Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-
214882
PROSPECTUS SUPPLEMENT
(To Prospectus Dated February 13, 2017)

Up to $75,000,000
Common Stock
In accordance with the terms of the Controlled Equity Offering
SM
sales agreement, dated May 19, 2016, we entered into with Cantor Fitzgerald & Co., or Cantor Fitzgerald, we may offer and sell shares of our common stock having an aggregate offering price of up to an additional $75.0 million from time to time through Cantor Fitzgerald, acting as sales agent.
Our common stock is traded on the Nasdaq Global Market, or Nasdaq, under the symbol “CLDX”. On November 7, 2017, the last reported sales price of our common stock on Nasdaq was $2.62 per share.
Sales of our common stock, if any, under this prospectus supplement and the accompanying prospectus may be made in sales deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the Securities Act, including sales made directly on or through Nasdaq or any other existing trading market for our common stock. Cantor Fitzgerald is not required to sell any specific number or dollar amount of securities, but will act as a sales agent using commercially reasonable efforts consistent with its normal trading and sales practices, on mutually agreed terms between Cantor Fitzgerald and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Cantor Fitzgerald will be entitled to compensation at a fixed commission rate equal to 3.0% of the gross sales price per share sold. In connection with the sale of our common stock on our behalf, Cantor Fitzgerald will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor Fitzgerald will be deemed to be underwriting commissions or discounts.
Investing in our common stock involves risks. Before buying any shares, you should read the discussion of material risks of investing in our common stock in “Risk Factors” beginning on page S-5 of this prospectus supplement, and in the risks discussed under similar headings in the documents incorporated by reference in this prospectus supplement and accompanying prospectus, as they may be amended, updated or modified periodically in our reports filed with the Securities and Exchange Commission.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement and accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.

The date of this prospectus supplement is November 9, 2017.
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
In this prospectus supplement, “Celldex,” “we,” “us,” “our” or “ours” refer to Celldex Therapeutics, Inc. and its consolidated subsidiary.
This prospectus supplement and the accompanying prospectus relate to the offering of shares of our common stock. Before buying any of the shares of common stock offered hereby, we urge you to carefully read this prospectus supplement and the accompanying prospectus, together with the information incorporated herein by reference as described under the headings “Where You Can Find More Information” and “Incorporation of Documents by Reference.” These documents contain important information that you should consider when making your investment decision. This prospectus supplement contains information about the common stock offered hereby and may add, update or change information in the accompanying prospectus.
You should rely only on the information that we have provided or incorporated by reference in this prospectus supplement and the accompanying prospectus. We have not, and Cantor Fitzgerald has not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it.
We are not, and Cantor Fitzgerald is not, making offers to sell or solicitations to buy our common stock in any jurisdiction in which an offer or solicitation is not authorized or in which the person making that offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make an offer or solicitation. You should assume that the information in this prospectus supplement and the accompanying prospectus or any related free writing prospectus is accurate only as of the date on the front of the document and that any information that we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus supplement, the accompanying prospectus or any related free writing prospectus, or any sale of a security.
This document is in two parts. The first part is this prospectus supplement, which adds to and updates information contained in the accompanying prospectus. The second part, the prospectus, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus, you should rely on the information in this prospectus supplement. However, if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference into this prospectus supplement—the statement in the document having the later date modifies or supersedes the earlier statement as our business, financial condition, results of operations and prospects may have changed since the earlier dates.
This prospectus supplement and the accompanying prospectus contain summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been or will be filed as exhibits to the registration statement of which this prospectus is a part or as exhibits to documents incorporated by reference herein, and you may obtain copies of those documents as described below under the headings “Where You Can Find More Information” and “Incorporation of Documents by Reference.”
“Celldex Therapeutics” and our design logo used in this prospectus supplement and the accompanying prospectus are our trademarks. This prospectus supplement and the accompanying prospectus may also include other trademarks, tradenames and service marks that are the property of their respective holders. Solely for convenience, trademarks and tradenames referred to in this prospectus supplement and the accompanying prospectus may appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or that the applicable holder will not assert its rights, to these trademarks and tradenames.
S-
1
Table of Contents
PROSPECTUS SUPPLEMENT SUMMARY
The following summary of our business highlights some of the information contained elsewhere in or incorporated by reference into this prospectus supplement. Because this is only a summary, however, it does not contain all of the information that may be important to you. You should carefully read this prospectus supplement and the accompanying prospectus, including the documents incorporated by reference, which are described under “Incorporation of Documents by Reference” and “Where You Can Find More Information” in this prospectus supplement. You should also carefully consider the matters discussed in the section titled “Risk Factors” in this prospectus supplement and in the accompanying prospectus and in other periodic reports incorporated by reference herein.
Our Company
We are a biopharmaceutical company focused on the development and commercialization of several immunotherapy technologies and other cancer-targeting biologics. Our drug candidates, including antibodies, antibody-drug conjugates and other protein-based therapeutics, are derived from a broad set of complementary technologies which have the ability to engage the human immune system and/or directly inhibit tumors to treat specific types of cancer or other diseases.
Our latest stage drug candidate, glembatumumab vedotin (also referred to as CDX-011) is a targeted antibody-drug conjugate in a randomized, Phase 2b study for the treatment of triple negative breast cancer and a Phase 2 study for the treatment of metastatic melanoma. Varlilumab (also referred to as CDX-1127) is an immune modulating antibody that is designed to enhance a patient’s immune response against cancer. We established proof of principle in a Phase 1 study with varlilumab, which supported the initiation of combination studies in various indications. We also have a number of earlier stage drug candidates in clinical development, including CDX-3379, a human monoclonal antibody designed to block the activity of ErbB3 (HER3) in solid tumors; CDX-014, an antibody-drug conjugate targeting renal and ovarian cancers; CDX-1401, a targeted immunotherapeutic aimed at antigen presenting cells, or APCs, for cancer indications; and, CDX-301, an immune cell mobilizing agent and dendritic cell growth factor. Our drug candidates address market opportunities for which we believe current therapies are inadequate or non-existent.
We are building a fully integrated, commercial-stage biopharmaceutical company that develops important therapies for patients with unmet medical needs. Our program assets provide us with the strategic options to either retain full economic rights to our innovative therapies or seek favorable economic terms through advantageous commercial partnerships. This approach allows us to maximize the overall value of our technology and product portfolio while best ensuring the expeditious development of each individual product.
The following table reflects Celldex-sponsored clinical studies that we are actively pursuing at this time. All programs are currently fully owned by Celldex.
|
Product (generic)
|
|
Indication/Field
|
|
Status
|
|
Sponsor
|
|
Glembatumumab vedotin
|
|
Triple negative breast cancer
|
|
Phase 2b
|
|
Celldex
|
|
Glembatumumab vedotin
|
|
Metastatic melanoma (with varlilumab or CPI
1
)
|
|
Phase 2
|
|
Celldex
|
|
Varlilumab
|
|
Multiple solid tumors (with nivolumab)
|
|
Phase 2
|
|
Celldex
2
|
|
CDX-3379
|
|
Head and neck squamous cell cancer (with cetuximab)
|
|
Phase 2
3
|
|
Celldex
|
|
CDX-014
|
|
Renal cell carcinoma
|
|
Phase 1
|
|
Celldex
|
|
CDX-1140
|
|
Multiple solid tumors
|
|
Phase 1
3
|
|
Celldex
|
1
checkpoint inhibitor;
2
BMS collaboration;
3
expected to initiate by year-end 2017
We also routinely work with external parties, such as government agencies, to collaboratively advance our drug candidates. The following pipeline reflects clinical trials of our drug candidates being actively pursued by outside organizations. In addition to the studies listed below, we also have an Investigator Initiated Research (IIR) program with seven studies ongoing with our drug candidates and additional studies currently under consideration.
|
Product (generic)
|
|
Indication/Field
|
|
Status
|
|
Sponsor
|
|
Glembatumumab vedotin
|
|
Uveal melanoma
|
|
Phase 2
|
|
NCI (CRADA)
|
|
Glembatumumab vedotin
|
|
Squamous cell lung cancer
|
|
Phase 2
|
|
PrECOG, LLC
|
|
CDX-1401/CDX-301
|
|
Malignant melanoma
|
|
Phase 2
|
|
NCI (CRADA)
|
|
CDX-1401/atezolizumab/SGI-110
|
|
Ovarian cancer
|
|
Phase 1
|
|
NCI (CRADA)
|
S-
2
Table of Contents
Corporate Information
We are a Delaware corporation organized in 1983. Our principal executive offices are located at Perryville III Building, 53 Frontage Road, Suite 220, Hampton, New Jersey 08827 and our telephone number is (908) 200-7500. Our corporate website is
www.celldex.com
. The information on our website is not incorporated by reference into this prospectus.
S-
3
Table of Contents
THE OFFERING
|
Common stock offered by us
|
|
Shares of our common stock having an aggregate offering price of up to $75.0 million.
|
|
|
|
|
|
Common stock to be outstanding after this offering
|
|
Up to 160,734,432 shares, assuming sales at a price of $2.62 per share, which was the closing price of our common stock on Nasdaq on November 7, 2017. The actual number of shares issued will vary depending on the sales price under this offering.
|
|
|
|
|
|
Plan of Distribution
|
|
“At the market offering” that may be made from time to time through our sales agent, Cantor Fitzgerald. See “Plan of Distribution” beginning on page S-12 of this prospectus supplement.
|
|
|
|
|
|
Use of Proceeds
|
|
We currently intend to use the net proceeds from this offering, if any, for working capital and other general corporate purposes. See “Use of Proceeds” on page S-9 of this prospectus supplement.
|
|
|
|
|
|
Risk Factors
|
|
Investing in our common stock involves a high degree of risk. See “Risk Factors” beginning on page S-5 of this prospectus supplement and under similar headings in the other documents that are filed after the date hereof and incorporated by reference in this prospectus supplement for a discussion of factors to consider before deciding to purchase shares of our common stock.
|
|
|
|
|
|
The Nasdaq Global Market symbol
|
|
“CLDX”
|
The total number of shares of common stock to be outstanding immediately after this offering is based on 132,108,478 shares of common stock issued and outstanding as of September 30, 2017, which does not include the following, all as of September 30, 2017:
·
11,582,818 shares issuable upon the exercise of outstanding stock options with a weighted-average exercise price of $9.54 per share; and
·
7,780,663 shares reserved for future issuance under our equity compensation plans.
Unless otherwise stated, all information in this prospectus supplement:
·
assumes no exercise of outstanding options to purchase common stock and no issuance of shares available for future issuance under our equity compensation plans; and
·
reflects all currency in U.S. dollars.
S-
4
Table of Contents
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the risks described under “Risk Factors” in the accompanying prospectus and our Annual Report on Form 10-K for the year ended December 31, 2016, respectively, as updated by any other document that we subsequently file with the Securities and Exchange Commission and that is incorporated by reference into this prospectus supplement and the accompanying prospectus, as well as the risks described below and all of the other information contained in this prospectus supplement and the accompanying prospectus, and incorporated by reference into this prospectus supplement and the accompanying prospectus, including our financial statements and related notes, before investing in our securities. These risks and uncertainties are not the only ones facing us, and there may be additional matters that we are unaware of or that we currently consider immaterial. All of these could adversely affect our business, business prospects, cash flow, results of operations and financial condition. In such case, the trading price of our common stock could decline, and you could lose all or part of your investment in our common stock.
Risks Related to this Offering
Management will have broad discretion as to the use of the proceeds from this offering, and we may not use the proceeds effectively.
Because we have not designated the amount of net proceeds received by us from this offering to be used for any particular purpose, our management will have broad discretion as to the application of the net proceeds from this offering and could use them for purposes other than those contemplated at the time of the offering. Our management may use the net proceeds for corporate purposes that may not improve our financial condition or market value.
If you purchase shares of common stock sold in this offering, you will experience immediate and substantial dilution in the book value per share of the common stock you purchase.
Because the price per share of our common stock being offered may be higher than the book value per share of our common stock, you may suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering. In addition, we have a significant number of options and restricted stock outstanding. If the holders of these securities exercise them or become vested in them, as applicable, you may incur further dilution.
You may experience future dilution as a result of future equity offerings or if we elect to pay milestones, if any, due to former Kolltan holders in shares of our common stock.
To raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering. In addition, in the event that certain specified preclinical and clinical development milestones related to Kolltan’s development programs and/or Celldex’s development programs and certain commercial milestones related to Kolltan’s drug candidates are achieved, we will be required to pay Kolltan’s stockholders milestone payments of up to $172.5 million, which milestone payments may be made, at our sole election, in cash, in shares of our common stock or a combination of both, subject to provisions of the merger agreement. If we elect to issue shares of our common stock, you will experience further dilution.
Sales of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress the market price of our common stock and impair our ability to raise capital through the sale of additional equity securities. We have agreed, without the prior written consent of Cantor Fitzgerald. and subject to certain exceptions set forth in the sales agreement, not to sell or otherwise dispose of any common stock or securities convertible into or exchangeable for shares of common stock, warrants or any rights to purchase or acquire common stock during the period beginning on the fifth trading day immediately prior to the delivery of any placement notice delivered by us to Cantor Fitzgerald and ending on the fifth trading day immediately following the final settlement date with respect to the shares sold pursuant to such notice. We have further agreed, subject to certain exceptions set forth in the sales agreement, not to sell or otherwise dispose of any common stock or securities convertible into or exchangeable for shares of common stock, warrants or any rights to purchase or acquire common stock in any other “at-the-market” or continuous equity transaction prior to the termination of the sales agreement with Cantor Fitzgerald. Therefore, it is possible that we
S-
5
Table of Contents
could issue and sell additional shares of our common stock in the public markets. We cannot predict the effect that future sales of our common stock would have on the market price of our common stock
.
Our share price has been and could remain volatile.
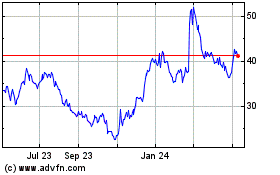
The market price of our common stock has historically experienced and may continue to experience significant volatility. From January 2015 through September 30, 2017, the market price of our common stock has fluctuated from a high of $32.82 per share in the first quarter of 2015, to a low of $2.20 per share in the second quarter of 2017. Our progress in developing and commercializing our products, the impact of government regulations on our products and industry, the potential sale of a large volume of our common stock by stockholders, our quarterly operating results, changes in general conditions in the economy or the financial markets and other developments affecting us or our competitors could cause the market price of our common stock to fluctuate substantially with significant market losses. If our stockholders sell a substantial number of shares of common stock, especially if those sales are made during a short period of time, those sales could adversely affect the market price of our common stock and could impair our ability to raise capital. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. This volatility has affected the market prices of securities issued by many companies for reasons unrelated to their operating performance and may adversely affect the price of our common stock. In addition, we could be subject to a securities class action litigation as a result of volatility in the price of our stock, which could result in substantial costs and diversion of management’s attention and resources and could harm our stock price, business, prospects, results of operations and financial condition.
Because we do not intend to declare cash dividends on our shares of common stock in the foreseeable future, stockholders must rely on appreciation of the value of our common stock for any return on their investment.
We have never declared or paid cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. In addition, the terms of any existing or future debt agreements may preclude us from paying dividends. As a result, we expect that only appreciation of the price of our common stock, if any, will provide a return to investors in this offering for the foreseeable future.
S-
6
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements represent our management’s judgment regarding future events. In many cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “plan,” “expect,” “anticipate,” “estimate,” “predict,” “intend,” “potential” or “continue” or the negative of these terms or other words of similar import, although some forward-looking statements are expressed differently. All statements other than statements of historical fact included in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein regarding our financial position, business strategy and plans or objectives for future operations are forward-looking statements. Without limiting the broader description of forward-looking statements above, we specifically note that statements regarding potential drug candidates, their potential therapeutic effect, the possibility of obtaining regulatory approval, our expected timing for completing clinical trials and clinical trial milestones for our drug candidates, our ability or the ability of our collaborators to manufacture and sell any products, market acceptance or our ability to earn a profit from sales or licenses of any drug candidate or to discover new drugs in the future are all forward-looking in nature.
There are a number of important factors that could cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include, but are not limited to:
·
our ability to successfully complete research and further development, including animal, preclinical and clinical studies, and, if we obtain regulatory approval, commercialization of glembatumumab vedotin (also referred to as CDX-011) and other drug candidates and the growth of the markets for those drug candidates;
·
our ability to raise sufficient capital to fund our clinical studies and to meet our liquidity needs, on terms acceptable to us, or at all. If we are unable to raise the funds necessary to meet our liquidity needs, we may have to delay or discontinue the development of one or more programs, discontinue or delay ongoing or anticipated clinical trials, license out programs earlier than expected, raise funds at significant discount or on other unfavorable terms, if at all, or sell all or part of our business;
·
our ability to negotiate strategic partnerships, where appropriate, for our programs, which may include, glembatumumab vedotin;
·
our ability to realize the anticipated benefits from the acquisition of Kolltan and to operate the combined business efficiently;
·
our ability to manage multiple clinical trials for a variety of drug candidates at different stages of development;
·
the cost, timing, scope and results of ongoing safety and efficacy trials of glembatumumab vedotin, and other preclinical and clinical testing;
·
the cost, timing, and uncertainty of obtaining regulatory approvals for our drug candidates;
·
the availability, cost, delivery and quality of clinical management services provided by our clinical research organization partners;
·
the availability, cost, delivery and quality of clinical and commercial-grade materials produced by our own manufacturing facility or supplied by contract manufacturers, suppliers and partners, who may be the sole source of supply;
·
our ability to develop and commercialize products before competitors that are superior to the alternatives developed by such competitors;
·
our ability to develop technological capabilities, including identification of novel and clinically important targets, exploiting our existing technology platforms to develop new drug candidates and expand our focus to broader markets for our existing targeted immunotherapeutics;
·
our ability to adapt our proprietary antibody-targeted technology, or APC Targeting Technology™, to develop new, safe and effective therapeutics for oncology and infectious disease indications; and
S-
7
Table of Contents
·
our ability to protect our intellectual property rights, including the ability to successfully defend patent oppositions filed against a European patent related to technology we use in varlilumab, and our ability to avoid intellectual property litigation, which can be costly and divert management time and attention.
You should also consider carefully the statements set forth in the section entitled “Risk Factors” in this prospectus supplement and in our Annual Report on Form 10-K for the year ended December 31, 2016, respectively, as updated by any other document that we subsequently filed with the Securities and Exchange Commission and that is incorporated by reference into this prospectus supplement, which address various factors that could cause results or events to differ from those described in the forward-looking statements. All subsequent written and oral forward-looking statements attributable to us or to persons acting on our behalf are expressly qualified in their entirety by the applicable cautionary statements. We have no plans to update these forward-looking statements.
S-
8
Table of Contents
USE OF PROCEEDS
The amount of proceeds from this offering will depend upon the number of shares of our common stock sold and the market price at which they are sold. There can be no assurance that we will be able to sell any shares under or fully utilize the sales agreement with Cantor Fitzgerald as a source of financing. We currently expect to use the net proceeds from this offering for working capital and other general corporate purposes. Until we use the net proceeds of this offering, we intend to invest the funds in short-term, investment grade, interest-bearing securities.
The amount and timing of actual expenditures for the purposes set forth above may vary based on several factors, and our management will retain broad discretion as to the ultimate allocation of the proceeds.
S-
9
Table of Contents
PRICE RANGE OF OUR COMMON STOCK
Our common stock currently trades on Nasdaq under the symbol “CLDX”. The following table sets forth for the periods indicated the high and low sale prices per share for our common stock, as reported by Nasdaq.
|
Fiscal Period
|
|
High
|
|
Low
|
|
|
Year Ending December 31, 2017
|
|
|
|
|
|
|
First Quarter
|
|
$
|
4.02
|
|
$
|
3.05
|
|
|
Second Quarter
|
|
3.65
|
|
2.20
|
|
|
Third Quarter
|
|
3.14
|
|
2.27
|
|
|
Fourth Quarter (through November 7, 2017)
|
|
3.26
|
|
2.31
|
|
|
Year Ended December 31, 2016
|
|
|
|
|
|
|
First Quarter
|
|
$
|
15.61
|
|
$
|
2.96
|
|
|
Second Quarter
|
|
5.13
|
|
3.40
|
|
|
Third Quarter
|
|
4.83
|
|
3.23
|
|
|
Fourth Quarter
|
|
5.02
|
|
2.85
|
|
|
Year Ended December 31, 2015
|
|
|
|
|
|
|
First Quarter
|
|
$
|
32.82
|
|
$
|
17.81
|
|
|
Second Quarter
|
|
30.28
|
|
23.62
|
|
|
Third Quarter
|
|
28.08
|
|
10.11
|
|
|
Fourth Quarter
|
|
18.62
|
|
10.15
|
|
On November 7, 2017 the closing price of our common stock, as reported by Nasdaq, was $2.62 per share. We have not paid any dividends on our common stock since our inception and do not intend to pay any dividends in the foreseeable future.
S-
10
Table of Contents
DILUTION
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the price per share you pay in this offering and our pro forma net tangible book value per share after this offering. We calculate net tangible book value per share by dividing our net tangible book value, which is tangible assets less total liabilities, by the number of outstanding shares of our common stock.
Our net tangible book value as of September 30, 2017 was approximately $60.4 million or $0.46 per share. Net tangible book value per share after this offering gives effect to the sale of $75.0 million of common stock in this offering at an assumed offering price of $2.62 per share, which was the closing price of our common stock as reported on Nasdaq on November 7, 2017, after deducting offering commissions and estimated expenses payable by us. Our net tangible book value as of September 30, 2017, after giving effect to this offering as described above, would have been approximately $133.1 million, or $0.83 per share of common stock. This represents an immediate increase in pro forma net tangible book value of $0.37 per share to existing stockholders and an immediate dilution of $1.79 per share to new investors purchasing our common stock in this offering. The following table illustrates the per share dilution:
|
Assumed public offering price per share
|
|
|
|
$
|
2.62
|
|
|
Net tangible book value per share as of September 30, 2017
|
|
$
|
0.46
|
|
|
|
|
Increase in net tangible book value per share attributable to this offering
|
|
$
|
0.37
|
|
|
|
|
Pro forma net tangible book value per share as of September 30, 2017, after giving effect to this offering
|
|
|
|
$
|
0.83
|
|
|
Dilution per share to investors participating in this offering
|
|
|
|
$
|
1.79
|
|
The above table is based on 132,108,478 shares of our common stock issued and outstanding as of September 30, 2017, which does not include the following:
·
11,582,818 shares issuable upon the exercise of outstanding stock options with a weighted-average exercise price of $9.54 per share; and
·
7,780,663 shares reserved for future issuance under our equity compensation plans.
S-
11
Table of Contents
PLAN OF DISTRIBUTION
On May 19, 2016, we entered into a Controlled Equity Offering
SM
sales agreement with Cantor Fitzgerald & Co., or Cantor, under which we issued and sold shares of our common stock having an aggregate gross sales price of approximately $60.0 million under the sales agreement prospectus dated May 19, 2016. Pursuant to this prospectus supplement, we may offer and sell an additional $75.0 million in aggregate gross sales price of our common stock from time to time through Cantor acting as agent pursuant to the sales agreement. This summary of the material provisions of the sales agreement does not purport to be a complete statement of its terms and conditions. A copy of the sales agreement is filed with the SEC and is incorporated by reference into the registration statement of which this prospectus supplement is a part. See “Where You Can Find More Information” below.
Upon delivery of a placement notice and subject to the terms and conditions of the sales agreement, Cantor may sell our common stock by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act, including sales made directly on Nasdaq or any other existing trading market for our common stock. We or Cantor may suspend or terminate the offering of our common stock upon notice and subject to other conditions.
We will pay Cantor in cash, upon each sale of our common stock pursuant to the sales agreement, a commission in an amount equal to 3.0% of the aggregate gross proceeds from each sale of our common stock. Because there is no minimum offering amount required as a condition to this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. We have agreed to reimburse a portion of Cantor’s expenses, including legal fees, in connection with this offering up to a maximum of $50,000. We estimate that the total expenses for the offering, excluding compensation and expense reimbursement payable to Cantor under the terms of the sales agreement, will be approximately $110,000.
Settlement for sales of common stock will occur on the second business day following the date on which any sales are made, or on some other date that is agreed upon by us and Cantor in connection with a particular transaction, in return for payment of the net proceeds to us. There is no arrangement for funds to be received in an escrow, trust or similar arrangement. Sales of our common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and Cantor may agree upon.
Cantor will act as sales agent on a commercially reasonable efforts basis consistent with its normal trading and sales practices and applicable state and federal laws, rules and regulations and the rules of Nasdaq. In connection with the sale of the common stock on our behalf, Cantor will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor will be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification and contribution to Cantor against certain civil liabilities, including liabilities under the Securities Act.
The offering of our common stock pursuant to the sales agreement will terminate upon the termination of the sales agreement as permitted therein. We or Cantor may each terminate the sales agreement at any time upon ten (10) days’ prior notice.
Cantor and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates, for which services they may in the future receive customary fees. To the extent required by Regulation M, Cantor will not engage in any market making activities involving our common stock while the offering is ongoing under this prospectus supplement.
This prospectus supplement in electronic format may be made available on a website maintained by Cantor, and Cantor may distribute this prospectus supplement electronically.
S-
12
Table of Contents
LEGAL MATTERS
Lowenstein Sandler LLP, New York, New York, will provide us with an opinion as to the validity of the shares of common stock offered by this prospectus supplement and the accompanying prospectus. Cantor Fitzgerald & Co. is being represented in connection with this offering by Cooley LLP, New York, New York.
EXPERTS
The financial statements and management’s assessment of the effectiveness of internal control over financial reporting (which is included in Management’s Report on Internal Control over Financial Reporting) incorporated in this prospectus supplement by reference to the Annual Report on Form 10-K for the year ended December 31, 2016 have been so incorporated in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. We have also filed a registration statement on Form S-3, including exhibits, under the Securities Act with respect to the securities offered by this prospectus supplement and the accompanying prospectus. This prospectus supplement and the accompanying prospectus are a part of the registration statement but do not contain all of the information included in the registration statement or the exhibits. You may read and copy the registration statement and any other document that we file at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington D.C. 20549. You can call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference room. You can also find our public filings with the SEC on the Internet at a web site maintained by the SEC located at
http://www.sec.gov
.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus supplement and the accompanying prospectus certain information. This means that we can disclose important information to you by referring you to those documents that contain the information. The information we incorporate by reference is considered a part of this prospectus supplement and the accompanying prospectus, and later information we file with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any future filings we make with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, on or after the date of this prospectus supplement (other than information “furnished” under Items 2.02 or 7.01 (or corresponding information furnished under Item 9.01 or included as an exhibit)) of any Current Report on Form 8-K or otherwise “furnished” to the SEC, unless otherwise stated) until this offering is completed:
·
Our Annual Report on Form 10-K for the fiscal year ended December 31, 2016, filed on March 14, 2017;
·
Our Quarterly Reports on Form 10-Q for the quarters ended March 31, 2017, June 30, 2017 and September 30, 2017 filed on May 9, 2017, August 8, 2017 and November 7, 2017, respectively;
·
Our Current Reports on Form 8-K or Form 8-K/A filed on February 7, 2017, March 1, 2017, March 14, 2017, May 9, 2017, June 16, 2017, July 5, 2017, August 8, 2017, September 12, 2017, October 3, 2017, November 7, 2017 and November 9, 2017 (other than information “furnished” under Items 2.02 or 7.01 (or corresponding information furnished under Item 9.01 or included as an exhibit));
·
Our Definitive Proxy Statement on Schedule 14A, filed with the SEC on April 28, 2017 (other than the portions thereof which are furnished and not filed); and
·
The description of our common stock contained in our Registration Statement on Form 8-A, filed on November 8, 2004, as amended by Form 8-A/A filed on October 22, 2007 and March 7, 2008.
You may request a copy of these filings, at no cost, by writing to or telephoning us at the following address:
Corporate Secretary
Celldex Therapeutics, Inc.
S-
13
Table of Contents
Perryville III Building, 53 Frontage Road, Suite 220,
Hampton, New Jersey 08827
(908) 200-7500
Any statement contained in this prospectus supplement or in a document incorporated or deemed to be incorporated by reference into this prospectus supplement will be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained in this prospectus supplement or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus supplement modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement.
You should rely only on information contained in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus supplement and the accompanying prospectus or incorporated by reference in this prospectus supplement and the accompanying prospectus. We are not making offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
S-
14
Table of Contents
PROSPECTUS

CELLDEX THERAPEUTICS, INC.
$250,000,000
Common Stock
Preferred Stock
Warrants
Depositary Shares
Units
Celldex Therapeutics, Inc. may offer, issue and sell from time to time, together or separately, in one or more offerings, any combination of:
·
our common stock,
·
our preferred stock, which we may issue in one or more series,
·
warrants,
·
depositary shares, and
·
units,
up to a maximum aggregate offering price of $250,000,000.
This prospectus provides a general description of the securities we may offer. Each time we sell securities, we will provide specific terms of the securities offered in a supplement to this prospectus (which includes an at-the-market offering prospectus). The prospectus supplement may also add, update or change information contained in this prospectus. You should read this prospectus and the accompanying prospectus supplement, as well as the documents incorporated or deemed incorporated by reference in this prospectus, carefully before you make your investment decision. Our common stock is traded on the NASDAQ Global Market under the symbol “CLDX.” On February 8, 2017, the last reported sale price of our common stock on the NASDAQ Global Market was $3.42 per share. You are urged to obtain current market quotations of the common stock. Each prospectus supplement will indicate if the securities offered thereby will be listed on any securities exchange.
This prospectus may not be used to sell securities unless accompanied by a prospectus supplement.
We may offer to sell these securities on a continuous or delayed basis, through agents, dealers or underwriters, or directly to purchasers. The prospectus supplement for each offering of securities will describe in detail the plan of distribution for that offering. If our agents or any dealers or underwriters are involved in the sale of the securities, the applicable prospectus supplement will set forth the names of the agents, dealers or underwriters and any applicable commissions or discounts. Our net proceeds from the sale of securities will also be set forth in the applicable prospectus supplement. For general information about the distribution of securities offered, please see “Plan of Distribution” in this prospectus.
Investing in our securities involves risks. Before making an investment decision, you should carefully review the information contained in this prospectus under the heading “Risk Factors” beginning on page 3 of this prospectus.
Table of Contents
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION OR REGULATORY BODY HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is February 13, 2017.
Table of Contents
ABOUT THIS PROSPECTUS
This prospectus is a part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, utilizing a “shelf” registration process. Under this shelf registration process, we may, from time to time, sell any combination of the securities described in this prospectus in one or more offerings.
The registration statement containing this prospectus, including the exhibits to the registration statement, provides additional information about us and the securities offered under this prospectus. You should read the registration statement and the accompanying exhibits for further information. The registration statement, including the exhibits and the documents incorporated or deemed incorporated herein by reference, can be read and are available to the public over the Internet at the SEC’s website at
http://www.sec.gov
as described under the heading “Where You Can Find More Information.”
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities pursuant to this prospectus, we will provide a prospectus supplement (which term includes, as applicable, the at-the-market offering prospectus filed with the registration statement of which this prospectus forms a part) containing specific information about the terms of a particular offering by us. That prospectus supplement may include a discussion of any risk factors or other special considerations that apply to those securities. The prospectus supplement may add, update or change information in this prospectus. If the information in the prospectus is inconsistent with a prospectus supplement, you should rely on the information in that prospectus supplement. You should read both this prospectus and, if applicable, any prospectus supplement. See “Where You Can Find More Information” for more information.
You should rely only on the information incorporated by reference or provided in this prospectus or any prospectus supplement. We have not authorized any dealer, salesman or other person to give any information or to make any representation other than those contained or incorporated by reference in this prospectus or any prospectus supplement. You must not rely upon any information or representation not contained or incorporated by reference in this prospectus or any prospectus supplement. This prospectus and any prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor do this prospectus and any prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information contained in this prospectus or any prospectus supplement is accurate on any date subsequent to the date set forth on the front of such document or that any information we have incorporated by reference is correct on any date subsequent to the date of the document incorporated by reference, even though this prospectus and any prospectus supplement is delivered or securities are sold on a later date.
Unless this prospectus indicates otherwise or the context otherwise requires, the terms “we,” “our,” “us,” “Celldex” or the “Company” as used in this prospectus refer to Celldex Therapeutics, Inc. and its subsidiaries, except that such terms refer to only Celldex Therapeutics, Inc. and not its subsidiaries in the sections entitled “Description of Common Stock,” “Description of Preferred Stock,” “Description of Warrants,” “Description of Depositary Shares,” and “Description of Units.”
PROSPECTUS SUMMARY
We are
a biopharmaceutical company focused on the development and commercialization of several immunotherapy technologies for the treatment of cancer and other difficult-to-treat diseases. Our drug candidates are derived from a broad set of complementary technologies which have the ability to utilize the human immune system and enable the creation of therapeutic agents. We are using these technologies to develop targeted immunotherapeutics comprised of protein-based molecules such as vaccines, antibodies and antibody-drug conjugates that are used to treat specific types of cancer or other diseases.
Our latest stage drug candidate, glembatumumab vedotin (also referred to as CDX-011) is a targeted antibody-drug conjugate in a randomized, Phase 2b study for the treatment of triple negative breast cancer and a Phase 2 study for the treatment of metastatic melanoma. Varlilumab (also referred to as CDX-1127) is an immune modulating antibody that is designed to enhance a patient’s immune response against their cancer. We established proof of concept in a Phase 1 study with varlilumab, which has allowed several combination studies to begin in
1
Table of Contents
various indications. We also have a number of earlier stage drug candidates in clinical development, including CDX-1401, a targeted immunotherapeutic aimed at antigen presenting cells, or APCs, for cancer indications, CDX-301, an immune cell mobilizing agent and dendritic cell growth factor, and CDX-014, an antibody drug conjugate targeting TIM-1. Our drug candidates address market opportunities for which we believe current therapies are inadequate or non-existent. As discussed below, we recently acquired Kolltan Pharmaceuticals, Inc. thereby expanding our pipeline of drug candidates.
We are building a fully integrated, commercial-stage biopharmaceutical company that develops important therapies for patients with unmet medical needs. Our program assets provide us with the strategic options to either retain full economic rights to our innovative therapies or seek favorable economic terms through advantageous commercial partnerships. This approach allows us to maximize the overall value of our technology and product portfolio while best ensuring the expeditious development of each individual product.
Recent Developments
On November 29, 2016, we consummated the transactions contemplated by that certain Agreement and Plan of Merger dated as of November 1, 2016 by and among Celldex, Kolltan Pharmaceuticals, Inc., a Delaware corporation, Connemara Merger Sub 1 Inc. a Delaware corporation and a wholly-owned subsidiary of Celldex and Connemara Merger Sub 2 LLC., a Delaware limited liability company and a wholly-owned subsidiary of Celldex. Upon consummation of the transactions, Kolltan became a wholly-owned subsidiary of Celldex.
Prior to the merger, Kolltan was a privately-held clinical-stage company focused on the discovery and development of novel, antibody-based drug candidates targeting reception tyrosine kinases, or RTKs. Kolltan’s programs include: (i) KTN0158, a humanized monoclonal antibody that is a potent inhibitor of KIT activation and receptor dimerization in tumor cells and mast cells, which is currently in a Phase 1 dose escalation study in refractory gastrointestinal stromal tumors (GIST); (ii) KTN3379, a human monoclonal antibody designed to block the activity of ErbB3 (HER3), which recently completed a Phase 1b study with combination cohorts where meaningful responses and stable disease were observed in cetuximab (Erbitux
®
) refractory patients in head and neck squamous cell carcinoma and in BRAF-mutant non-small cell lung cancer (NSCLC); and (iii) a multi-faceted TAM program, a broad antibody discovery effort underway to generate antibodies that modulate the TAM family of RTKs, comprised of Tyro3, AXL and MerTK, which are expressed on tumor-infiltrating macrophages, dendritic cells and some tumors. Research supports TAMs having broad application and potential across immuno-oncology and inflammatory diseases.
Under the terms of the Merger Agreement, upon consummation of the transactions contemplated by the Merger Agreement, Kolltan’s investors received, in exchange for their share and debt interests in Kolltan, an aggregate of 18,257,996 shares of Celldex’s common stock with a calculated value of $62.5 million, based on the average closing price of Celldex’s stock for the five trading day period ending on October 28, 2016, the third calendar day prior to the date of the Merger Agreement, as adjusted downward pursuant to the terms of the Merger Agreement. The Merger Agreement provides that the number of shares that can be issued at the closing can be increased or decreased by no more than 5% in either direction based on the comparable average closing prices over the five trading days prior to the closing date. Therefore, because the average closing price of Celldex’s stock over the five trading days prior to the closing date was higher than the comparable average closing prices over the five trading days prior to the date of the Merger Agreement, there was a full 5% downward adjustment in the number of shares issued at closing. In addition, following closing, certain officers of Kolltan will receive an aggregate of 437,901 shares of Celldex’s common stock in lieu of cash severance obligations owed to them by Kolltan. In addition, in the event that certain specified preclinical and clinical development milestones related to Kolltan’s development programs and/or Celldex’s development programs and certain commercial milestones related to Kolltan’s product candidates are achieved, Celldex will be required to pay Kolltan’s stockholders milestone payments of up to $172.5 million, which milestone payments may be made, at Celldex’s sole election, in cash, in shares of Celldex’s common stock or a combination of both, subject to NASDAQ listing requirements and provisions of the Merger Agreement. The number of shares of Celldex common stock issued in connection with a milestone payment, if any, will be determined based on the average closing price per share of Celldex common stock for the five trading day period ending three calendar days prior to the achievement of such milestone. Pursuant to applicable NASDAQ listing rules, we are required to obtain stockholder approval of such issuances of our common stock to the extent that such issuances exceed 19.9% of our common stock outstanding prior to the merger. If we do not obtain stockholder approval of such common stock issuances, we may elect to pay the milestone consideration in cash to maintain compliance with applicable NASDAQ listing standards. We may still decide to pay cash even if we obtain stockholder approval.
Corporate Information
We are a Delaware corporation organized in 1983. Our principal executive offices are located at Perryville III Building, 53 Frontage Road, Suite 220, Hampton, New Jersey 08827 and our telephone number is (908) 200-7500. Our corporate website is www.celldex.com. The information on our website is not incorporated by reference into this prospectus.
2
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the documents that we incorporate by reference, contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Any statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and may be forward-looking. These statements are often, but not always, made through the use of words or phrases such as “anticipate,” “estimate,” “plans,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend” and similar words or phrases. Accordingly, these statements involve estimates, assumptions and uncertainties, which could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the risk factors discussed in this prospectus or discussed in documents incorporated by reference in this prospectus.
Forward-looking statements are subject to known and unknown risks and uncertainties, which change over time, and are based on management’s expectations and assumptions at the time the statements are made, and are not guarantees of future results. Our actual results may differ materially from those expressed or anticipated in the forward-looking statements for many reasons including the factors described in the section entitled “Risk Factors” in this prospectus and in any risk factors described in a supplement to this prospectus or in other filings.
You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date on which they were made. We undertake no obligation to publicly revise any forward-looking statement to reflect circumstances or events after the date of this prospectus or to reflect the occurrence of unanticipated events. You should, however, review the factors and risks we describe in the reports we file from time to time with the SEC after the date of this prospectus. We undertake no obligation to revise or update the forward-looking statements contained in this prospectus at any time. All forward-looking statements are qualified in their entirety by this cautionary statement.
RISK FACTORS
Investing in our securities involves significant risks. Before making an investment decision, you should carefully consider the risks and other information we include or incorporate by reference in this prospectus and any prospectus supplement. In particular, you should consider the risk factors under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K, or 10-K/A, as applicable, as may be revised or supplemented by our subsequent Quarterly Reports on Form 10-Q or Current Reports on Form 8-K, each of which are on file with the SEC and are incorporated herein by reference, and which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. The risks and uncertainties we have described are not the only ones facing our company. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also affect our business operations. Additional risk factors may be included in a prospectus supplement relating to a particular offering of securities. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. The trading price of our securities could decline due to any of these risks, and you may lose all or part of your investment. This prospectus is qualified in its entirety by these risk factors.
RATIOS OF COMBINED FIXED CHARGES AND PREFERRED STOCK DIVIDENDS TO EARNINGS
The following table sets forth our consolidated ratios of earnings to combined fixed charges and preferred stock dividends for the years ended December 31, 2015, 2014, 2013, 2012 and 2011. We do not have any outstanding shares of preferred stock and therefore have not paid any preferred stock dividends.
3
Table of Contents
Ratios of Combined Fixed Charges and Preferred Stock Dividends to Earnings
|
|
|
Nine months
ended
September
30, 2016
|
|
Year ended December 31,
|
|
|
|
|
2016
|
|
2015
|
|
2014
|
|
2013
|
|
2012
|
|
2011
|
|
|
|
|
(1
|
)
|
(1
|
)
|
(1
|
)
|
(1
|
)
|
(1
|
)
|
(1
|
)
|
(1) Due to our losses from continuing operations for the nine months ended September 30, 2016 and for the years ended December 31, 2015, 2014, 2013, 2012 and 2011 earnings were insufficient to cover fixed charges by $98.1 million, $129.5 million, $122.4 million, $81.4 million, $58.1 million, and $43.3 million, respectively. For this reason, no ratios are provided.
USE OF PROCEEDS
Unless otherwise provided in the applicable prospectus supplement to this prospectus used to offer specific securities, we expect to use the net proceeds from any offering of securities by us for general corporate purposes, which may include acquisitions, capital expenditures, investments, and the repayment, redemption or refinancing of all or a portion of any indebtedness or other securities outstanding at a particular time, to fund our operations until we receive FDA approval of our products and are able to commercialize our products and to make substantial investments to establish sales, marketing, quality control, and regulatory compliance capabilities in anticipation of FDA approval of our products. Pending the application of the net proceeds, we expect to invest the net proceeds in short-term, interest-bearing instruments with a maturity of three months or less at the date of purchase and consist primarily of investments in money market mutual funds with commercial banks and financial institutions or other investment-grade securities. Such investments may include depositing such net proceeds into, and maintaining cash balances with, financial institutions in excess of insured limits.
DESCRIPTIONS OF SECURITIES WE MAY OFFER
This prospectus contains summary descriptions of the common stock, preferred stock, warrants, depositary shares and units that we may offer and sell from time to time. The preferred stock may also be exchangeable for and/or convertible into shares of common stock or another series of preferred stock. When one or more of these securities are offered in the future, a prospectus supplement will explain the particular terms of the securities and the extent to which these general provisions may apply. These summary descriptions and any summary descriptions in the applicable prospectus supplement do not purport to be complete descriptions of the terms and conditions of each security and are qualified in their entirety by reference to our third restated certificate of incorporation, as amended, our by-laws and by applicable Delaware law and any other documents referenced in such summary descriptions and from which such summary descriptions are derived. If any particular terms of a security described in the applicable prospectus supplement differ from any of the terms described herein, then the terms described herein will be deemed superseded by the terms set forth in that prospectus supplement.
We may issue securities in book-entry form through one or more depositaries, such as The Depository Trust Company, Euroclear or Clearstream, named in the applicable prospectus supplement. Each sale of a security in book-entry form will settle in immediately available funds through the applicable depositary, unless otherwise stated. We will issue the securities only in registered form, without coupons, although we may issue the securities in bearer form if so specified in the applicable prospectus supplement. If any securities are to be listed or quoted on a securities exchange or quotation system, the applicable prospectus supplement will say so.
DESCRIPTION OF COMMON STOCK
As of November 30, 2016 we are authorized to issue up to 297,000,000 shares of common stock, $.001 par value per share. As of November 30, 2016, approximately 119,507,692 shares of common stock were outstanding. All outstanding shares of our common stock are fully paid and non-assessable. Our common stock is listed on the NASDAQ Global Market under the symbol “CLDX”.
4
Table of Contents
Dividends
The Board of Directors may, out of funds legally available, at any regular or special meeting, declare dividends to the holders of shares of our common stock as and when they deem expedient, subject to the rights of holders of the preferred stock, if any.
Voting
Each share of common stock entitles the holders to one vote per share on all matters requiring a vote of the stockholders, including the election of directors. No holders of shares of common stock shall have the right to vote such shares cumulatively in any election for the board of directors.
Rights Upon Liquidation
In the event of our voluntary or involuntary liquidation, dissolution, or winding up, the holders of our common stock will be entitled to share equally in our assets available for distribution after payment in full of all debts and after the holders of preferred stock, if any, have received their liquidation preferences in full.
Miscellaneous
No holders of shares of our common stock shall have any preemptive rights to subscribe for, purchase or receive any shares of any class, whether now or hereafter authorized, or any options or warrants to purchase any such shares, or any securities convertible into or exchanged for any such shares, which may at any time be issued, sold or offered for sale by Celldex.
Anti-Takeover Provisions
Certain provisions in our third restated certificate of incorporation, as amended, and applicable Delaware corporate, as well as our shareholder rights agreement, may have the effect of discouraging a change of control of Celldex, even if such a transaction is favored by some of our stockholders and could result in stockholders receiving a substantial premium over the current market price of our shares. The primary purpose of these provisions is to encourage negotiations with our management by persons interested in acquiring control of our corporation. These provisions may also tend to perpetuate present management and make it difficult for stockholders owning less than a majority of the shares to be able to elect even a single director.
Computershare Trust Company, N.A. is presently the transfer agent and registrar for our common stock.
DESCRIPTION OF PREFERRED STOCK
At November 30, 2016, the Company had authorized preferred stock comprised of 3,000,000 shares of Class C Preferred Stock of which 350,000 shares has been designated as Class C-1 Junior Participating Cumulative Preferred Stock, the terms of which are to be determined by our Board of Directors. As of November 30, 2016, there was no preferred stock outstanding.
Class C Preferred Stock
This section describes the general terms and provisions of our Class C Preferred Stock. The applicable prospectus supplement will describe the specific terms of the shares of preferred stock offered through that prospectus supplement, as well as any general terms described in this section that will not apply to those shares of preferred stock.
Our board of directors has been authorized to provide for the issuance of the 2,650,000 unissued and undesignated shares of our Class C Preferred Stock In general, our third restated certificate of incorporation, as amended, authorizes our board of directors to issue new shares of our common stock or preferred stock without further stockholder action, provided that there are sufficient authorized shares.
5
Table of Contents
With respect to each series of our Class C Preferred Stock, our board of directors has the authority to fix the following terms:
·
the designation of the series;
·
the number of shares within the series;
·
whether dividends are cumulative and, if cumulative, the dates from which dividends are cumulative;
·
the rate of any dividends, any conditions upon which dividends are payable, and the dates of payment of dividends;
·
whether interests in the shares of preferred stock will be represented by depositary shares as more fully described below under “Description of Depositary Shares”;
·
whether the shares are redeemable, the redemption price and the terms of redemption;
·
the amount payable to you for each share you own if we dissolve or liquidate;
·
whether the shares are convertible or exchangeable, the price or rate of conversion or exchange, and the applicable terms and conditions;
·
any restrictions on issuance of shares in the same series or any other series;
·
voting rights applicable to the series of preferred stock; and
·
any other rights, priorities, preferences, restrictions or limitations of such series.
The rights with respect to any shares of our Class C Preferred Stock will be subordinate to the rights of our general creditors. Shares of our Class C Preferred Stock that we issue in accordance with their terms will be fully paid and nonassessable, and will not be entitled to preemptive rights unless specified in the applicable prospectus supplement.
Our ability to issue preferred stock, or rights to purchase such shares, could discourage an unsolicited acquisition proposal. For example, we could impede a business combination by issuing a series of preferred stock containing class voting rights that would enable the holders of such preferred stock to block a business combination transaction. Alternatively, we could facilitate a business combination transaction by issuing a series of preferred stock having sufficient voting rights to provide a required percentage vote of the stockholders. Additionally, under certain circumstances, our issuance of preferred stock could adversely affect the voting power of the holders of our common stock. Although our board of directors is required to make any determination to issue any preferred stock based on its judgment as to the best interests of our stockholders, our board of directors could act in a manner that would discourage an acquisition attempt or other transaction that some, or a majority, of our stockholders might believe to be in their best interests or in which stockholders might receive a premium for their stock over prevailing market prices of such stock. Our board of directors does not at present intend to seek stockholder approval prior to any issuance of currently authorized stock, unless otherwise required by law or applicable stock exchange requirements.
Terms of the Preferred Stock That We May Offer and Sell to You
We summarize below some of the provisions that will apply to the preferred stock that we may offer to you unless the applicable prospectus supplement provides otherwise. This summary may not contain all information that is important to you. You should read the prospectus supplement, which will contain additional information and which may update or change some of the information below. Prior to the issuance of a new series of preferred stock, we will further amend our third restated certificate of incorporation, as amended, designating the stock of that series and the terms of that series. We will file a copy of the certificate of designation that contains the terms of each new series
6
Table of Contents
of preferred stock with the SEC each time we issue a new series of preferred stock. Each certificate of designation will establish the number of shares included in a designated series and fix the designation, powers, privileges, preferences and rights of the shares of each series as well as any applicable qualifications, limitations or restrictions. You should refer to the applicable certificate of designation as well as our third restated certificate of incorporation, as amended, before deciding to buy shares of our preferred stock as described in the applicable prospectus supplement.
Our board of directors has the authority, without further action by the stockholders, to issue preferred stock in one or more series and to fix the number of shares, dividend rights, conversion rights, voting rights, redemption rights, liquidation preferences, sinking funds, and any other rights, preferences, privileges and restrictions applicable to each such series of preferred stock.
The issuance of any preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the common stock. The ability of our board of directors to issue preferred stock could discourage, delay or prevent a takeover or other corporate action.
The terms of any particular series of preferred stock will be described in the prospectus supplement relating to that particular series of preferred stock, including, where applicable:
·
the designation, stated value and liquidation preference of such preferred stock;
·
the number of shares within the series;
·
the offering price;
·
the dividend rate or rates (or method of calculation), the date or dates from which dividends shall accrue, and whether such dividends shall be cumulative or noncumulative and, if cumulative, the dates from which dividends shall commence to cumulate;
·
whether interests in the shares of preferred stock will be represented by depositary shares as more fully described below under “Description of Depositary Shares”);
·
any redemption or sinking fund provisions;
·
the amount that shares of such series shall be entitled to receive in the event of our liquidation, dissolution or winding-up;
·
the terms and conditions, if any, on which shares of such series shall be convertible or exchangeable for shares of our stock of any other class or classes, or other series of the same class;
·
the voting rights, if any, of shares of such series; the status as to reissuance or sale of shares of such series redeemed, purchased or otherwise reacquired, or surrendered to us on conversion or exchange;
·
the conditions and restrictions, if any, on the payment of dividends or on the making of other distributions on, or the purchase, redemption or other acquisition by us or any subsidiary, of the common stock or of any other class of our shares ranking junior to the shares of such series as to dividends or upon liquidation;
·
the conditions and restrictions, if any, on the creation of indebtedness by us or by any subsidiary, or on the issuance of any additional stock ranking on a parity with or prior to the shares of such series as to dividends or upon liquidation; and
·
any additional dividend, liquidation, redemption, sinking or retirement fund and other rights, preferences, privileges, limitations and restrictions of such preferred stock.
7
Table of Contents
The description of the terms of a particular series of preferred stock in the applicable prospectus supplement will not be complete. You should refer to the applicable amendment to our third restated certificate of incorporation, as amended, for complete information regarding a series of preferred stock.
The preferred stock will, when issued against payment of the consideration payable therefor, be fully paid and nonassessable. Unless otherwise specified in the applicable prospectus supplement, each series of preferred stock will, upon issuance, rank senior to the common stock and on a parity in all respects with each other outstanding series of preferred stock. The rights of the holders of our preferred stock will be subordinate to that of our general creditors.
DESCRIPTION OF WARRANTS
We summarize below some of the provisions that will apply to the warrants unless the applicable prospectus supplement provides otherwise. This summary may not contain all information that is important to you. The complete terms of the warrants will be contained in the applicable warrant certificate and warrant agreement. These documents have been or will be included or incorporated by reference as exhibits to the registration statement of which this prospectus is a part. You should read the warrant certificate and the warrant agreement. You should also read the prospectus supplement, which will contain additional information and which may update or change some of the information below.
General
We may issue, together with other securities or separately, warrants to purchase common stock, preferred stock or other securities. We may issue the warrants under warrant agreements to be entered into between us and a bank or trust company, as warrant agent, all as set forth in the applicable prospectus supplement. The warrant agent would act solely as our agent in connection with the warrants of the series being offered and would not assume any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants.
The applicable prospectus supplement will describe the following terms, where applicable, of warrants in respect of which this prospectus is being delivered:
·
the title of the warrants;
·
the designation, amount and terms of the securities for which the warrants are exercisable and the procedures and conditions relating to the exercise of such warrants;
·
the designation and terms of the other securities, if any, with which the warrants are to be issued and the number of warrants issued with each such security;
·
the price or prices at which the warrants will be issued;
·
the aggregate number of warrants;
·
any provisions for adjustment of the number or amount of securities receivable upon exercise of the warrants or the exercise price of the warrants;
·
the price or prices at which the securities purchasable upon exercise of the warrants may be purchased;
·
if applicable, the date on and after which the warrants and the securities purchasable upon exercise of the warrants will be separately transferable;
·
if applicable, a discussion of the material U.S. federal income tax considerations applicable to the warrants;
8
Table of Contents
·
any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants;
·
the date on which the right to exercise the warrants shall commence and the date on which the right shall expire;
·
if applicable, the maximum or minimum number of warrants which may be exercised at any time;
·
the identity of the warrant agent;
·
any mandatory or optional redemption provision;
·
whether the warrants are to be issued in registered or bearer form;
·
whether the warrants are extendible and the period or periods of such extendibility;
·
information with respect to book-entry procedures, if any; and
·
any other terms of the warrants.
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including the right to receive dividends, if any, or payments upon our liquidation, dissolution or winding-up or to exercise voting rights, if any.
Exercise of Warrants
Each warrant will entitle the holder thereof to purchase such number of shares of common stock or preferred stock or other securities at the exercise price as will in each case be set forth in, or be determinable as set forth in, the applicable prospectus supplement. Warrants may be exercised at any time up to the close of business on the expiration date set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void. Warrants may be exercised as set forth in the applicable prospectus supplement relating to the warrants offered thereby. Upon receipt of payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will, as soon as practicable, forward the purchased securities. If less than all of the warrants represented by the warrant certificate are exercised, a new warrant certificate will be issued for the remaining warrants.
Enforceability of Rights of Holders of Warrants
Each warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, that holder’s warrant(s).
Modification of the Warrant Agreement
The warrant agreement will permit us and the warrant agent, without the consent of the warrant holders, to supplement or amend the agreement in the following circumstances:
·
to cure any ambiguity;
9
Table of Contents
·
to correct or supplement any provision which may be defective or inconsistent with any other provisions; or
·
to add new provisions regarding matters or questions that we and the warrant agent may deem necessary or desirable and which do not adversely affect the interests of the warrant holders.
DESCRIPTION OF DEPOSITARY SHARES
We summarize below some of the provisions that will apply to depositary shares unless the applicable prospectus supplement provides otherwise. This summary may not contain all information that is important to you. The complete terms of the depositary shares will be contained in the depositary agreement and depositary receipt applicable to any depositary shares. These documents have been or will be included or incorporated by reference as exhibits to the registration statement of which this prospectus is a part. You should read the depositary agreement and the depositary receipt. You should also read the prospectus supplement, which will contain additional information and which may update or change some of the information below.
General
We may, at our option, elect to offer fractional or multiple shares of common stock or preferred stock, rather than single shares of common stock or preferred stock (to be set forth in the prospectus supplement relating to such depositary shares). In the event we elect to do so, depositary receipts evidencing depositary shares will be issued to the public.
The shares of common stock or any class or series of preferred stock represented by depositary shares will be deposited under a deposit agreement among us, a depositary selected by us, and the holders of the depositary receipts. The depositary will be a bank or trust company having its principal office in the United States and having a combined capital and surplus of at least $50,000,000. Subject to the terms of the deposit agreement, each owner of a depositary share will be entitled, in proportion to the applicable fraction of a share of common stock or preferred stock represented by such depositary share, to all the rights and preferences of the shares of common stock or preferred stock represented by the depositary share, including dividend, voting, redemption and liquidation rights.
The depositary shares will be evidenced by depositary receipts issued pursuant to the deposit agreement. Depositary receipts will be distributed to those persons purchasing the fractional shares of common stock or the related class or series of preferred shares in accordance with the terms of the offering described in the related prospectus supplement.
DESCRIPTION OF UNITS
We may issue units comprised of one or more of the other securities described in this prospectus in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date. The applicable prospectus supplement may describe:
·
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately;
·
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units;
·
the terms of the unit agreement governing the units;
·
United States federal income tax considerations relevant to the units; and
10
Table of Contents
·
whether the units will be issued in fully registered global form.
This summary of certain general terms of units and any summary description of units in the applicable prospectus supplement do not purport to be complete and are qualified in their entirety by reference to all provisions of the applicable unit agreement and, if applicable, collateral arrangements and depositary arrangements relating to such units. The forms of the unit agreements and other documents relating to a particular issue of units will be filed with the SEC each time we issue units, and you should read those documents for provisions that may be important to you.
PLAN OF DISTRIBUTION
We may sell the securities covered hereby from time to time pursuant to underwritten public offerings, direct sales to the public, negotiated transactions, block trades or a combination of these methods. A distribution of the securities offered by this prospectus may also be effected through the issuance of derivative securities, including without limitation, warrants and subscriptions. We may sell the securities to or through underwriters or dealers, through agents, or directly to one or more purchasers. We may distribute securities from time to time in one or more transactions:
·
at a fixed price or prices, which may be changed;
·
at market prices prevailing at the time of sale;
·
at prices related to such prevailing market prices;
·
at varying prices determined at the time of sale; or
·
at negotiated prices.
·
A prospectus supplement or supplements will describe the terms of the offering of the securities, including:
·
the name or names of the underwriters, dealers or agents participating in the offering, if any;
·
the purchase price of the securities sold by us to any underwriter or dealer and the net proceeds we expect to receive from the offering;
·
any over-allotment options under which underwriters may purchase additional securities from us;
·
any agency fees or underwriting discounts or commissions and other items constituting agents’ or underwriters’ compensation;
·
any public offering price;
·
any discounts or concessions allowed or reallowed or paid to dealers; and
·
any securities exchange or market on which the securities may be listed.
Only underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
If underwriters are used in the sale, they will acquire the securities for their own account and may resell the securities from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to
11
Table of Contents
certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement, other than securities covered by any over-allotment option. Any public offering price and any discounts or commissions or concessions allowed or reallowed or paid to dealers may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions and other compensation we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We may provide agents and underwriters with indemnification against civil liabilities related to this offering, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
All securities we may offer, other than common stock, will be new issues of securities with no established trading market. Any agents or underwriters may make a market in these securities, but will not be obligated to do so and may discontinue any market making at any time without notice. We cannot guarantee the liquidity of the trading markets for any securities. There is currently no market for any of the offered securities, other than our common stock which is listed on the NASDAQ Global Market. We have no current plans for listing of the preferred stock, warrants or subscription rights on any securities exchange or quotation system; any such listing with respect to any particular preferred stock, warrants or subscription rights will be described in the applicable prospectus supplement or other offering materials, as the case may be.
Any underwriter may engage in overallotment, stabilizing transactions, short covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
Any agents and underwriters who are qualified market makers on the NASDAQ Global Market may engage in passive market making transactions in the securities on the NASDAQ Global Market in accordance with Regulation M, during the business day prior to the pricing of the offering, before the commencement of offers or sales of the securities. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
LEGAL MATTERS
Unless otherwise indicated in the applicable prospectus supplement, the validity of the securities offered hereby will be passed upon for us by Lowenstein Sandler LLP, New York, New York. If the validity of the securities offered hereby in connection with offerings made pursuant to this prospectus are passed upon by counsel for the
12
Table of Contents
underwriters, dealers or agents, if any, such counsel will be named in the prospectus supplement relating to such offering.
EXPERTS
The financial statements and management’s assessment of the effectiveness of internal control over financial reporting (which is included in Management’s Report on Internal Control over Financial Reporting) incorporated in this prospectus by reference to the Annual Report on Form 10-K/A for the year ended December 31, 2015 have been so incorporated in reliance on the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The financial statements of Kolltan Pharmaceuticals, Inc., incorporated in this prospectus by reference to Celldex Therapeutics, Inc.’s Current Report on Form 8-K/A dated February 7, 2017, have been so incorporated in reliance upon the report of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3, including exhibits, under the Securities Act of which this prospectus forms a part. This prospectus does not contain all of the information set forth in the registration statement. This prospectus contains descriptions of certain agreements or documents that are exhibits to the registration statement. The statements as to the contents of such exhibits, however, are brief descriptions and are not necessarily complete, and each statement is qualified in all respects by reference to such agreement or document. For further information about us, please refer to the registration statement and the documents incorporated by reference in this prospectus.
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at
http://www.sec.gov
. The SEC’s website contains reports, proxy statements and other information regarding issuers, such as Celldex Therapeutics, Inc., that file electronically with the SEC. You may also read and copy any document we file with the SEC at the SEC’s Public Reference Room, located at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of its Public Reference Room. We make available free of charge through our web site our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, Proxy Statements on Schedule 14A and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the SEC. Our website address is
http://www.celldextherapeutics.com
. Please note that our website address is provided as an inactive textual reference only. Information contained on or accessible through our website is not part of this prospectus or the prospectus supplement, and is therefore not incorporated by reference unless such information is otherwise specifically referenced elsewhere in this prospectus or the prospectus supplement.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we have filed with the SEC, which means that we can disclose important information to you by referring you to those documents. Any information that we file subsequently with the SEC will automatically update this prospectus. We incorporate by reference into this prospectus the information contained in the documents listed below, which is considered to be a part of this prospectus:
·
Our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, filed with the SEC on February 23, 2016;
·
Our Annual Report on Form 10-K/A for the fiscal year ended December 31, 2015, filed with the SEC on February 25, 2016;
·
Our Quarterly Reports on Form 10-Q for the fiscal quarters ended March 31, 2016, June 30, 2016 and September 30, 2016, filed on May 5, 2016, August 8, 2016 and November 7, 2016, respectively;
·
Our Current Reports on Form 8-K or 8-K/A filed with the SEC on February 23, 2016, March 7, 2016, May 19, 2016, June 9, 2016, August 11, 2016, November 1, 2016, November 29, 2016, as amended by Form 8-K/A filed on February 7, 2017, December 5, 2016, and December 14, 2016 (in each case, not including any information furnished under Items 2.02 or 7.01 of Form 8-K, including the related exhibits, which information is not incorporated by reference herein);
13
Table of Contents
·
Our Definitive Proxy Statement on Schedule 14A, filed with the SEC on April 21, 2016 (other than the portions thereof which are furnished and not filed); and
·
The description of our common stock contained in our Registration Statement on Form 8-A, filed on November 8, 2004, as amended by Form 8-A/A filed on October 22, 2007 and March 7, 2008.
We also incorporate by reference all documents we file under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (a) after the initial filing date of the registration statement of which this prospectus is a part and before the effectiveness of the registration statement and (b) after the effectiveness of the registration statement and before the filing of a post-effective amendment that indicates that the securities offered by this prospectus have been sold or that deregisters the securities covered by this prospectus then remaining unsold. The most recent information that we file with the SEC automatically updates and supersedes older information. The information contained in any such filing will be deemed to be a part of this prospectus, commencing on the date on which the document is filed.
Nothing in this prospectus shall be deemed to incorporate information furnished but not filed with the SEC pursuant to Item 2.02 or 7.01 of Form 8-K.
We will furnish without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request, a copy of any documents incorporated by reference other than exhibits to those documents. Requests should be addressed to:
Corporate Secretary
Celldex Therapeutics, Inc.
Perryville III Building, 53 Frontage Road, Suite 220,
Hampton, New Jersey 08827
(908) 200-7500
14
Table of Contents

Celldex Therapeutics, Inc.
Up to $75,000,000
Common Stock
PROSPECTUS SUPPLEMENT

November 9, 2017
Celldex Therapeutics (NASDAQ:CLDX)
Historical Stock Chart
From Mar 2024 to Apr 2024
Celldex Therapeutics (NASDAQ:CLDX)
Historical Stock Chart
From Apr 2023 to Apr 2024