritional and dietary products. In March of 2017, we completed development of our CBD products and commenced the distribution of these products. Our distribution strategy includes selling to private label retailers, distributors, and consumers through retail outlets. Our products are primarily sold to private label distributors which represented approximately 100%, 100% and 73.56% of our sales during the year ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, respectively.
Product Development
Our products are available as oral spray or tincture products and are designed to provide faster and more efficient absorption than pill or capsule formulas. Each product we offer is based upon the research of Edgar Ward, our Chief Executive Officer, President, and Sole Director, with the assistance of chemists, which we employ. For the year ended December 31, 2016 and nine (9) months ended September 30, 2017, we paid an aggregate of $44,625 and $29,468 to our in house chemists who assisted with the development of our CBD products.
Our products are and, in the future, will continue to be identified by Mr. Ward based upon suggestions from our customers, and from industry and market research he conducts on an ongoing basis. We do not employ medical professionals and our management does not have experience in the healthcare industry or in the treatment of disease. Our products have not been confirmed in any respect by the U.S. Food and Drug Administration or any other governmental agency, and may not produce the results intended.
Once developed, our products are manufactured at our facility in Coconut Creek, Florida. We obtain all raw materials and ingredients for our products from third party suppliers. For all orders we receive, we manufacture, package, label and ship the product to the customer. Our products are primarily sold to private label distributors who sell the products we manufacture under their own brand names. We do not enter into long term contracts with our private label distributors and all sales are made by purchase order. Our private label distributors are not obligated to order any amount of products from us and can discontinue purchasing our product at any time.
Our Hemp Based Products
In March of 2017, we completed development of our CBD
(Cannabidiol)
oil products. Our products have no THC and are parasite-free. Sales of our CBD products represented approximately seventy five percent (75%) of our sales during the nine (9) month period ended September 30, 2017.
Our CBD oil is derived from the seeds and mature stalks of the Cannabis Sativa plant which includes all parts and varieties of the cannabis sativa plant, which contain a tetrahydrocannabinol concentration (“THC”) that does not exceed 0.3 percent on a dry-weight basis." Under 21 U.S.C. § 802(16), the seeds (incapable of germination) and the mature stalks of the
Cannabis Sativa
plant, together with products made from these parts, are known as “Hemp Finished Products” and are exempted from the definition of cannabis and are legal for manufacture and over-the-counter sale to consumers.
5
On December 14, 2016, the DEA issued a final rule effective January 13, 2017 creating a separate Administration Controlled Substances Code Number for “Marihuana Extract” under Schedule I, defining it as “an extract containing one or more cannabinoids,” and stated that “all extracts that contain CBD will also contain at least small amounts of other cannabinoids.” Subsequent thereto, the DEA clarified that: (i) the new drug code (7350) does not include materials or products that are excluded from the definition of marijuana set forth in the Controlled Substances Act (“CSA”), (ii) the new drug code includes only those extracts that fall within the CSA definition of marijuana and (iii) if a product consisted solely of parts of the cannabis plant excluded from the CSA definition of marijuana, such product would not be included in the new drug code (7350) or in the drug code for marijuana (7360).
The CSA definition of marijuana states that, “Marijuana
does not include the mature stalks of such plant, fiber produced from such stalks, oil or cake made from the seeds of such plant, any other compound, manufacture, salt, derivative, mixture, or preparation of such mature stalks (except the resin extracted therefrom), fiber, oil, or cake, or the sterilized seed of such plant which is incapable of germination."
Because our CBD products are Hemp Finished Products derived from the seeds and mature stalks of the cannabis plant, our products are excluded from the CSA definition of marijuana, and as such, our operations are not impacted by the December 14, 2016 final rule.
Despite the foregoing, our operations are potentially subject to a complex web of Federal and state regulations that are evolving at a rapid rate. The DEA and other agencies may change their rules at any time. Should we become subject to DEA or other enforcement proceedings we would have to cease operations.
HempGenix Spray
On March 16, 2017, we completed development of our HempGenix product which is available in an oral spray and tincture drops. HempGenix represented seventy percent (70%) of our product sales for the nine (9) months ended September 30, 2017. HempGenix contains CBD oil and is designed to:
·
Support Pain Relief
·
Increase Energy & Focus
·
Aid in Sleep
·
Reduce Stress & Increase Relaxation
·
Aid in Weight-Loss
Hemp CBD Spray
On March 16, 2017, we completed development of our NutraHemp product
which is available in an oral spray and tincture drops. NutraHemp contains CBD oil and is designed to:
·
Support Pain Relief
·
Increase Energy & Focus
·
Aid in Sleep
·
Reduce Stress & Increase Relaxation
·
Aid in Weight-Loss
6
E-Vape Spray
On March 18, 2017, we completed development of our E-Vape product line which is available as a cartridge to be used with a battery operated vape inhaler. E-Vape contains CBD and is designed to:
·
Support Health and Wellness
·
Support Pain Relief
·
Increase Energy & Focus
·
Aid in Sleep
·
Reduce Stress & Increase Relaxation
·
Aid in Weight-Loss
Non-CBD Products
We presently manufacture and distribute the non-hemp based oral spray products below:
Sleep Support Spray
Our Sleep Spray represents approximately 12.4% of our product sales and is our highest selling product after our HempGenix product. Our Sleep Spray contains Melatonin, GABA, and Valerian Root. Our Sleep Spray is designed to support a healthy sleep cycle and improve the quality of restful sleep. The retail price of our Sleep Spray is $9.95 per .25 (¼) ounce.
Energy Boost Spray
Our Energize Spray contains B complex Vitamins, B12. Energize Spray is designed to increase energy and restore vigor and vitality. The retail price of our Energize Spray is $9.95 per .25 (¼) ounce.
Garcinia Cambogia Weight Loss Spray
Our Appetite and Weight Management Spray contains Garcinia Cambogia. Garcinia Cambogia Spray is designed to suppress the appetite and boost metabolism. The retail price of our Weight Loss Spray is $17.95 per three (3) pack of three (3) .25 (¼) ounce bottles.
Headache & Pain Spray
Our Headache and Pain Spray contains Turmacin, a natural anti-inflammatory. Our Headache and Pain Spray is designed to relieve headaches and pain. The retail price of our Headache and Pain Spray is $9.95 per .25 (¼) ounce.
Spa Treatment Hair, Skin & Nails Spray
Our Hair, Skin and Nails Spray contains Biotin, MSM, and Collagen. Our Hair, Skin and Nails Spray is designed to nourish and encourage hair, skin and nail growth. The retail price of our Hair, Skin and Nails Spray is $9.95 per .25 (¼) ounce.
7
Revenues
Our product revenues for our current products for the nine (9) months ended September 30, 2017 and years ended December 31, 2016 and December 31, 2015 and are summarized below.
|
|
|
|
|
Product
|
Nine (9) Months Ended 9/30/2017
|
Twelve (12) Months Ended 12/31/2016
|
Twelve (12) Months Ended 12/31/2015
|
|
Hemp Based Sprays
|
$
693,459
|
0
|
0
|
|
E-Vape
|
0
|
0
|
0
|
|
Non-Hemp Sleep Support
|
$
128,415
|
$
54,178
|
$
44,196
|
|
Non-Hemp Energy Boost Spray
|
$
70,842
|
$
152,820
|
$
45,756
|
|
Non-Hemp Garcinia Cambogia
Weight Loss Spray
|
$
64,597
|
$
16,892
|
$
42,543
|
|
Non-Hemp Headache & Pain Spray
|
$
62,983
|
$
103
|
0
|
|
Non-Hemp Spa Treatment Hair, Skin & Nails Spray
|
$
9,431
|
$
1,301
|
$
1,511
|
|
Total Sales by Product
|
$
1,029,727
|
$
225,293
|
$
134,006
|
Order Processing
Once developed, our products are manufactured at our facility in Coconut Creek, Florida. We obtain all raw materials and ingredients for our products from third party suppliers. For all orders we receive, we manufacture, package, label and ship the product to the customer. Our products are primarily sold to private label distributors who sell the products we manufacture under their own brand names. We do not enter into long term contracts with our private label distributors and all sales are made by purchase order. Our private label distributors are not obligated to order any amount of products from us and can discontinue purchasing our product at any time. We ship the product ordered within forty-five (45) days to our private label distributors, thirty (30) days to retail customers and within thirty (30) days to wholesale and third party (non-private label) distributors. All orders are shipped by freight delivery at the cost of the customer. All orders placed by My Daily Choice and our other three (3) private label distributors are by purchase order. We require a deposit of fifty (50%) upon an order being places. The balance must be paid by the purchaser prior to shipping.
Distributors
Our products are sold primarily through five (5) private-label distributors. Our three largest distributors, Breadfruit Tree, Inc, Organic By Nature and My Daily Choice represented approximately 73.56% of our total sales during the nine (9) month period ended September 30, 2017. As a result, our revenues are highly concentrated and we are dependent on orders from these three (3) distributors. Should any of these three (3) distributors cease ordering product from us, our revenues would decline or, if either decreased orders, our revenues and results of operations will be negatively affected.
8
We had direct sales of $194,807 and sales to private label distributors in the amount of $834,920 in the nine (9) month period ended September 30, 2017. Our product sales to private label distributors for the nine (9) month period ended September 30, 2017 and years ended December 31, 2016 and 2015 are summarized below:
|
|
|
|
|
|
|
|
Top Current Distributors
|
Sales by Distributor
for the (9) Months
Ended 9/30/2017
|
Percentage
of Total
Sales by Distributor
for the (9) Months
Ended 9/30/2017
|
Sales by
Distributor
for the Year
Ended 12/31/2016
|
Percentage
Of Total Sales
by Distributor
for the Year Ended 12/31/2016
|
Sales by Distributor
for the Year
Ended 12/31/2015
|
Percentage
Of Total Sales
By Distributor
for the Year
Ended
12/31/2015
|
|
My Daily Choice
(1)
|
127,359
|
13.58%
|
204,039
|
95.95%
|
119,514
|
94.74%
|
|
Breadfruit Tree Inc. doing business as NF Skin
(2)
|
562,538
|
59.98%
|
4,277
|
2.01%
|
0
|
0%
|
|
Life Bloom Organics
|
12,900
|
1.38%
|
0
|
0%
|
0
|
0%
|
|
Organic by Nature
|
150,728
|
16.07%
|
0
|
0%
|
0
|
0%
|
|
Oxzgen – 5 Linx, Inc.
|
84,394
|
9.0%
|
0
|
0%
|
0
|
0%
|
|
Other Distributor Sales
|
0
|
0%
|
4,325
|
2.03%
|
6,630
|
5.25%
|
|
Total Sales by Distributors
|
834,920
|
100.0%
|
212,641
|
100.0%
|
126,144
|
100.0%
|
(1) My Daily Choice is an Idaho company controlled by Josh Zwagil who holds 244,514 of our restricted common shares which he received for services rendered to us. As of the date hereof, Mr. Zwagil owns 244,514 restricted common shares
(2) BreadFruit Tree Inc. is a Florida corporation doing business as NF Skin, which is controlled by F. Bruce Hutson who holds 200,000 of our restricted common shares which he received for the price of $0.10 per share or an aggregate of $20,000. As of the date hereof, Mr. Hutson holds 200,000 restricted shares of our common stock.
Product Quality
In developing our products, we require:
·
ingredients that are supported with a certificate of analysis, publicly available scientific research and references which our Chief Executive Officer reviews with a chemist who assists in developing our final products;
·
ingredients that are combined so that their effectiveness is not impaired;
ingredients in our non-hemp based products that are in dosage levels that fall within tolerable upper intake levels established for healthy people by the Institute of Medicine of the National Academies;
·
products that do not contain adulterated ingredients such as ephedra, androstenedione, aspartame, steroids, or human growth hormones; and
·
formulations that have a minimum one-year shelf life.
9
Our Growth Strategy
Our primary growth strategy is to:
·
increase our product distribution and sales through increased private label distributors;
·
increase our margins by focusing on increasing our manufacturing capabilities while seeking operating efficiencies in our operations;
·
continue to conduct additional testing of the safety and efficacy of our products and formulate new products using CBD oil and other ingredients;
·
increase our sales through direct consumer sales, and
·
increase awareness of our products by increasing our marketing and branding opportunities through social media and sponsorships.
Return and Refund Policy
We will exchange any product found to be defective. A written exchange request must be submitted when a customer returns defective or damaged products. Purchasers can apply for a refund in the full amount of purchased products within ten (10) days of purchase. If the purchasers are not satisfied with our products for any reason, they can return products and request for an exchange. All shipping fees for product exchanges or returns must be paid by the purchaser. Historically, product returns as a percentage of our net sales have been nominal.
Our Core Marketing Strategy
Our core marketing strategy is to brand our manufacturing capabilities to private label distributors as the “must have” for Nutraceutical Sprays and tinctures for companies targeting the health conscious and workout enthusiasts as customers. We seek to be known as The Natural Spray Company, that creates high quality products for distributors and consumers in the health conscious and athletic markets. We believe that our marketing mix of social media promotions and providing sample products for our private label distributors to use is an optimal strategy to increase sales.
In 2016, we launched an advanced website at www.nutrafuels.com, seeking to tap into the social networking world and to further our product brand, direct consumer sales, private label distributor opportunities, and consumer awareness.
Patents and Trademarks
We received federal trademark registration for the expression “Spray your way to a healthier day!” that we use, or intend to use, to distinguish ourselves from others. All trademark registrations are protected for an initial period of five (5) years and then are renewable after five (5) years, if still in use, and every ten (10) years thereafter. We hold the following trade names from the U.S. Patent and Trademark office:
·
OralPro NutraSpray
·
NutraSpray
·
NRG X Spray
·
Micro Blast Body Slim
·
Micro Blast
·
Body Slim
·
NutraHemp CBD
·
Spray your way to a healthier day!
10
Material Agreements
On April 10, 2017, we entered into a strategic alliance agreement with Hall Global LLC (“HG”), a Florida limited liability company controlled by Michael Anderson. The agreement has a term of three (3) years and provides that for three (3) years and three (3) months we will manufacture products for HG in exchange for thirty-three point thirty-three percent (33.33%) of the proceeds of such products. Under the terms of the agreement, payment must be made to us within thirty (30) days after the end of each month. In connection with the agreement, on April 10, 2017 we issued 250,000 shares of our restricted common stock to Michael R. Anderson for services rendered as our Chief Scientific Officer. Additionally, we agreed to issue two million (2,000,000) shares of our restricted common stock to Mr. Anderson upon certain equipment being placed at our manufacturing facility. The agreement renews annually with additional consideration of one million (1,000,000) of our common shares. Additionally, we agreed to issue two hundred and fifty thousand (250,000) common shares to Mr. Anderson for our use and distribution of certain technologies including patented Blast Cap and Nutritional drinking straws.
Employees
We have ten (10) full time employees as follows:
·
Our Chief Executive Officer, President and Sole Director, Edgar Ward who oversees our day to day operations;
·
Three (3) full time Chemists;
·
One (1) supervisor of our manufacturing facility;
·
Four (4) employees who assist in our manufacturing facility; and
·
One (1) Executive Assistant.
Neil Catania, our Vice President, works closely with Edgar Ward and provides us with approximately ten (10) hours per month of services. We have no other part time employees. We hire independent contractors on an as needed basis.
None of our employees are employed under a collective bargaining agreement. We believe we have an excellent relationship with our employees and independent contractors.
Manufacturing
We manufacture one hundred percent (100%) of our products. By manufacturing our own products, we believe that we maintain better control over product quality and availability while also reducing production costs. We lease an aggregate of six thousand four hundred (6,400) square feet of office and warehouse space at 6601 Lyons Rd, Suites L-6&7, Coconut Creek, Florida 33073 Approximately five thousand eight hundred (5,800) square feet at his location is used for manufacturing, storage and distribution of our products.
On June 6, 2017, we entered into an agreement to lease nineteen thousand eight hundred and thirty one (19,831) square feet in Deerfield Beach, FL 33441. We plan to use seventeen thousand eight hundred (17,800) square feet of the new Deerfield Beach location for manufacturing, storage and distribution of our products. We expect to occupy this location in January 2018.
Our manufacturing process generally consists of the following operations: (i) sourcing ingredients for products, (ii) warehousing raw ingredients, (iii) efficacy testing and measuring ingredients for inclusion in products, and (iv) blending using automatic equipment. The next step, bottling and packaging, involves filling, capping, coding, labeling and placing the product in packaging with appropriate tamper-evident features then sending the packaged product to our customers.
11
Food and Drug Administration ("FDA") requires companies manufacturing homeopathic medicines to have their facilities certified as Good Manufacturing Practices ("GMPs"). Our non-hemp based nutritional products are subject to FDA regulation. Our manufacturing facility has been fully compliant with its GMP certification. Our quality control program seeks to ensure the superior quality of our products and that they are manufactured in accordance with current GMP. Our processing methods are monitored closely to ensure that only quality ingredients are used and to ensure product purity. Periodically, we retain the services of outside GMP audit firms to assist in our efforts to comply with GMPs.
Sources and Availability of Raw Materials
We obtain the raw materials for our Hemp Finished Products from two state licensed suppliers located in Kentucky. These raw materials consist of Cannabidiol rich oil and isolate. We obtain the raw materials from our non-hemp based products from several ingredient suppliers located in throughout the U.S. These raw materials consist of naturally derived vitamins and nutrients.
Raw materials used by us are available from a variety of suppliers. We maintain a good relationship with our suppliers and do not anticipate that any of our suppliers will terminate their relationship with us in the near term. We have ongoing relationships with secondary and tertiary suppliers. In the event, we are unable to obtain any of our raw materials from our suppliers; we believe that we could obtain alternative sources of any raw materials from other suppliers. We do not have contracts with our suppliers and we order our raw materials on an as needed basis. We have not experienced any material adverse effects on our business as a result of shortages of raw materials or packaging materials used in the manufacturing of our products. An unexpected interruption or a shortage in supply of raw materials could adversely affect our business derived from these products.
Backlog of Orders
We have no backlog of orders.
Seasonal Aspect of our Business
None of our products are affected by seasonal factors.
Status of any Publicly Announced New Product or Service
We do not have any publicly announced new product or service.
Competitive Business Conditions
The nutritional and dietary supplement industries are highly competitive. Nutritional supplements include vitamins, minerals, dietary supplements, herbs, botanicals and compounds derived therefrom. Numerous manufacturers and distributors compete with us for customers throughout the United States in the packaged nutritional supplement industry selling products to retailers such as mass merchandisers, drug store chains, independent pharmacies and health food stores. We are also vulnerable to competition from companies that can purchase similar products to our products and private label them with their own brand name.
Many of our indirect competitors are substantially larger, have more experience than us, have longer operating histories, and have materially greater financial and other resources than us.
Costs and Effects of Compliance with Environmental Laws
We are in a business that involves the use of raw materials in a manufacturing process, however, it is unlikely that such materials are likely to result in the violation of any existing environmental rules and/or regulations. Further, we do not own any real property that could lead to liability as a landowner. Therefore, we do not anticipate that there will be any material costs associated with compliance with environmental laws and regulations.
12
Government Approvals
We are not required to obtain governmental approval of our products.
Product Liability Insurance
We maintain commercial liability, including product liability coverage, and property insurance. Our policy provides for a general liability of five million dollars ($5,000,000) per occurrence, and five million dollars ($5,000,000) annual aggregate coverage which includes our main corporate facility. We carry property coverage on our main office facility to cover our legal liability, tenant’s improvements, business property, and inventory.
Government Regulation
Our operations are potentially subject to a complex web of Federal and state regulations that are evolving at a rapid rate. The DEA and FDA may change rules or enforcement proceedings at any time. We do not believe that current rules and enforcement have a significant potential impact because CBD does not cause the "high" associated with the THC in marijuana. As the legal landscape and understanding about the differences in medical cannabinoids unfolds, it will be increasingly important to distinguish “marijuana” certain CBD products (with noted varying degrees of psychotropic effects and deficits in executive function) from Hemp Finished Products.
The principal uncertainties are whether regulators will, at any time, attempt to treat Hemp Finished Products similarly to THC products.
Some states are considering various taxation of marijuana-related products. These considerations seem to range from routine sales taxes to taxes similar to those imposed on tobacco products. It is unclear whether products like those we offer containing no CBD would fall under these tax plans if and when they are imposed.
IRS section 280E prevents cannabis companies from deducting expenses from their income, except for those considered cost of goods sold. No deduction or credit is allowed for any amount paid or incurred during the taxable year in carrying on any trade or business if such trade or business (or the activities which comprise such trade or business) consists of trafficking in controlled substances (within the meaning of schedule I and II of the Controlled Substances Act) which is prohibited by Federal law or the law of any State in which such trade or business is conducted. If this section is enforced against the Company even though its products contain no THC or other illegal substance, it could create operating and cash flow problems in the future.
The formulation, manufacturing, packaging, labeling, advertising, and distribution of our non-hemp based products are subject to regulation by one or more federal agencies, principally the FDA, the Federal Trade Commission ("FTC"), and, to a lesser extent, the Consumer Product Safety Commission ("CPSC"), the United States Department of Agriculture (“USDA”), and the Environmental Protection Agency (“EPA”). Our activities are also regulated by various governmental agencies for the states and localities in which our products are sold, as well as by governmental agencies in certain countries outside the United States in which our products are sold. Among other matters, regulation by the FDA and FTC are concerned with product safety and claims made with respect to a product's ability to provide health-related benefits. Specifically, the FDA, under the Federal Food, Drug, and Cosmetic Act, ("FDCA"), regulates the formulation, manufacturing, packaging, labeling, distribution and sale of food, including dietary supplements, and over-the-counter drugs. The FTC regulates the advertising of these products. The National Advertising Division ("NAD") of the Council of Better Business Bureaus oversees an industry sponsored, self-regulatory system that permits competitors to resolve disputes over advertising claims. The NAD has no enforcement authority of its own, but may refer matters that appear to violate the Federal Trade Commission Act or the FDCA to the FTC or the FDA for further action, as appropriate.
13
Federal agencies, primarily the FDA and the FTC, have a variety of procedures and enforcement remedies available to them including initiating investigations, issuing warning letters and cease and desist orders, requiring corrective labeling or advertising, requiring consumer redress (for example, requiring that a company offer to repurchase products previously sold to consumers), seeking injunctive relief or product seizures, imposing civil penalties, or commencing criminal prosecution. In addition, certain state agencies have similar authority. These federal and state agencies have in the past used these remedies in regulating participants in the food, dietary supplement and over-the-•counter drug industries, including the imposition of civil penalties in the millions of dollars against a few industry participants.
The Dietary Supplement Health and Education Act ("DSHEA") was enacted in 1994, amending the FDCA. We believe DSHEA is generally favorable to consumers and to the dietary supplement industry. DSHEA establishes a statutory class of "dietary supplements”, which includes vitamins, minerals, herbs, amino acids and other dietary ingredients for human use to supplement the diet. Dietary ingredients marketed in the United States before October 15, 1994 may be marketed without the submission of a "new dietary ingredient" ("NDI") premarket notification to the FDA. Dietary ingredients not marketed in the United States before October 15, 1994 may require the submission, at least seventy-five (75) days before marketing of an NDI notification containing information establishing that the ingredient is reasonably expected to be safe for its intended use. Among other things, DSHEA prevents the FDA from regulating dietary ingredients in dietary supplements as "food additives" and allows the use of statements of nutritional support on product labels and in labeling. The FDA has issued final regulations under DSHEA and has issued draft guidance on NDI notification requirements. Further guidance and regulations are expected. Several bills to amend DSHEA in ways that would make this law less favorable to consumers and industry have been proposed in Congress.
The Nutrition Labeling and Education Act of 1990 ("NLEA”) amended the FDCA to establish additional requirements for ingredient and nutrition labeling and labeling claims for foods. If the NLEA labeling requirements change at a future time, we may need to revise our product labeling. Our non-CBD products are classified as dietary supplements. The FDA has concluded that THC and CBD products are excluded from the definition of a dietary supplement. The FDA issued a Final Rule on GMPs for dietary supplements on June 22, 2007. The GMPs cover manufacturers and holders of finished dietary supplement products, including dietary supplement products manufactured outside the United States that are imported for sale into the United States. Among other things, the new GMPs: (a) require identity testing on all incoming dietary ingredients, call for a "scientifically valid system" for ensuring finished products meet all specifications, (b) include requirements related to process controls, including statistical sampling of finished batches for testing and requirements for written procedures, and (c) require extensive recordkeeping.
We have reviewed the GMPs and have taken steps to ensure compliance. While we believe we are in compliance, there can be no assurance that our operations or those of our suppliers will be in compliance in all respects at all times. Additionally, there is a potential risk of increased audits as the FDA and other regulators seek to ensure compliance with the GMP’s.
On December 22, 2006, Congress passed the Dietary Supplement and Nonprescription Drug Consumer Protection Act, which went into effect on December 22, 2007. The law requires, among other things, that companies that manufacture or distribute nonprescription drugs or dietary supplements report serious adverse events allegedly associated with their products to the FDA and institute recordkeeping requirements for all adverse events (serious and non-serious). There is a risk that consumers, the press and government regulators could misinterpret reported serious adverse events as evidence of causation by the ingredient or product complained of, which could lead to additional regulations, banned ingredients or products, increased insurance costs and a potential increase in product liability litigation, among other things.
The Consumer Product Safety Improvement Act of 2008 ("CPSIA") primarily addresses children's product safety but also improves the administrative process of the CPSC. Among other things, the CPSIA requires testing and certification of certain products and enhances the CPSC's authority to order recalls.
14
The FDA Food Safety Modernization Act ("FSMA"), enacted January 4, 2011, amended the FDCA to significantly enhance the FDA's authority over various aspects of food regulation. The FSMA granted the FDA mandatory recall authority when the FDA determines if there is reasonable probability that a food is adulterated or misbranded and that the use of, or exposure to, the food will cause serious adverse health consequences or death to humans or animals. Other changes include the FDA's expanded access to records; the authority to suspend food facility registrations and require high risk imported food to be accompanied by a certification; stronger authority to administratively detain food; the authority to refuse admission of an imported food if it is from a foreign establishment to which a U.S. inspector is refused entry for an inspection; and the requirement that importers verify that the foods they import meet domestic standards.
One of the FSMA's more significant changes is the requirement of hazard analysis and
risk-based preventive controls ("HARBPC") for all food facilities required to register with the FDA, except dietary supplement facilities in compliance with both GMPs and the serious adverse event reporting requirements. Although dietary supplement facilities are exempt from the HARBPC requirements, dietary ingredient facilities might not qualify for the exemption. The HARBPC requirements, which the FDA has yet to propose, are expected to be onerous because facilities will have to develop and implement preventive controls to assure that identified hazards are significantly minimized or prevented, monitor the effectiveness of the preventive controls and maintain numerous records related to the HARBPC. The HARBPC requirements may increase the costs of dietary ingredients and/or affect our ability to obtain dietary ingredients.
As required by Section 113(b) of the FSMA, the FDA published in July 2011, a draft guidance document clarifying when the FDA believes a dietary ingredient is an NDI, when a manufacturer or distributor must submit an NDI premarket notification to the FDA, the evidence necessary to document the safety of an NDI and the methods for establishing the identity of an NDI. The draft guidance, if implemented as proposed, could have a material impact on our operations. Although our industry has strongly objected to several aspects of the draft guidance, it is unclear whether the FDA will make changes to the final guidance. In addition, it is possible that the FDA will begin taking enforcement actions consistent with the interpretations in the draft guidance before issuing a final version.
The new FSMA requirements, as well as the FDA enforcement of the NDI guidance as written, could require us to incur additional expenses, which could be significant, and negatively impact our business in several ways, including, but not limited to, the detention and refusal of admission of imported products, the injunction of manufacturing of any dietary ingredients or dietary supplements until the FDA determines that such ingredients or products are in compliance and the potential imposition of fees for re-inspection of noncompliant facilities. Each of these events would increase our liability and could have a material adverse effect on our financial
condition, results of operations or cash flows.
The FTC and the FDA have pursued a coordinated effort to challenge what they consider to be unsubstantiated and unsafe weight-loss products, and have also coordinated enforcement against dietary supplement claims in other areas, including children's products. Their efforts to date have focused on manufacturers and marketers as well as media outlets, and have resulted in a significant number of investigations and enforcement actions, some resulting in civil penalties of several million dollars under the Federal Trade Commission Act. We expect that the FTC and the FDA will continue to focus on health-related claims for dietary supplements and foods which could cause our non-CBD products to be the subject of an FTC/FDA inquiry.
15
ITEM 1A. RISK FACTORS
Risks Related to our Financial Condition.
We are dependent on the sale of our securities to fund our operations.
During the years ended December 31, 2015, December 31, 2016, and nine (9) months ended September 30, 2017, we received $1,191,700, $936,000,
and $1,375,500 from the sale of our securities.
For the years ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, our revenues were $134,006, $225,293 and $1,029,727, respectively from the sale of our products. Our cash on hand as of the day of this Form 10 Registration Statement is $216,000. Our operating expenses are presently approximately $115,000 per month or $1,380,000 annually which consist of rent, advertising, salaries and other general and administrative expenses. Once this Form 10 Registration Statement becomes effective, our monthly expenses will increase to $121,250 or $1,455,000 annually because of our operating expenses of $115,000 per month ($1,380,000 annually) and costs of being an SEC reporting company of $6,250 per month ($75,000 annually). We do not have any arrangements for future financing. We are dependent on the sale of our securities to help fund our operations. There is no assurance we will be able to obtain future funding for our operations from the sale of our securities. The future issuance of our securities will result in substantial dilution in the percentage of our common stock held by our then existing stockholders, and would likely have an adverse effect on any trading market for our common stock. Obtaining financing would be subject to a number of factors, including investor acceptance. These factors may adversely affect the timing, amount, terms, or conditions of any financing that we may obtain or make any additional financing unavailable to us. If we do not obtain additional financing to fund our future operations, our business could fail and you could lose your investment.
There is substantial doubt about our ability to continue as a going concern as a result of our limited operating history and financial resources, and if we are unable to generate significant revenue or secure financing we may be required to cease or curtail our operations.
For the years ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, we incurred net losses of $2,174,541, $2,098,771 and $23,202,212. As a result, our auditor has rendered an opinion that we may be unable to continue as a going concern. Our limited operating history and financial resources raises substantial doubt about our ability to continue as a going concern and our financial statements contain a going concern qualification. Our financial statements do not include adjustments that might result from the outcome of this uncertainty and if we are unable to generate significant revenue or secure financing we may be required to cease or curtail our operations.
We have limited historical performance for you to base an investment decision upon, and we may never become profitable.
For the years ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, our revenues were $134,006, $225,293 and $1,029,727. Accordingly, we have limited historical performance upon which you may evaluate our prospects for achieving our business objectives and becoming profitable in light of our operating losses and the risks, difficulties and uncertainties frequently encountered by companies with limited operations such as us. Accordingly, before investing in our common stock, you should consider the challenges, expenses and difficulties that we will face as an early stage company, and whether we will ever become profitable.
16
If we are unable to generate sufficient revenues for our operating expenses we will need financing, which we may be unable to obtain; should we fail to obtain sufficient financing, our potential revenues will be negatively impacted.
For the years ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, our revenues were $134,006, $225,293 and $1,029,727, respectively from the sale of our products. For the years ended December 31, 2015, December 31, 2016 and nine (9) months ended September 30, 2017, we incurred net losses of $2,174,541, $2,098,771 and $23,202,212.
Because we have limited revenues and lack historical financial data, including revenue data, our future revenues are unpredictable. Our operating expenses are presently approximately $115,000 per month or $1,380,000 annually to meet our existing operational costs, which consist of rent, advertising, salaries and other general and administrative expenses. Once this Form 10 Registration Statement becomes effective, our monthly expenses will increase to $121,250 or $1,455,000 annually because of our operating expenses of $115,000 per month ($1,380,000 annually) and costs of being an SEC reporting company of $6,250 per month ($75,000 annually).
As of the filing of this Form 10 Registration Statement, we had $216,000 of cash and cash equivalents for our operational needs. If we fail to generate sufficient revenues to meet our monthly operating costs of $121,250 we will not have available cash for our operating needs after approximately two (2) months. In the future, we may require additional debt or equity funding to continue our operations.
We intend to raise additional funds from an offering of our stock in the future; however, this offering may never occur, or if it occurs, we may be unable to raise the required funding. Further new offerings of our common shares will dilute our existing shareholders and your investment in our common shares. We do not have any plans or specific agreements for new sources of funding and we have no agreements for financing in place.
Our liabilities could adversely affect our ability to raise additional capital to fund our operations, limit our ability to react to changes in the economy or our industry and prevent us from meeting our obligations under our indebtedness.
As of September 30, 2017, our total liabilities were $8,824,895, consisting principally of $8,464,463 of a liability to issue shares of our common stock to our CEO under his employment agreement. Our liabilities could have important consequences for our investors, including: making it more difficult for us to make payments on indebtedness; increasing our vulnerability to general economic and industry conditions; requiring a substantial portion of cash flow from operations to be dedicated to the payment of principal and interest on indebtedness when our indebtedness become due. This reduces our ability to use our cash flow to fund our operations, capital expenditures and future business opportunities; limiting our ability and the ability of our subsidiaries to obtain additional financing for working capital, capital expenditures, product development, debt service requirements, acquisitions and general corporate or other purposes; and limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors which have fewer liabilities. We may incur substantial additional indebtedness in the future. If new indebtedness is added to our current debt levels, the related risks that we face could increase.
We may not be able to comply with the reporting obligations of the Securities & Exchange Commission which would have a negative impact on us.
We were previously a public company which filed reports with the Securities & Exchange Commission (“SEC”). We exited the reporting system, and at that time we were delinquent in meeting our periodic reporting obligations under the federal securities laws. Although we plan to comply with our periodic reporting obligations, there is no assurance that we will do so in the future because of the complexity of the federal securities laws, the accounting, auditing and legal costs of SEC reporting and management time required for such compliance.
17
Should we fail to comply with the SEC’s reporting requirements, we could be subject to SEC enforcement action that could result in penalties and fines against us. This would harm our financial condition and make it difficult or impossible for you to sell your shares. Further, should we not comply with the SEC reporting requirements, there will be limited public information available about us which could make it more difficult for you to sell your shares.
Risks Related to Our Business
We have been growing rapidly and expect to continue to invest in our growth for the foreseeable future. If we are unable to manage our growth effectively, our revenue and profits could be adversely affected.
We have experienced rapid growth in a relatively short period of time. Our revenue grew from $$225,293 for the year ended December 31, 2016 to $1,029,727 for the nine (9) months ended September 30, 2017.
We plan to continue to expand our operations, and we anticipate that further significant expansion which will be required. which will place additional demands on our resources and operations. Our future operating results depend to a large extent on our ability to manage this expansion and growth successfully. Sustaining our growth will place significant demands on our management as well as on our administrative, operational, and financial resources. To manage our growth, we must continue to improve our sales and manage our operational systems. If we are unable to manage our growth successfully, our revenue and profits could be harmed. Risks that we face in undertaking future expansion include:
·
effectively recruiting, integrating, training, and motivating a new employees, including our direct sales force, while retaining existing private label distributors and effectively executing our new business plan focusing on the sale of CBD products;
·
satisfying existing customers and attracting new customers of our products;
·
introducing new products and services;
·
Increasing our private label distributors
·
controlling expenses and investments in expanded operations including our new manufacturing facility;
·
implementing and enhancing our administrative, operational, and financial infrastructure, systems, and processes; and
·
addressing new products to meet consumer preferences.
A failure to manage our growth effectively could harm our business, operating results, financial condition. Further, due to our recent rapid growth, we have limited experience operating at our current scale and potentially at a larger scale, and as a result, it may be difficult for us to fully evaluate future prospects and risks. Our recent and historical growth should not be considered indicative of our future performance. We have encountered in the past, and will encounter in the future, risks and uncertainties frequently experienced by growing companies in rapidly changing industries. If our assumptions regarding these risks and uncertainties, which we use to plan and operate our business, are incorrect or change, or if we do not address these risks successfully, our financial condition and operating results could differ materially from our expectations, our growth rates may slow and our business would by adversely impacted.
18
Our operating results may fluctuate between periods, which makes our future results difficult to predict.
Our operating results have fluctuated in the past and may fluctuate in the future. Additionally, we have a limited operating history and in March of 2017, started selling CBD products which represent seventy five percent (75%) of our revenues for the nine (9) month period ended September 30, 2017, which makes it difficult to forecast our future results. As a result, you should not rely upon our past quarterly operating results as indicators of future performance. You should take into account the risks and uncertainties frequently encountered by companies in rapidly evolving markets. Our operating results in any given quarter can be influenced by numerous factors, many of which are unpredictable or are outside of our control, including:
·
our ability to generate significant revenue from our products;
·
our ability to maintain and grow our distributors and direct customer base;
·
the development and introduction of new products and services by us or our competitors;
·
increases in and timing of operating expenses that we may incur to grow and expand our operations and to remain competitive;
·
the impact of existing and new regulation of the CBD industry which is uncertain;
Our revenues are highly dependent upon three private label distributors, which represented
73.56%, 100%, and 100% of our revenues for the nine (9) months ended September 30, 2017 and years ended December 31, 2016 and 2015 and should this distributor reduce its orders from us or should we lose this distributor; our revenues and results of operations would be negatively affected which could cause you to lose your investment.
Our revenues are highly dependent on private label distributors which represented 73.56%,
100%, and 100% of our revenues for the nine (9) months ended September 30, 2017 and years ended December 31, 2016 and 2015. As a result, our revenues are highly concentrated. We have no agreement obligating this distributor to purchase our products. As such, this distributor can cease ordering products from us at any time without notice. Should this occur our revenues and results of operations will be negatively affected which could cause you to lose your investment in our common shares.
Any potential growth in the cannabis or cannabidiol-related industries continues to be subject to new and changing state and local laws and regulations.
Our products are made from Hemp Finished Products. Under 21 U.S.C. § 802(16), the seeds (incapable of germination) and the mature stalks of the Cannabis sativa plant, together with products made from these parts, are known as Hemp Finished Products and are exempted from the definition of cannabis and are legal. Continued development of the cannabis and cannabidiol related industries is dependent upon continued legislative legalization of cannabis and cannabidiol related products at the state level, and a number of factors could slow or halt progress in this area, even where there is public support for legislative action. Any delay or halt in the passing or implementation of legislation for the re-criminalization or restriction of cannabidiol at the state level could negatively impact our business because of the perception that it is related to cannabidiol. Additionally, changes in applicable state and local laws or regulations could restrict the products and services we offer or impose additional compliance costs on us or our customers. Violations of applicable laws, or allegations of such violations, could disrupt our business and result in a material adverse effect on our operations. We cannot predict the nature of any future laws, regulations, interpretations or applications, and it is possible that regulations may be enacted in the future that will have a material adverse effect on our business.
19
We recently entered the CBD Market and as a result, we are subject to numerous potential regulatory matters, which could negatively impact our operations.
The Drug Enforcement Administration (“DEA”) which enforces the controlled substances laws of the United States has issued various rules and announcements concerning various items considered to be marijuana extracts which may encompass Cannabinoids. The uncertainty involves the extent to which the DEA will try to restrict the marketing or distribution of hemp finished/CBD products which we manufacture and distribute. For the period ended September 30, 2017, 73.56% of our revenues were derived from the sale of CBD products. If the DEA were to take any action concerning our CBD products, it would have a negative impact on our revenues and financial condition.
Because we are subject to numerous laws and regulations we could incur substantial costs.
The manufacture, labeling and distribution of the products that we distribute is regulated by various federal, state and local agencies. These governmental authorities may commence regulatory or legal proceedings, which could restrict the permissible scope of our product claims or the ability to sell our products in the future. The FDA regulates our products to ensure that the products are not adulterated or misbranded.
We are subject to regulation by the DEA and other agencies as a result of our CBD products. The shifting compliance environment and the need to build and maintain robust systems to comply with different compliance in multiple jurisdictions increase the possibility that we may violate one or more of the requirements. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our financial results.
Failure to comply with FDA requirements may result in, among other things, injunctions, product withdrawals, recalls, product seizures, fines and criminal prosecutions. Our advertising is subject to regulation by the FTC under the FTCA. In recent years, the FTC has initiated numerous investigations of dietary and nutrition supplement products and companies. Additionally, some states also permit advertising and labeling laws to be enforced by private attorney generals, who may seek relief for consumers, seek class action certifications, seek class wide damages and product recalls of products sold by us. Any actions against us by governmental authorities or private litigants could have a material adverse effect on our business, financial condition and results of operations.
If the products we sell do not have the healthful effects intended, our business may suffer.
In general, our products contain food, nutritional supplements which are classified in the United States as “dietary supplements” which do not currently require approval from the FDA or other regulatory agencies prior to sale. Many of our products contain innovative ingredients or combinations of ingredients. There is little long term experience with human or other animal consumption of certain of these ingredients or combinations thereof in concentrated form. Our products could have certain side effects if not taken as directed or if taken by a consumer that has certain medical conditions. Furthermore, there can be no assurance that any of the products, even when used as directed, will have the effects intended or will not have harmful side effects.
We may be exposed to material product liability claims, which could increase our costs and adversely affect our reputation and business.
As a manufacturer and distributor of products intended for human consumption, we are subject to product liability claims if the use of our products for others is alleged to have resulted in injury. Our products consist of vitamins, minerals, herbs and other ingredients that are classified as dietary and nutrition supplements and, in most cases, are not subject to pre-market regulatory approval in the United States or internationally. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur.
20
Our insurance coverage may not be sufficient to cover our legal claims or other losses that we may incur in the future.
We maintain insurance, including property, general and product liability, and workers’ compensation to protect ourselves against potential loss exposures. There is no assurance that our insurance will be sufficient to cover any claims that are asserted against us. In the future, insurance coverage may not be available at adequate levels or on adequate terms to cover potential losses, including on terms that meet our customer’s requirements. If insurance coverage is inadequate or unavailable, we may face claims that exceed coverage limits or that are not covered, which could increase our costs and adversely affect our operating results.
Our intellectual property rights are valuable, and any inability to protect them could reduce the value of our products and brand.
We manufacture our products primarily for third parties who sell the products under their own brand names. Our product formulations are not patented and there are numerous companies selling similar products. As such, third parties could copy our products or sell similar products to our distributors and/or customers.
Our competitors may have or develop equivalent or superior manufacturing and design skills, and may develop an enhancement to our formulations that will be patentable or otherwise protected from duplication by others. Further, we may be unable or unwilling to strictly enforce our intellectual property rights, including our trademarks, from infringement. Our inability to obtain and/or failure to enforce our intellectual property rights could diminish the value of our product offerings and have a material adverse effect on our business, prospects, results of operations, and financial condition.
Adverse publicity or consumer perception of our products and any similar products distributed by others could harm our reputation and adversely affect our sales and revenues.
We believe we are highly dependent upon positive consumer perceptions of the safety and quality of our products as well as similar products distributed by other nutrition supplement companies. Consumer perception of nutrition supplements and our products, in particular, can be substantially influenced by scientific research or findings, national media attention and other publicity about product use. Adverse publicity from these sources regarding the safety, quality or efficacy of nutritional supplements and our products could harm our reputation and results of operations. The mere publication of news articles or reports asserting that such products may be harmful or questioning their efficacy could have a material adverse effect on our business, financial condition and results of operations, regardless of whether such news articles or reports are scientifically supported or whether the claimed harmful effects would be present at the dosages recommended for such products.
The Diet and Nutritional Supplement industry is highly competitive, and our failure to compete effectively could adversely affect our market share, financial condition and future growth.
The Diet and Nutritional Supplement industry is highly competitive with respect to price, brand and product recognition and new product introductions. Several of our competitors are larger, more established and possess greater financial, personnel, distribution and other resources. We face competition (a) in the health food channel from a limited number of large nationally known manufacturers, private label brands and many smaller manufacturers of dietary and nutrition supplements; and (b) in the mass-market distribution channel from manufacturers, major private label manufacturers and others. Private label brands at mass-market chains represent substantial sources of income for these merchants and the mass-market merchants often support their own labels at the expense of other brands. As such, the growth of our brands within food, drug, and general mass-market merchants are highly competitive and uncertain. If we cannot compete effectively, we may not be profitable.
21
We may experience greater than expected product returns, which might adversely affect our sales and results of operations.
Product returns are a customary part of our business. Products may be returned for various reasons, including expiration dates or lack of sufficient sales volume. Any increase in product returns could reduce our results of operations.
A shortage in the supply of key raw materials used by our manufacturer could increase our costs or adversely affect our sales and revenues.
Our inability to obtain adequate supplies of raw materials in a timely manner or a material increase in the price of the raw materials used in our products could have a material adverse effect on our business, financial condition and results of operations.
The purchase of many of our products is discretionary, and may be negatively impacted by adverse trends in the general economy and make it more difficult for us to generate revenues.
Our business is affected by general economic conditions since our products are discretionary and we depend, to a significant extent, upon a number of factors relating to discretionary consumer spending. These factors include economic conditions and perceptions of such conditions by consumers, employment rates, the level of consumers' disposable income, business conditions, interest rates, consumer debt levels and availability of credit. Consumer spending on our products may be adversely affected by changes in general economic conditions.
We may not be able to anticipate consumer preferences and trends within the diet and nutritional industry, which could negatively affect acceptance of our products by retailers and consumers and result in a significant decrease in our revenues.
Our products must appeal to a broad range of consumers, whose preferences cannot be predicted with certainty and are subject to rapid change. Our products will need to successfully meet constantly changing consumer demands. If our products are not successfully received by our private label distributors and their customers, our business, financial condition, results of operations and prospects may be harmed.
Risks Related to Our Management
Should we lose the services of Edgar Ward, our founder, chief executive officer, president and sole director, our financial condition and proposed expansion may be negatively impacted.
Our future depends on the continued contributions of Edgar Ward, our founder, chief executive officer, president and sole director who would be difficult to replace. The services of Mr. Ward are critical to the management of our business and operations. Additionally, we do not maintain key man life insurance on Mr. Ward. Should we lose the services of Mr. Ward, and be unable to replace his services with equally competent and experienced personnel, our operational goals and strategies may be adversely affected, which will negatively affect our potential revenues.
Because we do not have an audit or compensation committee, shareholders will have to rely on the one member of our board of directors who is not independent to perform these functions.
We do not have an audit or compensation committee or board of directors as a whole, that is composed of independent directors. These functions are performed by our sole director. Because our Sole Director is not independent, there is a potential conflict between their or our interests and our shareholders’ interests since Edgar Ward, our Sole Board Member, is also our Chief Executive Officer and president who will participate in discussions concerning management compensation and audit issues that may affect management decisions. Until we have an audit committee or independent directors, there may less oversight of management decisions and activities and little ability for minority shareholders to challenge or reverse those activities and decisions, even if they are not in the best interests of minority shareholders.
22
Our vice president devotes limited time to our business, which may negatively impact our plan of operations, implementation of our business plan and our potential profitability.
Neil Catania, our vice president currently devotes only ten (10) hours to our business each month. Our Chief Executive Officer and President, Edgar Ward, devotes full time to our business however, there is no assurance he will be able to do so in the future. Management time devoted to our business activities in the future may be inadequate to implement our plan of operations and develop a profitable business.
Risks Related to Our Common Stock
Our chief executive officer, president and sole director has voting control over all matters submitted to a vote of our common stockholders, which will prevent our minority shareholders from having the ability to control any of our corporate actions.
As of the filing of this Form 10 Registration Statement, we had 80,248,561 shares of common stock outstanding, each entitled to one vote per common share. Our chief executive officer, president and sole director, Edgar Ward, holds 23,744,084 common shares directly, 1,000,000 shares indirectly and 1,000 Series A Preferred Shares which provide him with 500,000 votes per share or an aggregate of 500,000,000 votes on all matters submitted to our stockholders. As a result, Mr. Ward controls 524,744,084 of 580,248,561 votes or 90.428% of all votes and has the ability to determine the outcome of all matters submitted to our stockholders for approval, including the election of directors. Mr. Ward’s control of our voting securities may make it impossible to complete some corporate transactions without his support and may prevent a change in our control. In addition, this ownership could discourage the acquisition of our common stock by potential investors and could have an anti-
‐
takeover effect, possibly depressing the trading price of our common stock.
As of September 30, 2017, we had warrants convertible into an aggregate of 17,331,285 common shares outstanding and we will likely issue additional shares in the future to fund our operations.
The issuance of the shares in the future upon conversion of outstanding securities will result in substantial dilution in the percentage of our common stock held by our existing shareholders. Any securities sold more than twelve (12) months prior to the date hereof are currently eligible for resale under Rule 144. In general, persons holding restricted securities in a company not reporting with the Securities & Exchange Commission, including affiliates, must hold their shares for a period of at least twelve months. Additionally, affiliates may not sell more than one percent of the total issued and outstanding shares in any ninety (90) day period, and must resell the shares in an unsolicited brokerage transaction at the market price. If substantial amounts of our common stock are resold under Rule 144, prevailing market prices for our common stock will be reduced.
We may, in the future, issue additional securities which would reduce investors’ percent of ownership and may dilute our share value.
Our Articles of Incorporation authorize us to issue 499,990,000 shares of common stock. As of the filing of this Form 10 Registration Statement, we had
80,248,561 shares of common stock outstanding. Accordingly, we may issue up to an additional 419,741,439 shares of common stock. The future issuance of common stock may result in substantial dilution in the percentage of our common stock held by our then existing shareholders. We may value any common stock issued in the future on an arbitrary basis including for services or acquisitions or other corporate actions that may have the effect of diluting the value of the shares held by our stockholders, and might have an adverse effect on any trading market for our common stock. Additionally, we are authorized to issue 10,000 shares of preferred stock of which 1,000 shares are outstanding. As such, we may issue an additional 9,000 shares of preferred stock. Our board of directors may designate the rights, terms and preferences of our authorized but unissued preferred shares at its discretion including conversion and voting preferences without notice to our shareholders.
23
We are an "emerging growth company”, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an "emerging growth company”, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five (5) years, although we could lose that status sooner if our revenues exceed $1,000,000,000, if we issue more than $1,000,000,000 in non-convertible debt in a three (3) year period, or if the market value of our common stock held by non-affiliates exceeds $100,000,000 as of any April 30 before that time, in which case we would no longer be an emerging growth company as of the following April 30. We cannot predict whether investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2) of the JOBS Act, that allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
ITEM 2. FINANCIAL
INFORMATION.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with the audited annual and unaudited interim Financial Statements and related notes included in this Form 10 filing, as well as the sections entitled “Risk Factors” in of this filing, as well as other cautionary statements and risks described elsewhere in this filing. Our actual results could differ materially from those discussed in the forward-looking statements
Overview
NutraFuels, Inc, a Florida corporation (“us”, “we”, or “our”) was formed as a limited liability company in the state of Florida on April 1, 2010, to engage in the development and distribution of nutritional and dietary oral spray products. On December 3, 2012, we converted from a Limited Liability Company to a Florida Corporation.
We manufacture and distribute oral spray nutritional and dietary products. Our distribution strategy includes selling to private label customers retailers, distributors, and consumers through retail outlets.
Nine (9) Months Ended September 30, 2017 and 2016
We had revenues of $1,029,727 and $195,909 for the nine (9) months ended September 30, 2017 and 2016, respectively, or a four hundred twenty five point six percent (425.6%) increase. This increase primarily resulted from the development of and acceptance our Hemp Finished Products in the marketplace, which led to greater sales.
Cost of sales was $617,958 compared to $118,731 for the nine (9) months ended September 30, 2017 and 2016, respectively, or a four hundred twenty point five percent (420.5%) increase. This increase was directly related to our increase in revenues.
24
Gross margin was $411,769 and $77,178 for the nine (9) months ended September 30, 2017 and 2016, respectively, or a four hundred thirty three point five percent (433.5%) increase.
General and administrative expenses were $815,035 compared to $269,087 for the nine (9) months ended September 30, 2017 and 2016, respectively, an increase of two hundred two point nine percent (202.9%).
Stock based compensation $19,134,686 and $232,050 for the nine (9) months ended September 30, 2017 and 2016, respectively, or an eight thousand one hundred forty five point nine percent (8145.9%) increase.
Our interest expense was $3,341,655 compared to $320,094 for the nine (9) months ended September 30, 2017 and 2016, respectively, an increase of $3,021,561 or nine hundred forty three point nine percent (943.9%). This increase is due to the recording of induced conversion charges upon the conversion of our debt to equity at a rate below the then prevailing market price of our stock.
We recorded a net loss of ($23,202,212) compared to ($1,052,657) for the nine (9) months ended September 30, 2017 and 2016, respectively.
Years Ended December 31, 2016 and 2015
We had revenues of $225,293 and $134,006 for the years ended December 31, 2016 and 2015, respectively, or a sixty eight point one percent (68.1%) increase. This increase resulted from greater acceptance of our products in the marketplace, which led to greater sales.
Cost of sales was $195,195 compared to $115,905 for the years ended December 31, 2016 and 2015, respectively, or a sixty eight point four percent (68.4%) increase.
Gross margin was $30,098 and $11,997 for the years ended December 31, 2016 and 2015, respectively, or a sixty six point three percent (66.3%) increase.
General and administrative expenses were $660,214 compared to $662,784 for the years ended December 31, 2016 and 2015, respectively, a decrease of zero point four percent (0.4%).
Stock based compensation $513,196 and $640,030 for the years ended December 31, 2016 and 2015 , respectively, or a nineteen point eight percent (19.8%) increase.
Our interest expense was $611,444 compared to $431,097 for the years ended December 31, 2016 and 2015, respectively, an increase of forty one point eight percent (41.8%). This increase is due to the recording of induced conversion charges upon the conversion of our debt to equity at a rate below the then prevailing market price of our stock.
We recorded a net loss of ($2,098,771) compared to ($2,174,541) for the years ended December 31, 2016 and 2015, respectively.
Liquidity and Capital Resources Cash Flow Activities
Liquidity
As disclosed in Footnote 6 on page F-14, $8,464,463 in current liabilities is a liability to issue shares to our Chief Executive Officer, President and Director under his January 13, 2017, employment agreement requiring the Company to maintain his common stock ownership at 30% of total issued and outstanding shares of the Company. Mr. Ward's employment agreement was amended on October 10, 2017 to remove the anti-dilution provision. The Company has issued to Mr. Ward 6,674,837 shares of its common stock on November 27, 2017 which eliminated this.
25
We had no material debt that matured during the nine (9) month period ended September 30, 2017. We currently have enough cash to cover our operating needs for approximately (1) one month and should our revenues be insufficient to pay our operating expenses, we intend to raise additional funds from an offering of our stock in the future; however, this offering may never occur, or if it occurs, we may be unable to raise the required funding. Further new offerings of our common shares will dilute our existing shareholders and your investment in our common shares. We do not have any plans or specific agreements for new sources of funding and we have no agreements for financing in place.
Cash Flow Activities
Our cash increased $375,269 for the nine months ended September 30, 2017. We used $878,638 of cash in operating activities during the nine months.
Financing Activities
During the nine (9) months ended September 30, 2017, we funded our working capital requirements principally through the increase in our revenues for the period to 1,029,727 and proceeds from the sale of our common stock and exercise of options in the amount of $1,379,500.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires our management to make assumptions, estimates and judgments that affect the amounts reported in the financial statements, including the notes thereto, and related disclosures of commitments and contingencies, if any. We consider our critical accounting policies to be those that require the more significant judgments and estimates in the preparation of financial statements, including the following:
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Fair Value of Financial Instruments
Our financial instruments consist of cash and cash equivalents, prepaid expenses, payables and accrued expenses. Fair value estimates are made at a specific point in time, based on relevant market information about the financial instrument. These estimates are subjective in nature and involve uncertainties and matters of significant judgment and therefore cannot be determined with precision. We consider the carrying values of our financial instruments in the consolidated financial statements to approximate fair value, due to their short-term nature.
Revenue Recognition
Revenue is recognized when earned, generally at shipment of product. Revenue is recognized on a gross basis in accordance with ASC 605-45.
Property and Equipment
Property and equipment are recorded at cost, less accumulated depreciation. Depreciation is provided for using straight-line methods over the estimated useful lives of the respective assets.
26
Valuation of Long-Lived Assets
We periodically evaluate long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. If the estimated future cash flows (undiscounted and without interest charges) from the use of an asset were less than the carrying value, a write-down would be recorded to reduce the related asset to its estimated fair value.
Derivatives
The Company evaluates its convertible debt, options, warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for. The result of this accounting treatment is that under certain circumstances the fair value of the derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operations as other income or expense. Upon conversion or exercise of a convertible note containing an embedded derivative instrument, the instrument is marked to fair value at the conversion date and that fair value is reclassified to equity. The shares issued upon conversion of the note are recorded at their fair value with gain or loss recognition as applicable.
Equity instruments that are initially classified as equity that become subject to reclassification under this accounting standard are reclassified to liability at the fair value of the instrument on the reclassification date.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as defined in Regulation S-K Item 303(a)(4).
Recent Accounting Pronouncements
(See “Recently Issued Accounting Pronouncements” in Note 2m) of Notes to the Financial Statements.)
ITEM 3. PROPERTIES
We lease an aggregate of 6,400 square feet of office and warehouse space at 6601 Lyons Rd, Suites L-6&7, Coconut Creek, FL 33073, with base rent at $6,700 per month from Lyons Corporate Park for our executive offices. Approximately 5,800 square feet is used for manufacturing, storage and distribution. The lease term expires on December 31, 2018. On June 6, 2017, we entered into an agreement with Hillsboro Technology Center, LLC to lease an aggregate of 19,831 square feet at 448 Hillsboro Technology Drive, Deerfield Beach, FL 33441, with base rent at $13,220.67 per month plus 7.65% of common area and 13% of building operating expenses. We plan to use this location as a manufacturing, storage and distribution facility. The lease term commences on January 1, 2018 and terminates eighty-six (86) months thereafter.
ITEM 4. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following tables set forth the ownership, as of the date of this Registration Statement, of our common stock by each person known by us to be the beneficial owner of more than five percent (5%) of our outstanding common stock, our directors, and our executive officers and directors as a group. To the best of our knowledge, the persons named have sole voting and investment power with respect to such shares, except as otherwise noted. There are not any pending or anticipated arrangements that may cause a change in control.
27
The information presented below regarding beneficial ownership of our voting securities has been presented in accordance with the rules of the Securities and Exchange Commission and is not necessarily indicative of ownership for any other purpose. Under these rules, a person is deemed to be a "beneficial owner" of a security if that person has or shares the power to vote or direct the voting of the security or the power to dispose or direct the disposition of the security. A person is deemed to own beneficially any security as to which such person has the right to acquire sole or shared voting or investment power within sixty (60) days through the conversion or exercise of any convertible security, warrant, option or other right. More than one person may be deemed to be a beneficial owner of the same securities. The percentage of beneficial ownership by any person as of a particular date is calculated by dividing the number of shares beneficially owned by such person, which includes the number of shares as to which such person has the right to acquire voting or investment power within sixty (60) days, by the sum of the number of shares outstanding as of such date plus the number of shares as to which such person has the right to acquire voting or investment power within sixty (60) days. Consequently, the denominator used for calculating such percentage may be different for each beneficial owner. Except as otherwise indicated below and under applicable community property laws, we believe that the beneficial owners of our common stock listed below have sole voting and investment power with respect to the shares shown. The business address for these shareholders is 6601 Lyons Road, Suite L-6, Coconut Creek Florida 33037.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Class
|
Position
|
Amount of Beneficial Ownership
|
Direct Ownership
|
Indirect Ownership
|
Total
|
Percent of Class(1)
|
|
Common
|
Edgar Ward, Chief Executive Officer, Sole Director
|
23,744,084
|
23,744,084
|
1,000,000(3)
|
24,744,084
|
29.59%(2)
|
|
Common
|
Neil Catania, Vice President
|
8,770,571
|
8,770,571
|
0
|
|
10.93%
|
|
Common
|
Cede & Co
|
10,569,009
|
10,569,009
|
0
|
---
|
13.17%
|
|
Common
|
Craig Hetherington
|
4,107,511
|
4,107,511
|
0
|
--
|
5.11%
|
|
Total Common
|
All Officers and Directors as a Group (2 Persons)
|
32,514,655
|
32,514,655
|
1,000,000
|
---
|
40.52%
|
|
Preferred
|
Edgar Ward, Chief Executive Officer, Sole Director
|
1,000
|
1,000
|
0
|
---
|
100%
|
|
Preferred
|
Neil Catania, Vice President
|
0
|
0
|
0
|
---
|
0
|
|
Total Preferred
|
All Officers and Directors as a Group (2 Persons)
|
1,000
|
1,000
|
0
|
---
|
100%
|
(1) Based upon 80,248,561 common shares outstanding, as of the filing of this Form 10 Registration Statement.
(2) As a result of Mr. Ward’s ownership of 23,744,084 common shares directly, 1,000,000 shares indirectly and 1,000 Series A Preferred Shares, he holds 90.28% of the votes on all matters submitted to a vote of our stockholders.
(3) Represents 1,000,0000 shares held by Nicole Archon, Mr. Ward’s live-in girlfriend and a company employee which were issued on December 1, 2016 to Ms. Archon for services rendered to us.
28
ITEM 5. DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS, AND CONTROL PERSONS
The board of directors elect our executive officers annually. A majority vote of the directors who are in office are required to fill vacancies. Each director shall be elected for the term of one year, and until his successor is elected and qualified, or until his earlier resignation or removal. Our directors and executive officers are as follows:
|
|
|
|
|
|
Name
|
|
Age
|
|
Position
|
|
Edgar Ward
|
|
45
|
|
Chief Executive Officer, President, Director
|
|
Neil Catania
|
|
56
|
|
Vice President
|
Edgar Ward, Chief Executive Officer, President And Director
From April 1, 2010 to present, Edgar Ward has served as our Chief Executive Officer, President, and Director. From January 1, 2008, until June 30, 2010, Mr. Ward was CEO at SkyRockit Records.
Mr. Ward’s services to us include day to day operations of our manufacturing facility and management of our company.
As our chief executive officer, president and director Mr. Ward brings his experience in managing our day to day operations.
Neil Catania, Vice President
Neil Catania became our vice president on November 20, 2012. From May 2004 until Present, Neil Catania has been the chief executive officer of MND LLC, a financial services company located in New York.
Mr. Catania’s services to us include assisting Mr. Ward with our day to day operations. Neil Catania holds Series 7, Series 63, Series 24 and Series 55 licenses from the Financial Industry Regulatory Authority (“FINRA”).
As our Vice-President Mr. Catania brings his experience in the financial services industry and executive management to our day to day operations.
Legal Proceedings
No officer, director, or persons nominated for such positions, promoter or significant employee has been involved in the last ten (10) years in any of the following:
·
Any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two (2) years prior to that time;
·
Any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses);
·
Being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities;
·
Being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated;
·
Having any government agency, administrative agency, or administrative court impose an administrative finding, order, decree, or sanction against them as a result of their involvement in any type of business, securities, or banking activity;
29
·
Being the subject of a pending administrative proceeding related to their involvement in any type of business, securities, or banking activity; and/or
·
Having any administrative proceeding been threatened against you related to their involvement in any type of business, securities, or banking activity.
Other Directorships
None of our other officers and directors are directors of other Securities and Exchange Commission reporting companies.
Conflicts of Interest
Our Chief Executive Officer, President, and sole Director, Edgar Ward devotes his full time to our business under the terms of his employment contract. Our only other executive officer, Neal Catania is not obligated to commit his full time and attention to our business; accordingly, he may encounter a conflict of interest in allocating his time between our operations and those of other businesses. Neal Catania only devotes ten (10) hours each month to our business and is not contractually required to devote full time services to us. In the future, our officers and directors may engage in other business activities, investments and business opportunities that may be appropriate for presentation to us as well as other entities to which they owe a fiduciary duty. As a result, they may have conflicts of interest in determining to which entity a particular business opportunity should be presented. Our officers and directors may also in the future become affiliated with entities, engaged in business activities similar to those we intend to conduct. In general, officers and directors of a corporation are required to present business opportunities to a corporation if:
·
the corporation could financially undertake the opportunity;
·
the opportunity is within the corporation’s line of business; and
·
it would be unfair to the corporation and its stockholders not to bring the opportunity to the attention of the corporation.
Code of Ethics
We plan to adopt a Code of Ethics in the future, at the latest prior to our stock being listed on an exchange that requires us to have a formal Code of Ethics.
30
ITEM 6. EXECUTIVE COMPENSATION
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to our Principal Executive Officer, our two (2) most highly compensated executive officers who occupied such position at the end of our latest fiscal year and up to two (2) additional executive officers who would have been included in the table below except for the fact that they were not executive officers at the end of our latest fiscal year, by us, or by any third party where the purpose of a transaction was to furnish compensation, for all services rendered in all capacities to us for the years ended December 31, 2016 and 2015.
|
|
|
|
|
|
|
|
|
|
|
Name
|
Position
|
Year
|
Salary
|
Bonus/Stock Awards
|
Options
|
Non-Equity Incentive Plan Compensation
|
Non-qualified deferred compensation
|
All other compensation
|
Total
|
|
Edgar Ward
(1)
|
Chief Executive Officer, President,
Director
|
2016
2015
|
$115,295
$206,000
|
$240,000
|
0
0
|
0
0
|
0
0
|
0
0
|
$355,295
$206,000
|
|
Neil
Catania
(2)
|
Vice
President
|
2016
2015
|
$0
$0
|
$120,000
|
00
|
0
0
|
0
0
|
0
0
|
$120,000
0
|
(1) On November 27, 2017, February 13, 2017, and December 1, 2016, Edgar Ward received 6,674,837; 7,220,585 and 4,000,000 restricted common shares which we valued at a per share price of $1.27; $1.44 and $0.06 or an aggregate of $8,464,463; $ 10,376,614.63 and $ 240,000 for services rendered to us. For the year ended December 31, 2016, Edgar Ward received $115,295 in cash compensation.
(2) On December 1, 2016, Mr. Catania received 2,000,000 restricted shares which we valued at a per share price of $0.06 or an aggregate of $120,000 for services rendered to us for the period from January 1, 2014 through December 31, 2016.
Edgar Ward is our Sole Director. Our directors are not compensated for their service as directors.
Our board of directors determines the compensation paid to our executive officers based upon the years of service to us, whether services are provided on a full-time basis and the experience and level of skill required.
We may award our officers and directors shares of common stock as non-cash compensation as determined by the board of directors from time to time. The board will base its decision to grant Common Stock as compensation on the level of skill required to perform the services rendered and time committed to providing services to us.
At no time during the last fiscal year with respect to any person listed in the Table above was there:
·
Any outstanding option or other equity-based award re-priced or otherwise materially modified (such as by extension of exercise periods, the change of vesting or forfeiture conditions, the change or elimination of applicable performance criteria, or the change of the bases upon which returns are determined);
·
Any waiver or modification of any specified performance target, goal or condition to payout with respect to any amount included in non-stock incentive plan compensation or payouts;
·
Any option or equity grant;
·
Any non-equity incentive plan award made to a named executive officer;
·
Any nonqualified deferred compensation plans including nonqualified defined contribution plans; or
·
Any payment for any item to be included under All Other Compensation (column (i)) in the Summary Compensation Table.
31
Corporate Governance and Director Independence
Our Board of Directors has not established Audit, Compensation, and Nominating or Governance Committees as standing committees. The Board does not have an executive committee or any committees performing a similar function. We are not currently listed on a national securities exchange or in an inter- dealer quotation system that has requirements that a majority of the board of directors be independent. Our Sole Director has determined that he is not “independent” under the definition set forth in the listing standards of the NASDAQ Stock Market, Inc., which is the definition that the Board has chosen to use for the purposes of the determining independence, as the OTC Markets does not provide such a definition. Therefore, our Sole Director is not independent.
|
|
|
|
|
|
|
|
|
|
Name
|
Year Ended
|
Fees Earned or paid in cash ($)
|
Stock Awards ($)
|
Option Awards ($)
|
Non-equity incentive plan compensation ($)
|
Nonqualified deferred compensation earnings ($)
|
All other compensation ($)
|
Total ($)
|
|
Edgar Ward
Founder
Chief Executive Officer, Director
|
2016
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
|
Edgar Ward
Founder
Chief Executive Officer, Director
|
2015
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
Our directors have not received any compensation as reflected above.
ITEM 7. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
On August 13, 2010, we sold five percent (5%) of our membership interests and on October 18, 2010, we sold 11.5% membership interests to Neil Catania in exchange for payment of $65,000.
On November 15, 2012, we entered into a note agreement with Neil Catania with a principal amount of $160,000. The note bears interest at the rate of 10% per annum. On January 4, 2017, the note had an aggregate of $335,587 principal and interest outstanding which was converted into 1,342,349 restricted common shares on such date.
On November 26, 2012, we issued 4,607,100 shares of our common stock to Edgar Ward in exchange for membership interests when we converted from a Florida limited liability company to a Florida corporation.
On November 26, 2012, we issued 1,000 shares of our non-convertible Series A Preferred Shares to Mr. Ward which entitle him to 500,000 votes per share or an aggregate of 500,000,000 on all matters submitted to our common stockholders. We valued the 1,000 Series A shares at $1.00 per share or an aggregate of $1,000. As a result of Mr. Ward’s ownership of 17,069,247 common shares and 1,000 Series A preferred shares he holds an aggregate of 517,069,247 votes representing 90.17% of the votes on all matters submitted to a vote of our stockholders as of As of the filing of this Form 10 Registration Statement.
32
On November 26, 2012, Mr. Catania exchanged his 16.5% membership interests for 2,625,000 of our common shares when we converted from a limited liability company to a corporation. On May 17, 2013, we issued 428,571 common shares.
On February 15, 2013, we entered into a note agreement with Neil Catania with a principal amount of $50,000. The note bears interest at the rate of 10% per annum.
On May 17, 2013, we issued 714,285 shares and on September 3, 2013, we issued 1,000,000 shares to Edgar Ward for services rendered which we valued at $.35 per share.
On September 3, 2013, we issued an additional 350,000 common shares to Neil Catania which we valued at $.35 per share, for services.
On December 1, 2016, we issued 2,000,000 Common Shares to Neil Catania for services rendered.
On December 1, 2016, we issued 1,000,000 shares to Nicole Archon, the girlfriend who resides with our Chief Executive Officer, for services rendered. We valued these shares at $0.06 per share or an aggregate of $60,000.00.
On December 1, 2016, March 3, 2017 and November 27, 2017, we issued 4,000,000; 7,220,585 and 6,674,837 common shares to Edgar Ward for services rendered to us. We valued these shares at $0.06; $1.44 and $1.27 per share or an aggregate of $240,000; $10,376,614.63 and $8,464,463, respectively.
On January 4, 2017, the note had an aggregate of $103,663 principal and interest outstanding which was converted into 414,641 restricted common shares on such date. In March 2013, we entered into a line of credit agreement with Neil Catania with a principal amount of $405,000. The note bears interest at the rate of 0% per annum.
On January 4, 2017, Neil Catania converted principal and accrued interest due in the amount of $841,750 pursuant to a November 15, 2012 and July 26, 2016 convertible notes and a December 31, 2013 line of credit into our common shares at the price of $.25 per share or an aggregate of 3,367,000 shares. On December 1, 2016, we issued 2,000,000 shares to Neil Catania, VP of the Company, for services rendered. We valued these shares at $0.06 per share or an aggregate of $120,000.00.
On January 4, 2017, the note had an aggregate of $432,500 principal outstanding which was converted into 1,750,000 restricted common shares on such date.
On January 13, 2017, we entered into an employment agreement with Edgar Ward, our Chief Executive Officer, President and Director. The agreement has a term of five (5) years. Under the agreement, each year, Mr. Ward shall receive a base salary of two hundred fifty thousand Dollars ($250,000.00) per year beginning with the year ending December 31, 2017, and a bonus of one hundred thousand ($100,000.00) each year for his services as our President and Director. We are required to reimburse all normal, usual and necessary expenses incurred by Mr. Ward in furtherance of the business, including reasonable travel and entertainment. Mr. Ward is entitled to a vacation of twenty-four (24) days per annum, in addition to holidays observed by the US. Mr. Ward is entitled to annual bonuses as determined by our Board of Directors.
On February 13, 2017, we issued 7,220,585 restricted common shares to Edgar Ward for services rendered. We valued these shares at a per share price of $1.44 or an aggregate of $10,376,615.
On January 6, 2017, we sold 200,000 units to Breadfruit Tree Inc., a Florida corporation, doing business as NF Skin, our distributor, and controlled by F. Bruce Hutson, for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $0.50 at any time until the two (2) year anniversary of the date of the investment. Sales to Breadfruit Tree, Inc. represented 0%, 2.01% and 59.98 % of our sales during the years ended December 31, 2015, December 31, 2016 and nine (9) month period ended September 30, 2017, respectively.
33
On June 9, 2016, we issued Josh Zwagil 244,514 restricted common shares for new business development services. We valued these shares at $0.11 per share, or an aggregate of $26,896. Josh Zwagil holds all shares he received. Mr. Zwagil controls My Daily Choice, our distributor. Sales to My Daily Choice represented 29.03%, 95.55% and 73.56% of our sales during the years ended December 31, 2015, December 31, 2016 and nine (9) month period ended September 30, 2017, respectively.
Other than described above, we have not entered into any material transactions with any director, executive officer, and promoter, beneficial owner of five percent (5%) or more of our common stock, or family members of such persons in the prior two (2) years.
Corporate Governance and Director Independence
Our Board of Directors has two directors and has not established Audit, Compensation, and Nominating or Governance Committees as standing committees. The Board does not have an executive committee or any committees performing a similar function. We are not currently listed on a national securities exchange or in an inter-dealer quotation system that has requirements that a majority of the board of directors be independent. The Board has determined that no members of the Board are “independent” under the definition set forth in the listing standards of the NASDAQ Stock Market, Inc., which is the definition that the Board has chosen to use for the purposes of the determining independence. Therefore, none of our current Board members are independent.
ITEM 8. LEGAL
PROCEEDINGS
We are not a party to any legal proceedings.
ITEM 9. MARKET PRICE
OF AND DIVIDENDS ON THE REGISTRANTS COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market for Common Stock
Our common stock, par value $0.0001 per share (the "Common Stock"), has been quoted with the symbol “NTFU” on the OTC Markets since May 19, 2014.
Trading in stocks quoted on the OTC Markets is often thin and is characterized by wide fluctuations in trading prices due to many factors that may have little to do with a company’s operations or business prospects.
OTC Markets securities are not listed or traded on the floor of an organized national or regional stock exchange. Instead, OTC Markets securities transactions are conducted through a telephone and computer network connecting dealers in stocks. OTC Markets issuers are traditionally smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange.
34
Set forth below are the range of high and low prices for our common stock from the OTC Markets OTC Pinks for the periods indicated. The quotations reflect inter-dealer prices, without retail mark-up, mark-down or commissions and may not necessarily represent actual transactions:
|
|
|
|
|
Period
|
|
|
|
|
Year 2015
|
High
|
|
Low
|
|
First Quarter Ended 03/31/15
|
$1.94
|
|
$0.40
|
|
Second Quarter Ended 06/30/15
|
$0.51
|
|
$0.30
|
|
Third Quarter Ended 09/30/15
|
$0.52
|
|
$0.10
|
|
Fourth Quarter Ended 12/31/15
|
$0.25
|
|
$0.10
|
|
Year 2016
|
High
|
|
Low
|
|
First Quarter Ended 03/31/16
|
$0.40
|
|
$0.10
|
|
Second Quarter Ended 06/30/16
|
$0.24
|
|
$0.11
|
|
Third Quarter Ended 09/30/16
|
$0.15
|
|
$0.03
|
|
Fourth Quarter Ended 12/31/16
|
$0.56
|
|
$0.03
|
|
Year 2017
|
High
|
|
Low
|
|
First Quarter 03/31/17
|
$2.40
|
|
$0.35
|
|
Second Quarter 06/30/17
|
$0.76
|
|
$0.40
|
|
Third Quarter 09/30/17
|
$0.55
|
|
$0.23
|
Transfer Agent
Our transfer agent is VStock Transfer LLC located at 18 Lafayette Place, Woodmere, NY 11598. Its telephone number is 212-
‐
828-
‐
8436 and its website is located at
http://www.vstocktransfer.com.
VStock Transfer is registered as a transfer agent with the Securities and Exchange Commission under the Securities Exchange Act of 1934, as amended.
Holders
As of the date of this Form 10 Registration Statement, we had 80,248,561 shares of common stock outstanding and eighty-six (86) record holders of our common stock.
Dividends
We have not paid any dividends to the holders of our common stock and we do not expect to pay any such dividends in the foreseeable future as we expect to retain our future earnings for use in the operation and expansion of our business.
Securities Authorized for Issuance Under Equity Compensation Plans
We presently do not have any equity based or other long-term incentive programs. In the future, we may adopt and establish an equity-based or other long-term incentive plan if it is in our best interest and our shareholders to do so.
Penny Stock Considerations
Our shares will be "penny stocks", as that term is generally defined in the Securities Exchange Act of 1934 to mean equity securities with a price of less than $5.00. Thus, our shares will be subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in certain transactions involving a penny stock.
Under the penny stock regulations, a broker-dealer selling a penny stock to anyone other than an established customer must make a special suitability determination regarding the purchaser and must receive the purchaser's written consent to the transaction prior to the sale, unless the broker-dealer is otherwise exempt.
35
In addition, under the penny stock regulations, the broker-dealer is required to:
·
Deliver, prior to any transaction involving a penny stock, a disclosure schedule prepared by the Securities and Exchange Commission relating to the penny stock market, unless the broker-dealer or the transaction is otherwise exempt;
·
Disclose commissions payable to the broker-dealer and our registered representatives and current bid and offer quotations for the securities;
·
Send monthly statements disclosing recent price information pertaining to the penny stock held in a customer's account, the account's value, and information regarding the limited market in penny stocks; and
·
Make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction, prior to conducting any penny stock transaction in the customer's account.
Because of these regulations, broker-dealers may encounter difficulties in their attempt to sell shares of our common stock, which may affect the ability of the Selling Stockholder or other holders to sell their shares in the secondary market, and have the effect of reducing the level of trading activity in the secondary market. These additional sales practice and disclosure requirements could impede the sale of our securities, if our securities become publicly traded. In addition, the liquidity for our securities may be decreased, with a corresponding decrease in the price of our securities.
Our shares are subject to such penny stock rules and our shareholders will, in all likelihood, find it difficult to sell their securities.
Sales of Our Common Stock Under Rule 144
We presently have 80,248,561 common shares outstanding. Of these shares 47,734,106 common shares are held by non-affiliates and 32,514,455 common shares are held by affiliates, which Rule 144 of the Securities Act of 1933 defines as restricted securities.
In general, non-affiliates holding restricted securities of SEC reporting companies must hold their shares for a period of at least six (6) months and non-affiliates who hold restricted securities of companies which are not SEC reporting companies must hold their shares for a period of at least twelve months. Persons who are affiliates of either reporting or non-reporting companies must file a Form 144 with the SEC prior to sale, may not sell more than one percent of the total issued and outstanding shares in any 90-day period, and must resell the shares in an unsolicited brokerage transaction at the market price.
Registration Rights
There are no agreements that require us to register securities under the Securities Act.
Dividends
We have not declared any cash dividends on our common stock since our inception and do not anticipate paying such dividends in the foreseeable future. We plan to retain any future earnings for use in our business. Any decisions as to future payments of dividends will depend on our earnings and financial position and such other facts, as the Board of Directors deems relevant.
36
Stock Re-Purchases
We have not made re-purchases of shares of our common stock since our inception and we do not currently have any publicly-announced repurchase plans in effect.
Proposed Public Offerings
There are no securities proposed to be, publicly offered by us.
Penny Stock Considerations
Our shares are "penny stocks", as that term is generally defined in the Securities Exchange Act of 1934 to mean equity securities with a price of less than $5.00. Thus, our shares are subject to rules that impose sales practice and disclosure requirements on broker-dealers who engage in certain transactions involving a penny stock.
Under the penny stock regulations, a broker-dealer selling a penny stock to anyone other than an established customer must make a special suitability determination regarding the purchaser and must receive the purchaser's written consent to the transaction prior to the sale, unless the broker-dealer is otherwise exempt.
In addition, under the penny stock regulations, the broker-dealer is required to:
·
Deliver, prior to any transaction involving a penny stock, a disclosure schedule prepared by the Securities and Exchange Commission relating to the penny stock market, unless the broker-dealer or the transaction is otherwise exempt;
·
Disclose commissions payable to the broker-dealer and our registered representatives and current bid and offer quotations for the securities;
·
Send monthly statements disclosing recent price information pertaining to the penny stock held in a customer's account, the account's value, and information regarding the limited market in penny stocks; and
·
Make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written agreement to the transaction, prior to conducting any penny stock transaction in the customer's account.
Because of these regulations, broker-dealers may encounter difficulties in their attempt to sell shares of our Common Stock, which may affect the ability of selling shareholders or other holders to sell their shares in the secondary market, and have the effect of reducing the level of trading activity in the secondary market. These additional sales practice and disclosure requirements could impede the sale of our securities, if our securities become publicly traded. In addition, the liquidity for our securities may be decreased, with a corresponding decrease in the price of our securities. Our shares in all probability will be subject to such penny stock rules and our shareholders will, in all likelihood, find it difficult to sell their securities.
37
ITEM 10.
RECENT SALES OF UNREGISTERED SECURITIES
In the prior three years, we offered and sold securities below. None of the issuances involved underwriters, underwriting discounts or commissions. We relied upon Sections 4(2) of the Securities Act, and Rule 506 of the Securities Act of 1933, as amended for the offer and sale of the securities.
We believed these exemptions were available because:
·
We are not a blank check company;
·
We filed a Form D, Notice of Sales, with the SEC;
·
Sales were not made by general solicitation or advertising;
·
All certificates had restrictive legends;
·
Sales were made to persons with a pre-existing relationship to our Chief Executive Officer and · Sole Director, Edgar Ward; and
·
Sales were made to investors who represented that they were accredited investors.
In connection with the above transactions, although some of the investors may have also been accredited, we provided the following to all investors:
·
Access to all our books and records;
·
Access to all material contracts and documents relating to our operations;
·
The opportunity to obtain any additional information, to the extent we possessed such information, necessary to verify the accuracy of the information to which the investors were given access; and
·
Prospective investors were invited to review at our offices at any reasonable hour, after reasonable advance notice, any materials available to us concerning our business.
On February 17, 2015, we sold a promissory note in the amount of $25,000 to Jerry O’Leary. The note included options to purchase 25,000 of our common shares at the price of $.20 per share or an aggregate of $5,000. On January 4, 2017, Mr. O’Leary converted the amount due of $25,000 into 100,000 of our common shares at the price of $.25 per share. On February 23, 2017, we issued 25,000 shares of our common stock to Jerry O’Leary in exchange for $5,000 for the exercise of the 25,000 options.
On October 4, 2016, we sold 500,000 units to Jerry O’Leary for the aggregate price of $50,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 4, 2017, we sold 500,000 units to Jerry O’Leary for the aggregate price of $50,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On April 21, 2015, we sold a promissory note in the amount of $250,000 to William Ferri. The note accrued interest at 10% and included options to purchase 250,000 common shares at the price of $.20 per share. On January 4, 2017, William Ferri converted the principal and accrued interest due in the amount of $275,000 into our common shares at the price of $.25 per share or an aggregate of 1,100,000 shares. On May 12, 2017, Mr Ferri exercised the 250,000 options at an exercise price of $.20 per share or an aggregate of $50,000.
38
On April 19, 2016, we sold a promissory note in the amount of $38,000 to Richard Scott Lohan. The note accrued interest at 10% and included warrants to purchase 100,000 common shares at the price of $.00 per share. On May 10, 2016, we repaid the principal due of $38,000. On April 19, 2016, Mr. Lohan exercised the 100,000 warrants at an exercise price of $0.00 per share or an aggregate of $0. On June 22, 2016, we sold an additional promissory note in the amount of $27,000 to Mr. Lohan. The note accrued interest at 10% and included warrants to purchase 70,000 common shares at the price of $.00 per share. On January 4, 2017, Mr. Lohan converted the principal due of $27,000 into 270,000 common shares and accrued interest of $11,700 into 117,000 common shares at the price of $.10 per share. On June 22, 2016, Mr. Lohan exercised the 100,000 warrants at an exercise price of $0.00 per share or an aggregate of $0.
On January 5, 2017, we sold 243,000 shares to Richard Scott Lohan for the aggregate price of $12,000 or $.05 per share.
Mr. Lohan was issued 700,000 shares instead of 70,000 shares in conjunction with the exercise of the June 22, 2016 cashless warrants. He elected to convert the June 22, 2016 promissory note and accrued interest for 387,000 common shares and pay $12,000 in cash for the remaining 243,000 common shares of the 630,000 over-issuance instead of returning those shares.
On June 23, 2016, we amended a noted dated June 7, 2013, whereby we are obligated to pay Craig Hetherington the sum of $100,000 plus interest at the rate of 10%. Under the terms of the amended note, the note’s maturity date was July 15, 2017.
On August 27, 2016, we amended an August 26, 2013 promissory note, whereby we are obligated to pay Craig Hetherington the sum of $100,000 plus interest at the rate of 15%. Under the terms of the amended note, the note’s maturity date was July 15, 2016.
On January 4, 2017, Craig Heatherington converted principal and accrued interest due in the amount of $882,235 pursuant to a June 7, 2013 and an August 26, 2013 and a March 26, 2014 and a June 23, 2014 convertible notes into our common shares at the price of $.25 per share or an aggregate of 3,528,940 shares.
On October 22, 2015, we sold 100,000 units to James Laurain for the aggregate price of $100,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On November 13, 2015, we sold 100,000 units to James Laurain for the aggregate price of $100,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On November 25, 2015, we sold 200,000 units to James Laurain for the aggregate price of $200,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 10, 2015, we sold 150,000 units to James Laurain for the aggregate price of $15,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On March 9, 2016, we sold 50,000 units to James Laurain for the aggregate price of $200,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
39
On March 18 2016, we sold 450,000 units to James Laurain for the aggregate price of $45,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On May 18, 2016, we sold 50,000 units to James Laurain for the aggregate price of $5,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On June 9, 2016, we sold a promissory note in the amount of $20,000 to James Laurain. The note accrued interest at 10%. On December 7, 2016, Mr. Laurain converted the principal and accrued interest due in the amount of $20,628 into our common shares at the price of $.25 per share or an aggregate of 82,512 shares.
On July 26, 2016, we sold a promissory note in the amount of $20,000 to James Laurain. The note accrued interest at 10%. On December 7, 2016, Mr. Laurain converted the principal and accrued interest due in the amount of $20,367 into our common shares at the price of $.25 per share or an aggregate of 81,468 shares.
On December 8, 2016, we sold 550,000 units to James Laurain for the aggregate price of $55,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 3, 2017, we sold 75,000 units to James Laurain for the aggregate price of $7,500 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 28, 2016, we sold 250,000 units to Michael Farr for the aggregate price of $25,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On June 23, 2016, we sold a promissory note in the amount of $25,000 to Michael Farr. The note accrued interest at 10% and included 70,000 common shares. On August 23, 2016, we repaid the note and accrued interest.
On August 29, 2016, we sold a promissory note in the amount of $35,000 to Michael Farr. The note accrued interest at 10% and included 100,000 common shares. On October 12, 2016, we repaid the note and accrued interest.
On September 21, 2016, we sold a promissory note in the amount of $40,000 to Michael Farr. The note accrued interest at 10% and included 100,000 common shares. On November 21, 2016, we repaid the note and issued 160,400 common shares to Mr. Farr.
On October 13, 2016, we sold a promissory note in the amount of $60,000 to Michael Farr. The note accrued interest at 10% and included 200,000 common shares. On December 28, 2016, Mr. Farr converted the note into 660,000 common shares.
On November 23, 2016, we sold a promissory note in the amount of $65,000 to Michael Farr. The note accrued interest at 10% and included 200,000 common shares. On January 4, 2017, Mr. Farr converted the note into 300,000 common shares.
On January 6, 2017, we sold 1,300,000 units to FMG Holdings LLC for the aggregate price of $130,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On May 18, 2017, we sold 150,000 units to FMG Holdings LLC for the aggregate price of $30,000
or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
40
On July 28, 2017, we sold 100,000 units to FMG Holdings LLC for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On October 15, 2015, we sold 500,000 units to Jerry Thompson for the aggregate price of $50,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On November 19, 2015, we sold 200,000 units to Jerry Thompson for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 16, 2015, we sold 250,000 units to Jerry Thompson for the aggregate price of $25,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 21, 2016, we sold 150,000 units to Jerry Thompson for the aggregate price of $15,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On February 5, 2016, we sold 200,000 units to Jerry Thompson for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On February 23, 2016, we sold 65,000 units to Jerry Thompson for the aggregate price of $6,500 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On March 4, 2016, we sold 200,000 units to Jerry Thompson for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On March 18, 2016, we sold 150,000 units to Jerry Thompson for the aggregate price of $15,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On July 6, 2016, we sold a promissory note in the amount of $15,000 to Jerry Thompson. The note accrued interest at 10% and included 15,000 common shares. On January 4, 2017, Mr. Thompson converted the principal and accrued interest outstanding note into 61,368 common shares.
On December 30, 2016, we sold 100,000 units to Jerry Thompson for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 3, 2017, we sold 195,000 units to Jerry Thompson for the aggregate price of $19,500 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On June 4, 2015, we sold 100,000 Units to G&C Investment Corp, a Florida corporation controlled by Jorge Garrido. Each Unit consists of one (1) share of common stock and one (1) warrants for the per share price of $100,000 or an aggregate of $100,000. The warrants were exercisable at $.10 per share and exercised at the time of the investment.
41
On August 14, 2015, we entered into a promissory note, whereby we are obligated to pay Ann Noble the sum of $25,000 plus interest at the rate of 10%. Under the terms of the amended note, the note’s maturity date is August 14, 2016. The note was converted into 250,000 shares of our common stock at the price of $.10 per share on August 14, 2016.
On April 3, 2015, we issued 30,000 shares of our common stock to Barbara Ludwig for an aggregate of $6,000 or the per share price of $.20 per share.
On August 14, 2016, Barbara Ludwig converted principal due in the amount of $20,000 pursuant to an August 14, 2015 convertible note into our common shares at the price of .10 per share or an aggregate of 200,000 shares.
On March 23, 2017, we sold 114,286 units to Barbara Ludwig for the aggregate price of $40,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 1, 2016, Dennis Poland converted principal and accrued interest due in the amount of $55,000 pursuant to a February 20, 2014 convertible note into our common shares at the price of .10 per share or an aggregate of 550,000 shares.
On December 1, 2016, James Stewart converted principal and accrued interest due in the amount of $28,849.05 pursuant to an August 14, 2015 convertible note into our common shares at the price of .10 per share or an aggregate of 287,285 shares.
On January 4, 2017, Neil Catania converted principal and accrued interest due in the amount of $841,750 pursuant to a November 15, 2012 and July 26, 2016 convertible notes and a December 31, 2013 line of credit into our common shares at the price of $.25 per share or an aggregate of 3,367,000 shares. On December 1, 2016, we issued 2,000,000 shares to Neil Catania, VP of the Company, for services rendered. We valued these shares at $.06 per share or an aggregate of $120,000.00.
On January 4, 2017,
John Hampton converted principal and accrued interest due in the amount of $147,583 pursuant to an August 27, 2014 and an October 3, 2014 convertible notes into our common shares at the price of $.25 per share or an aggregate of 590,332 shares.
On January 4, 2017, Michael Smyth converted principal and accrued interest due in the amount of $70,680 pursuant to a November 15, 2012 convertible note into our common shares at the price of $.25 per share or an aggregate of 282,720 shares.
On January 4, 2017, Donald Brennick converted principal and accrued interest due in the amount of $28,395 pursuant to an August 26, 2015 convertible note into our common shares at the price of $.10 per share or an aggregate of 283,950 shares.
On January 30, 2017, we issued 250,000 shares to Bernadine Cawley for services rendered to us. We valued these shares at $.40 per share or an aggregate of $100,000.00.
On May 10, 2017, we sold 375,000 units to Bernadine Cawley for the aggregate price of $75,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $0.50 at any time until the two (2) year anniversary of the date of the investment.
On October 13, 2015, we sold 100,000 units to Tom and Carol Perrine for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
42
On November 17, 2015, we sold 100,000 units to Tom and Carol Perrine for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 29, 2016, we sold 100,000 units to Tom and Carol Perrine for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On October 22, 2015, we sold 102,000 units to Alan Maurer for the aggregate price of $10,200 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On October 22, 2015, we sold 50,000 units to Gordon Langston for the aggregate price of $5,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 9, 2017, we sold 250,000 units to Gordon Langston for the aggregate price of $25,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On November 5, 2015, we sold 50,000 units to Barclay Armitage for the aggregate price of $5,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 8, 2016, we sold 75,000 units to Barclay Armitage for the aggregate price of $7,500 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On November 17, 2015, we sold 50,000 units to Michael Ward for the aggregate price of $5,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 7, 2015, we sold 50,000 units to David Knudtson for the aggregate price of $5,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 15, 2015, we sold 200,000 units to William Rodriguez for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 9, 2017, we sold 405,000 units to William Rodriguez for the aggregate price of $40,500 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 17, 2017, we sold 200,000 units to William Rodriguez for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On December 15, 2015, we sold 50,000 units to Nathaniel Rodriguez for the aggregate price of $5,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
43
On February 19, 2016, we sold 75,000 units to Kerry McDonald for the aggregate price of $7,500 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On February 23, 2016, we sold 250,000 units to Thomas Jacobsen for the aggregate price of $25,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On April 5, 2016, we sold 100,000 units to Laura & Anthony Suttora for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On May 2, 2016, we sold 200,000 units to Anthony Monteleone for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On May 6, 2016, we sold 100,000 units to Leon English for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 7, 2017, we sold 100,000 units to Leon English for the aggregate price of $10,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On June 1, 2016, we sold 400,000 units to Dominant Holdings LLC, a Massachusetts limited liability company controlled by Kelly Benson, for the aggregate price of $40,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $0.50 at any time until the two (2) year anniversary of the date of the investment.
On July 16, 2016, we sold 400,000 units to Dominant Holdings LLC for the aggregate price of $40,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On August 1, 2016, we sold 300,000 units to Dominant Holdings LLC for the aggregate price of $30,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On August 19, 2016, we sold 300,000 units to Dominant Holdings LLC for the aggregate price of $30,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On July 25, 2016, we sold 150,000 units to John Berning for the aggregate price of $15,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 20, 2017, we sold 150,000 units to John Berning for the aggregate price of $15,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On July 27, 2016, we sold 500,000 units to Paul & Cheryl Botts for the aggregate price of $50,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
44
On January 11, 2017, we sold 500,000 units to Paul & Cheryl Botts for the aggregate price of $50,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 6, 2017, we sold 500,000 units to Jeff Luccesi for the aggregate price of $50,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 6, 2017, we sold 300,000 units to STF Partners, LP, a New York limited partnership controlled by Sharyn Frankel, for the aggregate price of $30,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $0.50 at any time until the two (2) year anniversary of the date of the investment.
On January 6, 2017, we sold 200,000 units to Breadfruit Tree Inc., a Florida corporation, doing business as NF Skin, our distributor, and controlled by F. Bruce Hutson, for the aggregate price of $20,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 9, 2017, we sold 300,000 units to Davis Pallen for the aggregate price of $30,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On January 9, 2017, we sold 1,500,000 units to Forage Complete LLC, an Idaho limited liability company controlled by Cody Jensen, for the aggregate price of $150,000 or $.10 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On May 17, 2017, we sold 200,000 units to Forage Complete LLC for the aggregate price of $40,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On July 28, 2017, we sold 100,000 units to Forage Complete LLC for the aggregate price of $20,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On February 23, 2017, we sold 41,666 units to Patricia Gleason for the aggregate price of $25,000 or $.60 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On February 23, 2017, we sold 83,333 units to David Corcoran for the aggregate price of $50,000 or $.60 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On April 24, 2017, we sold 500,000 units to Gregory Ross for the aggregate price of $100,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On May 21, 2017, we sold 50,000 units to James Rutledge for the aggregate price of $10,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
45
On May 24, 2017, we sold 575,000 units to EW Strategies LLC, a Georgia limited liability company controlled by Greg Schantz, for the aggregate price of $115,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On July 7, 2017, we sold 250,000 units to Wolbers Family Trust, a trust controlled by Jennifer Wolbers, for the aggregate price of $50,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $0.50 at any time until the two (2) year anniversary of the date of the investment.
On July 19, 2017, we sold 150,000 units to Peter Mazza for the aggregate price of $30,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On August 1, 2017, we sold 50,000 units to David Dickman for the aggregate price of $10,000 or $.20 per unit. Each unit consists of one (1) share of common stock and one (1) warrant to purchase one (1) share of common stock at the price of $.50 at any time until the two (2) year anniversary of the date of the investment.
On October 1, 2015, we issued 30,000 of our common stock to Peter Cianci in exchange for services rendered. We valued these shares at the price of $.17 per share or an aggregate of $5,10.
On October 1, 2015, we issued 40,000 shares to Five Star Labs, LLC, a Florida limited liability company controlled by Eric Caprarese for services rendered. We valued these shares at $.17 per share or an aggregate of $6,800.00.
On October 1, 2015, we issued 1,000,000 shares to Osprey Capital Advisors, LLC a Florida limited liability company controlled by Terence M. Taylor, for services rendered. We valued these shares at $.183 per share or an aggregate of $183,000.00.
On December 1, 2015, we issued 1,000,000 shares to Osprey Capital Advisors, LLC a Florida limited liability company controlled by Terence M. Taylor, for services rendered. We valued these shares at $.122 per share or an aggregate of $122,000.00.
On December 16, 2015 and January 2, 2016, we issued 75,000 shares to WT Consulting, LLC a Florida limited liability company controlled by William Hirschy, for services rendered. We valued these shares at $.3467 per share or an aggregate of $52,000.
On January 4, 2016, we issued 100,000 shares to Patagonia Global Trading, LLC, a Florida limited liability company controlled by David Zirulnikoff, for services rendered. We valued these shares at $.10 per share or an aggregate of $10,000.00.
On January 2, 2017, we issued 50,000 shares to Patagonia Global Trading, a Florida entity, for services rendered. We valued these shares at $.25 per share or an aggregate of $12,500.00.
On June 9, 2016, we issued Josh Zwagil 244,514 shares for new business development. We valued these shares at $.11 per share, or an aggregate of $26,896.54.
On December 1, 2016, we issued 4,000,000 shares to Edgar Ward, CEO of the Company, for services rendered. We valued these shares at $.06 per share or an aggregate of $240,000.00.
On March 3, 2017, we issued 7,220,585 shares to Edgar Ward, CEO of the Company, for services rendered. We valued these shares at $1.45 per share or an aggregate of $10,453,315. On November 27, 2017, we issued 6,674,837 shares to Edgar Ward, CEO of the Company, for services rendered. We valued these shares at $1.27 per share or an aggregate of $8,464,463.
On December 1, 2016, we issued 1,000,000 shares to Nicole Archon for services rendered. We valued these shares at $.06 per share or an aggregate of $60,000.00.
46
On December 14, 2016, we issued 249,999 shares to Venture Capital Group, LLC a Delaware limited liability company controlled by William Stern, for services rendered. We valued these shares at an aggregate $.0972 per share or an aggregate of $24,300.00.
On January 17, 2017, we issued 100,000 shares to Hamilton & Associates Law Group a Florida law firm controlled by Brenda Hamilton, Esq., for services rendered. We valued these shares at an aggregate $.10 per share or an aggregate of $10,000.00.
On January 30, 2017, we issued 400,000 shares to Anthony Procelli, for services rendered. We valued these shares at $.40 per share or an aggregate of $160,000.00.
On January 30, 2017, we issued 50,000 shares to Patrick Kilcooley, for services rendered. We valued these shares at $.40 per share or an aggregate of $20,000.00.
On January 30, 2017, we issued 300,000 shares to Daniel Ryan, for services rendered. We valued these shares at $.40 per share or an aggregate of $120,000.00.
On February 1, 2017, we issued 50,000 shares to Sylvan Eudes, for services rendered. We valued these shares at $.12 per share or an aggregate of $6,000.00.
On April 10, 2017, we issued 250,000 shares of our restricted common stock to Michael R. Anderson for services rendered. We valued these shares at $.69 per share or an aggregate of $172,500.00.
On July 17, 2017, we issued 100,000 shares to Kenneth Duchin, for services rendered. We valued these shares at $.80 per share or an aggregate of $80,000.00.
On October 14, 2014, we issued 60,000 shares of our common stock to Uptick Capital LLC for services rendered to us. We valued these shares at an aggregate $48,000 or $.80 per share.
On March 5, 2015, we issued 60,000 shares of our common stock to Uptick Capital LLC for services rendered to us. We valued these shares at an aggregate $116,400 or $1.94 per share.
On April 14, 2015, we entered into a consulting agreement with Benchmark Advisory Partners, LLC, a Florida limited liability company controlled by Timothy Conner. In consideration for future consulting services, we agreed to pay a fixed fee of three hundred thousand restricted shares of our stock, payable in one hundred thousand share installments on April 14, 2015, June 14, 2015, and August 14, 2015. We paid the initial 100,000 common shares. We valued these shares at an aggregate $51,000 or $.51 per share. The agreement was terminated in June 2015.
47
ITEM 11. DESCRIPTION
OF SECURITIES
The following description is a summary of the material terms of the provisions of our Articles of Incorporation and Bylaws as they relate to our capital structure. The Articles of Incorporation and Bylaws are available for inspection upon request and are filed as Exhibits to this Form 10 Registration Statement.
We are authorized to issue 499,990,000 shares of common stock, $.0001 par value per share, and 10,000 shares of preferred stock. As of the date of this Form 10 Registration Statement there are 80,248,561 shares of our common stock issued and outstanding held by 86 stockholders of record, and 1,000 shares of preferred stock outstanding held by one (1) holder, Edgar Ward, our chief executive officer, president and sole director.
Common Stock
Each share of our common stock entitles the holder to one (1) vote, either in person or by proxy, at meetings of shareholders. The shareholders are not permitted to vote their shares cumulatively. Accordingly, the holders of more than fifty percent (50%) of the total voting rights on matters presented to our common stockholders can elect all of our directors and, in such event, the holders of the remaining minority shares will not be able to elect any such directors. The vote of the holders of a majority of the holders entitled to vote on matters submitted to our common stockholders including of our Series A Preferred Shares described below is sufficient to authorize, affirm, ratify, or consent to such act or action, except as otherwise provided by law.
To date, we have paid no cash dividends on our shares of common stock. Any future disposition of dividends will be at the discretion of our Board of directors and will depend upon, among other things, our future earnings, operating and financial condition, capital requirements, and other factors. We have no present plans for future cash or stock dividends. We intend to retain future earnings, if any, to provide funds for operation of our business.
Holders of our common stock have no preemptive rights. All outstanding shares of our common stock are, and the common stock to be issued upon completion of the Offering upon issuance will be validly issued, fully paid and non-assessable.
Upon our liquidation or dissolution, the assets legally available for distribution to holders of shares of the common stock, after payment of all of our obligations, are distributable ratably among the holders of the then outstanding common stock.
All shares of common stock outstanding are validly issued, fully paid and non-assessable.
Preferred Stock
We are authorized to issue 10,000 shares of preferred stock with a par value of $.0001 per share. We have designated 1,000 shares of our preferred stock as Series A Shares. All outstanding Series A Preferred Shares are validly issued, fully paid and non-assessable.
The Series A Shares have the following rights and preferences:
·
Each one (1) share is entitled to 500,000 votes per share on all matters submitted to our common stockholders.
·
The Series A Shares are not convertible into common shares;
·
The holders of the Series A Shares are not entitled to receive dividends or any distribution upon our liquidation or dissolution;
·
The holders of the Series A Shares cannot assign or sell the shares; and
·
The Series A Shares are redeemable in whole by us for the price of $1000 at the option of the holder.
48
So long as any of the Series A Shares are outstanding, we cannot take the following actions without the consent of the holders of 100% of the Series A Shares: amend, alter, waive or repeal, whether by merger consolidation, combination, reclassification or otherwise, the Articles of Incorporation or Bylaws; or create, authorize or issue any class, series or shares of any class of capital stock. The rights and preferences of the Series A Share cannot be amended without the consent of 100% of the holders of the Series A Shares.
As of the date of this Form 10 Registration Statement, we had 1,000 Series A shares outstanding which are held by Edgar Ward, our chief executive officer, president and sole director. The 1,000 shares held by Mr. Ward entitle him to 500,000 votes per share, or a total of 500,000,000 votes, on all matters submitted to our stockholders. As of the date hereof, we have 80,248,561 common shares outstanding. As a result, Mr. Ward has the ability to determine the outcome of all matters submitted to a vote of our stockholders.
Our board of directors may designate the authorized but unissued shares of the Preferred Stock with such rights and privileges as the board of directors may determine subject to the rights granted to the holders of the Series A Shares as described above. As such, our board of directors may issue an additional 9,000 preferred shares and designate conversion, voting and other rights and preferences without notice to our shareholders and without shareholder approval if it obtains the consent of Mr. Ward.
FLORIDA ANTI-TAKEOVER LAWS
As a Florida corporation, we are subject to certain anti-takeover provisions that apply to public corporations under Florida law. Pursuant to Section 607.0901 of the Florida Business Corporation Act, or the Florida Act, a publicly held Florida corporation may not engage in a broad range of business combinations or other extraordinary corporate transactions with an interested shareholder without the approval of the holders of two-thirds of the voting shares of the corporation (excluding shares held by the interested shareholder), unless:
·
the transaction is approved by a majority of disinterested directors before the shareholder becomes an interested shareholder;
·
the interested shareholder has owned at least 80% of the corporation’s outstanding voting shares for at least five years preceding the announcement date of any such business combination;
·
the interested shareholder is the beneficial owner of at least 90% of the outstanding voting shares of the corporation, exclusive of shares acquired directly from the corporation in a transaction not approved by a majority of the disinterested directors; or
·
the consideration paid to the holders of the corporation’s voting stock is at least equal to certain fair price criteria.
An interested shareholder is defined as a person who together with affiliates and associates beneficially owns more than 10% of a corporation’s outstanding voting shares. We have not made an election in our amended Articles of Incorporation to opt out of Section 607.0901. In addition, we are subject to Section 607.0902 of the Florida Act which prohibits the voting of shares in a publicly held Florida corporation that are acquired in a control share acquisition unless (i) our board of directors approves such acquisition prior to its consummation or (ii) after such acquisition, in lieu of prior approval by our board of directors, the holders of a majority of the corporation’s voting shares, exclusive of shares owned by officers of the corporation, employee directors or the acquiring party, approve the granting of voting rights as to the shares acquired in the control share acquisition. A control share acquisition is defined as an acquisition that immediately thereafter entitles the acquiring party to 20% or more of the total voting power in an election of directors.
49
ITEM 12.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our Articles and Bylaws subject to the provisions of Florida law contain provisions which allow the corporation to indemnify any person against liabilities and other expenses incurred as the result of defending or administering any pending or anticipated legal issue in connection with service to us if it is determined that person acted in good faith and in a manner which he/she reasonably believed was in the best interest of the corporation. Insofar, indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers, and controlling persons. We have been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
ITEM 13. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
See “Item 15. Financial Statements and Exhibits.”
ITEM 14. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 15. FINANCIAL STATEMENTS AND EXHIBITS
(a) Financial Statements.
The financial statements and related notes are included as part of this Form 10 registration statement as indexed in the appendix on page F-1 through F-16.
(b) Exhibits required by Item 601 of Regulation S-K
Where You Can Find More Information
We are not required to deliver an annual report to our stockholders unless our directors voluntarily determine to issue and deliver an annual report. We have filed with the Securities and Exchange Commission this registration statement on Form 10 under the Securities Act of 1934 with respect to the registration of our common stock under Section 12G.
You may review a copy of the registration statement at the Securities and Exchange Commission’s public reference room at 100 F Street, N.E. Washington, D.C. 20549 on official business days during the hours of 10 a.m. to 3 p.m. You may obtain information on the operation of the public reference room by calling the Securities and Exchange Commission at 1-800-SEC-0330. You may also read and copy any materials we file with the Securities and Exchange Commission at the Securities and Exchange Commission’s public reference room. Our filings and the registration statement can also be reviewed by accessing the Securities and Exchange Commission’s website at http://www.sec.gov.
50
INDEX TO FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm
F-2
Balance Sheets
F-3
Statements of Operations
F-4
Statements of Changes in Stockholders’ Deficit
F-5
Statements of Changes in Cash Flows
F-6
Notes to Financial Statements
F-7
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Shareholders of NutraFuels, Inc.
Coconut Creek, Florida
We have audited the accompanying balance sheets of NutraFuels, Inc. (the “Company”) at December 31, 2016 and 2015, and the related statements of operations, shareholders’ deficit and cash flows for the years then ended. The Company’s management is responsible for these financial statements. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of NutraFuels, Inc. at December 31, 2016 and 2015, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 3 to the financial statements, the Company has sustained recurring losses from operations and has working capital and accumulated deficits that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are described in Note 3. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
/s/ Daszkal Bolton LLP
Fort Lauderdale, Florida
November 1, 2017
F-2
NUTRAFUELS, INC.
Balance Sheets
|
|
|
|
|
|
|
ASSETS
|
September 30, 2017
|
|
December 31, 2016
|
|
December 31, 2015
|
|
CURRENT ASSETS
|
(unaudited)
|
|
|
|
|
|
Cash
|
$
387,402
|
|
$
12,133
|
|
$
17,144
|
|
Accounts receivable
|
115,240
|
|
-
|
|
162
|
|
Inventory, net of reserve of $0; $0 and $14,378
|
259,961
|
|
94,404
|
|
97,427
|
|
Prepaid expenses and other current assets
|
229,016
|
|
152,040
|
|
270,270
|
|
Total current assets
|
991,619
|
|
258,577
|
|
385,003
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT
|
|
|
|
|
|
|
Furniture, fixtures and equipment
|
372,540
|
|
296,447
|
|
283,491
|
|
Leasehold improvements
|
146,285
|
|
112,285
|
|
108,935
|
|
Total Property and Equipment
|
518,825
|
|
408,732
|
|
392,426
|
|
Less accumulated depreciation
|
(265,683)
|
|
(214,842)
|
|
(154,341)
|
|
|
|
|
|
|
|
|
Property and equipment, net
|
253,142
|
|
193,890
|
|
238,085
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Assets
|
$
1,244,761
|
|
$
452,467
|
|
$
623,088
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
Accounts payable
|
$
99,481
|
|
$
19,335
|
|
$
33,042
|
|
Accrued expenses
|
8,624,463
|
|
724,492
|
|
470,255
|
|
Customer deposits
|
86,951
|
|
34,996
|
|
34,997
|
|
Convertible debt net of discount of $0; $249,338 and 162,160
|
-
|
|
1,132,251
|
|
900,663
|
|
Convertible debt - related party
|
-
|
|
210,000
|
|
210,000
|
|
Notes payable, net of discount of $0
|
-
|
|
-
|
|
55,000
|
|
Notes payable - related party
|
14,000
|
|
432,000
|
|
452,500
|
|
|
|
|
|
|
|
|
Total current liabilities
|
8,824,895
|
|
2,553,074
|
|
2,156,457
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ DEFICIT
|
|
|
|
|
|
|
Preferred stock, $0.0001 par value, authorized 10,000 shares; 1,000
shares issued and outstanding
|
-
|
|
-
|
|
-
|
|
Common stock, $0.0001 par value, authorized 499,990,000 shares;
73,723,724 and 45,890,912 and 29,014,114 shares issued and
outstanding
|
7,372
|
|
4,589
|
|
2,901
|
|
Additional paid-in capital
|
24,744,510
|
|
7,024,608
|
|
5,494,763
|
|
Accumulated deficit
|
(32,332,016)
|
|
(9,129,804)
|
|
(7,031,033)
|
|
|
|
|
|
|
|
|
Total stockholders’ deficit
|
(7,580,134)
|
|
(2,100,607)
|
|
(1,533,369)
|
|
Total Liabilities and Stockholders’ Deficit
|
$
1,244,761
|
|
$
452,467
|
|
$
623,088
|
The accompanying notes are an integral part of the financial statements
F-3
NUTRAFUELS, INC.
Statements of Operations
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
Year Ended December 31,
|
|
|
2017
|
|
2016
|
|
2016
|
|
2015
|
|
|
(unaudited)
|
|
(unaudited)
|
|
|
|
|
|
Revenue
|
$
1,029,727
|
|
$
195,909
|
|
$
225,293
|
|
$
134,006
|
|
|
|
|
|
|
|
|
|
|
Cost of sales
|
617,958
|
|
118,731
|
|
195,195
|
|
115,905
|
|
|
|
|
|
|
|
|
|
|
Gross Profit
|
411,769
|
|
77,178
|
|
30,098
|
|
18,101
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES:
|
|
|
|
|
|
|
|
|
Advertising and promotion
|
51,151
|
|
21,493
|
|
53,619
|
|
198,424
|
|
Administrative salaries
|
221,330
|
|
238,553
|
|
235,295
|
|
204,500
|
|
Non cash compensation
|
19,134,686
|
|
232,050
|
|
513,196
|
|
640,030
|
|
General and administrative expenses
|
815,035
|
|
269,087
|
|
660,214
|
|
662,784
|
|
Depreciation expense
|
50,841
|
|
45,160
|
|
60,501
|
|
55,807
|
|
Total operating expenses
|
20,273,043
|
|
806,343
|
|
1,522,825
|
|
1,761,545
|
|
LOSS FROM OPERATIONS
|
(19,861,274)
|
|
(729,165)
|
|
(1,492,727)
|
|
(1,743,444)
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME AND (EXPENSE)
|
|
|
|
|
|
|
|
|
Other income
|
717
|
|
(3,398)
|
|
5,400
|
|
-
|
|
Interest expense
|
(3,341,655)
|
|
(320,094)
|
|
(611,444)
|
|
(431,097)
|
|
Total other expense
|
(3,340,938)
|
|
(323,492)
|
|
(606,044)
|
|
(431,097)
|
|
Net loss before income taxes
|
(23,202,212)
|
|
(1,052,657)
|
|
(2,098,771)
|
|
(2,174,541)
|
|
Income tax expense
|
-
|
|
-
|
|
-
|
|
-
|
|
Net loss
|
$
(23,202,212)
|
|
$
(1,052,657)
|
|
$
(2,098,771)
|
|
$
(2,174,541)
|
|
Loss per weighted average common shares outstanding, basic and diluted
|
($0.33)
|
|
($0.03)
|
|
($0.06)
|
|
($0.09)
|
|
|
|
|
|
|
|
|
|
|
Number of weighted average common shares outstanding, basic and diluted
|
70,266,530
|
|
33,689,465
|
|
33,689,465
|
|
23,583,399
|
The accompanying notes are an integral part of the financial statements
F-4
NUTRAFUELS, INC.
Statements of Changes in Stockholders’ Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number
Shares
Pfd
|
|
Number
Shares
Common
|
|
Par
Amount Pfd
|
|
Par
Amount
Common
|
|
Additional
Paid-in
Capital
|
|
Accumulated
Deficit
|
|
Total
Stockholder
s’
Deficit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE,
January 1, 2015
|
1,000
|
|
22,282,114
|
|
$
-
|
|
$
2,228
|
|
$
3,904,935
|
|
$
(4,856,492)
|
|
$
(949,329)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares issued for cash
|
-
|
|
2,452,000
|
|
-
|
|
245
|
|
334,956
|
|
-
|
|
335,201
|
|
Shares issued for services
|
-
|
|
3,305,000
|
|
-
|
|
331
|
|
909,969
|
|
-
|
|
910,300
|
|
Shares issued for issuance of debt
|
-
|
|
275,000
|
|
-
|
|
27
|
|
274,973
|
|
-
|
|
275,000
|
|
Warrants exercised for cash
|
-
|
|
700,000
|
|
-
|
|
70
|
|
69,930
|
|
-
|
|
70,000
|
|
Net loss
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(2,174,541)
|
|
(2,174,541)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE
, December 31, 2015
|
1,000
|
|
29,014,114
|
|
-
|
|
2,901
|
|
5,494,763
|
|
(7,031,033)
|
|
(1,533,369)
|
|
Shares issued for cash
|
-
|
|
5,615,000
|
|
-
|
|
562
|
|
560,937
|
|
-
|
|
561,499
|
|
Shares issued for services
|
-
|
|
7,719,513
|
|
-
|
|
772
|
|
512,425
|
|
-
|
|
513,197
|
|
Shares issued for issuance of debt
|
-
|
|
1,195,000
|
|
-
|
|
119
|
|
80,569
|
|
-
|
|
80,688
|
|
Shares issued for debt conversion
|
-
|
|
2,347,285
|
|
-
|
|
235
|
|
375,914
|
|
-
|
|
376,149
|
|
Net loss
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(2,098,771)
|
|
(2,098,771)
|
|
Balance
, December 31, 2016
|
1,000
|
|
45,890,912
|
|
-
|
|
4,589
|
|
7,024,608
|
|
(9,129,804)
|
|
(2,100,607)
|
|
Shares issued for cash
|
-
|
|
9,707,285
|
|
-
|
|
971
|
|
1,323,529
|
|
-
|
|
1,324,500
|
|
Options exercised for cash
|
-
|
|
275,000
|
|
-
|
|
27
|
|
54,973
|
|
-
|
|
55,000
|
|
Shares issued for debt conversion
|
-
|
|
10,129,942
|
|
-
|
|
1,013
|
|
5,613,858
|
|
-
|
|
5,614,871
|
|
Shares issued for services
|
-
|
|
7,720,585
|
|
-
|
|
772
|
|
10,727,543
|
|
-
|
|
10,728,315
|
|
Net loss
|
-
|
|
-
|
|
-
|
|
-
|
|
-
|
|
(23,202,212)
|
|
(23,202,212)
|
|
Balance
, September 30, 2017 (unaudited)
|
1,000
|
|
73,723,724
|
|
$
0
|
|
$
7,372
|
|
$
24,744,510
|
|
$
(32,332,015)
|
|
$
(7,580,134)
|
The accompanying notes are an integral part of the financial statements
F-5
NUTRAFUELS, INC.
Statements of Changes in Cash Flows
|
|
|
|
|
|
|
|
|
|
Nine Months Ended September 30,
|
|
Year Ended December 31,
|
|
|
2017
|
|
2016
|
|
2016
|
|
2015
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
(Unaudited)
|
|
(Unaudited)
|
|
|
|
|
|
Net loss
|
$
(23,202,212)
|
|
$
(1,052,657)
|
|
$
(2,098,771)
|
|
$
(2,174,541)
|
|
Adjustments to reconcile net loss to net cash used in
operating activities:
|
|
|
|
|
|
|
|
|
Stock compensation
|
10,728,315
|
|
180,107
|
|
513,196
|
|
640,030
|
|
Depreciation
|
50,841
|
|
45,160
|
|
60,501
|
|
55,807
|
|
Amortization of stock based compensation
|
217,524
|
|
-
|
|
|
|
|
|
Amortization of debt discount
|
531,876
|
|
194,214
|
|
250,128
|
|
290,551
|
|
Induced conversion expense
|
3,116,500
|
|
-
|
|
141,300
|
|
-
|
|
Inventory valuation allowance
|
-
|
|
-
|
|
-
|
|
(193,824)
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
(Increase) decrease in accounts receivable
|
(115,240)
|
|
162
|
|
162
|
|
1,517
|
|
Decrease (Increase) in subscription receivable
|
-
|
|
5,000
|
|
5,000
|
|
(5,000)
|
|
(Increase) decrease in inventory
|
(165,557)
|
|
5,860
|
|
3,023
|
|
(27,426)
|
|
(Increase) decrease in prepaid expenses and other assets
|
(76,976)
|
|
59,865
|
|
123,230
|
|
-
|
|
Increase (decrease) in accounts payable
|
80,146
|
|
9,180
|
|
(13,707)
|
|
(968)
|
|
Increase in customer deposits
|
51,955
|
|
-
|
|
-
|
|
-
|
|
Increase (decrease) in accrued expenses
|
7,904,190
|
|
(36,596)
|
|
289,233
|
|
269,174
|
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
(878,638)
|
|
(589,705)
|
|
(726,705)
|
|
(1,144,680)
|
|
|
|
|
|
|
|
|
|
|
CASH FLOW FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
Purchase of property and equipment
|
(110,093)
|
|
(16,306)
|
|
(16,306)
|
|
(44,929)
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
(110,093)
|
|
(16,306)
|
|
(16,306)
|
|
(44,929)
|
|
|
|
|
|
|
|
|
|
|
CASH FLOW FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
Common stock issued for cash
|
1,324,500
|
|
446,500
|
|
556,500
|
|
340,200
|
|
Options exercised for cash
|
55,000
|
|
-
|
|
|
|
|
|
Cash proceeds from debt issuance
|
-
|
|
235,000
|
|
345,000
|
|
549,000
|
|
Cash proceeds from debt issuance - related parties
|
-
|
|
-
|
|
34,500
|
|
302,500
|
|
Repayment of debt
|
-
|
|
(65,500)
|
|
(148,000)
|
|
-
|
|
Repayment of debt - related party
|
(15,500)
|
|
(15,000)
|
|
(50,000)
|
|
(10,000)
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
1,364,000
|
|
601,000
|
|
738,000
|
|
1,181,700
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash
|
375,269
|
|
(5,011)
|
|
(5,011)
|
|
(7,909)
|
|
|
|
|
|
|
|
|
|
|
CASH
, beginning of period
|
12,133
|
|
17,144
|
|
17,144
|
|
25,053
|
|
|
|
|
|
|
|
|
|
|
CASH
, end of period
|
$
387,402
|
|
$
12,133
|
|
$
12,133
|
|
$
17,144
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
|
|
|
|
|
|
|
|
Interest paid in cash
|
$
1,843
|
|
$
1,753
|
|
$
11,821
|
|
$
2,351
|
|
Taxes paid in cash
|
$
-
|
|
$
-
|
|
$
-
|
|
$
-
|
|
Non-Cash Financing Activities:
|
|
|
|
|
|
|
|
|
Debt discount from beneficial conversion feature
|
$
-
|
|
$
-
|
|
$
-
|
|
$
370,000
|
|
Shares issued for the issuance of debt
|
$
-
|
|
$
31,600
|
|
$
80,688
|
|
$
275,000
|
|
Shares issued for prepaid expenses
|
$
-
|
|
$
-
|
|
$
-
|
|
$
270,270
|
|
Shares issued for the conversion of debt
|
$
2,403,343
|
|
$
-
|
|
$
234,849
|
|
$
-
|
The accompanying notes are an integral part of the financial statements
F-6
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(1) NATURE OF OPERATIONS
NutraFuels, Inc. (“We”, or the “Company”) is the producer and distributor of nutritional supplements that uses micro molecular formulae and a utilization of an oral spray to provide faster and more efficient absorption.
(2) BASIS OF PRESENTATION AND USE OF ESTIMATES
a) Basis of Presentation
The accompanying audited annual and unaudited interim financial statements have been prepared in accordance with Generally Accepted Accounting Principles ("GAAP") in the United States of America ("U.S.") as promulgated by the Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") and with the rules and regulations of the U.S. Securities and Exchange Commission ("SEC"). In our opinion, the accompanying unaudited interim financial statements contain all adjustments (which are of a normal recurring nature) necessary for a fair presentation. Operating results for the nine months ended September 30, 2017 are not necessarily indicative of the results that may be expected for the year ending December 31, 2017.
b) Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from these estimates.
c) Cash and Equivalents
Cash equivalents are highly liquid investments with an original maturity of three months or less.
d) Inventories
Inventories are stated at cost utilizing the weighted average method of valuation and consist of raw materials and finished goods.
e) Allowance for Doubtful Accounts
We establish a reserve for bad debts through a review of several factors including historical collection experience, current aging status of the customer accounts, and financial condition of our customers.
f) Property and Equipment
All property and equipment are recorded at cost and depreciated over their estimated useful lives, generally three to twelve years, using the straight-line method. Upon sale or retirement, the cost and related accumulated depreciation are eliminated from their respective accounts, and the resulting gain or loss is included in the results of operations. Repairs and maintenance charges, which do not increase the useful lives of the assets, are charged to operations as incurred.
g) Revenue Recognition
Our financial statements are prepared under the accrual method of accounting. Revenues are recognized when persuasive evidence of an arrangement exists, services have been rendered, the sales price is fixed or determinable, and collectability is reasonably assured. This occurs only when the ordered product is shipped to the customer.
F-7
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(2) BASIS OF PRESENTATION AND USE OF ESTIMATES, continued
h) Income Taxes
We follow the provisions of ASC 740-10, Accounting for Uncertain Income Tax Positions. When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. In accordance with the guidance of ASC 740-10, the benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above should be reflected as a liability for unrecognized tax benefits in the accompanying consolidated balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination.
As of September 30, 2017, the tax years 2016, 2015 and 2014 for the Company remains open for IRS audit. The Company has received no notice of audit or any notifications from the IRS for any of the open tax years.
i) Net Loss Per Share
Basic loss per share excludes dilution and is computed by dividing the loss attributable to stockholders by the weighted-average number of shares outstanding for the period. Diluted loss per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that shared in our earnings. Diluted loss per share is computed by dividing the loss available to stockholders by the weighted average number of shares outstanding for the period and dilutive potential shares outstanding unless consideration of such dilutive potential shares would result in anti-dilution.
j) Financial Instruments and Fair Value Measurements
ASC 825 requires disclosures of the fair value of financial instruments. The carrying value of our current financial instruments, which include cash, accounts payable and accrued liabilities approximates their fair values because of the short-term maturities of these instruments.
FASB ASC 820 “Fair Value Measurement” clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. It also requires disclosure about how fair value is determined for assets and liabilities and establishes a hierarchy for which these assets and liabilities must be grouped, based on significant levels of inputs as follows:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Quoted prices in active markets for similar assets and liabilities and inputs that are observable for the asset or liability.
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
F-8
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(2) BASIS OF PRESENTATION AND USE OF ESTIMATES, continued
k) Impairment of Long-Lived Assets
A long-lived asset is tested for impairment whenever events or changes in circumstances indicate that its carrying value amount may not be recoverable. An impairment loss is recognized when the carrying amount of the asset exceeds the sum of the undiscounted cash flows resulting from its use and eventual disposition. The impairment loss is measured as the amount by which the carrying amount of the long-lived assets exceeds its fair value.
l) Related Party Transactions
All transactions with related parties are in the normal course of operations and are measured at the exchange amount.
m) Recent Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers,” which requires companies to identify contractual performance obligations and determine whether revenue should be recognized at a point in time or over time based on when control of goods and services transfer to a customer. As a result, we do not expect significant changes in the presentation of our financial statements. This ASU is effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period, and entities are permitted to apply either prospectively or retrospectively; early adoption is permitted. We do not expect adoption of this guidance to have a material effect on our financial position, results of operations and cash flows.
In February 2016, the FASB issued ASU 2016-02, “Leases” which, for operating leases, requires a lessee to recognize a right-of-use asset and a lease liability, initially measured at the present value of the lease payments, in its balance sheet. The standard also requires a lessee to recognize a single lease cost, calculated so that the cost of the lease is allocated over the lease term, on a generally straight-line basis. The ASU is effective for public companies for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early adoption is permitted. The adoption of ASU 2016-02 is expected to result in the recognition of right of use assets and associated obligations on our balance sheet.
(3) LIQUIDITY AND GOING CONCERN CONSIDERATIONS
Our financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the settlement of liabilities and commitments in the normal course of business. We sustained a net loss of approximately $2.1 and $23.2 million for the year ended December 31, 2016and nine months ended September 30, 2017, respectively, and have an accumulated deficit of approximately $23.3 million and a negative working capital of approximately $7.8 million at September 30, 2017. These conditions raise substantial doubt about our ability to continue as a going concern.
Failure to successfully continue to grow operational revenues could harm our profitability and materially adversely affect our financial condition and results of operations. We face all of the risks inherent in a new business, including the need for significant additional capital, management’s potential underestimation of initial and ongoing costs, and potential delays and other problems in connection with establishing sales channels.
We are continuing our plan to further grow and expand operations and seek sources of capital to pay our contractual obligations as they come due. Management believes that its current operating strategy will provide the opportunity for us to continue as a going concern as long as we are able to obtain additional financing; however, there is no assurance this will occur. The accompanying consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
The independent auditors’ report on our consolidated financial statements for the years ended December 31, 2016 and 2015 contain explanatory paragraphs expressing substantial doubt as to our ability to continue as a going concern.
F-9
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(4) CONVERTIBLE DEBT
On March 26, 2014, we issued a $290,000 convertible note. The note bears interest at 10%, with an initial maturity of March 26, 2015 (subsequently amended to January 15, 2016), and is convertible into shares of our common stock at $1.00 per share. The investor also received warrants to purchase 500,000 shares of our common stock at $0.50 per share with a two-year exercise term.
We evaluated the warrants for derivative accounting consideration under ASC Topic 815-40, Derivatives and Hedging – Contracts in Entity’s own stock, and concluded that the warrants meet the criteria for classification in shareholders’ equity. We allocated the proceeds received to the debt, stock, and warrants based on their relative fair values and determined the fair value of the warrants using a Black-Scholes option pricing model.
On June 23, 2014, we issued a $30,000 convertible note. The note bears interest at 10%, matures on June 23, 2015, and is convertible into shares of our Company at $1.00 per share. Because the market price for our common stock on the date of the note exceeded the note’s conversion price of $1.00 per share, we recognized a beneficial conversion feature of $21,600 as a discount on the note which was amortized as additional interest.
We evaluated the conversion features embedded in the two notes payable described above for derivative accounting in accordance with ASC 815-40, Derivatives and Hedging embedded in the modified notes payable for derivative accounting in accordance with the criteria for classification in shareholders’ equity.
On August 27, 2014, we issued a $50,000 convertible note. The note bears interest at 10%, had an initial maturity of January 2, 2015 (subsequently extended to January 15, 2016), and is convertible into shares of our common stock at $1.00 per share. The investor also received 50,000 shares of our common stock. Because the market price for our common stock on the date of the note exceeded the note’s conversion price of $1.00 per share, we recognized a beneficial conversion feature of $32,927 as a discount on the note which was amortized as additional interest.
During October 2014, we issued a $60,000 convertible note. The note bears interest at 10%, had an initial maturity of November 2, 2014 (subsequently extended to January 15, 2016) and is convertible into shares of our common stock at $1.00 per share. The investor also received 150,000 shares of our common stock. Because the market price for our common stock on the date of the note exceeded the note’s conversion price of $1.00 per share, we recognized a beneficial conversion feature of $40,500 as a discount on the note which was amortized as additional interest.
In February 2015, we sold 25,000 units to an investor in exchange for $25,000. The 25,000 units consist of: (i) 25,000 shares of our common stock; (ii) 2-year options to purchase 25,000 shares of our common stock at $0.20, and (iii) a 2-year convertible promissory note in the amount of $25,000. The note is non-interest bearing and is convertible into shares of our common stock at the higher of (a) twenty five cents ($.25) or (b) fifty percent (50%) of the average closing price of our shares as reported by the OTC Markets for the 10 trading days prior to the day of conversion.
The conversion rights embedded in the note are accounted for as a derivative financial instrument because of the beneficial conversion feature embedded therein. The beneficial conversion feature was valued and recorded at the date of issuance at fair value, and recorded as a debt discount.
In April 2015, we sold 250,000 units to an investor in exchange for $250,000. The 250,000 units consist of: (i) 250,000 shares of our common stock; (ii) 2-year options to purchase 250,000 shares of our common stock at $0.20, and (iii) a 2-year convertible promissory note in the amount of $250,000. The note bears 10% interest and is convertible into shares of our common stock at the higher of (a) twenty-five cents ($.25) or (b) fifty percent (50%) of the average closing price of our shares as reported by the OTC Markets for the 10 trading days prior to the day of conversion.
The conversion rights embedded in the note are accounted for as a derivative financial instrument because of the beneficial conversion feature embedded therein. The beneficial conversion feature was valued and recorded at the date of issuance at fair value, and recorded as a debt discount.
F-10
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(4) CONVERTIBLE DEBT, continued
In August 2015, we entered into convertible promissory notes with four individual investors for a total amount of $95,000. The notes are interest bearing at a fixed rate of ten percent (10%) and are convertible into shares at $0.10 per share.
In April 2016 we entered into a one year 10% note for $38,000 cash, which included the issuance of 100,000 shares of common stock, valued at $14,000. We paid this loan back in full in June 2016.
In June 2016 we entered into two one year 10% notes for $47,000 cash, which included the issuance of 90,000 shares of common stock, valued at $9,900. In June 2016 we entered into a short term 10% note for $25,000 cash, which included the issuance of 70,000 shares of common stock, valued at $7,700.
In July 2016 we entered into two one year 10% notes for $35,000 cash, which included the issuance of 35,000 shares of common stock, valued at $4,588.
In August 2016, we extended the maturity of our $100,000 promissory note to August 26, 2017.
In the third quarter 2016 we entered into two short term 10% notes for $75,000 cash, which included the issuance of 350,000 shares of common stock, valued at $21,500.
In the fourth quarter 2016 we entered into two short term 10% notes for $125,000 cash, which included the issuance of 400,000 shares of common stock, valued at $23,000.
During 2016 we issued 2,347,285 shares of common stock for the conversion of debt and accrued interest totaling $234,849 and recorded an induced conversion expenses of $141,300 for debt that was converted at less than the stated conversion rate.
In January 2017 we issued 10,129,942 shares of common stock for the conversion of debt and accrued interest totaling $2,403,343 and recorded an induced conversion charge of $3,116,500 for debt that was converted at a discount to the stated conversion rate.
(5) NOTES PAYABLE - RELATED PARTY
On November 15, 2012, we issued a $160,000 convertible note. The note bears interest at 10% with an initial maturity of November 15, 2014 (subsequently extended to November 15, 2017), and is convertible into shares of our common stock at $1.00 per share.
On February 15, 2013 we issued a $50,000 convertible note. The note bears interest at 10%, with original maturity of May 15, 2014 (subsequently modified to November 15, 2017), and is convertible into shares of our common stock at $1.00 per share.
During 2016 we received $102,500 in cash and repaid $73,000 in cash under a non-interest bearing line of credit from an officer. During the first nine months of 2017 we repaid $14,500 of the remaining balance.
In January 2017 we issued 3,367,000 shares of common stock to convert $841,750 of debt and accrued interest to a related party.
In the April 2017 we repaid $10,000 in cash to a related party on a short-term non interest bearing basis. In July 2017 we repaid $5,500 in cash to a related party on a short-term non interest bearing basis.
F-11
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(6) STOCKHOLDERS’ EQUITY
On January 1, 2015 we issued 1,000,000 shares of our common stock to four individuals for future services, ending on January 31, 2018. We recorded prepaid consulting fees in the amount of $400,000. The unamortized balance was $76,614; $140,540 and $270,270 at September 30, 2017; December 31 2016 and 2015, respectively.
During February 2015, we issued 25,000 shares of our common stock as a debt discount, valued at $25,000.
During March 2015, we issued 60,000 shares of our common stock for consulting services rendered to us. We valued these shares at $1.94 per share, the closing stock price on the date of issuance.
During April 2015, we issued 100,000 shares of our common stock for consulting services rendered to us. We valued these shares at $0.51 per share, the closing stock price on the date of issuance.
During April 2015, we issued 250,000 shares of our common stock as a debt discount, valued at $250,000, the stated value on the convertible promissory note.
During June 2015, we issued 200,000 shares of our common stock and warrants to purchase 200,000 shares of our common stock for $110,000 in cash.
During August 2015, we received $40,000 in exchange for 400,000 shares of our common stock for the exercise of warrants.
During September 2015, we received $30,000 upon the exercise of warrants to purchase 300,000 shares of our common stock.
On July 18, 2015, we entered into an agreement for consulting services. We agreed to pay a $2,000 per month retainer for services, as well as 25,000 restricted shares per month.
In July 2015, we entered into an amended agreement in which we agreed to issue warrants to acquire approximately 4,500,000 shares of our common stock. In connection with this agreement we recorded a prepaid expense of $2,239,211. In 2015 this agreement was cancelled for non-performance and the warrants were cancelled and the associated prepaid expense was reversed.
On August 1, 2015, we entered into a consulting agreement in which we agreed to issue shares of our common stock in exchange for services rendered on a transactional basis. On October 1, 2015 we issued 30,000 shares.
On August 1, 2015, we entered into an agreement for future consulting services. We agreed to issue restricted shares on a transactional basis. We issued 40,000 shares on October 1, 2015.
During the fourth quarter 2015 we issued 2,302,000 shares of common stock and 2,377,000 warrants to purchase our common stock in exchange for $230,200 in cash.
During the first quarter 2016 we issued 1,915,000 shares of common stock and 1,915,000 warrants to purchase our common stock in exchange for $191,500 in cash. During the first quarter 2016 we issued 175,000 shares of common stock in exchange for services valued at $36,000.
During the second quarter 2016 we issued 850,000 shares of common stock and 850,000 warrants to purchase our common stock in exchange for $85,000 in cash. During the second quarter 2016 we issued 244,514 shares of common stock in exchange for services valued at $26,897. During the second quarter 2016 we issued 260,000 shares of common stock valued at $31,600 as debt discount.
F-12
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(6) STOCKHOLDERS’ EQUITY, continued
During the third quarter 2016 we issued 1,650,000 shares of common stock and 1,650,000 warrants to purchase our common stock in exchange for $165,000 in cash. During the third quarter 2016 we issued 285,000 shares of common stock valued at $26,088 as debt discount. During the third quarter 2016 we issued 450,000 shares of common stock for the conversion of $45,000 of convertible debt.
During the fourth quarter 2016 we issued 1,150,000 shares of common stock and 1,150,000 warrants to purchase our common stock in exchange for $115,000 in cash. During the fourth quarter 2016 we issued 7,299,999 shares of common stock in exchange for services valued at $450,300. During the fourth quarter 2016 we issued 650,000 shares of common stock valued at $23,000 as debt discount. During the fourth quarter 2016 we issued 1,897,285 shares of common stock for the conversion of $189,849 of convertible debt and accrued interest.
In the first quarter 2017 we issued 6,762,942 shares of common stock to convert $1,561,593 of convertible debt and accrued interest and 3,367,000 shares of common stock to convert $841,750 of debt and accrued interest to a related party. In February 2017 we issued 275,000 shares of common stock in exchange for $55,000 in cash for the exercise of options. During the first quarter 2017 we issued 6,957,285 shares of common stock and 6,957,285 warrants to purchase our common stock in exchange for $774,500 in cash.
During the second quarter 2017 we issued 1,850,000 shares of common stock and 1,850,000 warrants to purchase our common stock in exchange for $370,000 in cash. During the second quarter 2017 we issued 250,000 shares of common stock in exchange for $50,000 in cash for an option exercise.
During the third quarter 2017 the Company issued 900,000 shares of common stock in exchange for $180,000 in cash
On January 13, 2017 we entered into an employment agreement with our President which includes an anti-dilution provision which requires us to maintain his share ownership in our Company at 30%, reduced by any shares he sells. These shares are required to be issued on January 2 of each year. On February 13, 2017 we issued 7,220,585 shares associate with the anti-dilution rights, which were valued at $10,453,315. At September 30, 2017, we have recorded a liability to issue 6,674,837 shares valued at $8,464,463. This employment agreement was amended on October 10, 2017, to remove the anti-dilution provision.
Our warrants and options outstanding are:
|
|
|
|
|
|
|
By Exercise Price:
|
September 30, 2017
|
|
December 31, 2016
|
|
December 31, 2015
|
|
Options - $0.20
|
-
|
|
275,000
|
|
305,000
|
|
Warrants - $0.35
|
2,750,000
|
|
-
|
|
-
|
|
Warrants - $0.50
|
14,342,000
|
|
7,867,000
|
|
2,802,000
|
|
Warrants - $0.75
|
114,286
|
|
-
|
|
-
|
|
Warrants - $1.00
|
124,999
|
|
-
|
|
-
|
|
Total outstanding
|
17,331,285
|
|
8,142,000
|
|
3,107,000
|
On January 13, 2017 we entered into an employment agreement with our President which includes an anti-dilution provision which requires us to maintain his share ownership in our Company at 30%, reduced by any shares he sells. These shares are required to be issued on January 2 of each year. On February 13, 2017 we issued 7,220,585 shares associate with the anti-dilution rights, which were valued at $10,453,315. At September 30, 2017, we have recorded a liability to issue 6,674,837 shares valued at $8,464,463. This employment agreement was amended on October 10, 2017, to remove the anti-dilution provision.
F-13
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(7) INCOME TAXES
We recognize deferred tax assets and liabilities for the tax effects of differences between the financial statements and tax basis of assets and liabilities. A valuation allowance is established to reduce the deferred tax assets if it is more likely than not that a deferred tax asset will not be realized.
The components of income tax provision (benefit) related to continuing operations are as follows at December 31, 2016 and 2015:
|
|
|
|
|
|
2016
|
|
2015
|
|
Current
|
$
-
|
|
$
-
|
|
Deferred
|
$
-
|
|
$
-
|
|
Total tax provision
|
$
-
|
|
$
-
|
The following is a reconciliation of the effective income tax rate with the statutory income tax rate at December 31, 2016 and 2015:
|
|
|
|
|
|
|
|
2016
|
|
2015
|
|
U.S. Federal statutory income tax rate
|
(34.0)%
|
|
(34.0)%
|
|
State income tax, net of federal benefit
|
(1.9)%
|
|
(1.9)%
|
|
Temporary differences, net
|
0.0%
|
|
0.0%
|
|
Valuation allowance
|
35.9%
|
|
35.%
|
|
Effective tax rate
|
0.0%
|
|
0.0%
|
The net deferred tax assets and liabilities included in the financial statements consist of the following amounts at December 31, 2016 and 2015:
|
|
|
|
|
Deferred tax assets
|
2016
|
|
2015
|
|
Net operating loss carry forwards
|
$
3,079,075
|
|
$
2,115,668
|
|
Stock based compensation
|
207,859
|
|
425,167
|
|
Other
|
647
|
|
-
|
|
Less: valuation allowance
|
(2,872,726)
|
|
(2,491,808)
|
|
Total
|
414,855
|
|
49,027
|
|
Deferred tax liabilities
|
|
|
|
|
Stock based compensation
|
(414,855)
|
|
(48,744)
|
|
Depreciation
|
-
|
|
(283)
|
|
Net deferred tax asset
|
$
-
|
|
$
-
|
F-14
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(7) INCOME TAXES (continued)
The change in valuation allowance was $752,829 and $780,008 for the years ended December 31, 2016 and 2015, respectively. We recorded a 100% valuation allowance related to the deferred tax asset for the loss from operations. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the period in which temporary differences become deductible.
In accordance with the provisions of ASC 740: Income Taxes, we record a liability for uncertain tax positions when it is probable that a loss has been incurred and the amount can be reasonably estimated. At December 31, 2016 and 2015, we have no liabilities for uncertain tax positions. We continually evaluate expiring statutes of limitations, audits, proposed settlements, changes in tax law and new authoritative rulings.
(8) COMMITMENTS AND CONTINGENCIES
a) Real Property Lease
We lease our office and warehouse facilities under an operating lease in Coconut Creek, Florida. The lease expires in February 2018. The minimum monthly lease payments required for the remaining term of the lease are $7,360.
In June 2017 we entered into a new lease for a new additional facility located in Deerfield Beach, Florida. This lease begins on January 1, 2018 and expires on March 1, 2025. The minimum monthly lease payments required begin at $13,220.
b) Contractual Obligations
During January 2014, we were granted a license to market nutritional supplements under the TapouT XT name to retail locations worldwide. Under the license agreement, we were required to pay a royalty fee to Nutra Evolution of 12.5% of net sales. The agreement provided us with an initial test period of four years, until January 31, 2018, to distribute the product. We paid $85,000 in conjunction with the license. At the expiration of this four year period, we had the option to extend the license for three (3) consecutive three (3) year terms.
The agreement originally required the company to pay minimum royalties of $400,000 during the first contract year; $750,000 during the second contract year and $1,000,000 each year thereafter. We terminated the license agreement during the second quarter 2015 and no longer are obligated to pay the minimum royalties.
c) Other
We are subject to asserted claims and liabilities that arise in the ordinary course of business. We maintain insurance policies to mitigate potential losses from these actions. In the opinion of management, the amount of the ultimate liability with respect to those actions will not materially affect our financial position or results of operations.
F-15
NUTRAFUELS, INC.
Notes to Financial Statements
(Information with respect to the nine months ended September 30, 2017 is unaudited)
(9) CONCENTRATIONS OF CREDIT RISK
a) Cash
We maintain its cash in bank deposit accounts, which may, at times, may exceed federally insured limits. The Company had cash balance in excess of FDIC insured limits at September 30, 2017 of $137,048 and no cash balance in excess of FDIC insured limits at December 31, 2016 and 2015.
b) Revenue
Our principal customers are comprised of five (5) separate independent private label resellers. Should we lose one or more of these resellers our revenue would decline significantly.
(10) SUBSEQUENT EVENTS
a) Stockholders’ Equity
On November 27, 2017 the Company issued 6,674,837 shares of common stock valued at $8,464,463 to the CEO pursuant to the anti-dilution clause contained in his employment agreement. This obligation for shares to be issued had been accrued through September 30, 2017.
F-16
EXHIBITS
Exhibit 3
.1
Articles of Organization of Nutrafuels, LLC, a Florida Limited Liability Company (i)
Exhibit 3
.2
Certificate of Conversion from a Florida Limited Liability Company to a Florida Corporation (i)
Exhibit 3.3
Articles of Incorporation of Nutrafuels, Inc., a Florida Corporation (i)
Exhibit 3.4
Certificate of Designation of Series A Preferred Shares (i)
Exhibit 3.5
Bylaws of Nutrafuels, Inc (ii)
Exhibit 10.1
Employment Agreement with Edgar Ward, dated October 10, 2017 (i)
Exhibit 10.2
Agreement with Neil Catania dated October 9, 2017 (i)
Exhibit 10.3
Agreement with JZ Marketing – Josh Zwagil, dated August 16, 2017 (i)
Exhibit 10.4
Agreement with Patagonia Global Trading – David Zirulnikoff, dated December 7, 2015 (i)
Exhibit 10.5
Agreement with Bernadette Cawley (i)
Exhibit 10.6
Agreement with Anthony Procelli (i)
Exhibit 10.7
Agreement with Patrick Kilcooley (i)
Exhibit 10.8
Agreement with Daniel Ryan (i)
Exhibit 10.9
Agreement with Michael R. Anderson, dated April 10, 2017 (i)
Exhibit 10.10
Agreement with Kenneth Duchin, dated February 23, 2017 (i)
Exhibit 10.11
Agreement with CFN Media, dated December 5, 2016 (i)
Exhibit 10.12
Agreement with Nicole Archon (i)
Exhibit 10.13
Agreement with Venture Capital Group LLC, dated December 14, 2016 (i)
Exhibit 10.14
Agreement with Sylvan Eudes (i)
Exhibit 10.15
Agreement with Peter Ciarci, dated August 1, 2015 (i)
Exhibit 10.16
Agreement with Five Star Labs LLC, dated August 1, 2015 (i)
Exhibit 10.17
Agreement with Osprey Capital Advisors, dated October 1, 2015 (i)
Exhibit 10.18
Agreement with WT Consulting, dated July 18, 2015 (i)
Exhibit 10.19
Agreement with Uptick Capital, dated October 14, 2014 (i)
Exhibit 10.20
Agreement with Benchmark Advisory Partners LLC, dated April 14, 2015(i)
Exhibit 10.21
Agreement with Sullivan Media, dated August 25, 2015 (i)
Exhibit 10.22
Neil Catania Note Agreement in the amount of $160,000 (i)
Exhibit 10.23
Neil Catania Note Agreement in the amount of $50,000 (i)
Exhibit 99.1
Form of Purchase Order
(i)
filed herewith
(ii)
filed as an exhibit to the Form 10 Registration Statement filed with the Securities and Exchange Commission on November 1, 2017.
51
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities Exchange Act of 1934, each of the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
NUTRAFUELS, INC.
|
|
|
|
|
|
|
|
Name:
|
Title:
|
Date:
|
|
By:
/s/Edgar Ward
Edgar Ward
|
President, Chief Executive Officer, Chief Financial Officer, Director
|
December 4, 2017
|
|
|
|
|
52
NutraLife Biosciences (CE) (USOTC:NLBS)
Historical Stock Chart
From Mar 2024 to Apr 2024
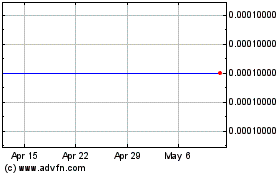
NutraLife Biosciences (CE) (USOTC:NLBS)
Historical Stock Chart
From Apr 2023 to Apr 2024