|
PROSPECTUS SUPPLEMENT
|
Filed Pursuant to Rule 424(b)(5)
|
|
(To Prospectus dated July 2, 2014)
|
Registration
Nos. 333-196976 and 333-217408
|

4,285,715 Shares of Common Stock
We are offering 4,285,715 shares of our
common stock, par value $0.0001 per share (“common stock”).
Our common stock is listed on The NASDAQ Capital
Market under the symbol “ADMP.” On April 19, 2017, the last reported sale price of our common stock on The NASDAQ
Capital Market was $4.50 per share.
Investing in our securities involves significant
risks. See “Risk Factors” beginning on page S-11 of this prospectus supplement and on page 6 of the accompanying prospectus
and the documents incorporated by reference herein.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement
or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
|
Per Share
|
|
Total
|
|
Price to the public
|
|
|
$
|
3.50
|
|
|
|
$
|
15,000,002.50
|
|
|
Underwriting discounts and commissions
(1)
|
|
|
$
|
0.21
|
|
|
|
$
|
900,000.15
|
|
|
Proceeds, before expenses, to us
(2)
|
|
|
$
|
3.29
|
|
|
|
$
|
14,100,002.35
|
|
|
|
(1)
|
For
additional information about the expenses for which we have agreed to reimburse the underwriters
in connection with this offering, see “Underwriting” on page S-27 of this
prospectus supplement.
|
|
|
(2)
|
We
estimate the total expenses of this offering will be approximately $245,000.
|
We have granted the underwriters an option for a
period of 30 days to purchase up to an additional 642,857 shares of common stock, to cover over-allotments, if any.
The underwriters expect to deliver the shares of common stock on or
about April 26, 2017, subject to customary closing conditions.
Sole Book-Running
Manager
RAYMOND JAMES
Co-Manager
MAXIM GROUP
LLC
The date of this
prospectus supplement is April 21, 2017.
TABLE OF CONTENTS
|
|
Page
|
|
PROSPECTUS
SUPPLEMENT
|
|
|
|
|
|
ABOUT
THIS PROSPECTUS SUPPLEMENT
|
S-1
|
|
PROSPECTUS
SUPPLEMENT SUMMARY
|
S-2
|
|
RISK
FACTORS
|
S-11
|
|
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
|
S-20
|
|
USE
OF PROCEEDS
|
S-21
|
|
DILUTION
|
S-22
|
|
CAPITALIZATION
|
S-24
|
|
MARKET
FOR OUR COMMON STOCK
|
S-26
|
|
UNDERWRITING
|
S-27
|
|
LEGAL
MATTERS
|
S-34
|
|
EXPERTS
|
S-34
|
|
WHERE
YOU CAN FIND MORE INFORMATION
|
S-34
|
|
INCORPORATION
OF DOCUMENTS BY REFERENCE
|
S-34
|
PROSPECTUS
|
ABOUT THIS PROSPECTUS
|
1
|
|
ABOUT THE COMPANY
|
2
|
|
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
|
5
|
|
RISK FACTORS
|
6
|
|
USE OF PROCEEDS
|
23
|
|
THE SECURITIES WE MAY OFFER
|
23
|
|
DESCRIPTION OF CAPITAL STOCK
|
24
|
|
DESCRIPTION OF WARRANTS
|
28
|
|
DESCRIPTION OF UNITS
|
31
|
|
PLAN OF DISTRIBUTION
|
31
|
|
LEGAL MATTERS
|
34
|
|
EXPERTS
|
35
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
35
|
|
INCORPORATION OF DOCUMENTS BY REFERENCE
|
35
|
You should rely only on this prospectus supplement, the accompanying
prospectus and the information incorporated or deemed to be incorporated by reference in this prospectus supplement and the accompanying
prospectus. We have not authorized anyone to provide you with information that is in addition to or different from that contained
or incorporated by reference in this prospectus supplement and the accompanying prospectus. If anyone provides you with different
or inconsistent information, you should not rely on it. This prospectus supplement, the accompanying prospectus and any related
free writing prospectus, if any, do not constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction
to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. You should not assume that the information
contained or incorporated by reference in this prospectus supplement or the accompanying prospectus is accurate as of any date
other than as of the date of this prospectus supplement or the accompanying prospectus, as the case may be, or in the case of the
documents incorporated by reference, the date of such documents regardless of the time of delivery of this prospectus supplement
and the accompanying prospectus or any sale of our securities. Our business, financial condition, liquidity, results of operations
and prospects may have changed since those dates.
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement is part of the registration
statement on Form S-3 that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration
process to register sales of our securities, under the Securities Act of 1933, as amended, or the Securities Act, and was declared
effective by the SEC on July 2, 2014. This document consists of two parts. The first part is this prospectus supplement, including
the documents incorporated by reference, which describes the specific terms of this offering. The second part is the accompanying
prospectus filed with the SEC as part of the registration statement that was declared effective by the SEC on July 2, 2014,
including the documents incorporated by reference, that gives more general information, some of which may not apply to this offering.
Generally, when we refer only to the “prospectus,” we are referring to both parts combined. This prospectus supplement
may add to, update or change information in the accompanying prospectus and the documents incorporated by reference into this prospectus
supplement or the accompanying prospectus.
If information in this prospectus supplement is
inconsistent with any document incorporated by reference that was filed with the SEC before the date of this prospectus supplement,
you should rely on this prospectus supplement. This prospectus supplement, the accompanying prospectus and the documents incorporated
into each by reference include important information about us, the securities being offered and other information you should know
before investing in our securities. You should also read and consider information in the documents to which we have referred you
in the section of this prospectus entitled “Where You Can Find More Information.”
We sometimes refer to the shares of common stock
offered hereby as the “securities.”
We further note that the representations, warranties
and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference into this
prospectus supplement or the accompanying prospectus were made solely for the benefit of the parties to such agreement, including,
in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation,
warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made.
Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state
of our affairs.
Unless otherwise indicated, all information contained
or incorporated by reference in this prospectus supplement and the accompanying prospectus concerning our industry in general or
any portion thereof, including information regarding our general expectations and market opportunity, is based on management’s
estimates using internal data, data from industry related publications, consumer research and marketing studies and other externally
obtained data.
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights selected information appearing
elsewhere in this prospectus supplement or in the accompanying prospectus or incorporated by reference into this prospectus supplement
and the accompanying prospectus, and does not contain all of the information that may be important to you or that you should consider
before investing in our common stock. Before making an investment decision, you should read this prospectus supplement, the accompanying
prospectus and the information incorporated by reference herein in their entirety, including “Risk Factors” beginning
on page S-11 of this prospectus supplement and on page 6 of the accompanying prospectus.
Company Overview
Adamis Pharmaceuticals Corporation (“we,” “us,” “our,” “Adamis”
or the “company”) is a specialty biopharmaceutical company focused on developing and commercializing products in the
therapeutic areas of respiratory disease and allergy. We are currently developing several products in the allergy and respiratory
markets, including our Epinephrine Injection pre-filled syringe, or PFS, product for use in the emergency treatment of acute allergic
reactions, including anaphylaxis; albuterol (APC-2000) and fluticasone (APC-4000) Dry Powder Inhaler, or DPI, products for
the treatment of bronchospasm and asthma, respectively; and beclomethasone (APC-1000), a metered dose inhaler product for the treatment
of asthma. Our goal is to create low cost therapeutic alternatives to existing treatments. Consistent across all specialty
pharmaceuticals product lines, we intend to submit Section 505(b)(2) New Drug Applications, or NDAs, or Section 505(j) Abbreviated
New Drug Applications, or ANDAs, to the U.S. Food and Drug Administration, or FDA, whenever possible, in order to potentially reduce the
time to market and to save on costs, compared to those associated with Section 505(b)(1) NDAs for new drug products.
Our subsidiary, U.S.
Compounding, Inc., or USC, which we acquired in April 2016 and which is registered as a drug compounding
outsourcing facility under Section 503B of the U.S. Food, Drug & Cosmetic Act, as amended, or FDCA, and the U.S.
Drug Quality and Security Act, or DQSA, provides compounded sterile prescription drugs, and certain nonsterile drugs, to
patients, physician clinics, hospitals, surgery centers and other clients throughout most of the United States. USC’s
offerings include, among others, injectable corticosteroids, hormone replacement therapies, hospital outsourcing
formulations, urological preparations, ophthalmic preparations, topical compounds for pain and men’s and
women’s health formulations. USC also provides certain veterinary pharmaceutical products for animals.
To
achieve our goals and support our overall strategy, we will need to raise a substantial amount of funding and make significant
investments in, among other things, new product development and working capital.
The
current status of our development programs is as follows:
Product Portfolio
|
Specialty
Pharmaceutical Products
|
|
Target
Indication
|
|
Development
Status
|
|
Epinephrine PFS
|
|
Anaphylaxis
|
|
Submitted NDA
|
|
Dry Powder Inhaler Products
|
|
|
|
|
|
Fluticasone (APC-4000)
|
|
Asthma
|
|
Phase
3 ready
(1)(2)
|
|
Albuterol (APC-2000)
|
|
Bronchospasm
|
|
Phase
3 ready
(1)(2)
|
|
Metered Dose Inhaler Product
|
|
|
|
|
|
Beclomethasone (APC-1000)
|
|
Asthma
|
|
Phase
3 ready
(1)(2)
|
|
|
(1)
|
Represents the next anticipated development
or regulatory stage for the product candidate that we may pursue following completion
of product development, assuming that we have the financial resources to pursue any of
these opportunities. There are no assurances that we will pursue these opportunities,
for financial or other reasons.
|
|
|
(2)
|
Following completion of product development,
a single Phase 3 trial, without previous Phase 1 or Phase 2 trials, is the anticipated
next product development stage. We intend to conduct additional trials, such as pharmacokinetic,
or PK, and/or dose escalation studies in connection with the Phase 3 trial.
|
We have not received regulatory approval for any
of the above drugs or products.
Anaphylaxis; Epinephrine Pre-Filled Syringe
Our most advanced product candidate, the Epinephrine Injection USP 1:1000 0.3mg Pre-filled Single Dose Syringe,
or the Epinephrine PFS, is a pre-filled syringe designed to deliver a premeasured 0.3 mg dose of epinephrine for the treatment
of anaphylaxis. The American Academy of Allergy Asthma and Immunology, or AAAAI, defines anaphylaxis as a serious life-threatening
allergic reaction. The most common anaphylactic reactions are to foods, insect stings, medications and latex. According to information
published by AAAAI reporting on findings from a 2009-2010 study, up to 8% of U.S. children under the age of 18 had a food allergy,
and approximately 38% of those with a food allergy had a history of severe reactions. Anaphylaxis requires immediate medical treatment,
which may include an injection of epinephrine.
We estimate that sales of prescription epinephrine
products in 2016 were approximately $1.2 billion, based on industry data. We cannot provide any assurances concerning any possible
future rates of annual growth or whether annual prescription sales will decline or grow. The market for prescription epinephrine
products is increasingly competitive, and a number of factors have resulted in, and could continue to result in, downward pressure
on the pricing of, and revenues from sales of, prescription epinephrine products such as our Epinephrine PFS product.
We believe that there is an opportunity for a simple, lower-cost, pre-filled syringe to compete in this market.
Our Epinephrine PFS product will allow users to administer a pre-measured epinephrine dose quickly with a syringe that we believe
will be familiar to many potential users. Auto-injectors are spring-loaded auto-injector devices, and if not administered properly,
there is a risk that they could misfire or be misused. We expect to introduce the Epinephrine PFS product at a price point reflecting
a discount to the price of the leading products. We believe that a lower-priced option may be attractive to individuals that pay
cash for their epinephrine products, professional users such as hospitals and first responders, and military and prison systems.
We believe that the Epinephrine PFS product candidate, if approved and commercially introduced, may acquire
a share of the market based on the price differential between the expected price of the Epinephrine PFS and the price at which
the current market-leading product is currently sold, which may motivate purchasers and reimbursing payors to choose the lower
cost alternative. We believe that the Epinephrine PFS has the potential to compete successfully, although there can be no assurance
that this will be the case. If our product candidate is approved and successfully launched, competitors may reduce or otherwise
modify the pricing of their existing products. In addition, a generic version of the market-leading auto-injector product was recently
introduced at a significantly lower price than the market-leading product, other competing products have been introduced or prices
on existing competing products have been reduced, and if additional competing products are introduced in the future, including
generic or bioequivalent, or A/B rated, versions of one or more existing spring-loaded auto-injector devices, at lower prices than
the current market leading products, the competitive success of our product could be adversely affected.
On May 28, 2014, we submitted a Section 505(b)(2) NDA application to the FDA for approval of our Epinephrine
PFS product candidate. We received a complete response letter, or CRL, from the FDA on March 27, 2015. A CRL is issued by
the FDA’s Center for Drug Evaluation and Research when it has completed its review of a file and questions remain that preclude
the approval of the NDA in its current form. We resubmitted the NDA on December 4, 2015. On June 3, 2016, we received
a second CRL from the FDA regarding our resubmitted NDA. The CRL indicated that the FDA determined that it could not approve the
NDA in its present form. The agency indicated that in order to support approval of the product candidate, the Company must
expand its human factors studies. The CRL indicated that new human factors studies would need to provide additional, adequate and
satisfactory data and information concerning, among other things, use of the product candidate in different use environments
and by different kinds of users and user groups. The CRL included comments on certain other aspects of the product candidate
and the materials and data submitted as part of the NDA. The CRL indicated that the agency had reserved comment, if any, on the
proposed labeling for the product candidate until the application was otherwise approvable. The FDA indicated that the NDA
will remain open until the issues identified in the CRL are resolved.
On December 15, 2016, we resubmitted our NDA to
the FDA. The resubmission was intended to address the issues raised by the FDA in the June 2016 CRL. On January 19, 2017, we announced
that the FDA had accepted for review our resubmitted NDA. The FDA indicated that it considered the resubmission to be a complete
response to the CRL. The agency continues to request certain additional information relating to some of the other comments contained
in the CRL, which we intend to provide.
There are no assurances that the FDA will approve the resubmitted NDA and grant marketing approval for Epinephrine
PFS. If the FDA approves the NDA, we hope to receive an approval in time to permit first commercial sales to commence sometime
in the third quarter of 2017, although there are no assurances that this will be the case. Under goals established in connection
with the Prescription Drug User Fee Act, or PDUFA, the FDA’s guidance for the review and acting on Class 2 NDA resubmissions
is six months from the date of receipt of the resubmission. However, the FDA’s review processes can extend beyond, and in
some cases significantly beyond, anticipated completion dates due to the timing of the FDA’s review process, issuance of
an additional CRL, FDA requests for additional data, information, materials or clarification, difficulties scheduling an advisory
committee meeting, FDA workload issues, extensions resulting from the submission of additional information or clarification regarding
information already in the submission within the last three months of the target PDUFA date, or other reasons. As a result, the
dates of regulatory approval, if obtained, and commercial introduction of our product could be delayed beyond our expectations.
Asthma and Bronchospasm
According to the National Institute of Health, or
NIH, asthma is a chronic lung disease that inflames and narrows the airways. Asthma causes recurring periods of wheezing, chest
tightness, shortness of breath, and coughing. Asthma affects people of all ages, but it most often starts during childhood. According
to information published by Centers for Disease Control & Prevention (CDC) reporting on findings from 2014, the number of people
in the U.S. with asthma is approximately 24 million and growing. We estimate that global sales of asthma and bronchospasm prescription
products were in excess of approximately $10.6 billion in 2016, based on industry data.
Dry Powder Inhaler (DPI) Device Platform
In December 2013, we acquired assets relating to 3M’s patented Taper dry powder inhaler, or DPI, technology
under development by 3M for the treatment of asthma and bronchospasm, for total consideration of $10 million. The Taper DPI technology
was under development by 3M as a device designed to efficiently deliver dry powder by utilizing a 3M proprietary microstructured
carrier tape. We are utilizing the Taper DPI assets to develop the DPI device. We believe that, if successfully developed,
the device can be utilized to deliver a variety of different drugs. We intend to utilize the DPI as a platform delivery device
for additional drugs that will compete in the respiratory markets, including combination products. Pursuant to our agreement with
3M, the microstructured carrier tape will be supplied by 3M under a separate supply agreement to be negotiated with 3M.
We believe that one advantage of the technology
is that it can deliver drug particles without the need for lactose or formulation excipients. The majority of current dry powder
products use lactose carrier excipients to enhance flowability; however, they have the disadvantage of increased bulk and require
a mechanism for detaching the drug from the surface of the lactose. Lactose carrier formulations require a complicated blending
process and delivery that is highly sensitive to excipient powder properties. To our knowledge, there are currently no excipient-free
dry powder inhalers in the U.S. market.
Asthma; Fluticasone
.
In light of competitive changes in the marketplace, we have prioritized two single compounded products (APC-2000 and 4000) over
a combination product that we were previously developing for the treatment of asthma to deliver the same active ingredients as
GlaxoSmithKline’s Advair® Diskus
®
, which combines fluticasone propionate and salmeterol xinafoate. As
a result, our first product candidate in development utilizing the DPI technology platform in our allergy and respiratory pipeline,
APC-4000, will deliver Fluticasone Propionate (Fluticasone) as a dry powder for inhalation for the treatment of asthma. The fluticasone
product candidate is designed to deliver the same active ingredient as GlaxoSmithKline’s Flovent® Diskus® for
the treatment of asthma. Fluticasone belongs to the family of medicines known as corticosteroids or steroids. It works by preventing
certain cells in the lungs and breathing passages from releasing substances that cause asthma symptoms. We estimate that Flovent®
Diskus
®
marketed by GlaxoSmithKline, generated more than $466 million in U.S. sales and $786 million in global
sales in 2016, based on GSK’s publicly announced results.
Bronchospasm; Albuterol
. Our second product
candidate that is in development using our DPI technology, albuterol (APC-2000), is a bronchodilator for the treatment or prevention
of bronchospasm. Bronchodilators are medicines that are breathed in through the mouth to open up the bronchial tubes (air passages)
in the lungs. Bronchodilators relieve cough, wheezing, shortness of breath, and troubled breathing by increasing the flow of air
through the bronchial tubes. We have had discussions with the FDA regarding regulatory approval requirements and intend
to have further discussions concerning, among other things, the appropriate regulatory pathway for the product under Section 505(j)
relating to ANDAs, Section 505(b)(2) or otherwise. Based on industry sources, we estimate that the annual worldwide sales of bronchodilators
are approximately $1.8 billion.
Total time to develop the DPI product candidates, including manufacture of the product, clinical trials and
FDA review, is expected to be approximately 24-30 months from inception of full product development efforts, assuming that
we are able to obtain adequate funding and that there are no unforeseen regulatory issues or other delays. As discussed further
below, product development time is subject to a number of risks and uncertainties, which can delay the actual development time
beyond the estimates described above.
We are currently preparing an investigational new
drug application, or IND, to be submitted to the FDA to begin human testing of both the albuterol and fluticasone DPIs. Assuming
receipt of sufficient funding, if product development is successful, and if clinical trials are initiated and successfully completed,
we intend to pursue an NDA under Section 505(b)(2) to seek approval for sale in the U.S. market. We also intend to seek to identify
opportunities to market DPI based products outside of the United States. We currently have no in-house manufacturing capabilities,
so we intend to rely on third-party contract manufacturers to manufacture the materials needed to produce DPI products.
Asthma; APC-1000 Metered Dose Inhaler
Our APC-1000 product candidate is a steroid hydrofluoroalkane, or HFA, metered dose inhaler product, for asthma.
Our product candidate, if developed and approved for marketing, will target a small niche within the larger market for respiratory
products. To date, we have not made any regulatory filings with the FDA for this product candidate. We estimate that the
annual global sales of prescription steroid HFA and similar products are approximately $3.0 billion, of which we intend to target
a smaller niche.
On February 24, 2015, we announced the result of our pharmacokinetic study, or PK study, comparing our beclomethasone
dipropionate HFA, 80 mcg Inhalation Aerosol, product, APC-1000, with Teva Respiratory, LLC’s Qvar® (Beclomethasone Dipropionate
HFA, 80 mcg Inhalation Aerosol) product. The study was a Phase I open label, randomized, single-dose, four-way crossover PK study
comparing APC-1000 to Qvar. Twenty-two healthy male and female subjects who met the study inclusion criteria were enrolled. The
study involved a screening period before randomization and four treatment periods each separated by a minimum of three days. Both
inhalation aerosols were administered to each subject for a total dose of 320 mcg BDP (4 inhalations). Twenty-one subjects completed
the study. One subject was withdrawn due to non-compliance. The purpose of this PK study was to compare the bioavailability of
APC-1000 to Qvar. The results showed the extent of absorption of APC-1000 to be equivalent to Qvar. Following discussions with
the FDA and additional consideration of the development pathway for the product candidate, we decided to conduct additional development
work for APC-1000 during 2016. We intend, depending on the outcome of several factors including results of additional development
work and obtaining additional funding that will be required to commence a trial, to submit an IND and initiate a dose escalation
and subsequent Phase 3 efficacy study during the second half of 2017, assuming that we are able to obtain adequate funding and
that there are no unforeseen regulatory issues or other delays. Product development time is subject to a number of risks
and uncertainties which can delay the actual development time beyond our estimates.
Our development plans concerning our allergy and respiratory product candidates, including APC-1000, are affected
by developments in the marketplace, including the introduction of potentially competing new products by our competitors. For example,
certain products that previously have been available by prescription only have been approved by the FDA and introduced for sale
over-the-counter without a prescription at a lower price than competing prescription products, and other new allergy or respiratory
products have been or could in the future also be approved as “branded generic” products or as over-the-counter products.
Such products could be sold at lower prices than prescription products, could adversely affect the willingness of health insurers
or other third party payors to reimburse patients for the cost of prescription products, and could adversely affect our ability
to successfully develop and market product candidates in our pipeline. As a result, our product development plans could be affected
by such considerations. The anticipated dates for development and introduction of products in our allergy and respiratory product
pipeline will depend on a number of factors, including the availability of adequate funding to support product development efforts.
We believe that should we decide to pursue such applications, we would be required to submit data for an application for approval
to market APC-1000 pursuant to Section 505(b)(2), although there are no assurances that this will be the case. We believe that
the next trial for APC-1000 would be a Phase 3 pivotal trial, potentially preceded by dose escalation studies, and we do not believe
that Phase 1 or Phase 2 trials would be required. Total time to develop APC-1000, including manufacture of the product, clinical
trials and FDA review, is expected to be approximately 24-30 months from inception of full product development efforts, assuming
that we are able to obtain adequate funding and that there are no unforeseen regulatory issues or other delays.
Factors that could affect the actual launch date for our allergy and respiratory product candidates, as well
as our other product candidates, include general market conditions, the outcome of discussions with the FDA concerning the number
and kind of clinical trials that the FDA will require before the FDA will consider regulatory approval of the applicable product
candidate, the outcome of discussions with the FDA concerning the regulatory approval pathway of the applicable product candidate,
any unexpected difficulties in licensing or sublicensing intellectual property rights that may be required for other components
of the product, patent infringement lawsuits relating to Paragraph IV certifications as part of any Section 505(b)(2) or ANDA submissions,
any unexpected difficulties in the ability of our suppliers to timely supply quantities for commercial launch of the product, any
unexpected delays or difficulties in assembling and deploying an adequate sales force to market the product, and receipt of adequate
funding to support product development and sales and marketing efforts.
Subject to several factors including the availability of sufficient funding, the success of future clinical
trials, obtaining required regulatory approvals and the absence of unexpected delays, we believe that up to four product candidates,
including Epinephrine PFS, could be ready for launch or launched before the end of 2019, although there can be no assurances that
this will be the case.
Prescription Compounded Medications
Overview.
Our USC subsidiary, which
is registered as a drug compounding outsourcing facility under Section 503B of the FDCA and the DQSA, provides compounded
prescription drugs, including compounded sterile drug preparations and certain non-sterile
drugs, to patients, physician clinics, hospitals, surgery centers and other clients throughout most of the United States.
Compounding is generally a practice in which a licensed pharmacist, a licensed physician, or, in the case
of an outsourcing facility, a person under the supervision of a licensed pharmacist, combines, mixes, or alters ingredients of
a drug to create a medication tailored to the needs of an individual patient whose health needs cannot be met by an FDA-approved
drug. USC’s offerings include, among others, injectable corticosteroids, hormone replacement therapies, hospital
outsourcing formulations, urological preparations, ophthalmic preparations, topical compounds for pain and
men’s and women’s health formulations. USC also provides certain veterinary pharmaceutical products for
animals, although those drugs are not subject to the DQSA.
USC sources raw materials from suppliers registered
with the FDA. Utilizing these raw material components, USC prepares and provides a broad range of customized stock keeping units
to meet the individual requirements of customers located throughout most of the United States.
Prior to the passage of the DQSA in 2017, compounding pharmacies in the United States were subject to varying
regulatory standards and it was not always clear whether the FDA or state boards of pharmacies were primarily responsible for their
oversight. Following a number of deaths and serious adverse reactions that were experienced by patients who had all received spinal
injections of certain drugs compounded at the same compounding pharmacy, the DQSA was enacted to ensure that pharmacies compounding
high risk drugs were held to universally high standards. The DQSA reinstated and amended Section 503A of the FDCA, and added a new section – Section 503B. Section 503A
set out certain federal requirements for compounded drugs, but left primary responsibility for the oversight of state-licensed
pharmacies to the states. Section 503B, however, gives primary jurisdiction to FDA to oversee over outsourcing facilities.
Under Section 503B, a compounder can voluntarily become an “outsourcing facility,” which is defined
as a facility at one geographic location or address that is engaged in the compounding of sterile drugs; has elected to register
as an outsourcing facility; and complies with all of the requirements of Section 503B. Drugs compounded by an outsourcing facility
can qualify for exemptions from the FDA approval requirements and the requirement to label products with adequate directions for
use, but not from current good manufacturing practice (cGMP) requirements. They are subject to FDA inspections on a risk-based
schedule and they must meet certain other conditions, such as reporting adverse events and providing FDA with certain information
about the products they compound. USC registered with the FDA as a Section 503B outsourcing facility in December 2013.
USC markets a portfolio of compounded preparations
for humans and animals, including sterile injectable and non-sterile integrative therapies, in therapeutic areas such as autoimmunity,
chronic infectious diseases, and endocrine and metabolic diseases. USC also offers customizable hormone replacement therapies and
a variety of weight loss and dermatology compounded formulations. Many of these formulations are offered in different formats than
other available alternatives, such as in suspension or topical. Many hospitals and surgery centers look to outsourcing facilities
to obtain medications in RTU format, with the specific packaging, volume, and strength often unique to individual facilities. Many
facilities and practitioners also look to outsourcing facilities when medications are on temporary backorder from the manufacturer
or are discontinued.
Compounding pharmacies and outsourcing facilities
combine different ingredients, some of which may be FDA-approved, to create specialized preparations prescribed by a physician.
Examples of compounded formulations include medications with alternative dosage strengths or unique dosage forms, such as topical
creams or gels, suspensions, or solutions with more tolerable drug delivery vehicles. A physician may also work together with a
pharmacist to repurpose or reformulate FDA-approved drugs via the compounding process to meet a patient’s specific medical
needs. Outsourcing facilities generally specialize in compounding ready-to-use unit dose medications and medications that may be
on commercial backorder from the traditional manufacturers. These compounds are distributed to hospitals, surgery centers, and
practitioners. Examples of compounded medications prepared by outsourcing facilities include sterile syringes used by hospital
and surgery center operating rooms, sterile injectables administered by the practitioner in the office, and unit-dosed sterile
and non-sterile medications.
Since we acquired USC in April 2016, we
have taken several measures intended to support the growth of the business including hiring additional personnel, expanding
sales channels, and strengthening our production processes. In addition, USC has, and after our acquisition of USC we
have, invested capital in efforts to comply with new and anticipated FDA regulations and other requirements applicable
to USC’s business and outsourcing facilities, to expand product offerings, enhance production capabilities,
improve warehouse space, develop new packaging, labeling and processing solutions, refine quality and safety measures, and
develop technology for the intake and management of customer orders. These regulations and requirements have increased and
are expected to continue to increase the costs for current and new products.
Recent Developments
Financial Condition
At December 31, 2016, we had cash and cash equivalents
of approximately $5.1 million, including approximately $1 million in restricted cash, accounts receivable of approximately $0.8
million and liabilities of approximately $12.5 million. As of March 31, 2017, we had approximately $1.4 million of cash and
cash equivalents, including restricted cash. We believe that our existing working capital and the proceeds of this offering should
be sufficient to fund our operations during the 2017 year. This estimate is based on our current planned operations and is subject
to changes in our plans and uncertainties inherent in our business, and we may need to seek to replenish our existing cash and
cash equivalents sooner than we expect.
Except with respect to revenues from the sales of
compounded pharmacy formulations by our USC subsidiary that we acquired in April 2016, since our fiscal 2010 year we have not generated
commercial revenues from marketing or selling any drugs or other products. Except with respect to revenues generated from the sale
of compounded pharmacy formulations, we do not expect to generate product revenue in the foreseeable future. We expect to continue
to incur operating losses as we continue to advance our product candidates through the development and regulatory process, incur
general and administrative costs and make capital or other expenditures relating to our business. We will need to generate significant
revenues to achieve profitability, and might never do so.
In the future, we will be dependent upon revenues
from commercialization of our product candidates, funding from third parties such as proceeds from debt or equity financings, funded
research and development payments or payments under collaborative agreements, in order to maintain our operations and meet our
obligations. There is no guarantee that we will generate significant revenues from the commercialization of our Epinephrine PFS
product or any of our product candidates, or that additional debt equity or other funding will be available to us on acceptable
terms, or at all. If we fail to generate adequate revenues or obtain additional funding when needed, we would be forced to scale
back or terminate our operations, or to seek to merge with or to be acquired by another company.
Company Information
We are incorporated under the laws of the State
of Delaware. Our principal executive offices are located at 11682 El Camino Real, Suite 300, San Diego, CA 92130, and our telephone
number is (858) 997-2400. Our website address is: www.adamispharmaceuticals.com. We have included our website address as a factual
reference and do not intend it to be an active link to our website. The information that can be accessed through our website is
not part of this prospectus, and investors should not rely on any such information in deciding whether to purchase our securities.
The Offering
|
|
|
Common stock offered by us pursuant to this prospectus supplement
|
|
4,285,715
shares of common stock.
|
|
|
|
|
|
Common stock to be outstanding after this offering
|
|
26,920,428
shares.
|
|
|
|
|
|
Over-allotment option
|
|
We have granted the underwriters
a 30-day option to purchase up to 642,857 additional shares of common stock at a price, after the underwriting discount, of
$3.29 per share, from us to cover over-allotments, if any.
|
|
|
|
|
|
Use of proceeds
|
|
We intend to use the net proceeds from the sale of the securities offered by this prospectus for general corporate purposes, which may include, without limitation, expenditures relating to research, development and clinical trials relating our products and product candidates, capital expenditures, hiring additional personnel, acquisitions of new technologies or products, the repayment, refinancing, redemption or repurchase of existing or future indebtedness or capital stock and working capital.
|
|
|
|
|
|
Dividend policy
|
|
We do not anticipate paying any cash dividends on our common stock.
|
|
|
|
|
|
NASDAQ Capital Market symbol
|
|
Our common stock is listed on The NASDAQ Capital Market under the symbol “ADMP.”
|
|
|
|
|
|
Risk factors
|
|
Investing in our securities involves significant risks. See “Risk Factors” beginning on page S-11 of this prospectus supplement and on page 6 of the accompanying prospectus and the documents incorporated by reference herein.
|
Unless we indicate otherwise, all information
in this prospectus, including the number of shares of common stock to be outstanding immediately after this offering as shown above,
is based on 21,991,543 shares of common stock outstanding as of December 31, 2016, and excludes:
|
|
●
|
4,320,409
shares of common stock issuable upon exercise of outstanding stock options under our equity incentive plans as of December 31,
2016, with exercise prices ranging from $2.50 to $11.39 and having a weighted average exercise price of $6.06 per share, and approximately
350,000 shares issuable upon the vesting of restricted stock units outstanding as of December 31, 2016, awarded under our
equity incentive plans;
|
|
|
●
|
294,781
shares of common stock issuable upon the exercise of outstanding warrants, as of December 31, 2016, other than the warrants
described in the bullet point below, at a weighted average exercise price of $8.00 per share;
|
|
|
●
|
625,013
shares of Series A-2 Convertible Preferred Stock outstanding as of December 31, 2016, convertible on a one-for-one basis
into 625,013 shares of common stock and outstanding warrants to purchase up to 1,418,439 and 1,183,432 shares of common stock
or Series A-1 Preferred Stock, and 1,724,137 shares of common stock or Series A-2 Preferred Stock, at an exercise price of $3.40,
$4.10 and $2.90 per share, respectively (subject to certain beneficial ownership limitations), that we issued in our August 2014,
January 2016 and July 2016 private placement transactions;
|
|
|
●
|
warrants
to purchase up to 3,573,255 shares of common stock outstanding as of December 31, 2016, at an exercise price of $2.98 per
share that we issued in our August 2016 financing transaction; and
|
|
|
●
|
1,000,000 shares of common stock issuable upon
the exercise of an outstanding warrant that we issued to Bear State Bank, N.A. as partial collateral to secure our
obligations
under
a
working capital loan to us of $2.0 million.
|
Additionally, unless we indicate otherwise, share
information in this prospectus does not reflect certain issuances, grants and actions that occurred after December 31, 2016
and during the quarter ended March 31, 2017, including (i) the grant of stock options exercisable to purchase up to 2,066,750
shares of common stock pursuant to our 2009 Equity Incentive Plan, (ii) the award of restricted stock units covering 950,000 shares
of common stock under our equity incentive plan, which vest seven years after the date of grant or earlier upon certain events
including a change of control of the company, (iii) issuance of 18,157 shares of common stock upon exercise of previously outstanding
warrants, and (iv) issuance of 625,013 shares of common stock upon the conversion of shares of Series A-2 Convertible Preferred
Stock, which shares of preferred stock are no longer outstanding.
Unless we specifically state otherwise, all
information in this prospectus supplement assumes no exercise by the underwriters of their option to purchase up to
an additional 642,857 shares of common stock to cover over-allotments, if any.
RISK FACTORS
Any investment in our common stock or other securities
involves a high degree of risk. Investors should carefully consider the risks described below, as well as the risks described in
the documents incorporated or deemed to be incorporated by reference herein, and all of the information contained in this prospectus
before deciding whether to purchase the securities offered hereby. Our business, financial condition, results of operations and
prospects could be materially and adversely affected by these risks if any of them actually occur. The risks and uncertainties
described below are not the only ones we face. Additional risks not currently known to us or other factors not perceived by us
to present significant risks to our business at this time also could adversely affect our business, operating results and financial
condition, as well as adversely affect the value of an investment in our securities. This prospectus also contains forward-looking
statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking
statements as a result of certain factors, including the risks we face as described below and elsewhere in this prospectus and
in the documents incorporated or deemed to be incorporated by reference herein.
Risks Related to this Offering
You will experience immediate and substantial dilution as a result
of this offering and may experience additional dilution in the future.
You will incur immediate and substantial
dilution as a result of this offering. After giving effect to the sale by us of up to 4,285,715 shares offered in this
offering a public offering price of $3.50 per share, and after deducting the underwriters’ discounts and commissions
and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $2.99 per
share. In addition, in the past, we issued shares of convertible preferred stock as well as options and warrants to acquire
shares of common stock and preferred stock. To the extent these securities are ultimately exercised or converted, you will
sustain additional future dilution. In addition, exercise of warrants that we issued in our past financing transactions, or
exercise of other outstanding options or warrants, or additional shares of common stock or other securities that we may issue
in the future in connection with additional financing transactions, could result in there being a significant number of
additional shares outstanding and dilution to our stockholders.
Because we will have broad discretion and flexibility in how the
net proceeds from this offering are used, we may use the net proceeds in ways in which you disagree.
We currently intend to use the net proceeds from
this offering for general corporate purposes, which include, without limitation, expenditures relating to research, development
and clinical trials relating our products and product candidates, capital expenditures, hiring additional personnel, acquisitions
of new technologies or products, the repayment, refinancing, redemption or repurchase of existing or future indebtedness or capital
stock and working capital. We may also use the proceeds to acquire or invest in complementary products, services, technologies
or other assets, although we have no agreements or understandings with respect to any acquisitions or investments at this time.
See “Use of Proceeds” on page S-23 of this prospectus supplement. Other than as described in the “Use of Proceeds”
section, we have not allocated specific amounts of the net proceeds from this offering for any of the foregoing purposes. Accordingly,
our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying
on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part
of your investment decision, to assess whether the net proceeds are being used appropriately. It is possible that the net proceeds
will be invested in a way that does not yield a favorable, or any, return for you. The failure of our management to use such funds
effectively could have a material adverse effect on our business, financial condition, operating results and cash flow.
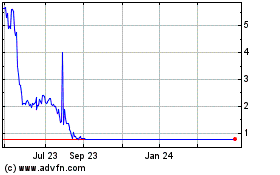
The price of our common stock may be volatile.
The market price of our common stock may
fluctuate substantially. For example, from January 1, 2015 through April 15, 2017, the market price of our common stock has
fluctuated between $2.50 and $10.12. The price of our common stock that will prevail in the market after this offering may be
higher or lower than the price that you have paid, depending on many factors, some of which are beyond our control and may
not be related to our operating performance. Market prices for securities of early-stage pharmaceutical, biotechnology and
other life sciences companies have historically been particularly volatile. Some of the factors that may cause the market
price of our common stock to fluctuate include:
|
|
●
|
relatively
low trading volume, which can result in significant volatility in the market price of our common stock based on a relatively smaller
number of trades and dollar amount of transactions;
|
|
|
●
|
the
timing and results of our current and any future preclinical or clinical trials of our product candidates;
|
|
|
●
|
our
ability to successfully expand sales of our compounded pharmacy formulations;
|
|
|
●
|
the
entry into or termination of key agreements, including, among others, key collaboration and license agreements;
|
|
|
●
|
the
results and timing of regulatory reviews relating to the approval of our product candidates;
|
|
|
●
|
the
initiation of, material developments in, or conclusion of, litigation to enforce or defend any of our intellectual property rights;
|
|
|
●
|
failure
of any of our product candidates, if approved, to achieve commercial success;
|
|
|
●
|
general
and industry-specific economic conditions that may affect our research and development expenditures;
|
|
|
●
|
the
results of clinical trials conducted by others on products that would compete with our product candidates;
|
|
|
●
|
issues
in manufacturing our product candidates or any approved products;
|
|
|
●
|
the
loss of key employees;
|
|
|
●
|
the
introduction of technological innovations or new commercial products by our competitors;
|
|
|
●
|
changes
in estimates or recommendations by securities analysts, if any, who cover our common stock;
|
|
|
●
|
future
sales of our common stock;
|
|
|
●
|
period-to-period
fluctuations in our financial results;
|
|
|
●
|
publicity
or announcements regarding regulatory developments relating to our products;
|
|
|
●
|
period-to-period
fluctuations in our financial results, including our cash and cash equivalents balance, operating expenses, cash burn rate or
revenue levels;
|
|
|
●
|
common
stock sales in the public market by one or more of our larger stockholders, officers or directors;
|
|
|
●
|
our
filing for protection under federal bankruptcy laws;
|
|
|
●
|
a
negative outcome in any litigation or potential legal proceeding; or
|
|
|
●
|
other
potentially negative financial announcements, such as a review of any of our filings by the SEC, changes in accounting treatment
or restatement of previously reported financial results or delays in our filings with the SEC.
|
The stock markets in general have experienced substantial
volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations
may also adversely affect the trading price of our common stock. In the past, following periods of volatility in the market price
of a company’s securities, stockholders have often instituted class action securities litigation against those companies.
Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could
significantly harm our profitability and reputation.
Future sales of substantial amounts of our common stock, or the
possibility that such sales could occur, could adversely affect the market price of our common stock.
Future sales in the public market of our common
stock, including shares offered by the prospectus supplement or shares issued upon exercise of our outstanding stock options, warrants
or convertible securities, or the perception by the market that these issuances or sales could occur, could lower the market price
of our common stock or make it difficult for us to raise additional capital. As of December 31, 2016, we had 21,991,543 shares
of common stock issued and outstanding, substantially all of which we believe may be sold publicly, subject in some cases to volume
and other limitations, provisions or limitations in registration rights agreements, or prospectus-delivery or other requirements
relating to the effectiveness and use of registration statements registering the resale of such shares.
As of December 31, 2016, 4,320,409 shares of common
stock were issuable upon the exercise of outstanding stock options under our equity incentive plans at a weighted-average exercise
price of $6.06 per share, we had outstanding restricted stock units covering 350,000 shares of common stock, and we had outstanding
warrants to purchase shares of common stock as described in the next paragraph below. Subject to applicable vesting requirements,
upon exercise of these options or warrants or issuance of shares following vesting of the restricted stock units, the underlying
shares may be resold into the public market, subject in some cases to volume and other limitations or prospectus-delivery requirements
pursuant to registration statements registering the resale of such shares. In the case of outstanding options or warrants that
have exercise prices that are below the market price of our common stock from time to time, or upon issuance of shares following
vesting of restricted stock units, our stockholders would experience dilution upon the exercise of these options.
Some of our outstanding warrants may result in dilution to our
stockholders.
As of December 31, 2016, we had outstanding warrants,
other than the warrants described in the next sentence, to purchase 294,781 shares of common stock at a weighted average exercise
price of $8.00 per share. As of December 31, 2016, 1,418,439, 1,183,432, and 1,724,137 shares of common stock and/or Series A-1
or Series A-2 Preferred were issuable (subject to certain beneficial ownership limitations) upon exercise of warrants that
we issued in our August 2014, January 2016 and July 2016 private placement transactions at exercise prices of $3.40, $4.10 and
$2.90, per share, respectively. Moreover, as of December 31, 2016, 3,573,255 shares of common stock were issuable at an exercise
price of $2.98 per share upon exercise of warrants issued in our August 2016 financing transaction and 1,000,000 shares of common
stock were issuable upon the exercise of warrants issued to Bear State Bank, N.A. to secure our Adamis Working Capital Line loan
obligation of $2.0 million. In the event of exercise of warrants that have exercise prices that are below the market price of our
common stock from time to time, our stockholders would experience dilution upon the exercise of such warrants.
Our principal stockholders have significant influence over us,
they may have significant influence over actions requiring stockholder approval, and your interests as a stockholder may conflict
with the interests of those persons.
Based on the number of outstanding shares of our
common stock held by our stockholders as of December 31, 2016, our directors, executive officers and their respective affiliates
as of such date owned approximately 6% of our outstanding shares of common stock and our largest stockholder owned approximately
7% of the outstanding shares of common stock as of such date. As a result, those stockholders have the ability to exert a significant
degree of influence with respect to the outcome of matters submitted to our stockholders for approval, including the election of
directors and any merger, consolidation or sale of all or substantially all of our assets. The interests of these persons may not
always coincide with our interests or the interests of our other stockholders. This concentration of ownership could harm the market
price of our common stock by (i) delaying, deferring or preventing a change in corporate control, (ii) impeding a merger,
consolidation, takeover or other business combination involving us, or (iii) discouraging a potential acquirer from making
a tender offer or otherwise attempting to obtain control of us. The significant concentration of stock ownership may adversely
affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Risks Related to our Business
We have incurred losses since our inception, and we anticipate
that we will continue to incur losses. We may never achieve or sustain profitability.
We incurred net losses of approximately $19.4 million
for the year ended year ended December 31, 2016, and a net loss of approximately $13.6 million for the year ended December
31, 2015. From inception through December 31, 2016, we have an accumulated deficit of approximately $88.5 million. These losses
will increase as we continue our research and development activities, seek regulatory approvals for our product candidates and
commercialize any approved products. These losses will cause, among other things, our stockholders’ equity and working capital
to decrease. Any future earnings and cash flow from operations of our business are dependent on our ability to further develop
our products and on revenue and profitability from sales of products.
There can be no assurance that we will be able to
generate sufficient product revenue to become profitable at all or on a sustained basis. We expect to have quarter-to-quarter fluctuations
in revenue and expenses, some of which could be significant, due in part to variations in expenses and activities relating to research,
development, clinical trial, marketing and manufacturing. If our product candidates fail in clinical trials or do not gain regulatory
approval, or if our products do not achieve market acceptance, we may never become profitable. As we commercialize and market products,
we will need to incur expenses for product marketing and brand awareness and conduct significant research, development, testing
and regulatory compliance activities that, together with general and administrative expenses, could result in substantial operating
losses for the foreseeable future. Even if we do achieve profitability, we may not be able to sustain or increase profitability
on a quarterly or annual basis.
We may never commercialize any of our product candidates that
are subject to regulatory approval or earn a profit.
We have not received regulatory approval for any
drugs or products. Since our fiscal 2010 year, except for revenues from sales of compounded pharmacy formulations after our acquisition
of USC in 2016, we have not generated commercial revenue from marketing or selling any drugs or other products. We expect to incur
substantial net losses for the foreseeable future. We may never be able to commercialize any of our product candidates that are
subject to regulatory approval or be able to generate revenue from sales of such products. Because of the risks and uncertainties
associated with developing and commercializing our specialty pharmaceuticals and other product candidates, we are unable to predict
when we may commercially introduce such products, the extent of any future losses or when we will become profitable, if ever.
There is no assurance that the FDA will grant marketing approval
for our Epinephrine PFS product candidate.
On December 16, 2016, we resubmitted to the FDA our NDA relating to our Epinephrine PFS product candidate
for the emergency treatment of anaphylaxis. The FDA accepted the resubmitted NDA for review and, pursuant to the Prescription
Drug User Fee Act, provided us with an agency goal action date of six months from date of receipt, for the agency’s response
to the resubmission. However, there are no assurances that the FDA will grant marketing approval for our Epinephrine PFS
product by such date, or at all. The FDA’s review processes can extend beyond, and in some cases significantly beyond,
anticipated completion dates due to the timing of the FDA’s review process, issuance by the FDA of a CRL, FDA requests for
additional data, information, materials or clarification, difficulties scheduling an advisory committee meeting, FDA workload issues,
extensions resulting from the submission of additional information or clarification regarding information already in the submission
within the last three months of the target PDUFA date, or other reasons. Failure of the FDA to grant marketing approval for
our Epinephrine PFS product candidate could have a material adverse effect on our business, financial conditions, results of operations
and the market price of our common stock.
Our auditors have expressed substantial doubt about our ability
to continue as a going concern, which may hinder our ability to obtain further financing.
Our audited financial statements for the year ended
December 31, 2016, were prepared under the assumption that we would continue our operations as a going concern. Our independent
registered public accounting firm has included a “going concern” explanatory paragraph in its report on our financial
statements for the year ended December 31, 2016, indicating that we have incurred recurring losses from continuing operations and
is dependent on additional financing to fund operations, and that these factors raise substantial doubt about our ability to continue
as a going concern. Uncertainty concerning our ability to continue as a going concern may hinder our ability to obtain future financing.
Continued operations and our ability to continue as a going concern are dependent on our ability to obtain additional funding in
the near future and thereafter, and there are no assurances that such funding will be available at all or will be available in
sufficient amounts or on reasonable terms. Our financial statements do not include any adjustments that may result from the outcome
of this uncertainty. Without additional funds from debt or equity financings, sales of assets, sales or out-licenses of intellectual
property or technologies, or other transactions or sources, we will exhaust our resources and will be unable to continue operations.
If we cannot continue as a viable entity, our stockholders would likely lose most or all of their investment in us.
Even if they are approved and commercialized, if our potential
products are unable to compete effectively with current and future products targeting similar markets as our potential products,
our commercial opportunities will be reduced or eliminated.
The markets for our PFS Epinephrine product candidate, our proposed DPI inhaler products and other allergy
and respiratory product candidates, are intensely competitive and characterized by rapid technological progress. We face competition
from numerous sources, including major biotechnology and pharmaceutical companies worldwide. Many of our competitors have substantially
greater financial and technical resources, and development, production and marketing capabilities, than we do. Our Epinephrine
PFS product candidate, if approved for marketing, will compete with a number of other currently marketed epinephrine products for
use in the emergency treatment of acute allergic reactions, including anaphylaxis. Certain companies have established technologies
that may be competitive with our product candidates and any future products that we may develop or acquire. Some of these products
may use different approaches or means to obtain results, which could be more effective or less expensive than our products for
similar indications. In addition, many of these companies have more experience than we do in pre-clinical testing, performance
of clinical trials, manufacturing, and obtaining FDA and foreign regulatory approvals. They may also have more brand name exposure
and expertise in sales and marketing. We also compete with academic institutions, governmental agencies and private organizations
that are conducting research in the same fields.
Competition among these entities to recruit and
retain highly qualified scientific, technical and professional personnel and consultants is also intense. As a result, there is
a risk that one or more of our competitors will develop a more effective product for the same indications for which we are developing
a product or, alternatively, bring a similar product to market before we can do so. Failure to successfully compete will adversely
impact the ability to raise additional capital and ultimately achieve profitable operations.
We have incurred significant indebtedness, which will require
substantial cash to service and which subjects us to certain financial requirements and business restrictions.
As
we have previously disclosed in our SEC filings, in connection with our acquisition of USC, we assumed approximately $5,722,000
principal amount of debt obligations under several loan agreements and related loan and security agreements and documents relating
to working capital, equipment and real property loan obligations of USC, referred to collectively as the Loan Documents, and we
agreed to become an additional co-borrower under the Loan Documents. The lender in all of the USC Loan Documents was First Federal
Bank and/or its successor Bear State Bank, referred to as Lender or the Bank. In November 2016, we entered into amendments of
these loan agreements with the Bank. Pursuant to the amended Loan Documents, we are required to make current periodic interest
and principal payments in an amount of approximately $55,000 per month, and the amount of required interest payments is subject
to change depending on future changes in interest rates. We also previously entered into a loan and security agreement with the
Lender, referred to as the Adamis Working Capital Line, pursuant to which we may borrow, and have borrowed, up to an aggregate
of $2,000,000 to provide working capital to USC, subject to the terms and conditions of those loan documents. Interest on amounts
borrowed under the Adamis Working Capital Line accrues at a rate equal to the prime interest rate, as defined in the agreement.
Interest payments are required to be made quarterly. As amended, the entire outstanding principal balance, and all accrued and
unpaid interest and all other sums payable under the Adamis Working Capital Line loan documents, are due and payable on March 1,
2018, or sooner upon the occurrence of certain events as provided in the loan agreement and related documents. Our obligations
under the Adamis Working Capital Line are secured by certain collateral, including without limitation our interest in amounts
that we have loaned to USC; a warrant that we issued to the Lender to purchase up to 1,000,000 shares of common stock at an exercise
price equal to par value per share, only exercisable by Lender if we are in default under the loan documents and if the Lender
delivers a notice to us and we do not cure the default within the applicable cure period; and our Certificate of Deposit, or the
CD, with the Lender of approximately $1,000,000. Further, if at any time before the repayment of the loan, the value of the sum
of (i) the amount of the funds in the CD, plus (ii) the product of: (A) the number of unexercised shares under the warrant multiplied
by (B) the value of our common stock, falls below the product of (Y) 1.5 multiplied by (Z) the outstanding principal balance of
the note evidencing the Adamis Working Capital Line, then following delivery of a notice from the Bank to us, we will either:
(1) amend the warrant or provide an additional warrant to provide Lender with rights to purchase additional shares of common stock;
or (2) reduce the principal balance of the note to bring us in compliance with the requirements set forth above, and failure to
comply with this requirement after notice from Lender is an event of default under the loan documents. The amended Loan Documents
include a variety of representations, warranties and covenants that we are required to comply with. If we do not comply with the
provisions of such agreements and documents and the Bank declares an event of default, the Bank would be entitled to accelerate
the maturity date of the loans, the principal and accrued interest would become due and payable, and the Bank could elect to exercise
its remedies as a secured creditor under the loan documents and applicable law.
Our
ability to make scheduled payments on our indebtedness depends on our future performance and ability to raise additional capital
if required, which is subject to economic, financial, competitive and other factors, some of which are beyond our control. If
we are unable to generate sufficient cash to service our debt, we may be required to adopt one or more alternatives, such as selling
assets, attempting to restructure our debt or obtaining additional capital through sales of equity or incurrence of additional
debt on terms that may be onerous or highly dilutive to our stockholders. Our ability to engage in any of these activities would
depend on the capital markets and our financial condition at such time, and we may not be able to do so when needed, on desirable
terms or at all, which could result in a default on our debt obligations. Additionally, the Loan Documents contain various restrictive
covenants, including, among others, our obligation to deliver to the Bank certain financial and other information, our obligation
to comply with certain notice and insurance requirements, and our inability, without the Bank’s prior consent, to dispose
of certain of our assets, incur certain additional indebtedness, enter into certain merger, acquisition or change of control transactions,
pay certain dividends or distributions on or make certain repurchases of our capital stock or incur any lien or other encumbrance
on our assets, subject to certain permitted exceptions. Any failure by us to comply with any of these covenants, subject to certain
cure periods, or to make all payments under the debt instruments when due, would cause us to be in default under the applicable
debt instrument. In the event of any such default, the Bank may be able to foreclose on the assets that secure the debt or declare
all borrowed funds, together with accrued and unpaid interest, immediately due and payable, thereby potentially causing all of
our available cash to be used to pay our indebtedness or forcing us into bankruptcy or liquidation if we do not then have sufficient
cash available. Any such event or occurrence could severely and negatively impact our business, financial conditions or results
of operations.
USC’s business and results of operations have been adversely affected by certain regulatory matters.
Problematic FDA inspections, warning letters or other negative communications from the FDA or state regulatory authorities, and
federal or state proceedings alleging non-compliance with FDA requirements or other applicable federal or state requirements, could
adversely affect our business, financial condition and results of operations.
USC’s business and results of
operations have been adversely affected by certain regulatory matters relating to its compounded drugs. Compounding
pharmacies have historically been subject to FDA inspections on an irregular basis, if at all, and are now as
outsourcing facilities subject to FDA inspections on a risk-based schedule in accordance with DQSA Section 503B(b)(4).
Observations by the FDA of potentially violative conditions during inspections will be reported to facility management
at the close of the inspection on FDA Form 483. If the violative practices are not corrected to the satisfaction of the
FDA, the Form 483 may be followed by a Warning Letter or other enforcement actions as the FDA deems warranted. In March
2014 and August 2015, USC received Form 483s following FDA inspections of its outsourcing facility, each noting a number of
observations relating to USC’s facility and practices.
Following
the receipt of the August 2015 Form 483, USC suspended production of sterile drugs and voluntarily recalled all lots of sterile
drugs compounded and packaged by USC that remained within expiry, due to the FDA’s concern over a lack of sterility assurance.
This was a voluntary recall and voluntary suspension of sterile production, and USC determined there was no evidence that any drugs
were defective. The recall did not pertain to any non-sterile drugs prepared by USC. USC responded to the August 2015 Form
483by implementing a number of corrective actions, including reviewing and enhancing quality control and production systems
.
In
December 2015, the FDA stated that it did not object to USC’s resumption of production and distribution of sterile drugs.
In March 2016, USC received another letter from the FDA indicating that the voluntary action was designated a class II recall.
Class II means that the probability of serious adverse health consequences is remote. USC
resumed production and sale
of compounded sterile drugs in March - April 2016.
Following
the suspension of sterile production and the voluntary recall, state pharmacy regulatory agencies in certain states also initiated
inquiries or took other actions regarding sales of USC products in such states, and some of those proceedings are ongoing. Resolution
of these proceedings, or any future proceedings by the FDA or state regulatory agencies alleging violation of applicable federal
or state laws or regulations, could require significant time and financial resources, and an adverse outcome in one or more of
these proceedings could adversely affect our business, results of operations and financial condition.
The suspension of sterile production, recall, remediation efforts, and resumption of sterile production
adversely affected USC’s relationships with certain of its customers, who following the suspension of sterile compounding
were required to purchase needed drugs from other pharmacies or outsourcing facilities, and its relationships with certain third
parties who previously assisted in distributing USC’s drugs, had a material adverse effect on USC’s revenues, income,
and financial condition for 2015 and 2016, and may continue to adversely affect revenues and income from sale of drugs by USC
in future periods.
We are subject to FDA inspection on an ongoing basis and cannot predict whether a future inspection will result
in the issuance of another FDA Form 483, warning letter or other enforcement-related communications from the FDA or state regulatory
authorities. We could be subject to additional regulatory action by the FDA and civil or criminal enforcement action by the Department
of Justice under the FDCA, Federal False Claims Act, or other applicable statutes, as well as related private actions, as a result
of previous, current or future FDA observations. USC’s suppliers and customers may negatively consider any enforcement actions
when deciding to award contracts or continue or renew agreements. Any such actions could significantly disrupt USC’s business
and harm its and our reputation, resulting in a material adverse effect on our business, results of operations and financial condition.
USC’s compounded preparations and the pharmacy compounding
industry are subject to regulatory and customer scrutiny, which may impair our growth and sales.
Compounded drugs are not FDA-approved. As a 503B outsourcing facility, USC’s compounded formulations
are not subject to the FDA drug approval process. This means that FDA does not verify the safety, or effectiveness of compounded
drugs. Consumers and health professionals rely on the drug approval process to ensure that drugs are safe and effective and made
in accordance with Federal quality standards. Compounded drugs also lack an FDA finding of manufacturing quality before such drugs
are marketed. Drugs available through branded and generic drug companies have been approved for marketing and sale by the FDA and
are subject to many more requirements than drugs compounded in outsourcing facilities. In addition, some compounding pharmacies
have been the subject of widespread negative media coverage in recent years. As a result, some physicians may be hesitant
to prescribe, and some patients or potential customers may be hesitant to purchase and use, compounded drugs. Other reasons
physicians may be unwilling to prescribe or patients may be unwilling to use USC’s compounded formulations could include
the following, among others: applicable law limits our ability to discuss the efficacy or safety of USC’s formulations
with potential users to the extent applicable data is available; and our compounded preparations are primarily sold on a cash-pay
basis and reimbursement may or may not be available from third-party payors, including the private payors and government programs
such as Medicare and Medicaid programs. Failure by physicians, patients, other potential customers, or third-party payors,
to accept compounded drugs could substantially limit USC’s market and cause its and our business and operations to suffer.
Formulations prepared and dispensed by compounding
pharmacies contain FDA-approved ingredients, but are not themselves approved by the FDA. As a 503B outsourcing facility, USC’s
compounded formulations are not subject to the FDA approval process. The drug products available through branded and generic drug
companies have been approved for marketing and sale by the FDA and are required to be manufactured in facilities compliant with
cGMP standards. In addition, certain compounding pharmacies have been the subject of widespread negative media coverage in recent
years, and the actions of these pharmacies have resulted in increased scrutiny of compounding pharmacy activities from the FDA
and state governmental agencies. For example, the FDA has in the past requested that a number of compounding pharmacies conduct
a recall of all non-expired, purportedly sterile drug products and cease sterile compounding operations due to lack of sterility
assurance, and additional compounding pharmacies have suspended sterile production or voluntarily recalled certain sterile compounding
products after an FDA inspection of the relevant facilities. As a result, some physicians may be hesitant to prescribe, and some
patients or potential customers may be hesitant to purchase and use, these compounded formulations. Other reasons physicians may
be unwilling to prescribe or patients may be unwilling to use USC’s compounded formulations could include the following,
among others: applicable law limits our ability to discuss the efficacy or safety of USC’s formulations with potential users
to the extent applicable data is available; and our compounded preparations are primarily sold on a cash-pay basis and reimbursement
may or may not be available from third-party payors, including the government Medicare and Medicaid programs. Failure by physicians,
patients, other potential customers, or third-party payors, to accept compounded formulations could substantially limit USC’s
market and cause its and our business and operations to suffer. An incident similar to the fungal meningitis outbreak in 2012,
which was caused by a compounding pharmacy, could cause USC’s customers to reduce their use of outsourced compounded medications
significantly or even stop using outsourced compounded medications altogether. States have in the past enacted, and could in the
future enact, regulations prohibiting or restricting the use of outsourcing compounded medication service providers in response
to such incidents. Such prohibitions or restrictions on outsourced compounded preparations by states, or reduced customer demand
as a result of an incident with compounded medication providers, could have a material adverse effect on USC’s and our business,
results of operations and financial condition.
We expect increased competition in the future regarding USC’s
compounded pharmacy products. If we fail to respond to such competition successfully, USC’s and our business, results of
operations and financial condition could be materially and adversely affected.
The pharmaceutical and pharmacy industries are highly
competitive. We compete against other registered outsourcing facilities, branded drug companies, generic drug companies, regional
compounders that provide patient-specific compounding that decide to expand to 503B outsourcing, large hospitals and integrated
delivery networks, other compounding pharmacies, and new entrants to the industry.
Many competitors that market and sell compounded drugs have longer operating histories and may have greater
financial, marketing and other resources than we do. We are significantly smaller than some of such competitors, and we may
lack the financial and other resources needed to develop, produce, distribute, market and commercialize any of USC’s formulations
or compete for market share in these sectors. These potential competitors could leverage existing resources and experience
operating in industries that are subject to significant regulatory oversight in order to overcome certain barriers to entry. Consequently,
competitors may be able to develop products and services competitive with, or superior to, USC’s products and services. Furthermore,
we may not be able to differentiate USC’s compounded drugs and services from those of our competitors, successfully develop
or introduce new services—on a timely basis or at all—that are less costly than those of our competitors or offer customers
payment and other commercial terms as favorable as those offered by our competitors. We expect competition to intensify as
technology advances, such as those in the field of robotics and automation, and consolidation continues. Also, new developments
by pharmaceutical manufacturers, such as increasing the number of abbreviated new drug applications, to cover less frequently used
drug formulations, could render some or most of USC’s products or services obsolete. In addition, the drug products
available through branded and generic drug companies with which USC’s formulations compete have been approved for marketing
and sale by the FDA and are required to be manufactured in facilities compliant with cGMP standards. Although USC is required
to compound its drugs in compliance with cGMP, its drugs are not required to be, and have not been, approved for marketing and
sale by the FDA. As a result, some physicians may be unwilling to prescribe, and some patients may be unwilling to use, USC’s
drugs. Increased competition could reduce revenue and gross profit and otherwise materially adversely affect our business,
results of operations and financial condition.
Biotechnology and related pharmaceutical technologies have undergone and continue to be subject to rapid and
significant change. Our future success will depend in large part on our ability to maintain a competitive position with respect
to these technologies. Products developed by our competitors, including FDA-approved drugs and compounded drugs created by
other pharmacies and outsourcing facilities, could render USC’s products and technologies obsolete or unable to compete effectively.
Other competitive factors include the safety and efficacy of a product, the size of the market for a product, the timing of market
entry relative to competitive products, the availability of alternative compounded formulations or approved drugs, the price of
a product relative to alternative products, the availability of third-party reimbursement, the success of sales and marketing efforts,
brand recognition and the availability of scientific and technical information about a product.
If we fail to comply with the rules under the Sarbanes-Oxley Act
of 2002 related to disclosure controls and procedures, or, if we discover material weaknesses and other deficiencies in our internal
controls over financial reporting, our stock price could decline and raising capital could be more difficult. Prior to its acquisition
by us, USC was a private company and has not previously been subject to the Sarbanes-Oxley Act Of 2002, the rules and regulations
of the SEC or other corporate governance requirements.
Our management is responsible for establishing and
maintaining adequate internal control over our financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. In the
future, our management may determine that our disclosure controls and procedures are ineffective or that there are one or more
material weaknesses in our internal controls over financial reporting, resulting in a reasonable possibility that a material misstatement
to the annual or interim financial statements would not have been prevented or detected. A material weakness is a deficiency, or
a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a
material misstatement of our financial statements will not be prevented or detected on a timely basis. Accordingly, a material
weakness increases the risk that the financial information we report contains material errors. Any system of internal controls,
however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances
that the objectives of the system are met.
In addition, as a smaller reporting company,
our independent registered public accounting firm has never performed an evaluation of our internal control over financial reporting
in accordance with the provisions of the Sarbanes-Oxley Act because no such evaluation has been required. Had our independent registered
public accounting firm performed an evaluation of our internal control over financial reporting in accordance with the provisions
of the Sarbanes-Oxley Act, significant deficiencies or material weaknesses may have been identified.
Efforts to correct
any material weaknesses or deficiencies that may be identified could require significant financial resources to address. Moreover,
if remedial measures are insufficient to address the deficiencies that are determined to exist, we may fail to meet our future
reporting obligations on a timely basis, our consolidated financial statements could contain material misstatements, we could be
required to restate our prior period financial results, our operating results may be harmed, and we could become subject to class
action litigation. Internal control deficiencies and ineffective disclosure controls and procedures could also cause investors
to lose confidence in our reported financial information. We can give no assurance that any material weaknesses or restatements
of financial results will not arise in the future due to a failure to implement and maintain adequate internal control over financial
reporting or adequate disclosure controls and procedures or circumvention of these controls. In addition, controls and procedures
may not be adequate to prevent or identify irregularities or errors or to facilitate the fair presentation of our consolidated
financial statements. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could
be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could
decline. Failure to comply with reporting requirements could also subject us to sanctions and/or investigations by the SEC, the
NASDAQ Stock Market or other regulatory authorities.
As disclosed elsewhere in this prospectus, in April
2016 we acquired USC. Prior to its acquisition by us, USC was a private company and has not been subject to the Sarbanes-Oxley
Act of 2002, the rules and regulations of the SEC, or other corporate governance requirements to which public reporting companies
may be subject. As a result, we are required to implement the appropriate internal control processes and procedures over USC’s
financial accounting and reporting. We may incur significant legal, accounting and other expenses in efforts to meet these requirements.
Implementing the controls and procedures at USC that are required to comply with the various applicable laws and regulations may
place a significant burden on our management and internal resources. The diversion of management’s attention and any difficulties
encountered in such an implementation could adversely affect our business, financial condition and operating results.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus supplement contains forward-looking statements. Such statements may include, without limitation, statements relating
to: our expectations concerning regulatory approvals for our products; our expectations for growth; estimates of future revenue;
our strategies and objectives; our sources and uses of cash; our liquidity needs; our ability to obtain sufficient funding to support
our planned activities; our current or planned clinical trials or research and development activities; product development timelines;
our future products; regulatory matters; anticipated dates for commencement of clinical trials; anticipated completion dates of
clinical trials; anticipated dates for meetings with regulatory authorities and submissions to obtain required regulatory marketing
approvals’ anticipated dates for commercial introduction of products; expense, profits, cash flow balance sheet and guidance
on future periods, such statements are not historical facts, but are based on our current expectations, estimates and beliefs
about our business and industry and other statements concerning our future operations
and activities. These forward-looking statements are based on our current expectations and projections about future events, and
they are subject to risks and uncertainties, known and unknown, that could cause actual results and developments to differ materially
from those expressed or implied in such statements.
In some cases, you can identify forward-looking
statements by terminology, such as “expects,” “anticipates,” “intends,” “estimates,”
“plans,” “believes,” “seeks,” “may,” “should”, “could”
or the negative of such terms or other similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties
that could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified
in their entirety by reference to the factors discussed throughout this prospectus supplement.
You should read this prospectus supplement and the
accompanying prospectus and the documents that we reference herein and therein and have filed as exhibits to the registration statement
of which this prospectus supplement is part, completely and with the understanding that our actual future results may be materially
different from what we expect. You should assume that the information appearing in this prospectus supplement and the accompanying
prospectus is accurate as of the date on the front cover of this prospectus supplement only. Because the risk factors referred
to elsewhere in the prospectus supplement could cause actual results or outcomes to differ materially from those expressed in any
forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements.
In addition, many forward-looking statements concerning our anticipated future business activities assume that we are able to obtain
sufficient funding in the near term and thereafter to support such activities and continue our operations and planned activities,
which may not be the case. Further, any forward-looking statement speaks only as of the date on which it is made, and except as
may be required by applicable law, we undertake no obligation to update any forward-looking statement to reflect events or circumstances
after the date on which the statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time
to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor
on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from
those contained in any forward-looking statements. We qualify all of the information presented in this prospectus supplement and
the accompanying prospectus, and particularly our forward-looking statements, by these cautionary statements.
USE OF PROCEEDS
We estimate that the net proceeds from this offering,
after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, will be approximately
$13,855,000 (or approximately $15,970,000 if the underwriters’ over-allotment option is exercised in full).
We currently intend to use the net proceeds from
this offering for general corporate purposes, which may include, without limitation, expenditures relating to research, development
and clinical trials relating our products and product candidates, capital expenditures, hiring additional personnel, acquisitions
of new technologies or products, the repayment, refinancing, redemption or repurchase of existing or future indebtedness or capital
stock and working capital. We may also use the proceeds to acquire or invest in complementary products, services, technologies
or other assets, although we have no agreements or understandings with respect to any acquisitions or investments at this time.
We have not determined the amounts we plan to spend
on any of the areas listed above or the timing of these expenditures. As a result, our management will have broad discretion to
allocate the net proceeds from this offering. Pending application of the net proceeds as described above, we expect to invest the
net proceeds in short-term, interest-bearing, investment-grade securities pursuant to our investment policy.
DILUTION
If you invest in our shares of common stock which
are a part of this offering, your investment will be diluted immediately to the extent of the difference between the public offering
price per share of common stock you purchase in this offering, and the net tangible book value per share of common stock immediately
after this offering.
Net tangible book value represents the amount of
our total tangible assets reduced by our total liabilities and preferred stock. Tangible assets equal our total assets less goodwill
and intangible assets. Net tangible book value per share represents our net tangible book value divided by the number of shares
of common stock outstanding. As of December 2016, our net tangible book value was $(496,798) and our net tangible book value per
share was $(0.023).
After giving effect to the sale of the shares
in this offering at the public offering price of $3.50 per share, and after deducting the estimated underwriting discounts and
commissions and estimated offering expenses payable by us, our as adjusted net tangible book value would have been
approximately $13,358,201 or approximately $0.51 per share of common stock, as of December 31, 2016. This represents an
immediate increase in net tangible book value of approximately $0.53 per share to existing stockholders and an immediate
dilution of approximately $2.99 per share to investors in this offering. The following table illustrates this calculation on
a per share basis.
|
Public
offering price per share
|
|
$
|
3.50
|
|
|
Net
tangible book value per share as of December 31, 2016
|
|
$
|
(0.023
|
)
|
|
Increase
in net tangible book value per share attributable to new investors
|
|
$
|
0.53
|
|
|
Adjusted
net tangible book value per share after giving effect to this offering
|
|
$
|
0.51
|
|
|
Dilution
in net tangible book value per share to new investors
|
|
$
|
2.99
|
|
If the underwriters exercise in full their
option to purchase 642,857 additional shares of our common stock at a public offering price of $3.50 per share, after
deducting estimated offering expenses payable by us, our as adjusted net tangible book value as of December 31, 2016 would
have been approximately $15.5 million or approximately $0.57 per share of common stock. This represents an immediate
increase in net tangible book value per share of approximately $0.60 per share to existing shareholders, and an immediate
dilution of approximately $2.93 per share to investors participating in this offering.
The calculation of net tangible book value as of
December 31, 2016 in the table above is based on 21,991,543 shares of common stock outstanding, and excludes the following:
|
|
●
|
4,320,409 shares of common stock issuable upon exercise of outstanding stock
options under our equity incentive plans with exercise prices ranging from $2.50 to $11.39 and having a weighted average exercise
price of $6.06 per share, and approximately 350,000 shares issuable upon the vesting of outstanding restricted stock units awarded
under our equity incentive plans;
|
|
|
●
|
294,781 shares of common stock issuable upon the exercise of outstanding warrants,
outstanding as of December 31, 2016, other than the warrants described in the bullet point below, at a weighted average exercise
price of $8.00 per share;
|
|
|
●
|
625,013 shares of Series A-2 Convertible Preferred Stock, outstanding as of
December 31, 2016, convertible on a one-for-one basis into 625,013 shares of common stock and outstanding warrants to purchase
up to 1,418,439 and 1,183,432 shares of common stock or Series A-1 Preferred Stock, and 1,724,137 shares of common stock or Series
A-2 Preferred Stock, at an exercise price of $3.40, $4.10 and $2.90 per share, respectively (subject to certain beneficial ownership
limitations), that we issued in our August 2014, January 2016 and July 2016 private placement transactions;
|
|
|
●
|
warrants to purchase up to 3,573,255 shares of common stock, outstanding as
of December 31, 2016, at an exercise price of $2.98 per share that we issued in our August 2016 financing transaction; and
|
|
|
●
|
1,000,000 shares of common stock issuable upon
the exercise of an outstanding warrant that we issued to Bear State Bank, N.A., as as partial collateral to secure our
obligations
under the
Adamis Working Capital Line of $2.0 million.
|
Additionally, the calculation in the above table
excludes and does not reflect certain issuances, grants and actions that occurred after December 31, 2016 and during the quarter
ended March 31, 2017, including (i) the grant of stock options exercisable to purchase up to 2,066,750 shares of common stock pursuant
to our 2009 Equity Incentive Plan, (ii) the award of restricted stock units covering 950,000 shares of common stock under our 2009
Equity Incentive Plan, which vest seven years after the date of grant or earlier upon certain events including a change of control
of the company, (iii) issuance of 18,157 shares of common stock upon exercise of previously outstanding warrants, and (iv) issuance
of 625,013 shares of common stock upon the conversion of shares of Series A-2 Convertible Preferred Stock, which shares of
preferred stock are no longer outstanding.
CAPITALIZATION
The following table sets forth our consolidated
cash and cash equivalents and capitalization as of December 31, 2016. Such information is set forth on the following basis:
|
|
●
|
on an as adjusted basis, giving effect to the sale of the securities in this
offering at a public offering price of $3.50 per share, after deducting estimated underwriting discounts and commissions and estimated
offering expenses.
|
You should read this table together with the section
of this prospectus supplement entitled “Use of Proceeds” and with the financial statements and related notes and the
other information that we incorporated by reference into this prospectus supplement and the accompanying prospectus, including
our Annual Report on Form 10-K and Quarterly Reports on Form 10-Q that we file from time to time with the SEC.
|
|
|
As of December 31, 2016
|
|
|
|
|
Actual
|
|
As Adjusted
|
|
|
|
|
|
(in thousands,
except per share amounts)
|
|
|
|
Cash
and cash equivalents
(1)
|
|
|
$
|
5,096
|
|
|
|
$
|
18,951
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
indebtedness
|
|
|
$
|
12,507
|
|
|
|
|
12,507
|
|
|
|
Stockholders’
equity:
|
|
|
|
|
|
|
|
|
|
|
|
|
Preferred
Stock, par value $0.0001 per share; 10,000,000 shares authorized; 0 and 1,009,021 issued and outstanding at December 31, 2016
and December 31, 2015, respectively; Series A-2 Convertible, 625,013 and 0 issued and outstanding at December 31, 2016, respectively.
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
Common Stock, par value
$0.0001 per share; 100,000,000 shares authorized; 22,299,083 issued, 21,991,543 outstanding at December 31, 2016;26,277,258
shares outstanding as adjusted
|
|
|
|
2
|
|
|
|
|
3
|
|
|
|
Additional
paid-in capital
|
|
|
$
|
113,742
|
|
|
|
|
127,596
|
|
|
|
Accumulated
Deficit
|
|
|
|
(88,459
|
)
|
|
|
|
(
88,459
|
)
|
|
|
Treasury
Stock, 307,540 Shares, at cost
|
|
|
|
(5
|
)
|
|
|
|
(5
|
)
|
|
|
Total
stockholders’ equity
|
|
|
$
|
25,280
|
|
|
|
|
39,135
|
|
|
|
Total
capitalization
|
|
|
$
|
25,280
|
|
|
|
|
39,135
|
|
|
|
|
(1)
|
Approximately
$1.0 million of which was restricted cash, held by Bear State Bank, N.A. as collateral to the $2.0 million Adamis Working Capital
Line.
|
|
|
|
|
The calculation in the table above excludes the
following as of December 31, 2016:
|
|
●
|
4,320,409 shares of common stock issuable upon exercise of outstanding stock
options under our equity incentive plans as of December 31, 2016, with exercise prices ranging from $2.50 to $11.39 and having
a weighted average exercise price of $6.06 per share, and approximately 350,000 shares issuable upon the vesting of outstanding
restricted stock units awarded under our equity incentive plans;
|
|
|
●
|
294,781 shares of common stock issuable upon the exercise of outstanding warrants,
as of December 31, 2016, other than the warrants described in the bullet point below, at a weighted average exercise price of $8.00
per share;
|
|
|
●
|
625,013 shares of Series A-2 Convertible Preferred Stock outstanding as of
December 31, 2016, convertible on a one-for-one basis into 625,013 shares of common stock and outstanding warrants to purchase
up to 1,418,439 and 1,183,432 shares of common stock or Series A-1 Preferred Stock, and 1,724,137 shares of common stock or Series
A-2 Preferred Stock, at an exercise price of $3.40, $4.10 and $2.90 per share, respectively (subject to certain beneficial ownership
limitations), that we issued in our August 2014, January 2016 and July 2016 private placement transactions;
|
|
|
●
|
warrants to purchase up to 3,573,255 shares of common stock outstanding as
of December 31, 2016, at an exercise price of $2.98 per share that we issued in our August 2016 financing transaction; and
|
|
|
●
|
1,000,000 shares of common stock issuable upon the exercise of an
outstanding warrant that we issued to Bear State Bank, N.A. as partial collateral to secure our obligations under the
Adamis
Working
Capital
Line of $2.0 million.
|
Additionally, the calculation in the above table
excludes and does not reflect certain issuances, grants and actions that occurred after December 31, 2016 and during the quarter
ended March 31, 2017, including (i) the grant of stock options exercisable to purchase up to 2,066,750 shares of common stock pursuant
to our 2009 Equity Incentive Plan, (ii) the award of restricted stock units covering 950,000 shares of common stock under our 2009
Equity Incentive Plan, which vest seven years after the date of grant or earlier upon certain events including a change of control
of the company, (iii) issuance of 18,157 shares of common stock upon exercise of previously outstanding warrants, and (iv) issuance
of 625,013 shares of common stock upon the conversion of shares of Series A-2 Convertible Preferred Stock, which shares of
preferred stock are no longer outstanding.
MARKET FOR OUR COMMON STOCK
Our common stock, $0.0001 par value, is listed on
the NASDAQ Capital Market under the symbol “ADMP.” The following table sets forth the range of high and low sales prices
for the common stock as reported for the periods indicated below.
|
|
|
High
|
|
Low
|
|
Fiscal 2015
|
|
|
|
|
|
|
|
|
|
|
|
First
Quarter
(January 2015 to March 2015)
|
|
|
$
|
7.07
|
|
|
|
$
|
3.83
|
|
|
Second
Quarter
(April 2015 to June 2015)
|
|
|
$
|
4.99
|
|
|
|
$
|
3.77
|
|
|
Third
Quarter
(July 2015 to September 2015)
|
|
|
$
|
4.63
|
|
|
|
$
|
3.25
|
|
|
Fourth Quarter
(
October
2015 to December 2015
)
|
|
|
$
|
5.56
|
|
|
|
$
|
3.76
|
|
|
Fiscal 2016
|
|
|
|
|
|
|
|
|
|
|
|
First
Quarter
(January 2016 to March 2016)
|
|
|
$
|
6.63
|
|
|
|
$
|
4.02
|
|
|
Second
Quarter
(April 2016 to June 2016)
|
|
|
$
|
10.12
|
|
|
|
$
|
2.62
|
|
|
Third
Quarter
(July 2016 to September 2016)
|
|
|
$
|
3.47
|
|
|
|
$
|
2.57
|
|
|
Fourth
Quarter
(October 2016 to December 2016)
|
|
|
$
|
3.35
|
|
|
|
$
|
2.50
|
|
|
Fiscal 2017
|
|
|
|
|
|
|
|
|
|
|
|
First
Quarter
(January 2017 to March 2017)
|
|
|
$
|
4.80
|
|
|
|
$
|
2.93
|
|
|
Second
Quarter
(
to
April 19, 2017)
|
|
|
$
|
4.70
|
|
|
|
$
|
4.30
|
|
As of April 19, 2017, we had approximately
92 common stock holders of record. The number of record holders was determined from the records of our transfer agent and does
not include beneficial owners of our common stock whose shares are held in the names of various security brokers, dealers, and
registered clearing agencies.
Dividend Policy
We have not previously declared or paid any dividends
on our common stock. The payment of dividends on our common stock in the future will depend on our profitability at the time, cash
available for those dividends, and such other factors as our board of directors may consider appropriate. We do not anticipate
paying dividends on our common stock in the foreseeable future.
UNDERWRITING
We entered into an underwriting agreement with the
underwriters named below. Raymond James & Associates, Inc., or Raymond James, is acting as the representative of the underwriters.
The underwriting agreement provides for the purchase of a specific number of shares of common stock by each of the underwriters.
The underwriters’ obligations are several, which means that each underwriter is required to purchase a specified number of
shares, but is not responsible for the commitment of any other underwriter to purchase shares. Subject to the terms and conditions
of the underwriting agreement, each underwriter has severally agreed to purchase the number of shares set forth opposite its name
below:
|
Underwriter
|
|
Number
of
Shares
|
|
Raymond
James & Associates, Inc.
|
|
|
3,214,285
|
|
|
Maxim
Group LLC
|
|
|
1,071,430
|
|
|
|
|
|
|
|
|
Total
|
|
|
4,285,715
|
|
The underwriters have agreed to purchase all of
the shares offered by this prospectus (other than those covered by the over-allotment option described below) if any are purchased.
The shares of common stock offered hereby should
be ready for delivery on or about April 26, 2017 against payment in immediately available funds.
The underwriters are offering the shares subject
to various conditions and may reject all or part of any order. The representative of the underwriters has advised us that the underwriters
propose to offer the shares directly to the public at the public offering price that appears on the cover page of this prospectus
supplement. In addition, the representative may offer some of the shares to other securities dealers at such price less a concession
of $0.126 per share. After the shares are released for sale to the public, the representative may change the offering price and other
selling terms at various times.
We have granted the underwriters an
over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus supplement,
permits the underwriters to purchase a maximum of 642,857 additional shares of common stock at a price of $3.50 per share
from us to cover over-allotments, if any. If the underwriters exercise all or part of this option, they will purchase shares
covered by the option at the public offering price that appears on the cover page of this prospectus supplement, less the
underwriting discounts and commissions. If this option is exercised in full, the total price to us before expenses will be
approximately $16,215,000, and the total net proceeds to us will be approximately $15,970,000. The underwriters have
severally agreed that, to the extent the over-allotment option is exercised, they will each purchase a number of additional
shares proportionate to the underwriter’s initial amount reflected in the foregoing table.
The following table provides information regarding
the amount of the discounts and commissions to be paid to the underwriters by us, before expenses:
|
|
|
Per Share
|
|
Total
Without Exercise of Over-Allotment Option
|
|
Total
With Full Exercise of Over-Allotment Option
|
|
Public
offering price
|
|
|
3.50
|
|
|
|
15,000,002
|
|
|
|
17,250,002
|
|
|
Underwriting
discounts and commissions
|
|
|
0.21
|
|
|
|
900,000
|
|
|
|
1,035,000
|
|
|
Proceeds,
before expenses, to us
|
|
|
$3.29
|
|
|
|
14,100,002
|
|
|
|
16,215,002
|
|
We estimate that our total expenses of the offering,
excluding the estimated underwriting discounts and commissions, will be approximately $245,000, which includes up to $100,000 that
we have agreed to reimburse the underwriters for the fees and expenses incurred by them in connection with the offering.
We have agreed to indemnify the underwriters against
certain liabilities, including liabilities under the Securities Act of 1933, and liabilities arising from breaches of representations
and warranties contained in the underwriting agreement, or to contribute to payments that the underwriters may be required to make
in respect of those liabilities.
We, and our officers and directors, have agreed
to a 90-day “lock-up” with respect to shares of our common stock and other of our securities that they beneficially
own, including securities that are convertible into shares of common stock and securities that are exchangeable or exercisable
for shares of common stock, subject to certain exceptions. This means that, subject to certain exceptions, for a period of 90 days
following the date of this prospectus supplement, we and such persons may not offer, sell, pledge or otherwise dispose of these
securities without the prior written consent of Raymond James. The terms of the lock-up agreements may be waived by the representative
of the underwriters at its discretion, although the representative has no present intention to waive or shorten the lock-up period.
Rules of the Securities and Exchange Commission
may limit the ability of the underwriters to bid for or purchase shares before the distribution of the shares is completed. However,
the underwriters may engage in the following activities in accordance with the rules:
|
|
●
|
Stabilizing transactions — The representative may make bids or purchases
for the purpose of pegging, fixing or maintaining the price of the shares, so long as stabilizing bids do not exceed a specified
maximum.
|
|
|
●
|
Over-allotments and syndicate covering transactions — The underwriters
may sell more shares of our common stock in connection with this offering than the number of shares than they have committed to
purchase. This over-allotment creates a short position for the underwriters. This short sales position may involve either “covered”
short sales or “naked” short sales. Covered short sales are short sales made in an amount not greater than the underwriters'
over-allotment option to purchase additional shares in this offering described above. The underwriters may close out any covered
short position either by exercising its over-allotment option or by purchasing shares in the open market. To determine how they
will close the covered short position, the underwriters will consider, among other things, the price of shares available for purchase
in the open market, as compared to the price at which they may purchase shares through the over-allotment option. Naked short sales
are short sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing
shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that, in the open
market after pricing, there may be downward pressure on the price of the shares that could adversely affect investors who purchase
shares in this offering.
|
|
|
●
|
Penalty bids — If the representative purchases shares in the open market
in a stabilizing transaction or syndicate covering transaction, it may reclaim a selling concession from the underwriters and selling
group members who sold those shares as part of this offering.
|
|
|
●
|
Passive market making — Market makers in the shares who are underwriters
or prospective underwriters may make bids for or purchases of shares, subject to limitations, until the time, if ever, at which
a stabilizing bid is made.
|
Similar to other purchase transactions, the underwriters’
purchases to cover the syndicate short sales or to stabilize the market price of our common stock may have the effect of raising
or maintaining the market price of our common stock or preventing or mitigating a decline in the market price of our common stock.
As a result, the price of the shares of our common stock may be higher than the price that might otherwise exist in the open market.
The imposition of a penalty bid might also have an effect on the price of the shares if it discourages resales of the shares.
Neither we nor the underwriters make any representation
or prediction as to the effect that the transactions described above may have on the price of the shares. These transactions may
occur on The NASDAQ Capital Market or otherwise. If such transactions are commenced, they may be discontinued without notice at
any time.
Electronic Delivery of Preliminary Prospectus: A
prospectus supplement in electronic format may be delivered to potential investors by one or more of the underwriters participating
in this offering. The prospectus supplement in electronic format will be identical to the paper version of such preliminary prospectus
supplement. Other than the prospectus supplement in electronic format, the information on any underwriter's website and any information
contained in any other website maintained by an underwriter is not part of this prospectus supplement, the accompanying prospectus
or the registration statement of which this prospectus supplement and the accompanying prospectus form a part.
Other Activities and Relationships: The underwriters
and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities
trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment,
hedging, financing and brokerage activities. The underwriters and certain of their affiliates have, from time to time, performed,
and may in the future perform, various commercial and investment banking and financial advisory services for us, for which they
received or will receive customary fees and expenses.
Notice to Non-U.S. Investors
Belgium
The offering is exclusively conducted under applicable
private placement exemptions and therefore it has not been and will not be notified to, and this document or any other offering
material relating to the shares has not been and will not be approved by, the Belgian Banking, Finance and Insurance Commission
(“Commission bancaire, financière et des assurances/Commissie voor het Bank, Financie en Assurantiewezen”).
Any representation to the contrary is unlawful.
Each underwriter has undertaken not to offer sell,
resell, transfer or deliver directly or indirectly, any units, or to take any steps relating/ancillary thereto, and not to distribute
or publish this document or any other material relating to the units or to the offering in a manner which would be construed as:
(a) a public offering under the Belgian Royal Decree of 7 July 1999 on the public character of financial transactions; or (b) an
offering of securities to the public under Directive 2003/71/EC which triggers an obligation to publish a prospectus in Belgium.
Any action contrary to these restrictions will cause the recipient and the Company to be in violation of the Belgian securities
laws.
France
Neither this prospectus supplement nor any other
offering material relating to the shares has been submitted to the clearance procedures of the Autorité des marchés
financiers in France. The shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the
public in France. Neither this prospectus supplement nor any other offering material relating to the shares has been or will be:
(a) released, issued, distributed or caused to be released, issued or distributed to the public in France; or (b) used in connection
with any offer for subscription or sale of the shares to the public in France. Such offers, sales and distributions will be made
in France only: (i) to qualified investors (investisseurs qualifiés) and/or to a restricted circle of investors (cercle
restreint d’investisseurs), in each case investing for their own account, all as defined in and in accordance with Articles
L.411-2, D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the French Code monétaire et financier; (ii) to
investment services providers authorised to engage in portfolio management on behalf of third parties; or (iii) in a transaction
that, in accordance with article L.411-2-II-1°-or-2°-or 3° of the French Code monétaire et financier and article
211-2 of the General Regulations (Règlement Général) of the Autorité des marchés financiers,
does not constitute a public offer (appel public à l’épargne). Such shares may be resold only in compliance
with Articles L.411-1, L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French Code monétaire et financier.
United Kingdom/Germany/Norway/The Netherlands
In relation to each Member State of the European
Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public
of any shares which are the subject of the offering contemplated by this prospectus supplement may not be made in that Relevant
Member State other than the offers contemplated in this prospectus supplement in name(s) of Member State(s) where prospectus will
be approved or passported for the purposes of a non-exempt offer once this prospectus supplement has been approved by the competent
authority in such Member State and published and passported in accordance with the Prospectus Directive as implemented in name(s)
of relevant Member State(s) except that an offer to the public in that Relevant Member State of any shares may be made at any time
under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a) to
legal entities which are authorised or regulated to operate in the financial markets or, if not so authorised or regulated, whose
corporate purpose is solely to invest in securities;
(b) to
any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total
balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last
annual or consolidated accounts;
(c) by
the representative to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive);
or
(d) in
any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares shall result
in a requirement for the publication by the Company or any underwriter of a prospectus pursuant to Article 3 of the Prospectus
Directive.
For the purposes of this provision, the expression
an “offer to the public” in relation to any shares in any Relevant Member State means the communication in any form
and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to
decide to purchase any shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive
in that Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant
implementing measure in each Relevant Member State.
Each underwriter has represented, warranted and
agreed that:
(a) it
has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement
to engage in investment activity (within the meaning of section 21 of the Financial Services and Markets Act 2000 (the FSMA)) received
by it in connection with the issue or sale of any shares in circumstances in which section 21(1) of the FSMA does not apply to
the Company; and
(b) it
has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to
the shares in, from or otherwise involving the United Kingdom.
Israel
In the State of Israel, the shares offered hereby
may not be offered to any person or entity other than the following:
(a) a fund
for joint investments in trust (i.e., mutual fund), as such term is defined in the Law for Joint Investments in Trust, 5754-1994,
or a management company of such a fund;
(b) a provident
fund as defined in Section 47(a)(2) of the Income Tax Ordinance of the State of Israel, or a management company of such a fund;
(c) an
insurer, as defined in the Law for Oversight of Insurance Transactions, 5741-1981, (d) a banking entity or satellite entity, as
such terms are defined in the Banking Law (Licensing), 5741-1981, other than a joint services company, acting for their own account
or for the account of investors of the type listed in Section 15A(b) of the Securities Law 1968;
(d) a company
that is licensed as a portfolio manager, as such term is defined in Section 8(b) of the Law for the Regulation of Investment Advisors
and Portfolio Managers, 5755-1995, acting on its own account or for the account of investors of the type listed in Section 15A(b)
of the Securities Law 1968;
(e) a company
that is licensed as an investment advisor, as such term is defined in Section 7(c) of the Law for the Regulation of Investment
Advisors and Portfolio Managers, 5755-1995, acting on its own account;
(f) a company
that is a member of the Tel Aviv Stock Exchange, acting on its own account or for the account of investors of the type listed in
Section 15A(b) of the Securities Law 1968;
(g) an
underwriter fulfilling the conditions of Section 56(c) of the Securities Law, 5728-1968;
(h) a venture
capital fund (defined as an entity primarily involved in investments in companies which, at the time of investment, (i) are primarily
engaged in research and development or manufacture of new technological products or processes and (ii) involve above-average risk);
(i) an
entity primarily engaged in capital markets activities in which all of the equity owners meet one or more of the above criteria;
and
(j) an
entity, other than an entity formed for the purpose of purchasing shares in this offering, in which the shareholders equity (including
pursuant to foreign accounting rules, international accounting regulations and U.S. generally accepted accounting rules, as defined
in the Securities Law Regulations (Preparation of Annual Financial Statements), 1993) is in excess of NIS 50 million.
Any offeree of the shares offered hereby in the
State of Israel shall be required to submit written confirmation that it falls within the scope of one of the above criteria. This
prospectus supplement will not be distributed or directed to investors in the State of Israel who do not fall within one of the
above criteria.
Italy
The offering of the shares offered hereby in Italy
has not been registered with the Commissione Nazionale per la Società e la Borsa (“CONSOB”) pursuant to Italian
securities legislation and, accordingly, the shares offered hereby cannot be offered, sold or delivered in the Republic of Italy
(“Italy”) nor may any copy of this prospectus supplement or any other document relating to the shares offered hereby
be distributed in Italy other than to professional investors (operatori qualificati) as defined in Article 31, second paragraph,
of CONSOB Regulation No. 11522 of 1 July, 1998 as subsequently amended. Any offer, sale or delivery of the shares offered hereby
or distribution of copies of this prospectus supplement or any other document relating to the shares offered hereby in Italy must
be made:
(a) by
an investment firm, bank or intermediary permitted to conduct such activities in Italy in accordance with Legislative Decree No.
58 of 24 February 1998 and Legislative Decree No. 385 of 1 September 1993 (the “Banking Act”);
(b) in
compliance with Article 129 of the Banking Act and the implementing guidelines of the Bank of Italy; and
(c) in
compliance with any other applicable laws and regulations and other possible requirements or limitations which may be imposed by
Italian authorities.
Sweden
This prospectus supplement has not been nor will
it be registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this prospectus
supplement may not be made available, nor may the shares offered hereunder be marketed and offered for sale in Sweden, other than
under circumstances which are deemed not to require a prospectus under the Financial Instruments Trading Act (1991: 980).
Switzerland
The shares offered pursuant to this prospectus supplement
will not be offered, directly or indirectly, to the public in Switzerland and this prospectus supplement does not constitute a
public offering prospectus as that term is understood pursuant to art. 652a or art. 1156 of the Swiss Federal Code of Obligations.
The company has not applied for a listing of the shares being offered pursuant to this prospectus supplement on the SWX Swiss Exchange
or on any other regulated securities market, and consequently, the information presented in this prospectus supplement does not
necessarily comply with the information standards set out in the relevant listing rules. The shares being offered pursuant to this
prospectus supplement have not been registered with the Swiss Federal Banking Commission as foreign investment funds, and the investor
protection afforded to acquirers of investment fund certificates does not extend to acquirers of shares.
Investors are advised to contact their legal, financial
or tax advisers to obtain an independent assessment of the financial and tax consequences of an investment in shares.
Canada
Notice to Canadian Residents
This document constitutes an “exempt offering
document” as defined in and for the purposes of applicable Canadian securities laws. No prospectus has been filed with any
securities commission or similar regulatory authority in Canada in connection with the offer and sale of the securities described
herein (the “Securities”). No securities commission or similar regulatory authority in Canada has reviewed or in any
way passed upon this document or on the merits of the Securities and any representation to the contrary is an offence.
Canadian
investors are advised that this document has been prepared in reliance on section 3A.3 of National Instrument 33-105
Underwriting
Conflicts
(“NI 33-105”). Pursuant to section 3A.3 of NI 33-105, this document is exempt from the requirement to
provide investors with certain conflicts of interest disclosure pertaining to “connected issuer” and/or “related
issuer” relationships as would otherwise be required pursuant to subsection 2.1(1) of NI 33-105.
Resale Restrictions
The offer and sale of the Securities in Canada is
being made on a private placement basis only and is exempt from the requirement to prepare and file a prospectus under applicable
Canadian securities laws. Any resale of Securities acquired by a Canadian investor in this offering must be made in accordance
with applicable Canadian securities laws, which may vary depending on the relevant jurisdiction, and which may require resales
to be made in accordance with Canadian prospectus requirements, a statutory exemption from the prospectus requirements, in a transaction
exempt from the prospectus requirements or otherwise under a discretionary exemption from the prospectus requirements granted by
the applicable local Canadian securities regulatory authority. These resale restrictions may under certain circumstances apply
to resales of the Securities outside of Canada.
Representations of Purchasers
Each Canadian investor who purchases the Securities
will be deemed to have represented to the issuer and to each dealer from whom a purchase confirmation is received, as applicable,
that the investor (i) is purchasing as principal, or is deemed to be purchasing as principal in accordance with applicable Canadian
securities laws, for investment only and not with a view to resale or redistribution; (ii) is an “accredited investor”
as such term is defined in section 1.1 of National Instrument 45-106
Prospectus Exemptions
(“NI 45-106”) or,
in Ontario, as such term is defined in section 73.3(1) of the
Securities Act
(Ontario); and (iii) is a “permitted
client” as such term is defined in section 1.1 of National Instrument 31-103
Registration Requirements, Exemptions and
Ongoing Registrant Obligations
.
Taxation and Eligibility for Investment
Any discussion of taxation and related matters contained
in this document does not purport to be a comprehensive description of all of the tax considerations that may be relevant to a
Canadian investor when deciding to purchase the Securities and, in particular, does not address any Canadian tax considerations.
No representation or warranty is hereby made as to the tax consequences to a resident, or deemed resident, of Canada of an investment
in the Securities or with respect to the eligibility of the Securities for investment by such investor under relevant Canadian
federal and provincial legislation and regulations.
Rights of Action for Damages or Rescission
Securities legislation in certain of the Canadian
jurisdictions provides certain purchasers of securities pursuant to an offering memorandum, including where the distribution involves
an “eligible foreign security” as such term is defined in Ontario Securities Commission Rule 45-501
Ontario Prospectus
and Registration Exemptions
and in Multilateral Instrument 45-107
Listing Representation and Statutory Rights of Action
Disclosure Exemptions
, as applicable, with a remedy for damages or rescission, or both, in addition to any other rights they
may have at law, where the offering memorandum, or other offering document that constitutes an offering memorandum, and any amendment
thereto, contains a “misrepresentation” as defined under applicable Canadian securities laws. These remedies, or notice
with respect to these remedies, must be exercised or delivered, as the case may be, by the purchaser within the time limits prescribed
under, and are subject to limitations and defences under, applicable Canadian securities legislation. In addition, these remedies
are in addition to and without derogation from any other right or remedy available at law to the investor.
Language of Documents
Upon receipt of this document, each Canadian investor
hereby confirms that it has expressly requested that all documents evidencing or relating in any way to the sale of the Securities
described herein (including for greater certainty any purchase confirmation or any notice) be drawn up in the English language
only.
Par la réception de ce document, chaque investisseur canadien confirme par les présentes qu’il a expressément
exigé que tous les documents faisant foi ou se rapportant de quelque manière que ce soit à la vente des valeurs
mobilières décrites aux présentes (incluant, pour plus de certitude, toute confirmation d’achat ou tout
avis) soient rédigés en anglais seulement.
LEGAL MATTERS
Certain legal matters in connection with the securities
offered hereby will be passed upon for us by Weintraub Tobin Chediak Coleman Grodin Law Corporation, Sacramento, California. Mintz,
Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York, is acting as counsel for the underwriters in connection with
this offering.
EXPERTS
The financial statements as of December 31, 2016
and 2015 and for the two years in the period ended December 31, 2016, incorporated by reference in this prospectus have been
so included in reliance on the report of Mayer Hoffman McCann P.C., an independent registered public accounting firm, given on
the authority of said firm as experts in auditing and accounting. The financial statements as of December 31, 2015 and 2014 and
for the two years in the period ended December 31, 2015, of U.S. Compounding, Inc., incorporated by reference in this prospectus
have been so included in reliance on the report of Hudson Cisne & Co. LLP, an independent public accounting firm, given on
the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy
statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s
website at
www.sec.gov.
Copies of certain information filed by us with the SEC are also available on our website at
www.adamispharmaceuticals.com
.
Our website is not a part of this prospectus and is not incorporated by reference in this prospectus. You may also read and copy
any document we file at the SEC’s Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC
at 1-800-SEC-0330 for further information on the operation of the Public Reference Room.
This prospectus is part of a registration statement
we filed with the SEC. This prospectus supplement and the accompany prospectus omit some information contained in the registration
statement in accordance with SEC rules and regulations. You should review the information and exhibits in the registration statement
for further information on us and our consolidated subsidiaries and the securities we are offering. Statements in this prospectus
supplement and the accompany prospectus concerning any document we filed as an exhibit to the registration statement or that we
otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review
the complete document to evaluate these statements. You can obtain a copy of the registration statement from the SEC at the address
listed above or from the SEC’s Internet site.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference”
the information we file with it, which means that we can disclose important information to you by referring you to those documents.
The information we incorporate by reference is an important part of this prospectus, and certain information that we will later
file with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below
(File No. 001-36242), as well as any future filings made with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange
Act from the date of the initial registration statement and prior to the effectiveness of this registration statement, and any
filings made after the date of this prospectus until we sell all of the securities under this prospectus, except that we do not
incorporate any document or portion of a document that was furnished and deemed by the rules of the SEC not to have been filed:
|
|
●
|
Annual Report on Form 10-K for the year ended December 31, 2016, filed on
March 30, 2017;
|
|
|
●
|
Current Reports on Form 8-K filed on January 11, 2017, February 13,
2017, and April 20, 2017; and
|
|
|
●
|
The description of our common stock contained in our Form 8-A filed on December
11, 2013.
|
You may request, and we will provide you with, a
copy of these filings, at no cost, by calling us at (858) 997-2400 or by writing to us at the following address:
Adamis Pharmaceuticals Corporation
11682 El Camino Real, Suite 300
San Diego, CA 92130
Attention: Corporate Secretary
PROSPECTUS

ADAMIS PHARMACEUTICALS CORPORATION
$50,000,000.00
Common Stock
Preferred Stock
Warrants
Units
We may offer and sell, from time to time in one
or more offerings, any combination of common stock, preferred stock, warrants, or units having an aggregate initial offering price
not exceeding $50,000,000. The preferred stock, warrants, and units may be convertible into or exercisable or exchangeable for
common stock, preferred stock or other securities of ours.
Each time we sell a particular class or series of
securities, we will provide specific terms of the securities offered in a supplement to this prospectus. We may also authorize
one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement may also
add, update or change information in this prospectus. You should read this prospectus and any prospectus supplement, as well as
the documents incorporated by reference or deemed to be incorporated by reference into this prospectus, carefully before you invest
in any securities.
This prospectus may not be used to offer or sell
our securities unless accompanied by a prospectus supplement relating to the offered securities.
Our common stock is presently listed on The NASDAQ
Capital Market under the symbol “ADMP.” On July 1, 2014, the last reported sale price of our common stock was $4.75.
The applicable prospectus supplement will contain information, where applicable, as to any other listing, if any, of the securities
covered by the applicable prospectus supplement.
These securities may be sold directly by us, through
dealers or agents designated from time to time, to or through underwriters or dealers or through a combination of these methods
on a continuous or delayed basis. See “Plan of Distribution” in this prospectus. We may also describe the plan of distribution
for any particular offering of our securities in a prospectus supplement. If any underwriters or agents are involved in the sale
of any securities in respect of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements
with them in a prospectus supplement. The net proceeds we expect to receive from any such sale will also be included in a prospectus
supplement.
Investing in our securities involves various
risks. See “Risk Factors” contained herein for more information on these risks. Additional risks will be described
in the related prospectus supplements under the heading “Risk Factors.” You should review that section of the related
prospectus supplements for a discussion of matters that investors in our securities should consider.
Neither the Securities and Exchange Commission
nor any state securities commission has approved or disapproved of these securities, or passed upon the adequacy or accuracy of
this prospectus or any accompanying prospectus supplement. Any representation to the contrary is a criminal offense.
The date of this prospectus is July 2, 2014.
TABLE OF CONTENTS
|
|
Page
|
|
|
|
|
PROSPECTUS
|
|
|
|
|
|
ABOUT THIS PROSPECTUS
|
1
|
|
ABOUT THE COMPANY
|
2
|
|
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
|
5
|
|
RISK FACTORS
|
6
|
|
USE OF PROCEEDS
|
23
|
|
THE SECURITIES WE MAY OFFER
|
23
|
|
DESCRIPTION OF CAPITAL STOCK
|
24
|
|
DESCRIPTION OF WARRANTS
|
28
|
|
DESCRIPTION OF UNITS
|
31
|
|
PLAN OF DISTRIBUTION
|
31
|
|
LEGAL MATTERS
|
34
|
|
EXPERTS
|
35
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
35
|
|
INCORPORATION OF DOCUMENTS BY REFERENCE
|
35
|
ABOUT THIS PROSPECTUS
This prospectus is part of a shelf registration
statement that we filed with the Securities and Exchange Commission (the “SEC”) using a “shelf” registration
process. Under this shelf registration process, we may sell any combination of the securities described in this prospectus in one
or more offerings from time to time having an aggregate initial offering price of $50,000,000. This prospectus provides you with
a general description of the securities we may offer. Each time we offer securities, we will provide you with a prospectus supplement
that describes the specific amounts, prices and terms of the securities we offer. We may also authorize one or more free writing
prospectuses to be provided to you that may contain material information relating to these offerings and securities. We may also
add, update or change in the prospectus supplement any of the information contained in this prospectus or in the documents that
we have incorporated by reference into this prospectus, including without limitation, a discussion of any risk factors or other
special considerations that apply to these offerings or securities or the specific plan of distribution. If there is any inconsistency
between the information in this prospectus and a prospectus supplement or information incorporated by reference having a later
date, you should rely on the information in that prospectus supplement or incorporated information having a later date. You should
read carefully both this prospectus and any prospectus supplement together with additional information described below under the
caption “Where You Can Find More Information.”
This prospectus does not contain all the information
provided in the registration statement we filed with the SEC. You should read both this prospectus, including the section titled
“Risk Factors,” and the accompanying prospectus supplement, together with the additional information described under
the heading “Where You Can Find More Information.”
You should rely only on the information contained
or incorporated by reference in this prospectus or a prospectus supplement. We have not authorized any other person to provide
you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. No
dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus,
any applicable prospectus supplement or any related free writing prospectus. This prospectus is not an offer to sell securities,
and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale is not permitted. You should assume
that the information appearing in this prospectus, any prospectus supplement or any related free writing prospectus, as well as
information we have previously filed with the SEC and incorporated by reference, is accurate as of the date on the front of those
documents only, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any related free
writing prospectus, or any sale of a security. Our business, financial condition, results of operations and prospects may have
changed since those dates.
Unless otherwise indicated, information in this
prospectus reflects a 1-for-17 reverse split of our outstanding common stock, effective at the close of business on December 12,
2013.
This prospectus contains summaries of certain provisions
contained in some of the documents described herein, but reference is made to the actual documents for complete information. All
of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have
been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus
is a part, and you may obtain copies of those documents as described below under “Where You Can Find More Information.”
THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF SECURITIES, UNLESS IT IS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
Unless otherwise stated or the context requires
otherwise, references in this prospectus to “Adamis,” the “company,” or the “Company,” “we,”
“us,” or “our” refer to Adamis Pharmaceuticals Corporation and our subsidiaries, taken together.
ABOUT THE COMPANY
Company Overview
We are an emerging pharmaceutical company focused
on combining specialty pharmaceuticals and biotechnology to provide innovative medicines for patients and physicians. We are currently
primarily focused on our specialty pharmaceutical products. We are currently developing four products in the allergy and respiratory
markets, including a dry powder inhaler technology that we recently acquired from 3M Company. Our goal is to create low cost therapeutic
alternatives to existing treatments. Consistent across all specialty pharmaceuticals product lines, we intend to pursue Section
505(b)(2) New Drug Application, or NDA, regulatory approval filings with the U.S. Food and Drug Administration, or FDA, whenever
applicable in order to reduce the time needed to get to market and to save on costs, compared to Section 505(b)(1) NDA filings
for new drug products. We also have a number of biotechnology product candidates and technologies, including therapeutic vaccine
and cancer product candidates and technologies for patients with unmet medical needs in the global cancer market. To achieve our
goals and support our overall strategy, we will need to raise a substantial amount of funding and make substantial investments
in equipment, new product development and working capital.
The current status of our development programs is
as follows:
Product Portfolio
|
Specialty
Pharmaceutical Products
|
|
Target
Indication
|
|
Development
Status
|
|
Epinephrine PFS
|
|
Anaphylaxis
|
|
Submitted NDA
|
|
APC-5000 DPI
|
|
Asthma/COPD
|
|
Phase 3 trial
(1)(2)
|
|
APC-1000
|
|
Asthma/COPD
|
|
Phase 3 trial
(1)(2)
|
|
APC-3000
|
|
Allergic Rhinitis
|
|
Phase 3 trial
(1)(2)
|
|
|
|
|
|
|
|
Biotechnology
Products
|
|
Target
Indication
|
|
Development
Status
|
|
TeloB-VAX (vaccine)
|
|
Prostate Cancer
|
|
Phase 2 trial
(1)
|
|
APC-100
|
|
Prostate Cancer
|
|
Phase 1 trial
(3)
|
|
APC-200
|
|
Prostate Cancer
|
|
Preclinical
|
|
APC-300
|
|
Prostate Cancer
|
|
Preclinical
|
|
|
(1)
|
Represents the next development or
regulatory stage that we intend to pursue, assuming that we have the financial resources
to pursue any of these opportunities. Even assuming the successful completion of one
or more offerings pursuant to the registration statement of which this prospectus is
a part, we may not have the financial resources to pursue these opportunities.
|
|
|
(2)
|
A single Phase 3 trial, without previous
Phase 1 or Phase 2 trials, is anticipated.
|
|
|
(3)
|
Phase 1/2a clinical trial has commenced.
|
We have not received regulatory approval for any
drugs or products. Since our fiscal 2010 year, we have not generated commercial revenues from marketing or selling any drugs or
other products.
Anaphylaxis; Epinephrine Pre-Filled Syringe
Our most advanced product candidate, the Epinephrine
Injection USP 1:1000 0.3mg Pre-filled Single Dose Syringe, or the Epinephrine PFS, is a simple syringe designed to deliver a premeasured
0.3 mg dose of epinephrine for the treatment of anaphylaxis. The American Academy of Allergy Asthma and Immunology, or AAAAI, defines
anaphylaxis as a serious life-threatening allergic reaction. The most common anaphylactic reactions are to foods, insect stings,
medications and latex. According to information published by AAAAI, up to 8% of U.S. children under the age of 18 have a food allergy,
and approximately 38% of those with a food allergy have a history of severe reactions. Anaphylaxis requires immediate medical treatment,
including an injection of epinephrine. We estimate that sales of prescription epinephrine products in 2013 were at least $900 million,
based on industry data. We cannot provide any assurances concerning any possible future rates of annual growth or whether annual
prescriptions will decline or grow. We believe that there is an opportunity for a simple, low-cost, intuitive pre-filled syringe
to compete in this market. With the help of our contract manufacturer, on May 28, 2014, we submitted an NDA to the FDA pursuant
to Section 505(b)(2) of the Food, Drug & Cosmetic Act, as amended, or FDCA, for approval for sale of our Epinephrine PFS
product. Assuming no unexpected regulatory delays, we hope to receive an approval by the end of the first calendar quarter of 2015.
Under goals established in connection with the Prescription Drug User Fee Act, or PDUFA, the FDA’s guidance for the review
and acting on standard NDA submissions that do not relate to new molecular entities, which we believe will be the case with our
Epinephrine PFS product, is ten months from the date of receipt of the submission. However, the FDA’s review processes can
extend beyond, and in some cases significantly beyond, anticipated completion dates due to FDA requests for additional information
or clarification, difficulties scheduling an advisory committee meeting, FDA workload issues or other reasons. As a result, the
dates of regulatory approval, if obtained, and commercial introduction of our product could be delayed beyond our expectations.
Asthma and COPD
According to the National Institute of Health, or
NIH, asthma is a chronic lung disease that inflames and narrows the airways. Asthma causes recurring periods of wheezing, chest
tightness, shortness of breath, and coughing. Asthma affects people of all ages, but it most often starts during childhood. According
to information published by AAAAI, the number of people in the U.S. with asthma is approximately 25 million and growing. COPD,
or chronic obstructive pulmonary disease, is a progressive disease that makes it difficult to breathe. COPD can cause coughing
that produces large amounts of mucus, wheezing, shortness of breath, chest tightness, and other symptoms. According to the NIH,
cigarette smoking is the leading cause of COPD. However, long-term exposure to other lung irritants such as air pollution, chemical
fumes, or dust may also contribute to COPD.
APC-5000 DPI. On
December 27, 2013, we acquired
assets relating to 3M’s patented Taper dry powder inhaler, or DPI, technology under development by 3M for the treatment of
asthma and COPD. The Taper DPI technology was under development by 3M as a device designed to efficiently deliver dry powder by
utilizing a 3M proprietary microstructured carrier tape. We intend to utilize the Taper DPI assets initially to develop a pre-metered
inhaler device, referred to as APC-5000 DPI, for the treatment of asthma and COPD to deliver the same active ingredients as GlaxoSmithKline’s
Advair Diskus
®
. The Advair Diskus
®
is a dry powder inhaler, or DPI, product that combines fluticasone propionate, or fluticasone
and salmeterol xinafoate, or salmeterol. Fluticasone belongs to the family of medicines known as corticosteroids or steroids. We
believe that, once developed, the device can be utilized to deliver a variety of different drug compounds.
Upon completion of product development and clinical
trials and if required regulatory approvals are obtained, we intend to commercially market the APC-5000 product to compete for
a share of the Advair Diskus market with a branded generic version utilizing the acquired technology. Pursuant to our agreement
with 3M, the microstructured carrier tape will be supplied by 3M under a separate supply agreement to be negotiated with 3M. We
are currently preparing an investigational new drug application, or IND, to be submitted to the FDA for approval to begin human
testing of APC-5000 DPI. Assuming receipt of sufficient funding and if clinical trials are initiated and successfully completed,
we intend to pursue an NDA under Section 505(b)(2) of the FDCA to seek approval for sale in the U.S. market. We also intend to
seek to identify opportunities to market APC-5000 DPI based products outside of the U.S. Pursuant to our August 1, 2013, agreement
with 3M, we made an initial payment of $3.0 million to 3M and acquired an exclusive license to the assets, and on December 27,
2013, we made a final payment to 3M of $7.0 million and the Taper DPI assets were transferred to us.
Additional Allergy Products; APC-1000 and APC-3000.
Additional product candidates in our allergy and respiratory product pipeline include a steroid hydrofluoroalkane, or HFA,
metered dose inhaler product, referred to as APC-1000, for asthma and COPD and an HFA pressurized metered dose nasal steroid for
the treatment of seasonal and perennial allergic rhinitis, referred to as APC-3000. Inhaled nasal steroid, or INS, products are
sold under prescription for seasonal allergic rhinitis. Our product candidates, if developed and approved for marketing, will target
a small niche within the larger market for INS products.
Cancer
We believe that there is a significant need for
new products and therapies for the treatment of prostate cancer and other forms of cancer.
TeloB-VAX.
In April 2011, we acquired exclusive
rights to patented telomerase-based cancer vaccine technology from the Regents of the University of California and the Dana-Farber/Harvard
Cancer Center. We intend to pursue development of the technology initially for what we believe may be a novel cell-based vaccine
product candidate for cancer, tentatively named TeloB-VAX. The technology is intended to activate the body’s natural defense
machinery to stimulate an immune response against one of nature’s most common tumor markers, telomerase reverse transcriptase,
or telomerase. We believe that a vaccine product, if developed, will utilize the patient’s own B cells as antigen producing
and antigen presenting cells. In a Phase 1 study completed at UCSD in castrate resistant prostate cancer patients, the vaccine
product candidate was shown to be safe and well tolerated. The vaccine was found to be immunogenic, and was shown to induce a specific
CD8 T cell response. More important, the T cells induced post-vaccination were shown to specifically kill prostate cancer cells.
This vaccine product candidate is covered by what we believe is a unique patented platform technology using a cancer antigen marker,
telomerase, that is increased in approximately 85% of all tumors. We believe that this technology may represent an opportunity
to program the immune system to mobilize killer lymphocytes to combat cancer cells, including progenitor cancer stem cells that
were shown to also express telomerase.
Prostate Cancer.
According to the American
Cancer Society, or ACS, and the National Cancer Institute, or NCI, prostate cancer is the second-most common cancer in American
men and the second leading cause of cancer death in American men. The ACS estimated that for 2013 in the United States, approximately
238,000 new cases of prostate cancer will be diagnosed and about 29,700 men will die of prostate cancer in 2013. In 2010, we licensed
patents and related intellectual property relating to three cancer drug candidates developed at the University of Wisconsin. We
believe these drug candidates, named APC-100, -200 and -300, may offer new treatment opportunities for prostate cancer.
APC-100 is the most advanced of the three drug candidates.
In animal studies conducted to date, APC-100 demonstrated anti-androgenic and anti-inflammatory activities against prostate tumors
growing in animal models and showed a strong safety profile in preclinical safety studies. In 2006, APC-100 was awarded the NCI
Rapid Award. The award is given for promising new drugs for the treatment of cancer and resulted in significant funding for research
and development of APC-100. APC-100 has demonstrated desirable pharmacological characteristics as an oral or injectable anti-inflammatory
and anti-androgenic drug candidate with multiple mechanisms of action. In studies conducted to date, APC-100 decreased secretion
of human PSA by human prostate cancer cells growing in mice and also increased the time-to-tumor progression and survival of mice
with prostate sensitive and castrate resistant tumors. In August 2011, we announced the enrollment of the first patient in a Phase
1/2a prostate cancer clinical study relating to the use of the APC-100 product to treat men with castrate-resistant prostate cancer.
The study began at the University of Wisconsin Carbone Cancer Center and was extended to the Wayne State University Karmanos Cancer
Institute. In the trial, each patient will be assessed for toxicity, biochemical responses (PSA), radiographic and clinical responses.
APC-200 is a drug candidate for both castrate-sensitive
and castrate resistant prostate cancer. In 2007, APC-200 was awarded the NCI Rapid Award. APC-200 blocks androgen-induced hydrogen
peroxide production and inflammation and inhibits mouse prostate cancer. In animal studies conducted to date, APC-200 was an excellent
inhibitor of chronic inflammation, also completely inhibiting oxidase mediated high rates of hydrogen peroxide production
in
vivo
and delaying prostate cancer progression and death in the standard mouse prostate cancer model. If we conclude pre-clinical
development activities, such as GMP manufacturing of drug substance and drug product, and pre-clinical safety, pharmacology and
toxicology studies, we anticipate that we would file and open an Adamis-sponsored IND relating to the clinical investigation of
oral APC-200 in PCa patients with castrate resistant prostate cancer, assuming adequate funding and no unexpected delays, although
there are no assurances that we will file or open such an IND.
APC-300 is a multi-targeted small molecule therapeutic
drug that we believe has the potential to demonstrate anti-inflammatory, pro-apoptotic anti-cancer activities for prostate cancer
patients, including men with advanced metastatic castrate resistance prostate cancer. In pre-clinical
in vitro
studies conducted
to date, APC-300 repeatedly demonstrated inhibition of human tumor cell growth and killed both castrate-sensitive and castrate-resistant
human prostate cancer tumors. It also materially decreased tumor volumes and suppressed local metastasis in human to mouse xenograft
models, where malignant human prostate, pancreas, or melanoma tumor tissue was grafted onto athymic immunosuppressed experimental
mice. For several reasons, including funding limitations, we have not yet developed a clinical protocol and other materials for
submission of an IND.
Corporate Background
Our principal executive offices are located at
11682 El Camino Real, Suite 300, San Diego, CA 92130, and our telephone number is (858) 997-2400. Our website address is:
www.adamispharmaceuticals.com
.
We have included our website address as a factual reference and do not intend it to be an active link to our website. The information
that can be accessed through our website is not part of this prospectus, and investors should not rely on any such information
in deciding whether to purchase our common stock.
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements.
Such statements may include, without limitation, statements relating to: our expectations for growth; estimates of future revenue;
our sources and uses of cash; our liquidity needs; our ability to obtain sufficient funding to support our planned activities;
our current or planned clinical trials or research and development activities; product development timelines; our future products;
regulatory matters; anticipated dates for commencement of clinical trials; anticipated completion dates of clinical trials; anticipated
dates for meetings with regulatory authorities and submissions to obtain required regulatory marketing approvals’ anticipated
dates for commercial introduction of products; expense, profits, cash flow balance sheet; guidance on future periods; and other
statements concerning our future operations and activities. Such forward-looking statements include those that express plans, anticipation,
intent, contingency, goals, targets or future development and/or otherwise are not statements of historical fact. These forward-looking
statements are based on our current expectations and projections about future events, and they are subject to risks and uncertainties,
known and unknown, that could cause actual results and developments to differ materially from those expressed or implied in such
statements.
In some cases, you can identify forward-looking
statements by terminology, such as “expects,” “anticipates,” “intends,” “estimates,”
“plans,” “believes,” “seeks,” “may,” “should”, “could”
or the negative of such terms or other similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties
that could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified
in their entirety by reference to the factors discussed throughout this prospectus.
You should read this prospectus and any accompanying
prospectus supplement and the documents that we reference herein and therein and have filed as exhibits to the registration statement
of which this prospectus is part, completely and with the understanding that our actual future results may be materially different
from what we expect. You should assume that the information appearing in this prospectus and any accompanying prospectus supplement
is accurate as of the date on the front cover of this prospectus or such prospectus supplement only. Because the risk factors referred
to elsewhere in the prospectus could cause actual results or outcomes to differ materially from those expressed in any forward-looking
statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. Further, any forward-looking
statement speaks only as of the date on which it is made, and except as may be required by applicable law, we undertake no obligation
to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to
reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict
which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor,
or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
We qualify all of the information presented in this prospectus and any accompanying prospectus supplement, and particularly our
forward-looking statements, by these cautionary statements.
RISK FACTORS
Any investment in our common stock or other securities
involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained
in this prospectus before deciding whether to purchase the securities offered hereby. Our business, financial condition, results
of operations and prospects could be materially and adversely affected by these risks if any of them actually occur. The risks
and uncertainties described below are not the only ones we face. Additional risks not currently known to us or other factors not
perceived by us to present significant risks to our business at this time also could adversely affect our business, operating results
and financial conditions, as well as adversely affect the value of an investment in our securities. This prospectus also contains
forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those anticipated
in these forward-looking statements as a result of certain factors, including the risks we face as described below and elsewhere
in this prospectus.
Risks Related to Our Business, Industry and Financial Condition
We may never commercialize any of our products or earn a profit.
We have not received regulatory approval for any
drugs or products. Since our fiscal 2010 year, we have not generated commercial revenues from marketing or selling, any drugs or
other products. We currently have no revenues from product sales, have not generated any revenue from operations for the last four
fiscal years, and expect to incur substantial net losses for the foreseeable future to further develop and commercialize our product
candidates and technologies. We may never be able to commercialize any of our product candidates or be able to generate revenues
from products sales. Because of the risks and uncertainties associated with developing and commercializing our specialty pharmaceuticals,
cancer and other product candidates, we are unable to predict when we may commercially introduce products, the extent of any future
losses or when we will become profitable, if ever. We may never successfully commercialize our product candidates, and our business
may fail.
Our auditors have expressed substantial doubt about our ability
to continue as a going concern, which may hinder our ability to obtain further financing.
Our audited financial statements for the year ended
March 31, 2014, were prepared under the assumption that we would continue our operations as a going concern. Our independent
registered public accounting firm has included a “going concern” explanatory paragraph in its report on our financial
statements for the years ended March 31, 2014 and 2013, indicating that we have sustained substantial losses from continuing
operations and have used, rather than provided, cash in our continuing operations, and that these factors raise substantial doubt
about our ability to continue as a going concern. Uncertainty concerning our ability to continue as a going concern may hinder
our ability to obtain future financing. Continued operations and our ability to continue as a going concern are dependent on our
ability to obtain additional funding in the near future and thereafter, and there are no assurances that such funding will be available
at all or will be available in sufficient amounts or on reasonable terms. Our financial statements do not include any adjustments
that may result from the outcome of this uncertainty. Without additional funds from debt or equity financings, sales of assets,
sales or out-licenses of intellectual property or technologies, or other transactions, we will exhaust our resources and will be
unable to continue operations. If we cannot continue as a viable entity, our stockholders would likely lose most or all of their
investment in us.
Our ability to raise capital is limited by applicable laws and
regulations.
This registration statement is a “shelf”
registration on Form S-3, which typically enables an issuer to raise additional capital on a more timely and cost effective basis
than through other means, such as registration of a securities offering under a Form S-1 registration statement. Our ability to
raise additional capital through the sale and issuance of our equity securities is limited by, among other things, Securities and
Exchange Commission rules and regulations. Under current Securities and Exchange Commission rules and regulations, to be eligible
to use a Form S-3 registration statement for primary offerings without restriction as to the amount of securities to be sold and
issued, the aggregate market value of our common equity held by non-affiliates (i.e., our “public float”) must be at
least $75 million at the time we file the Form S-3 (calculated pursuant to the General Instructions to Form S-3). Furthermore,
the Securities and Exchange Commission’s rules and regulations require that we periodically re-evaluate the value of our
public float (typically when we file our Annual Report on Form 10-K) to determine whether we continue to satisfy the foregoing
public float requirement. At the time that we filed the registration statement on Form S-3 of which this prospectus is a part,
we did not meet the $75 million public float requirement. If we do not meet the $75 million public float requirement at the time
that we file our next Annual Report on Form 10-K for the year ended March 31, 2015, or at another applicable re-evaluation
date, the amount we could raise through primary offerings of our securities in any 12-month period using a Form S-3 registration
statement would be limited to an aggregate of one-third of our public float. Moreover, the market value of all securities sold
by us under our Form S-3 registration statements during the 12-month period prior to any intended sale will be subtracted from
that amount to determine the amount we can then raise under our Form S-3 registration statements. If after we are or become subject
to the foregoing one-third limitation our public float increases to $75 million or more, such limitation would cease to apply until
we conduct our next re-evaluation.
We will require additional financing to continue as a going concern.
We incurred a net loss of approximately $8.2 million
for the year ended March 31, 2014, and a net loss of approximately $7.2 million for the year ended March 31, 2013. At
March 31, 2014, we had cash and cash equivalents of approximately $5.4 million, no accounts receivable and liabilities of
approximately $2.9 million. Absent additional funding, we believe that our cash and cash equivalents will be sufficient to fund
our operations only for a relatively short period of time. The development of our business will require substantial additional
capital in the future to commercialize our Epinephrine PFS product, proceed with development of the APC-5000 DPI product, and conduct
research and develop our cancer and vaccine technologies and other product candidates, as well as to fund our ongoing operations
and satisfy our obligations and liabilities. We have historically relied upon private sales of our equity or debt securities to
fund our operations. We currently have no credit facility or committed sources of capital. Delays in obtaining funding could adversely
affect our ability to develop and commercially introduce products and cause us to be unable to comply with our obligations under
outstanding instruments.
Our ability to obtain additional financing will
be subject to a number of factors, including market conditions, our operating performance and investor sentiment. If we are unable
to raise additional capital when required or on acceptable terms, we may have to significantly delay, scale back or discontinue
the development or commercialization of one or more of our product candidates, restrict our operations or obtain funds by entering
into agreements on unattractive terms, which would likely have a material adverse effect on our business, stock price and our relationships
with third parties with whom we have business relationships, at least until additional funding is obtained. If we do not have sufficient
funds to continue operations, we could be required to seek bankruptcy protection or other alternatives that would likely result
in our stockholders losing some or all of their investment in us.
Statements in this prospectus concerning our future plans and
operations are dependent on our ability to secure adequate funding and the absence of unexpected delays or adverse developments.
We may not be able to secure required funding.
The statements contained in this prospectus concerning
future events or developments or our future activities, such as concerning current or planned clinical trials, anticipated research
and development activities, anticipated dates for commencement of clinical trials, anticipated completion dates of clinical trials,
anticipated meetings with the FDA or other regulatory authorities concerning our product candidates, anticipated dates for submissions
to obtain required regulatory marketing approvals, anticipated dates for commercial introduction of products, and other statements
concerning our future operations and activities, are forward-looking statements that in each instance assume that we are able to
obtain sufficient funding in the near term and thereafter to support such activities and continue our operations and planned activities
in a timely manner. There can be no assurance that this will be the case. Also, such statements assume that there are no significant
unexpected developments or events that delay or prevent such activities from occurring. Failure to timely obtain sufficient funding,
or unexpected developments or events, could delay the occurrence of such events or prevent the events described in any such statements
from occurring which could adversely affect our business, financial condition and results of operations.
We have incurred losses since our inception, and we anticipate
that we will continue to incur losses. We may never achieve or sustain profitability.
We incurred net losses of approximately $8.2 million
for the fiscal year ended March 31, 2014, and a net loss of approximately $7.2 million for the year ended March 31, 2013.
From inception through March 31, 2014, we have an accumulated deficit of approximately $46.1 million. These losses will increase
as we continue our research and development activities, seek regulatory approvals for our product candidates and commercialize
any approved products. These losses will cause, among other things, our stockholders’ equity and working capital to decrease.
Any future earnings and cash flow from operations of our business are dependent on our ability to further develop our products
and on revenues and profitability from sales of products.
There can be no assurance that we will be able to
generate sufficient product revenue to become profitable at all or on a sustained basis. Even if we generate revenues, we expect
to have quarter-to-quarter fluctuations in revenues and expenses, some of which could be significant, due to research, development,
clinical trial, marketing and manufacturing expenses and activities. If our product candidates fail in clinical trials or do not
gain regulatory approval, or if our products do not achieve market acceptance, we may never become profitable. As we commercialize
and market products, we will need to incur expenses for product marketing and brand awareness and conduct significant research,
development, testing and regulatory compliance activities that, together with general and administrative expenses, could result
in substantial operating losses for the foreseeable future. Even if we do achieve profitability, we may not be able to sustain
or increase profitability on a quarterly or annual basis.
Our limited operating history may make it difficult to evaluate
our business and our future viability.
We are in the relatively early stage of operations
and development of our current products and product candidates and have only a limited operating history on which to base an evaluation
of our business and prospects. Even if we successfully obtain additional funding, we are subject to the risks associated with early
stage companies with a limited operating history, including: the need for additional financings; the uncertainty of research and
development efforts resulting in successful commercial products, as well as the marketing and customer acceptance of such products;
unexpected issues with the FDA or other federal or state regulatory authorities; regulatory setbacks and delays; competition from
larger organizations; reliance on the proprietary technology of others; dependence on key personnel; uncertain patent protection;
fluctuations in expenses; and dependence on corporate partners and collaborators. Any failure to successfully address these risks
and uncertainties could seriously harm our business and prospects. We may not succeed given the technological, marketing, strategic
and competitive challenges we will face. The likelihood of our success must be considered in light of the expenses, difficulties,
complications, problems and delays frequently encountered in connection with the growth of a new business, the continuing development
of new drug technology, and the competitive and regulatory environment in which we operate or may choose to operate in the future.
Many of our potential products and technologies are in early stages
of development.
The development of new pharmaceutical products is
a highly risky undertaking, and there can be no assurance that any future research and development efforts we might undertake will
be successful. Our potential products in the cancer and viral fields will require extensive additional research and development
before any commercial introduction, as will research and development work on our allergy and respiratory products. There can be
no assurance that any future research, development or clinical trial efforts will result in viable products or meet efficacy standards.
Future clinical or preclinical results may be negative or insufficient to allow us to successfully market our product candidates.
Obtaining needed data and results may take longer than planned or may not be obtained at all. Any such delays or setbacks could
have a material adverse effect on our ability to achieve our financial goals.
We rely on third parties to conduct our clinical trials. If these
third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be unable to obtain, or
may experience delays in obtaining, regulatory approval, or may not be successful in commercializing our planned and future products.
Like many companies our size, we do not have the
ability to conduct preclinical or clinical studies for our product candidates without the assistance of third parties who conduct
the studies on our behalf. These third parties are usually toxicology facilities and clinical research organizations, or CROs,
that have significant resources and experience in the conduct of pre-clinical and clinical studies. The toxicology facilities conduct
the pre-clinical safety studies as well as associated tasks connected with these studies. The CROs typically perform patient recruitment,
project management, data management, statistical analysis, and other reporting functions. We intend to rely on third parties to
conduct clinical trials of our product candidates and to use third party toxicology facilities and CROs for our pre-clinical and
clinical studies. We may also rely on academic institutions or clinical research organizations to conduct, supervise or monitor
some or all aspects of clinical trials involving our products.
Our reliance on these third parties for development
activities will reduce our control over these activities. If these third parties do not successfully carry out their contractual
duties or obligations or meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised
due to the failure to adhere to our clinical protocols or for other reasons, we may be required to replace them, and our clinical
trials may be extended, delayed or terminated. Although we believe there are a number of third-party contractors that we could
engage to continue these activities, replacing a third-party contractor may result in a delay of the affected trial.
Delays in the commencement or completion of clinical testing of
our product candidates could result in increased costs and delay our ability to generate significant revenues.
The actual timing of commencement and completion
of clinical trials can vary dramatically from our anticipated timing due to factors such as funding limitations, scheduling conflicts
with participating clinicians and clinical institutions, and the rate of patient enrollment. Clinical trials involving our product
candidates may not commence or be completed as forecast. Delays in the commencement or completion of clinical testing could significantly
impact our product development costs. We do not know whether current or planned clinical trials will begin on time or be completed
on schedule, if at all. The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
|
|
●
|
obtaining required funding;
|
|
|
●
|
obtaining regulatory approval to commence a clinical trial;
|
|
|
●
|
reaching agreement on acceptable terms with prospective contract research
organizations and clinical trial sites;
|
|
|
●
|
obtaining sufficient quantities of clinical trial materials for product candidates;
|
|
|
●
|
obtaining institutional review board approval to conduct a clinical trial
at a prospective site; and
|
|
|
●
|
recruiting participants for a clinical trial.
|
In addition, once a clinical trial has begun, it
may be suspended or terminated by us or the FDA or other regulatory authorities due to a number of factors, including:
|
|
●
|
failure to conduct the clinical trial in accordance with regulatory requirements;
|
|
|
●
|
inspection of the clinical trial operations or clinical trial site by the
FDA or other regulatory authorities resulting in the imposition of a clinical hold;
|
|
|
●
|
failure to achieve certain efficacy and/or safety standards; or
|
|
|
●
|
lack of adequate funding to continue the clinical trial.
|
Clinical trials require sufficient participant enrollment,
which is a function of many factors, including the size of the target patient population, the nature of the trial protocol, the
proximity of participants to clinical trial sites, the availability of effective treatments for the relevant disease, the eligibility
criteria for our clinical trials and competing trials. Delays in enrollment can result in increased costs and longer development
times. Our failure to enroll participants in our clinical trials could delay the completion of the clinical trials beyond current
expectations. In addition, the FDA could require us to conduct clinical trials with a larger number of participants than we may
project for any of our product candidates. As a result of these factors, we may not be able to enroll a sufficient number of participants
in a timely or cost-effective manner.
Furthermore, enrolled participants may drop out
of clinical trials, which could impair the validity or statistical significance of the clinical trials. A number of factors can
influence the discontinuation rate, including, but not limited to: the inclusion of a placebo in a trial; possible lack of effect
of the product candidate being tested at one or more of the dose levels being tested; adverse side effects experienced, whether
or not related to the product candidate; and the availability of numerous alternative treatment options that may induce participants
to withdraw from the trial.
We may be required to suspend, repeat or terminate our clinical
trials if the trials are not well designed, do not meet regulatory requirements or the results are negative or inconclusive, which
may result in significant negative repercussions on business and financial condition.
Before regulatory approval for a potential product
can be obtained, we must undertake clinical testing on humans to demonstrate the tolerability and efficacy of the product. We cannot
assure you that we will obtain authorization to permit product candidates that are in the preclinical development phase to enter
the human clinical testing phase. In addition, we cannot assure you that any authorized preclinical or clinical testing will be
completed successfully within any specified time period by us, or without significant additional resources or expertise to those
originally expected to be necessary. We cannot assure you that such testing will show potential products to be safe and efficacious
or that any such product will be approved for a specific indication. Further, the results from preclinical studies and early clinical
trials may not be indicative of the results that will be obtained in later-stage clinical trials. In addition, we or regulatory
authorities may suspend clinical trials at any time on the basis that the participants are being exposed to unacceptable health
risks.
We
are subject to the risk of clinical trial and product liability lawsuits.
The testing of human health care product candidates
entails an inherent risk of allegations of clinical trial liability, while the marketing and sale of approved products entails
an inherent risk of allegations of product liability and associated adverse publicity. We currently maintain liability insurance
coverage of $1,000,000. Such insurance is expensive, difficult to obtain and may not be available in the future on acceptable terms,
or at all. As we conduct additional clinical trials and introduce products into the United States market, the risk of adverse events
increases and our requirements for liability insurance coverage are likely to increase. We are subject to the risk that substantial
liability claims from the testing or marketing of pharmaceutical products could be asserted against us in the future. There can
be no assurance that we will be able to obtain or maintain insurance on acceptable terms, particularly in overseas locations, for
clinical and commercial activities or that any insurance obtained will provide adequate protection against potential liabilities.
An inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability
claims could inhibit our business.
Moreover, our current and future coverages may not
be adequate to protect us from all of the liabilities that we may incur. If losses from liability claims exceed our insurance coverage,
we may incur substantial liabilities that exceed our financial resources. In addition, a product or clinical trial liability action
against us would be expensive and time-consuming to defend, even if we ultimately prevailed. If we are required to pay a claim,
we may not have sufficient financial resources and our business and results of operations may be harmed. A product liability claim
brought against us in excess of our insurance coverage, if any, could have a material adverse effect upon our business, financial
condition and results of operations.
We do not have commercial-scale manufacturing capability, and
we lack commercial manufacturing experience. We will likely rely on third parties to manufacture and supply our product candidates.
We do not own or operate manufacturing facilities
for clinical or commercial production of product candidates. We do not have any experience in drug formulation or manufacturing,
and we lack the resources and the capability to manufacture any of our product candidates on a clinical or commercial scale. Accordingly,
we expect to depend on third-party contract manufacturers for the foreseeable future. Any performance failure on the part of our
contract manufacturers could delay clinical development, regulatory approval or commercialization of our current or future product
candidates, depriving us of potential product revenue and resulting in additional losses.
The manufacture of pharmaceutical products requires
significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls.
Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling up initial production.
These problems can include difficulties with production
costs and yields, quality control (including stability of the product candidate and quality assurance testing), shortages of qualified
personnel, and compliance with strictly enforced federal, state and foreign regulations. If our third-party contract manufacturers
were to encounter any of these difficulties or otherwise fail to comply with their obligations or under applicable regulations,
our ability to provide product candidates to patients in our clinical trials or commercially would be jeopardized. If we file an
application for marketing approval of the product and the FDA grants marketing approval, any delay or interruption in the supply
of product could delay the commercial launch of the product or impair our ability to meet demand for the product. Difficulties
in supplying products for clinical trials could increase the costs associated with our clinical trial programs and, depending upon
the period of delay, require us to commence new trials or qualify new manufacturers at significant additional expense, possibly
causing commercial delays or termination of the trials.
Our products can only be manufactured in a facility
that has undergone a satisfactory inspection by the FDA and other relevant regulatory authorities. For these reasons, we may not
be able to replace manufacturing capacity for our products quickly if we or our contract manufacturer(s) were unable to use manufacturing
facilities as a result of a fire, natural disaster (including an earthquake), equipment failure, or other difficulty, or if such
facilities were deemed not in compliance with the regulatory requirements and such non-compliance could not be rapidly rectified.
An inability or reduced capacity to manufacture our products would have a material adverse effect on our business, financial condition,
and results of operations.
We are subject to substantial government regulation, which could
materially adversely affect our business. If we do not receive regulatory approvals, we may not be able to develop and commercialize
our technologies.
We need FDA approval to market our proposed Epinephrine
PFS product and other products in the United States, and similar approvals from foreign regulatory authorities to market products
outside the United States. The production and marketing of our products and potential products and our ongoing research and development,
pre-clinical testing and clinical trial activities are subject to extensive regulation and review by numerous governmental authorities
in the United States and will face similar regulation and review for overseas approval and sales from governmental authorities
outside of the United States. The regulatory review and approval process, which may include evaluation of preclinical studies and
clinical trials of our products, as well as the evaluation of manufacturing processes and contract manufacturers’ facilities,
is lengthy, expensive and uncertain. We have limited experience in filing and pursuing applications necessary to gain regulatory
approvals. Many of the product candidates that we are currently developing must undergo rigorous pre-clinical and clinical testing
and an extensive regulatory approval process before they can be marketed. This process makes it longer, more difficult and more
costly to bring our potential products to market, and we cannot guarantee that any of our potential products will be approved.
Many products for which FDA approval has been sought by other companies have never been approved for marketing. In addition to
testing and approval procedures, extensive regulations also govern marketing, manufacturing, distribution, labeling, and record-keeping
procedures. If we or our collaboration partners do not comply with applicable regulatory requirements, such violations could result
in non-approval, suspensions of regulatory approvals, civil penalties and criminal fines, product seizures and recalls, operating
restrictions, injunctions, and criminal prosecution.
Regulatory authorities generally have substantial
discretion in the approval process and may either refuse to accept an application, or may decide after review of an application
that the data submitted is insufficient to allow approval of the proposed product. If regulatory authorities do not accept or approve
our applications, they may require that we conduct additional clinical, preclinical or manufacturing studies and submit that data
before regulatory authorities will reconsider such application. We may need to expend substantial resources to conduct further
studies to obtain data that regulatory authorities believe is sufficient. Depending on the extent of these studies, approval of
applications may be delayed by several years, or may require us to expend more resources than we may have available. It is also
possible that additional studies may not suffice to make applications approvable. If any of these outcomes occur, we may be forced
to abandon our applications for approval.
Failure to obtain FDA or other required regulatory
approvals, or withdrawal of previous approvals, would adversely affect our business. Even if regulatory approval of a product is
granted, this approval may entail limitations on uses for which the product may be labeled and promoted, or may prevent us from
broadening the uses of products for different applications.
Following regulatory approval of any of our drug candidates, we
will be subject to ongoing regulatory obligations and restrictions, which may result in significant expense and limit our ability
to commercialize our potential products.
With regard to our drug candidates, if any, approved
by the FDA or by another regulatory authority, we are held to extensive regulatory requirements over product manufacturing, labeling,
packaging, adverse event reporting, storage, advertising, promotion and record keeping. Regulatory approvals may also be subject
to significant limitations on the indicated uses or marketing of the drug candidates. Potentially costly follow-up or post-marketing
clinical studies may be required as a condition of approval to further substantiate safety or efficacy, or to investigate specific
issues of interest to the regulatory authority. Previously unknown problems with the drug candidate, including adverse events of
unanticipated severity or frequency, may result in restrictions on the marketing of the drug, and could include withdrawal of the
drug from the market. In addition, the law or regulatory policies governing pharmaceuticals may change. New statutory requirements
may be enacted or additional regulations may be enacted that could prevent or delay regulatory approval of our drug candidates.
We cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative
action, either in the United States or elsewhere. If we are not able to maintain regulatory compliance, we might not be permitted
to market our drugs and our business could suffer.
We intend to pursue Section 505(b)(2) regulatory approval filings
with the FDA for our products where applicable. Such filings involve significant costs, and we may also encounter difficulties
or delays in obtaining regulatory approval for our products.
We intend to pursue a Section 505(b)(2) regulatory
filing with the FDA in connection with our Epinephrine PFS, APC-1000, APC-3000 and APC-5000 DPI products and product candidates.
A Section 505(b)(2) NDA is a special type of NDA that enables the applicant to rely, in part, on the FDA’s findings of safety
and efficacy of an existing previously approved product, or published literature, in support of its application. Section 505(b)(2)
NDAs often provide an alternate path to FDA approval for new or improved formulations or new uses of previously approved products.
Such filings involve significant filing costs, including filing fees.
To the extent that a Section 505(b)(2) NDA
relies on clinical trials conducted for a previously approved drug product or the FDA’s prior findings of safety and effectiveness
for a previously approved drug product, the Section 505(b)(2) applicant must submit patent certifications in its Section 505(b)(2)
application with respect to any patents for the previously approved product on which the applicant’s application relies and
that are listed in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange
Book. Specifically, the applicant must certify for each listed patent that, in relevant part, (1) the required patent information
has not been filed; (2) the listed patent has expired; (3) the listed patent has not expired, but will expire on a particular
date and approval is not sought until after patent expiration; or (4) the listed patent is invalid, unenforceable or will
not be infringed by the proposed new product. A certification that the new product will not infringe the previously approved product’s
listed patent or that such patent is invalid or unenforceable is known as a Paragraph IV certification. If the applicant does not
challenge one or more listed patents through a Paragraph IV certification, the FDA will not approve the Section 505(b)(2)
NDA application until all the listed patents claiming the referenced product have expired.
If the Section 505(b)(2) NDA applicant has
provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the
owner of the referenced NDA for the previously approved product and relevant patent holders within 20 days after the Section 505(b)(2)
NDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement suit against the
Section 505(b)(2) applicant. Under the FDCA, the filing of a patent infringement lawsuit within 45 days of receipt of the
notification regarding a Paragraph IV certification automatically prevents the FDA from approving the Section 505(b)(2) NDA
until the earliest to occur of 30 months beginning on the date the patent holder receives notice, expiration of the patent, settlement
of the lawsuit, or until a court deems the patent unenforceable, invalid or not infringed.
If we rely in our Section 505(b)(2) regulatory filings
on clinical trials conducted, or the FDA’s prior findings of safety and effectiveness, for a previously approved drug product
that involves patents referenced in the Orange Book, then we will need to make the patent certifications or the Paragraph IV certification
described above. If we make a Paragraph IV certification and the holder of the previously approved product that we referenced in
our application initiates patent litigation within the time periods described above, then any FDA approval of our 505(b)(2) application
would be delayed until the earlier of 30 months, resolution of the lawsuit, or the other events described above. Accordingly, our
anticipated dates of commercial introduction of our Epinephrine PFS product and or other products would be delayed. In addition,
we would incur the expenses, which could be material, involved with any such patent litigation. As a result, we may invest a significant
amount of time and expense in the development of our product only to be subject to significant delay and patent litigation before
our product may be commercialized, if at all.
In addition, even if we submit a Section 505(b)(2)
application, such as we have submitted for the Epinephrine PFS product, and as we may submit for other future products, that relies
on clinical trials conducted for a previously approved product where there are no patents referenced in the Orange Book for such
other product with respect to which we have to provide certifications, we are subject to the risk that the FDA could disagree with
our reliance on the particular previously approved product that we chose to rely on, conclude that such previously approved product
is not an acceptable reference product, and require us instead to rely as a reference product on another previously approved product
that involves patents referenced in the Orange Book, requiring us to make the certifications described above and subjecting us
to additional delay, expense and the other risks described above.
If we fail to obtain acceptable prices or appropriate reimbursement
for our products, our ability to successfully commercialize our products will be impaired.
Government and insurance reimbursements for healthcare
expenditures play an important role for all healthcare providers, including physicians and pharmaceutical companies such as Adamis,
that plan to offer various products in the United States and other countries in the future. Physicians and patients may decide
not to order our products unless third-party payors, such as managed care organizations as well as government payors such as Medicare
and Medicaid, pay a substantial portion of the price of the products. Market acceptance and sales of our products and potential
products will depend in part on the extent to which reimbursement for the costs of such products will be available from government
health administration authorities, private health coverage insurers, managed care organizations, and other organizations. In the
United States, our ability to have our products eligible for Medicare, Medicaid or private insurance reimbursement will be an important
factor in determining the ultimate success of our products. If, for any reason, Medicare, Medicaid or the insurance companies decline
to provide reimbursement for our products, our ability to commercialize our products would be adversely affected.
Third-party payors may challenge the price of medical
and pharmaceutical products. Reimbursement by a third-party payor may depend on a number of factors, including a payor’s
determination that our product candidates are:
|
|
●
|
not experimental or investigational;
|
|
|
●
|
appropriate for the specific patient;
|
|
|
●
|
supported by peer-reviewed publications; and
|
|
|
●
|
included in clinical practice guidelines.
|
If purchasers or users of our products and related
treatments are not able to obtain appropriate reimbursement for the cost of using such products, they may forego or reduce such
use. Significant uncertainty exists as to the reimbursement status of newly approved pharmaceutical products, and there can be
no assurance that adequate third-party coverage will be available for any of our products. Even if our products are approved for
reimbursement by Medicare, Medicaid and private insurers, of which there can be no assurance, the amount of reimbursement may be
reduced at times or even eliminated. This would have a material adverse effect on our business, financial condition and results
of operations.
Legislative or regulatory reform of the healthcare system may
affect our ability to sell our products profitably.
In both the United States and certain foreign jurisdictions,
there have been and are expected to be a number of legislative and regulatory changes to the healthcare system in ways that could
impact our ability to sell our products profitably, including the Patient Protection and Affordable Care Act signed into law in
the United States in March 2010. Given the enactment of these laws and other federal and state legislation and regulations relating
to the healthcare system, it is still too early to determine their impact on the biotechnology and pharmaceutical industries and
our business. The U.S. Congress continues to consider issues relating to the healthcare system, and future legislation or regulations
may affect our ability to market and sell products on favorable terms, which would affect our results of operations, as well as
our ability to raise capital, obtain additional collaborators or profitably market our products. Such legislation or regulation
may reduce our revenues, increase our expenses or limit the markets for our products. In particular, we expect to experience pricing
pressures in connection with the sale of our products due to the influence of health maintenance and managed health care organizations
and additional legislative proposals.
We have limited sales, marketing and distribution experience.
We have limited experience in the sales, marketing,
and distribution of pharmaceutical products. There can be no assurance that we will be able to establish sales, marketing, and
distribution capabilities or make arrangements with our current collaborators or others to perform such activities or that such
efforts will be successful. If we decide to market any products directly, we must either acquire or internally develop a marketing
and sales force with technical expertise and with supporting distribution capabilities. The acquisition or development of a sales,
marketing and distribution infrastructure would require substantial resources, which may not be available to us or, even if available,
could divert the attention of our management and key personnel and have a negative impact on further product development efforts.
We may seek to enter into arrangements to develop and commercialize
our products. These collaborations, if secured, may not be successful.
We have entered into arrangements with third parties
regarding development and commercialization of some of our products and may in the future seek to enter into collaborative arrangements
to develop and commercialize some of our potential products both in North America and international markets. There can be no assurance
that we will be able to negotiate collaborative arrangements on favorable terms or at all or that our current or future collaborative
arrangements will be successful. The amount and timing of resources such third parties will devote to these activities may not
be within our control. There can be no assurance that such parties will perform their obligations as expected. There can be no
assurance that our collaborators will devote adequate resources to our products.
If our potential products are unable to compete effectively with
current and future products targeting similar markets as our potential products, our commercial opportunities will be reduced or
eliminated.
The markets for epinephrine products, our proposed
APC-5000 DPI inhaler product and other allergy and respiratory products, and cancer and vaccine products, are intensely competitive
and characterized by rapid technological progress. We face competition from numerous sources, including major biotechnology and
pharmaceutical companies worldwide. Many of our competitors have substantially greater financial and technical resources, and development,
production and marketing capabilities, than we do. Certain companies have established technologies that may be competitive with
our product candidates and any future products that we may develop or acquire. Some of these products may use different approaches
or means to obtain results, which could be more effective or less expensive than our products for similar indications. In addition,
many of these companies have more experience than we do in pre-clinical testing, clinical trials and manufacturing of compounds,
obtaining FDA and foreign regulatory approvals, and brand name exposure and expertise in sales and marketing. We also compete with
academic institutions, governmental agencies and private organizations that are conducting research in the same fields.
Competition among these entities to recruit and
retain highly qualified scientific, technical and professional personnel and consultants is also intense. As a result, there is
a risk that one or more of our competitors will develop a more effective product for the same indications for which we are developing
a product or, alternatively, bring a similar product to market before we can do so. Failure to successfully compete will adversely
impact the ability to raise additional capital and ultimately achieve profitable operations.
Our product candidates may not gain acceptance among physicians,
patients, or the medical community, thereby limiting our potential to generate revenues, which will undermine our future growth
prospects.
Even if our product candidates are approved for
commercial sale by the FDA or other regulatory authorities, the degree of market acceptance of any approved product candidate by
physicians, health care professionals and third-party payors, and our profitability and growth will depend on a number of factors,
including:
|
|
●
|
the ability to provide acceptable evidence of safety and efficacy;
|
|
|
●
|
pricing and cost effectiveness, which may be subject to regulatory control;
|
|
|
●
|
our ability to obtain sufficient third-party insurance coverage or reimbursement;
|
|
|
●
|
effectiveness of our or our collaborators’ sales and marketing strategy;
|
|
|
●
|
relative convenience and ease of administration;
|
|
|
●
|
the prevalence and severity of any adverse side effects; and
|
|
|
●
|
availability of alternative treatments.
|
If any product candidate that we develop does not
provide a treatment regimen that is at least as beneficial as the current standard of care or otherwise does not provide some additional
patient benefit over the current standard of care, that product will not achieve market acceptance and we will not generate sufficient
revenues to achieve profitability.
If we suffer negative publicity concerning the safety of our products
in development, our sales may be harmed and we may be forced to withdraw such products.
If concerns should arise about the safety of any
of our products that are marketed, regardless of whether or not such concerns have a basis in generally accepted science or peer-reviewed
scientific research, such concerns could adversely affect the market for these products. Similarly, negative publicity could result
in an increased number of product liability claims, whether or not these claims are supported by applicable law.
Our failure to adequately protect or to enforce our intellectual
property rights or secure rights to third party patents could materially harm our proprietary position in the marketplace or prevent
the commercialization of our products.
Our success depends in part on our ability to obtain
and maintain protection in the United States and other countries for the intellectual property covering or incorporated into our
technologies and products. The patents and patent applications in our existing patent portfolio are either owned by us or licensed
to us. Our ability to protect our product candidates from unauthorized use or infringement by third parties depends substantially
on our ability to obtain and maintain, or license, valid and enforceable patents. Due to evolving legal standards relating to the
patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these
patents, our ability to obtain and enforce patents is uncertain and involves complex legal and factual questions for which important
legal principles are unresolved.
There is a substantial backlog of patent applications
at the United States Patent and Trademark Office, or USPTO. There can be no assurance that any patent applications relating to
our products or methods will be issued as patents, or, if issued, that the patents will not be challenged, invalidated or circumvented
or that the rights granted thereunder will provide a competitive advantage. We may not be able to obtain patent rights on products,
treatment methods or manufacturing processes that we may develop or to which we may obtain license or other rights. Even if we
do obtain patents, rights under any issued patents may not provide us with sufficient protection for our product candidates or
provide sufficient protection to afford us a commercial advantage against our competitors or their competitive products or processes.
It is possible that no patents will be issued from any pending or future patent applications owned by us or licensed to us. Others
may challenge, seek to invalidate, infringe or circumvent any patents we own or license. Alternatively, we may in the future be
required to initiate litigation against third parties to enforce our intellectual property rights. The defense and prosecution
of patent and intellectual property claims are both costly and time consuming, even if the outcome is favorable to us. Any adverse
outcome could subject us to significant liabilities, require us to license disputed rights from others, or require us to cease
selling our future products.
In addition, many other organizations are engaged
in research and product development efforts that may overlap with our products. Such organizations may currently have, or may obtain
in the future, legally blocking proprietary rights, including patent rights, in one or more products or methods under development
or consideration by us. These rights may prevent us from commercializing technology, or may require us to obtain a license from
the organizations to use the technology. We may not be able to obtain any such licenses that may be required on reasonable financial
terms, if at all, and we cannot be sure that the patents underlying any such licenses will be valid or enforceable. As with other
companies in the pharmaceutical industry, we are subject to the risk that persons located in other countries will engage in development,
marketing or sales activities of products that would infringe our patent rights if such activities were conducted in the United
States.
Our patents also may not afford protection against
competitors with similar technology. We may not have identified all patents, published applications or published literature that
affect our business either by blocking our ability to commercialize our product candidates, by preventing the patentability of
our products or by covering the same or similar technologies that may affect our ability to market or license our product candidates.
Many companies have encountered difficulties in protecting and defending their intellectual property rights in foreign jurisdictions.
If we encounter such difficulties or are otherwise precluded from effectively protecting our intellectual property rights in either
the United States or foreign jurisdictions, our business prospects could be substantially harmed. In addition, because of funding
limitations and our limited cash resources, we may not be able to devote the resources that we might otherwise desire to prepare
or pursue patent applications, either at all or in all jurisdictions in which we might desire to obtain patents, or to maintain
already-issued patents.
We may become involved in patent litigations or other intellectual
property proceedings relating to our future product approvals, which could result in liability for damages or delay or stop our
development and commercialization efforts.
The pharmaceutical industry has been characterized
by significant litigation and other proceedings regarding patents, patent applications, and other intellectual property rights.
The situations in which we may become parties to such litigation or proceedings may include any third parties initiating litigation
claiming that our products infringe their patent or other intellectual property rights; in such case, we will need to defend against
such proceedings. For example, the field of generic pharmaceuticals is characterized by frequent litigation that occurs in connection
with the regulatory filings under Section 505(b)(2) of the FDCA and attempts to invalidate the patent of the reference drug.
The costs of resolving any patent litigation or
other intellectual property proceeding, even if resolved in our favor, could be substantial. Many of our potential competitors
will be able to sustain the cost of such litigation and proceedings more effectively than we can because of their substantially
greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other intellectual property
proceedings could have a material adverse effect on our ability to compete in the marketplace. Patent litigation and other intellectual
property proceedings may also consume significant management time.
In the event that a competitor infringes upon our
patent or other intellectual property rights, enforcing those rights may be costly, difficult, and time-consuming. Even if successful,
litigation to enforce our intellectual property rights or to defend our patents against challenge could be expensive and time-consuming
and could divert our management’s attention. We may not have sufficient resources to enforce our intellectual property rights
or to defend our patent or other intellectual property rights against a challenge. If we are unsuccessful in enforcing and protecting
our intellectual property rights and protecting our products, it could materially harm our business.
We depend on our officers. If we are unable to retain our key
employees or to attract additional qualified personnel, our product operations and development efforts may be seriously jeopardized.
Our success will be dependent upon the efforts of
a small management team and staff, including Dennis J. Carlo, Ph.D., our chief executive officer. The employment of Dr. Carlo
may be terminated at any time by either us or Dr. Carlo. We currently do not have key man life insurance policies covering any
of our executive officers or key employees. If key individuals leave us, we could be adversely affected if suitable replacement
personnel are not quickly recruited. There is competition for qualified personnel in all functional areas, which makes it difficult
to attract and retain the qualified personnel necessary for the operation of our business. Our success also depends in part on
our ability to attract and retain highly qualified scientific, commercial and administrative personnel. If we are unable to attract
new employees and retain existing key employees, the development and commercialization of our product candidates could be delayed
or negatively impacted.
We may experience difficulties in managing growth.
We are a small company. Future growth will impose
significant added responsibilities on members of management, including the need to identify, attract, retain, motivate and integrate
highly skilled personnel. We may increase the number of employees in the future depending on the progress of our development of
our products and technologies. Our future financial performance and our ability to compete effectively will depend, in part, on
our ability to manage any future growth effectively. To that end, we must be able to:
|
|
●
|
manage our clinical studies effectively;
|
|
|
●
|
integrate additional management, administrative, manufacturing and regulatory
personnel;
|
|
|
●
|
maintain sufficient administrative, accounting and management information
systems and controls; and
|
|
|
●
|
hire and train additional qualified personnel.
|
We may not be able to accomplish these tasks, and
our failure to accomplish any of them could harm our financial results.
There are significant limitations on our ability in the future
to utilize any net operating loss carry forwards for federal and state income tax purposes.
At March 31, 2014, we had net operating loss
carry forwards of approximately $129 million and $58 million for federal and state purposes, respectively. The net operating loss
carry forwards expire through the year 2031. At March 31, 2014, we also had research and development credit carry forwards
of approximately $2.8 million and $200,000 for federal and state purposes, respectively. The federal credits expire through the
year 2027 and the state credits expire through the year 2019. The Tax Reform Act of 1986, as amended, or the TRA, provides for
a limitation on the annual use of net operating loss and research and development tax credit carry forwards following certain ownership
changes that could limit our ability to utilize these carry forwards. We most likely have experienced various ownership changes,
as defined by the TRA, as a result of past financings and merger transactions. Accordingly, our ability to utilize some of all
of these carry forwards is likely limited. Additionally, U.S. tax laws limit the time during which these carry forwards may be
applied against future taxes, and as a result we may not be able to take full advantage of these carry forwards for federal income
tax purposes.
Risks Related to Our Common Stock
Provisions of our charter documents could discourage an acquisition
of our company that would benefit our stockholders and may have the effect of entrenching, and making it difficult to remove, management.
Provisions of our restated certificate of incorporation
and bylaws may make it more difficult for a third party to acquire control of us, even if a change of control would benefit our
stockholders. For example, shares of our preferred stock may be issued in the future without further stockholder approval, and
upon such terms and conditions, and having such rights, privileges and preferences, as our board of directors may determine, including,
for example, rights to convert into our common stock. The rights of the holders of our common stock will be subject to, and may
be adversely affected by, the rights of the holders of any of our preferred stock that may be issued in the future. The issuance
of our preferred stock could have the effect of making it more difficult for a third party to acquire control of us. This could
limit the price that certain investors might be willing to pay in the future for shares of our common stock and discourage those
investors from acquiring a majority of our common stock. Similarly, our bylaws require that any stockholder proposals or nominations
for election to our board of directors must meet specific advance notice requirements and procedures, which make it more difficult
for our stockholders to make proposals or director nominations. The existence of these charter provisions could have the effect
of entrenching management and making it more difficult to change our management. Furthermore, because we are incorporated in Delaware,
we are governed by the provisions of Section 203 of the Delaware General Corporation Law. These provisions may prohibit or
restrict large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining
with us, unless one or more exemptions from such provisions apply. These provisions under Delaware law could discourage potential
takeover attempts and could reduce the price that investors might be willing to pay for shares of our common stock in the future.
The price of our common stock may be volatile.
The market price of our common stock may fluctuate
substantially. For example, from April 2012 to June 17, 2014, the market price of our common stock, adjusted retroactively
to give effect to our 1-for-17 reverse split of the common stock in December 2013, has fluctuated between $3.74 to $17.85. The
price of our common stock that will prevail in the market after any offering made pursuant to this prospectus may be higher or
lower than the price that investors purchasing our securities in such an offering have paid, depending on many factors, some of
which are beyond our control and may not be related to our operating performance. Market prices for securities of early-stage pharmaceutical,
biotechnology and other life sciences companies have historically been particularly volatile. Some of the factors that may cause
the market price of our common stock to fluctuate include:
|
|
●
|
relatively low trading volume, which can result in significant volatility
in the market price of our common stock based on a relatively smaller number of trades and dollar amount of transactions;
|
|
|
●
|
the timing and results of our current and any future preclinical or clinical
trials of our product candidates;
|
|
|
●
|
the entry into or termination of key agreements, including, among others,
key collaboration and license agreements;
|
|
|
●
|
the results and timing of regulatory reviews relating to the approval of our
product candidates;
|
|
|
●
|
the initiation of, material developments in, or conclusion of, litigation
to enforce or defend any of our intellectual property rights;
|
|
|
●
|
failure of any of our product candidates, if approved, to achieve commercial
success;
|
|
|
●
|
general and industry-specific economic conditions that may affect our research
and development expenditures;
|
|
|
●
|
the results of clinical trials conducted by others on products that would
compete with our product candidates;
|
|
|
●
|
issues in manufacturing our product candidates or any approved products;
|
|
|
●
|
the loss of key employees;
|
|
|
●
|
the introduction of technological innovations or new commercial products by
our competitors;
|
|
|
●
|
changes in estimates or recommendations by securities analysts, if any, who
cover our common stock;
|
|
|
●
|
future sales of our common stock;
|
|
|
●
|
period-to-period fluctuations in our financial results;
|
|
|
●
|
publicity or announcements regarding regulatory developments relating to our
products;
|
|
|
●
|
period-to-period fluctuations in our financial results, including our cash
and cash equivalents balance, operating expenses, cash burn rate or revenue levels;
|
|
|
●
|
common stock sales in the public market by one or more of our larger stockholders,
officers or directors;
|
|
|
●
|
our filing for protection under federal bankruptcy laws;
|
|
|
●
|
a negative outcome in any litigation or potential legal proceeding; or
|
|
|
●
|
other potentially negative financial announcements, such as a review of any
of our filings by the SEC, changes in accounting treatment or restatement of previously reported financial results or delays in
our filings with the SEC.
|
The stock markets in general have experienced substantial
volatility that has often been unrelated to the operating performance of individual companies. These broad market fluctuations
may also adversely affect the trading price of our common stock. In the past, following periods of volatility in the market price
of a company’s securities, stockholders have often instituted class action securities litigation against those companies.
Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could
significantly harm our profitability and reputation.
Trading of our common stock is limited.
Trading of our common stock is limited, and trading
restrictions imposed on us by applicable regulations may further reduce our trading, making it difficult for our stockholders to
sell their shares.
Prior to the listing of our common stock on the
NASDAQ Capital Market, trading of our common stock was conducted on the OTCQB. The liquidity of our common stock is limited, not
only in terms of the number of shares that can be bought and sold at a given price, but also as it may be adversely affected by
delays in the timing of transactions and reduction in security analysts’ and the media’s coverage of us, if at all.
The foregoing factors may result in lower prices
for our common stock than might otherwise be obtained and could also result in a larger spread between the bid and asked prices
for our common stock. In addition, without a large public float, our common stock is less liquid than the stock of companies with
broader public ownership, and as a result, the trading price of our common stock may be more volatile. In the absence of an active
public trading market, an investor may be unable to liquidate his or her investment in our common stock. Trading of a relatively
small volume of our common stock may have a greater impact on the trading price of our stock than would be the case if our public
float were larger. We cannot predict the price at which our common stock will trade at any given time.
Our common stock may become subject to additional trading restrictions
as a “penny stock,” which could adversely affect the liquidity and price of such stock. If our common stock becomes
subject to the SEC’s penny stock rules, broker-dealers may experience difficulty in completing customer transactions and
trading activity in our securities may be adversely affected.
Prior to the listing of our common stock on the
NASDAQ Capital Market, our common stock was traded on the OTCQB. The OTCQB, the OTC Bulletin Board and Pink Sheets are viewed by
most investors as a less desirable, and less liquid, marketplace. As a result, an investor may find it more difficult to purchase,
dispose of or obtain accurate quotations as to the value of our common stock.
Unless our common stock is listed on a national
securities exchange, such as the NASDAQ Capital Market, our common stock may also be subject to the regulations regarding trading
in “penny stocks,” which are those securities trading for less than $5.00 per share, and that are not otherwise exempted
from the definition of a penny stock under other exemptions provided for in the applicable regulations. The following is a list
of the general restrictions on the sale of penny stocks:
|
|
●
|
Before the sale of penny stock by a broker-dealer to a new purchaser, the
broker-dealer must determine whether the purchaser is suitable to invest in penny stocks. To make that determination, a broker-dealer
must obtain, from a prospective investor, information regarding the purchaser’s financial condition and investment experience
and objectives. Subsequently, the broker-dealer must deliver to the purchaser a written statement setting forth the basis of the
suitability finding and obtain the purchaser’s signature on such statement.
|
|
|
●
|
A broker-dealer must obtain from the purchaser an agreement to purchase the
securities. This agreement must be obtained for every purchase until the purchaser becomes an “established customer.”
|
|
|
●
|
The Securities Exchange Act of 1934, or the Exchange Act, requires that before
effecting any transaction in any penny stock, a broker-dealer must provide the purchaser with a “risk disclosure document”
that contains, among other things, a description of the penny stock market and how it functions and the risks associated with such
investment. These disclosure rules are applicable to both purchases and sales by investors.
|
|
|
●
|
A dealer that sells penny stock must send to the purchaser, within 10 days
after the end of each calendar month, a written account statement including prescribed information relating to the security.
|
These requirements can severely limit the liquidity
of securities in the secondary market because fewer brokers or dealers are likely to be willing to undertake these compliance activities.
If our common stock is not listed on a national securities exchange, the rules and restrictions regarding penny stock transactions
may limit an investor’s ability to sell to a third party and our ability to raise additional capital. We make no guarantee
that market-makers will make a market in our common stock, or that any market for our common stock will continue.
Our principal stockholders have significant influence over us,
they may have significant influence over actions requiring stockholder approval, and your interests as a stockholder may conflict
with the interests of those persons.
Based on the number of outstanding shares of our
common stock held by our stockholders as of March 31, 2014, our directors, executive officers and their respective affiliates
owned approximately 23% of our outstanding shares of common stock and our largest stockholder owned approximately 16% of the outstanding
shares of our common stock. As a result, those stockholders have the ability to exert a significant degree of influence with respect
to the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation
or sale of all or substantially all of our assets. The interests of these persons may not always coincide with our interests or
the interests of our other stockholders. This concentration of ownership could harm the market price of our common stock by (i)
delaying, deferring or preventing a change in corporate control, (ii) impeding a merger, consolidation, takeover or other
business combination involving us, or (iii) discouraging a potential acquirer from making a tender offer or otherwise attempting
to obtain control of us. The significant concentration of stock ownership may adversely affect the trading price of our common
stock due to investors’ perception that conflicts of interest may exist or arise.
In preparing our consolidated financial statements, our management
determined that our disclosure controls and procedures, and that our internal controls over financial reporting, were ineffective
as of March 31, 2014, which could result in material misstatements in our financial statements. If we continue to fail to
comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or, if we discover additional
material weaknesses and other deficiencies in our internal controls over financial reporting, our stock price could decline and
raising capital could be more difficult.
Our management is responsible for establishing and
maintaining adequate internal control over our financial reporting, as defined in Rule 13a-15(f) under the Exchange Act. As
of March 31, 2014, our management determined that our disclosure controls and procedures were ineffective, and that there
was a material weakness in our internal controls over financial reporting, due to insufficient segregation of duties in our finance
and accounting function because of limited personnel, based on the absence of finance and accounting personnel other than the Chief
Financial Officer. This resulted in not ensuring appropriate segregation of duties between incompatible functions, and made it
more difficult to ensure review of financial reporting issues sufficiently in advance of the dates on which filings are required
to be made with the SEC and to ensure that financial information is adequately analyzed and reviewed on a timely basis to detect
misstatements. These above deficiencies represent a material weakness in our internal control over financial reporting given that
they result in a reasonable possibility that a material misstatement to the annual or interim financial statements would not have
been prevented or detected. In addition, management determined that our disclosure controls and procedures, and that our internal
controls over financial reporting, had several significant deficiencies which did not rise to the level of material weaknesses.
Because the material weaknesses and significant deficiencies identified by our management will require significant financial resources
to address, we expect to continue to experience these material weaknesses and significant deficiencies for the foreseeable future.
We intend to address the weaknesses identified above
by increasing the oversight and review procedures of the board of directors with regard to financial reporting, financial processes
and procedures and internal control procedures; where possible preparing and reviewing SEC filings farther in advance of required
filing dates; and when funding is available, hiring additional finance and accounting personnel. Nevertheless, there can be no
assurances that we will have enough financial resources to remedy our current material weaknesses and significant deficiencies.
If remedial measures that we intend to take are
insufficient to address the ineffectiveness of our disclosure controls and procedures and our internal controls over financial
reporting, or if other material weaknesses or significant deficiencies in our internal controls are discovered or occur in the
future and the ineffectiveness of our disclosure controls and procedures continues, we may fail to meet our future reporting obligations
on a timely basis, our consolidated financial statements may contain material misstatements, we could be required to restate our
prior period financial results, our operating results may be harmed, and we could become subject to class action litigation. Internal
control deficiencies and ineffective disclosure controls and procedures could also cause investors to lose confidence in our reported
financial information. We can give no assurance that the measures we plan to take in the future will remediate the ineffectiveness
of our disclosure controls and procedures or that any material weaknesses or restatements of financial results will not arise in
the future due to a failure to implement and maintain adequate internal control over financial reporting or adequate disclosure
controls and procedures or circumvention of these controls. In addition, even if we are successful in strengthening our controls
and procedures, in the future those controls and procedures may not be adequate to prevent or identify irregularities or errors
or to facilitate the fair presentation of our consolidated financial statements. If we cannot provide reliable financial reports
or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial
information, and the trading price of our common stock could decline.
Our stockholders may experience significant dilution as a result
of the sale of securities offered by this prospectus, or as a result of any additional financing using our securities, as the result
of the exercise or conversion of our outstanding securities.
To the extent that we raise additional funds through
the sale of securities offered by this prospectus, our stockholders may experience significant dilution. If additional funds are
raised through the issuance of preferred stock, holders of preferred stock would likely have rights that are senior to the rights
of holders of our common stock, and the agreements relating to any such issuance could contain covenants that would restrict our
operations.
We will need to raise significant additional capital
in order to maintain and continue our operations. To the extent that we raise additional funds by issuing equity securities or
securities convertible into or exercisable for equity securities, our stockholders may experience significant dilution. In addition,
conversion or exercise of other outstanding options, warrants or convertible securities could result in there being a significant
number of additional shares outstanding and dilution to our stockholders. Certain of our outstanding securities include anti-dilution
provision providing that, with certain exceptions, if we issue shares of common stock or options, warrants, convertible securities
or other common stock equivalents, at an effective price per share less than the conversion or exercise price of such securities,
the conversion or exercise price of such securities will be adjusted downward to equal the per share price of the securities issued
in such transaction, entitling the holders to pay a lower per share exercise price and/or to acquire a larger number of shares
upon exercise or conversion of such securities, which could result in dilution to our stockholders. As a result, sale of additional
equity or convertible securities at prices below certain levels could trigger anti-dilution provisions with respect to certain
securities we have previously sold.
We have not paid cash dividends on our common stock in the past
and do not expect to pay cash dividends on our common stock for the foreseeable future. Any return on investment may be limited
to the value of our common stock.
No cash dividends have been paid on our common stock,
and we do not expect to pay cash dividends on our common stock in the foreseeable future. Payment of dividends would depend upon
our profitability at the time, cash available for those dividends, and other factors as our board of directors may consider relevant.
If we do not pay dividends, our common stock may be less valuable because a return on a stockholder’s investment will only
occur if our stock price appreciates.
A sale of a substantial number of shares of our common stock may
cause the price of our common stock to decline and may impair our ability to raise capital in the future.
There have been and may continue to be periods when
our common stock could be considered “thinly-traded,” meaning that the number of persons interested in purchasing our
common stock at or near bid prices at any given time may be relatively small or non-existent. Finance transactions resulting in
a large amount of newly issued shares that become readily tradable, conversion of outstanding convertible notes or exercise of
outstanding warrants and sale of the shares issuable upon conversion of such notes or exercise of such warrants, or other events
that cause stockholders to sell shares, could place downward pressure on the trading price of our stock. In addition, the lack
of a robust resale market may require a stockholder who desires to sell a large number of shares of common stock to sell the shares
in increments over time to mitigate any adverse impact of the sales on the market price of our stock. If our stockholders sell,
or the market perceives that our stockholders intend to sell for various reasons, substantial amounts of our common stock in the
public market, the market price of our common stock could decline. Sales of a substantial number of shares of our common stock
may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable
or appropriate.
Sales of additional equity securities may adversely affect the
market price of our common stock.
We expect to incur research, development and selling,
general and administrative costs, and to satisfy our funding requirements we will need to sell additional equity securities, which
may be subject to registration rights, and warrants with anti-dilutive protective provisions. The sale or the proposed sale of
substantial amounts of our common stock or other equity securities in the public markets may adversely affect the market price
of our common stock, and our stock price may decline substantially. Our stockholders may experience substantial dilution and a
reduction in the price that they are able to obtain upon the sale of their shares. Also, new equity securities issued may have
greater rights, preferences or privileges than our existing common stock.
Because we expect to have broad discretion and flexibility in
how the net proceeds from any offering made by this prospectus are used, we may use the net proceeds in ways in which you disagree.
Except as otherwise provided in any applicable prospectus
supplement, we intend to use the net proceeds from the sale of the securities offered by this prospectus for general corporate
purposes, which may include working capital, capital expenditures, research and development expenditures, regulatory affairs expenditures,
clinical trial expenditures, acquisitions of new companies, products or technologies, and the repayment, refinancing, redemption
or repurchase of future indebtedness or capital stock. As a result, our management will have significant discretion and flexibility
in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of
these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds
are being used appropriately. It is possible that the net proceeds will be invested or used in a way that does not yield a favorable,
or any, return for us. The failure of our management to use such funds effectively could have a material adverse effect on our
business, financial condition, operating results and cash flow.
If securities or industry analysts do not publish research or
reports about our business, or if they change their recommendations regarding our stock adversely, our stock price and trading
volume could decline.
The trading market for our common stock will be
influenced by the research and reports that industry or securities analysts publish about us or our business. We may never obtain
substantial research coverage by industry or financial analysts. If no or few analysts commence or continue coverage of us, the
trading price of our stock would likely decrease. Even if we do obtain analyst coverage, if one or more of the analysts who cover
us downgrade our stock, our stock price would likely decline. If one or more of these analysts cease coverage of our company or
fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock
price or trading volume to decline.
The rights of the holders of common stock may be impaired by the
potential issuance of preferred stock.
Our restated certificate of incorporation gives
our board of directors the right to create new series of preferred stock. As a result, the board of directors may, without stockholder
approval, issue preferred stock with voting, dividend, conversion, liquidation or other rights which could adversely affect the
voting power and equity interest of the holders of common stock. Preferred stock, which could be issued with the right to more
than one vote per share, could be utilized as a method of discouraging, delaying or preventing a change of control. The possible
impact on takeover attempts could adversely affect the price of our common stock.
USE OF PROCEEDS
Except as otherwise provided in the applicable prospectus
supplement, we intend to use the net proceeds from the sale of the securities offered by this prospectus for general corporate
purposes, which may include without limitation working capital, capital expenditures, research and development expenditures, regulatory
affairs expenditures, clinical trial expenditures, acquisitions of new companies, technologies or products, and the repayment,
refinancing, redemption or repurchase of future indebtedness or capital stock.
The intended application of proceeds from the sale
of any particular offering of securities using this prospectus will be described in the accompanying prospectus supplement relating
to such offering. The precise amount and timing of the application of these proceeds will depend on our funding requirements and
the availability and costs of other funds. We may temporarily invest the net proceeds in investment-grade, interest-bearing securities
until they are used for their stated purpose. We have not determined the amount of net proceeds to be used specifically for such
purposes. As a result, management will retain broad discretion over the allocation of net proceeds.
THE SECURITIES WE MAY OFFER
The descriptions of the securities contained in
this prospectus, together with the applicable prospectus supplements, summarize all the material terms and provisions of the various
types of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities the
particular terms of the securities offered by that prospectus supplement. If we indicate in the applicable prospectus supplement,
the terms of the securities may differ from the terms we have summarized below. We will also include in the prospectus supplement
information, where applicable, about material United States federal income tax considerations relating to the securities, and the
securities exchange, if any, on which the securities will be listed.
We
may sell from time to time, in one or more offerings:
|
|
●
|
shares of our common stock;
|
|
|
●
|
shares of our preferred stock;
|
|
|
●
|
warrants to purchase any of the securities listed above; and/or
|
|
|
●
|
units consisting of any of the securities listed above.
|
The terms of any securities we offer will be determined
at the time of sale. We may issue securities that are exchangeable for or convertible into common stock or any of the other securities
that may be sold under this prospectus. When particular securities are offered, a supplement to this prospectus will be filed with
the SEC, which will describe the terms of the offering and sale of the offered securities, including the specific amounts, prices
and other important terms of the securities including, to the extent applicable:
|
|
●
|
Designation or classification
|
|
|
●
|
Aggregate principal amount or aggregate offering price;
|
|
|
●
|
Rates and times of payment of interest or dividends, if any;
|
|
|
●
|
Redemption, conversion or sinking fund terms, if any;
|
|
|
●
|
Voting or other rights, if any;
|
|
|
●
|
Conversion prices, if any; and
|
|
|
●
|
Important federal income tax considerations.
|
The prospectus supplement and any related free writing
prospectus also may supplement or, as applicable, add, update or change information contained in this prospectus or in documents
as we have incorporated by reference. However, no prospectus supplement or free writing prospectus will offer a security that is
not registered and described in this prospectus at the time of the effectiveness of the registration statement of which this prospectus
is a part.
The terms of any particular offering, the initial
offering price and the net proceeds to us will be contained in the prospectus supplement, information incorporated by reference
or free writing prospectus relating to such offering.
We may issue securities in book-entry form through
one or more depositaries, such as The Depository Trust Company, named in the applicable prospectus supplement. If any securities
are to be listed or quoted on a securities exchange or quotation system, the applicable prospectus supplement will say so.
DESCRIPTION OF CAPITAL STOCK
General
The following description of common stock and preferred
stock, together with the additional information we include in any applicable prospectus supplements or related free writing prospectuses,
summarizes the material terms and provisions of the common stock and preferred stock that we may offer under this prospectus but
is not complete. For the complete terms of our common stock and preferred stock, please refer to our restated certificate of incorporation,
as the same may be amended from time to time, any certificates of designation for our preferred stock, and our amended and restated
bylaws, as amended from time to time. The Delaware General Corporation Law, or DGCL, may also affect the terms of these securities.
While the terms we have summarized below will apply generally to any future common stock or preferred stock that we may offer,
we will describe the particular terms of any series of these securities in more detail in the applicable prospectus supplement.
If we so indicate in a prospectus supplement, the terms of any common stock or preferred stock we offer under that prospectus supplement
may differ from the terms we describe below.
As of the date of this prospectus, our authorized
capital stock consisted of 100,000,000 shares of common stock, $0.0001 par value per share, and 10,000,000 shares of preferred
stock, $0.0001 par value per share. Our board of directors may establish the rights and preferences of the preferred stock from
time to time. As of June 16, 2014, there were approximately 10,501,519 shares of our common stock outstanding and no shares of
preferred stock outstanding.
Common Stock
Holders of our common stock are entitled to one
vote per share. Our restated certificate of incorporation does not provide for cumulative voting. Subject to preferences that may
be applicable to any outstanding preferred stock, the holders of our common stock are entitled to receive ratably such dividends,
if any, as may be declared by our board of directors, or the Board, out of legally available funds. However, the current policy
of our board of directors is to retain earnings, if any, for the operation and expansion of the company. Upon liquidation, dissolution
or winding-up, the holders of our common stock are entitled to share ratably in all of our assets which are legally available for
distribution, after payment of or provision for all liabilities and the liquidation preference of any outstanding preferred stock.
The holders of our common stock have no preemptive, subscription, redemption or conversion rights.
Preferred Stock
Our restated certificate of incorporation provides
that the Board is authorized to provide for the issuance of shares of preferred stock in one or more series and, by filing a certificate
of designation pursuant to the applicable law of the State of Delaware, to establish from time to time for each such series the
number of shares to be included in each such series and to fix the designations, powers, rights and preferences of the shares of
each such series, and the qualifications, limitations and restrictions thereof, which may include, among others, dividend rights,
voting rights, liquidation preferences, conversion rights and preemptive rights. The authority of the Board with respect to each
series of preferred stock includes, but is not limited to, determination of the following:
|
|
●
|
the designation of the series, which may be by distinguishing number, letter
or title;
|
|
|
●
|
the number of shares of the series, which number the Board may thereafter
(except where otherwise provided in the certificate of designation) increase or decrease (but not below the number of shares thereof
then outstanding);
|
|
|
●
|
whether dividends, if any, shall be paid, and, if paid, the date or dates
upon which, or other times at which, such dividends shall be payable, whether such dividends shall be cumulative or noncumulative,
the rate of such dividends (which may be variable) and the relative preference in payment of dividends of such series;
|
|
|
●
|
the redemption provisions and price or prices, if any, for shares of the series;
|
|
|
●
|
the terms and amounts of any sinking fund or similar fund provided for the
purchase or redemption of shares of the series;
|
|
|
●
|
the amounts payable on shares of the series in the event of any voluntary
or involuntary liquidation, dissolution or winding up of the affairs of our corporation;
|
|
|
●
|
whether the shares of the series shall be convertible into shares of any other
class or series, or any other security, of our corporation or any other corporation, and, if so, the specification of such other
class or series of such other security, the conversion price or prices, or rate or rates, any adjustments thereto, the date or
dates on which such shares shall be convertible and all other terms and conditions upon which such conversion may be made;
|
|
|
●
|
restrictions on the issuance of shares of the same series or of any other
class or series; and
|
|
|
●
|
the voting rights, if any, of the holders of shares of the series.
|
Preferred stock may be issued in the future in connection
with acquisitions, financings, or other matters as the Board deems appropriate. In the event that any shares of preferred stock
are to be issued, a certificate of designation containing the rights, privileges and limitations of such series of preferred stock
may be filed with the Secretary of State of Delaware. The effect of such preferred stock is that, subject to federal securities
laws and Delaware law, the Board alone may be able to authorize the issuance of preferred stock, which could have the effect of
delaying, deferring or preventing a change in control of us without further action by the stockholders, and may adversely affect
the other rights of the holders of our common stock. The issuance of preferred stock with voting and conversion rights may also
adversely affect the voting power of holders of our common stock, including the loss of voting control to others.
Each series of preferred stock, if issued, will be
more fully described in the particular prospectus supplement that will accompany this prospectus, including redemption provisions,
rights in the event of our liquidation, dissolution or winding up, voting rights and rights to convert into common stock or other
securities. We do not have any shares of our preferred stock presently outstanding.
The purpose of authorizing the board of directors
to issue preferred stock and determine its rights and preferences is to eliminate delays associated with a shareholder vote on
specific issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible acquisitions
and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or of discouraging
a third party from acquiring, a majority of our outstanding voting stock. The effects of issuing preferred stock could include
one or more of the following:
|
|
●
|
decreasing the amount of earnings and assets available for distribution to
holders of common stock;
|
|
|
●
|
diluting the voting power of the common stock;
|
|
|
●
|
impairing the liquidation rights of the common stock; or
|
|
|
●
|
delaying, deferring or preventing changes in our control or management.
|
Anti-Takeover Effects of Certain Provisions of our Certificate of
Incorporation, Bylaws and the DGCL
Delaware Law
We are subject to Section 203 of the DGCL.
This provision generally prohibits a Delaware corporation from engaging in any business combination with any interested stockholder
for a period of three years following the date the stockholder became an interested stockholder, unless:
|
|
●
|
prior to such date, the board of directors approved either the business combination
or the transaction that resulted in the stockholder becoming an interested stockholder;
|
|
|
●
|
upon consummation of the transaction that resulted in the stockholder becoming
an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at
the time the transaction commenced, excluding for purposes of determining the number of shares outstanding those shares owned by
persons who are directors and also officers and by employee stock plans in which employee participants do not have the right to
determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
|
|
|
●
|
on or subsequent to such date, the business combination is approved by the
board of directors and authorized at an annual meeting or special meeting of stockholders and not by written consent, by the affirmative
vote of at least 66-2/3% of the outstanding voting stock that is not owned by the interested stockholder.
|
Section 203 defines a business combination to include:
|
|
●
|
any merger or consolidation involving the corporation and the interested stockholder;
|
|
|
●
|
any sale, transfer, pledge or other disposition of 10% or more of the assets
of the corporation involving the interested stockholder;
|
|
|
●
|
subject to certain exceptions, any transaction that results in the issuance
or transfer by the corporation of any stock of the corporation to the interested stockholder;
|
|
|
●
|
any transaction involving the corporation that has the effect of increasing
the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder;
or
|
|
|
●
|
the receipt by the interested stockholder of the benefit of any loans, advances,
guarantees, pledges or other financial benefits provided by or through the corporation.
|
In general, Section 203 defines an “interested
stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of a corporation, or
an affiliate or associate of the corporation and was the owner of 15% or more of the outstanding voting stock of a corporation
at any time within three years prior to the time of determination of interested stockholder status; and any entity or person affiliated
with or controlling or controlled by such entity or person.
These statutory provisions could delay or frustrate
the removal of incumbent directors or a change in control of our company. They could also discourage, impede, or prevent a merger,
tender offer, or proxy contest, even if such event would be favorable to the interests of stockholders.
Restated Certificate of Incorporation and Bylaw Provisions
Our restated certificate of incorporation and bylaws
contain provisions that could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying
or preventing a change in control, including changes a stockholder might consider favorable. In particular, the certificate of
incorporation and bylaws, as applicable, among other things:
|
|
●
|
permit the Board to issue up to 10,000,000 shares of preferred stock, without
further action by the stockholders, with any rights, preferences and privileges as they may designate;
|
|
|
●
|
provide that all vacancies on the Board, including newly created directorships,
may, except as otherwise required by law, or as determined otherwise by resolution of the Board, be filled by the affirmative vote
of a majority of directors then in office, even if less than a quorum
;
|
|
|
●
|
do not provide for cumulative voting rights (therefore allowing the holders
of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing
for election, if they should so choose);
|
|
|
●
|
set forth an advance notice procedure with regard to the nomination, other
than by or at the direction of the Board, of candidates for election as directors and with regard to business to be brought before
a meeting of stockholders; and
|
|
|
●
|
provide the Board with the ability to alter its bylaws without stockholder
approval.
|
Such provisions may have the effect of discouraging
a third-party from acquiring us, even if doing so would be beneficial to our stockholders. These provisions are intended to enhance
the likelihood of continuity and stability in the composition of the Board and in the policies formulated by them, and to discourage
some types of transactions that may involve an actual or threatened change in control of our company. These provisions are designed
to reduce our vulnerability to an unsolicited acquisition proposal and to discourage some tactics that may be used in proxy fights.
We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly
or unsolicited proposal to acquire or restructure our company outweigh the disadvantages of discouraging such proposals because,
among other things, negotiation of such proposals could result in an improvement of their terms.
However, these provisions could have the effect
of discouraging others from making tender offers for our shares that could result from actual or rumored takeover attempts. These
provisions also may have the effect of preventing changes in our management.
Transfer Agent and Registrar
The Transfer Agent and Registrar for our common
stock is First American Stock Transfer, Inc.
Indemnification of Directors and Officers
Section 145 of the DGCL provides, in general, that
a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or
is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action
by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent
of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another
enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably
incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such
person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action
or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action,
a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably
incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith
and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no
indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable
to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which
such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our bylaws provide that we will indemnify our directors
and officers, to the maximum extent permitted by the DGCL, or any other applicable law, except that we are not required to indemnify
any director or officer in connection with any proceeding initiated by such person unless (i) such indemnification is expressly
required to be made by law or the bylaws, (ii) the proceeding was authorized by the Board, or (iii) such indemnification is provided
by us pursuant to the powers vested in the company under the DGCL or any other applicable law. In addition, our bylaws provide
that we may indemnify our employees and other agents as set forth in the DGCL or any other applicable law. Our bylaws also provide
for the advancement of expenses incurred by a person who was or is a party or is threatened to be made a party to any threatened,
pending or completed proceeding by reason of the fact that the person is or was a director or officer of the company, or is or
was serving at the request of the company as a director or officer of another corporation, partnership, joint venture, trust or
other enterprise, prior to the final disposition of the proceeding, provided, however, that if the DGCL requires, an advancement
of expenses incurred by a director or officer in his or her capacity as a director or officer shall be made only upon delivery
to the company of an undertaking by or on behalf of the indemnitee to repay all amounts so advanced if it shall ultimately be determined
by final judicial decision from which there is no further right to appeal the indemnitee is not entitled to be indemnified for
such expenses under the bylaws. In addition, our restated certificate of incorporation provides that the liability of any of our
directors for monetary damages shall be eliminated to the fullest extent under applicable law. We carry officer and director liability
insurance with respect to certain matters, including matters arising under the Securities Act.
Disclosure of Commission Position on Indemnification for Securities
Act Liabilities
Insofar as indemnification for liabilities arising
under the Securities Act, may be permitted to our directors, officers and persons controlling us, we have been advised that it
is the Securities and Exchange Commission’s opinion that such indemnification is against public policy as expressed in the
Securities Act and is, therefore, unenforceable.
DESCRIPTION OF WARRANTS
The following description, together with the additional
information we may include in any applicable prospectus supplements, summarizes the material terms and provisions of the warrants
that we may offer under this prospectus and the related warrant agreements and warrant certificates. While the terms summarized
below will apply generally to any warrants that we may offer, we will describe the particular terms of any series of warrants in
more detail in the applicable prospectus supplement, information incorporated by reference or a free writing prospectus. If we
so indicate in the prospectus supplement, information incorporated by reference or free writing prospectus, the terms of any warrants
offered under that prospectus supplement, information incorporated by reference and free writing prospectus may differ from the
terms described below. If there are differences between that prospectus supplement, information incorporated by reference or free
writing prospectus and this prospectus, such prospectus supplement, information incorporated by reference or free writing prospectus
will control. Thus, the statements we make in this section may not apply to a particular series of warrants. Specific warrant agreements
will contain additional important terms and provisions and will be incorporated by reference as an exhibit to the registration
statement which includes this prospectus.
We may issue warrants for the purchase of common
stock and/or preferred stock, or units, in one or more series. We may issue warrants independently or together with common stock,
preferred stock and/or units, and the warrants may be attached to or separate from these securities.
We will evidence each series of warrants by warrant
certificates that we may issue under a separate agreement. We may enter into the warrant agreement with a warrant agent which may
be a bank or other institution that we select. We may also choose to act as our own warrant agent. We will indicate the name and
address of any such warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
We will describe in the applicable prospectus supplement,
information incorporated by reference or free writing prospectus the terms of the series of warrants, including:
|
|
●
|
the offering price and aggregate number of warrants offered;
|
|
|
●
|
the currency for which the warrants may be purchased;
|
|
|
●
|
if applicable, the designation and terms of the securities with which the
warrants are issued and the number of warrants issued with each such security or each principal amount of such security;
|
|
|
●
|
if applicable, the date on and after which the warrants and the related securities
will be separately transferable;
|
|
|
●
|
in the case of warrants to purchase common stock or preferred stock, the number
of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at
which these shares may be purchased upon such exercise;
|
|
|
●
|
the warrant agreement under which the warrants will be issued;
|
|
|
●
|
the effect of any merger, consolidation, sale or other disposition of our
business on the warrant agreement and the warrants;
|
|
|
●
|
anti-dilution provisions of the warrants, if any;
|
|
|
●
|
the terms of any rights to redeem or call the warrants;
|
|
|
●
|
any provisions for changes to or adjustments in the exercise price or number
of securities issuable upon exercise of the warrants;
|
|
|
●
|
the dates on which the right to exercise the warrants will commence and expire
or, if the warrants are not continuously exercisable during that period, the specific date or dates on which the warrants will
be exercisable;
|
|
|
●
|
the manner in which the warrant agreement and warrants may be modified;
|
|
|
●
|
the identities of the warrant agent and any calculation or other agent for
the warrants;
|
|
|
●
|
federal income tax consequences of holding or exercising the warrants;
|
|
|
●
|
the terms of the securities issuable upon exercise of the warrants;
|
|
|
●
|
information with respect to book-entry procedures, if any;
|
|
|
●
|
any securities exchange or quotation system on which the warrants or any securities
deliverable upon exercise of the warrants may be listed; and
|
|
|
●
|
any other specific terms, preferences, rights or limitations of or restrictions
on the warrants.
|
Unless otherwise described in an applicable prospectus
supplement, information incorporated by reference or free writing prospectus, before exercising their warrants, holders of warrants
will not have any of the rights of holders of the securities purchasable upon such exercise, including in the case of warrants
to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution
or winding up or to exercise voting rights, if any.
Exercise of Warrants
Each warrant will entitle the holder to purchase
the securities that we specify in the applicable prospectus supplement, information incorporated by reference or free writing prospectus,
at the exercise price that we describe therein. Unless we otherwise specify in the applicable prospectus supplement, information
incorporated by reference or free writing prospectus, holders of the warrants may exercise the warrants at any time up to 5:00 p.m.
Eastern Time on the expiration date that we set forth in the applicable prospectus supplement, information incorporated by reference
or free writing prospectus. After the close of business on the expiration date, unexercised warrants will become void.
A warrant will entitle the holder to purchase for
cash an amount of securities at an exercise price that will be stated in, or that will be determinable as described in, the applicable
prospectus supplement, information incorporated by reference or free writing prospectus. Warrants may be exercised, redeemed, as
set forth in the applicable offering material.
Until the warrant is properly exercised, no holder
of any warrant will be entitled to any rights of a holder of the securities purchasable upon exercise of the warrant and will not
be entitled to dividend payments, if any, or voting rights of the common stock or preferred stock purchasable upon such exercise.
Upon receipt of the required payment and the warrant
certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated
in the applicable prospectus supplement, information incorporated by reference or free writing prospectus, we will issue and deliver
the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised,
then we will issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable prospectus
supplement, information incorporated by reference or free writing prospectus, holders of the warrants may surrender securities
as all or part of the exercise price for warrants.
Warrant Agreement
We may issue the warrants in one or more series
under one or more warrant agreements, each to be entered into between us and a warrant agent, which may include a bank, trust company
or other financial institution as warrant agent. We may add, replace or terminate warrant agents from time to time. We may also
choose to act as our own warrant agent or may choose one of our subsidiaries to do so.
The warrant agent under a warrant agreement will
act solely as our agent in connection with the warrants issued under that agreement. Any holder of warrants may, without the consent
of any other person, enforce by appropriate legal action, on its own behalf, its right to exercise those warrants in accordance
with their terms.
Form, Exchange and Transfer
We may issue the warrants in registered form or
bearer form. Warrants issued in registered form, i.e., book-entry form, will be represented by a global security registered in
the name of a depository, which will be the holder of all the warrants represented by the global security. Those investors who
own beneficial interests in a global warrant will do so through participants in the depository’s system, and the rights of
these indirect owners will be governed solely by the applicable procedures of the depository and its participants. In addition,
we may issue warrants in non-global form, i.e., bearer form. If any warrants are issued in non-global form, warrant certificates
may be exchanged for new warrant certificates of different denominations, and holders may exchange, transfer or exercise their
warrants at the warrant agent’s office or any other office indicated in the applicable prospectus supplement, information
incorporated by reference or free writing prospectus.
No warrant agreement will be qualified as an indenture,
and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders of warrants issued
under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
DESCRIPTION OF UNITS
We may issue units comprised of one or more of the
other securities described in this prospectus in any combination. Each unit will be issued so that the holder of the unit is also
the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of
each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may
not be held or transferred separately, at any time or at any time before a specified date.
The following description is a summary of selected
provisions relating to units that we may offer. The summary is not complete. When units are offered in the future, a prospectus
supplement, information incorporated by reference or a free writing prospectus, as applicable, will explain the particular terms
of those securities and the extent to which these general provisions may apply. The specific terms of the units as described in
a prospectus supplement, information incorporated by reference, or free writing prospectus will supplement and, if applicable,
may modify or replace the general terms described in this section.
This summary and any description of units in the
applicable prospectus supplement, information incorporated by reference or free writing prospectus is subject to and is qualified
in its entirety by reference to the unit agreement, collateral arrangements and depositary arrangements, if applicable. We will
file each of these documents, as applicable, with the SEC and incorporate them by reference as an exhibit to the registration statement
of which this prospectus is a part on or before the time we issue a series of units. See “Where You Can Find More Information”
and “Incorporation of Documents by Reference” above for information on how to obtain a copy of a document when it is
filed.
The applicable prospectus supplement, information
incorporated by reference or free writing prospectus will describe:
|
|
●
|
the designation and terms of the units and of the securities comprising the
units, including whether and under what circumstances those securities may be held or transferred separately;
|
|
|
●
|
any unit agreement under which the units will be issued;
|
|
|
●
|
any provisions for the issuance, payment, settlement, transfer or exchange
of the units or of the securities comprising the units; and
|
|
|
●
|
whether the units will be issued in fully registered or global form.
|
The applicable provisions described in this section,
as well as those described under “Description of Capital Stock” and “Description of Warrants” above, will
apply to each unit and to each security included in each unit, respectively.
PLAN OF DISTRIBUTION
We may sell the securities being offered pursuant
to this prospectus to or through underwriters or dealers, through agents, or directly to one or more purchasers (including our
affiliates and shareholders), through a specific bidding or auction process, a rights offering or otherwise, through a combination
of these methods or through any other methods described in a prospectus supplement. The applicable prospectus supplement will describe
the terms of the offering of the securities, including:
|
|
●
|
the name or names of any underwriters, if any, and if required, any dealers
or agents;
|
|
|
●
|
the purchase price of the securities and the proceeds we will receive from
the sale;
|
|
|
●
|
any underwriting discounts and other items constituting underwriters’
compensation;
|
|
|
●
|
any discounts or concessions allowed or reallowed or paid to dealers; and
|
|
|
●
|
any securities exchange or market on which the securities may be listed.
|
The distribution of securities may be effected,
from time to time, in one or more transactions, including:
|
|
●
|
block transactions (which may involve crosses) and transactions on the Nasdaq
Capital Market or any other organized market where the securities may be traded;
|
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer
for its own account pursuant to a prospectus supplement;
|
|
|
●
|
ordinary brokerage transactions and transactions in which a broker-dealer
solicits purchasers;
|
|
|
●
|
sales “at the market” to or through a market maker or into an
existing trading market, on an exchange or otherwise; and
|
|
|
●
|
sales in other ways not involving market makers or established trading markets,
including direct sales to purchasers.
|
The securities may be sold at a fixed price or prices,
which may be changed, or at market prices prevailing at the time of sale, at prices relating to the prevailing market prices or
at negotiated prices. The consideration may be cash or another form negotiated by the parties.
We may also make direct sales through subscription
rights distributed to our existing shareholders on a pro rata basis, which may or may not be transferable. In any distribution
of subscription rights to our shareholders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed
securities directly to third parties or may engage the services of one or more underwriters, dealers or agents, including standby
underwriters, to sell the unsubscribed securities to third parties.
Some or all of the securities that we offer through
this prospectus may be new issues of securities with no established trading market. Any underwriters to whom we sell our securities
for public offering and sale may make a market in those securities, but they will not be obligated to do so and they may discontinue
any market making at any time without notice. Accordingly, we cannot assure you of the liquidity of, or continued trading markets
for, any securities that we offer.
Only underwriters named in the prospectus supplement
are underwriters of the securities offered by the prospectus supplement.
If underwriters are used in an offering, we will
execute an underwriting agreement with such underwriters and will specify the name of each underwriter and the terms of the transaction
(including any underwriting discounts and other terms constituting compensation of the underwriters and any dealers) in a prospectus
supplement. The securities may be offered to the public either through underwriting syndicates represented by managing underwriters
or directly by one or more investment banking firms or others, as designated. If an underwriting syndicate is used, the managing
underwriter(s) will be specified on the cover of the prospectus supplement. If underwriters are used in the sale, the offered securities
will be acquired by the underwriters for their own accounts and may be resold from time to time in one or more transactions, including
negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale, or under delayed
delivery contracts or other contractual commitments. Any public offering price and any discounts or concessions allowed or reallowed
or paid to dealers may be changed from time to time. Unless otherwise set forth in the prospectus supplement, the obligations of
the underwriters to purchase the offered securities will be subject to conditions precedent and the underwriters will be obligated
to purchase all of the offered securities if any are purchased.
We may grant to the underwriters options to purchase
additional securities to cover over-allotments, if any, at the public offering price, with additional underwriting commissions
or discounts, as may be set forth in a related prospectus supplement. The terms of any over-allotment option will be set forth
in the prospectus supplement for those securities.
If we use a dealer in the sale of the securities
being offered pursuant to this prospectus or any prospectus supplement, we or an underwriter will sell the securities to the dealer,
as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time
of resale. To the extent required, we will set forth in the prospectus supplement, document incorporated by reference or free writing
prospectus, as applicable, the name of the dealer and the terms of the transactions.
We may directly solicit offers to purchase the securities
and may make sales of securities directly to institutional investors or others. These persons may be deemed to be underwriters
with respect to any resale of the securities. To the extent required, the prospectus supplement, document incorporated by reference
or free writing prospectus, as applicable, will describe the terms of any such sales, including the terms of any bidding or auction
process, if used.
We may sell the securities directly or through agents
we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any
commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, any agent will
act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit
offers by institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement
pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the
conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
In connection with the sale of the securities, underwriters,
dealers or agents may receive compensation from us or from purchasers of the securities for whom they act as agents in the form
of discounts, concessions, commissions or other payments. Underwriters may sell the securities to or through dealers, and those
dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from
the purchasers for whom they may act as agents. Underwriters, dealers and agents that participate in the distribution of the securities,
and any institutional investors or others that purchase securities directly and then resell the securities, may be deemed to be
underwriters, and any discounts or commissions received by them from us and any profit on the resale of the securities by them
may be deemed to be underwriting discounts and commissions under the Securities Act. If such persons were deemed to be underwriters,
they may be subject to statutory liabilities under the Securities Act.
Any person participating in the distribution of
common stock registered under the registration statement that includes this prospectus will be subject to applicable provisions
of the Exchange Act, and the applicable SEC rules and regulations, including, among others, Regulation M, which may limit the timing
of purchases and sales of any of our common stock by any such person. Furthermore, Regulation M may restrict the ability of any
person engaged in the distribution of our common stock to engage in market-making activities with respect to our common stock.
These restrictions may affect the marketability of our common stock and the ability of any person or entity to engage in market-making
activities with respect to our common stock.
We may provide agents and underwriters with indemnification
against particular civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments
that the agents or underwriters may make with respect to such liabilities. Agents and underwriters may engage in transactions with,
or perform services for, us in the ordinary course of business.
In addition, we may enter into derivative transactions
with third parties (including the writing of options), or sell securities not covered by this prospectus to third parties in privately
negotiated transactions. If the applicable prospectus supplement indicates, in connection with such a transaction, the third parties
may, pursuant to this prospectus and the applicable prospectus supplement, sell securities covered by this prospectus and the applicable
prospectus supplement. If so, the third party may use securities borrowed from us or others to settle such sales and may use securities
received from us to close out any related short positions. We may also loan or pledge securities covered by this prospectus and
the applicable prospectus supplement to third parties, who may sell the loaned securities or, in an event of default in the case
of a pledge, sell the pledged securities pursuant to this prospectus and the applicable prospectus supplement. The third party
in such sale transactions will be an underwriter and will be identified in the applicable prospectus supplement or in a post-effective
amendment.
To facilitate an offering of a series of securities,
persons participating in the offering may engage in transactions that stabilize, maintain, or otherwise affect the market price
of the securities. This may include over-allotments or short sales of the securities, which involves the sale by persons participating
in the offering of more securities than have been sold to them by us. In those circumstances, such persons would cover such over-allotments
or short positions by purchasing in the open market or by exercising the over-allotment option granted to those persons. In addition,
those persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market
or by imposing penalty bids, whereby selling concessions allowed to underwriters or dealers participating in any such offering
may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these
transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail
in the open market. Such transactions, if commenced, may be discontinued at any time. We make no representation or prediction as
to the direction or magnitude of any effect that the transactions described above, if implemented, may have on the price of our
securities.
Any common stock sold pursuant to a prospectus supplement
will be eligible for quotation and trading on The NASDAQ Capital Market. Any underwriters to whom securities are sold by us for
public offering and sale may make a market in the securities, but such underwriters will not be obligated to do so and may discontinue
any market making at any time without notice.
In order to comply with the securities laws of some
states, if applicable, the securities offered pursuant to this prospectus will be sold in those states only through registered
or licensed brokers or dealers. In addition, in some states securities may not be sold unless they have been registered or qualified
for sale in the applicable state or an exemption from the registration or qualification requirement is available and complied with.
In compliance with the guidelines of the Financial
Industry Regulatory Authority (“FINRA”), the aggregate maximum discount, commission or agency fees or other items constituting
underwriting compensation to be received by any FINRA member or independent broker-dealer will not exceed 8% of any offering pursuant
to this prospectus and any applicable prospectus supplement, as the case may be.
If more than 10% of the net proceeds of any offering
of securities made under this prospectus will be received by FINRA members participating in the offering or affiliates or associated
persons of such FINRA members, the offering will be conducted in accordance with FINRA Conduct Rule 5110(h).
So long as the aggregate market value of our voting
and non-voting common equity held by non-affiliates is less than $75,000,000.00 and so long as required by the rules of the SEC,
the amount of securities we may offer hereunder will be limited such that the aggregate market value of securities sold by us during
a period of 12 calendar months cannot exceed one-third of the aggregate market value of the voting and non-voting common equity
held by non-affiliates.
To the extent required, this prospectus may be amended
or supplemented from time to time to describe a specific plan of distribution.
LEGAL MATTERS
The validity of the issuance of the securities offered
hereby will be passed upon for us by Weintraub Tobin Chediak Coleman Grodin, Law Corporation.
EXPERTS
The financial statements as of March 31, 2014
and 2013 and for the two years in the period ended March 31, 2014, included in this prospectus have been so included in reliance
on the report of Mayer Hoffman McCann P.C., an independent registered public accounting firm, appearing elsewhere herein, given
on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement
on Form S-3 under the Securities Act of 1933, as amended (“Securities Act”), with respect to the securities covered
by this prospectus. This prospectus and any prospectus supplement which form a part of the registration statement, do not contain
all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information
with respect to us and the securities covered by this prospectus, please see the registration statement and the exhibits filed
with the registration statement. Any statements made in this prospectus or any prospectus supplement concerning legal documents
are not necessarily complete and you should read the documents that are filed as exhibits to the registration statement or otherwise
filed with the SEC for a more complete understanding of the document or matter. A copy of the registration statement and the exhibits
filed with the registration statement may be inspected without charge at the Public Reference Room maintained by the SEC, located
at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation
of the Public Reference Room. The SEC also maintains an Internet website that contains reports, proxy and information statements
and other information regarding registrants that file electronically with the SEC. The address of the website is
http://www.sec.gov
.
We file annual, quarterly and current reports,
proxy statements and other information with the SEC. You may read, without charge, and copy the documents we file at the SEC’s
public reference rooms in Washington, D.C. at 100 F Street, NE, Room 1580, Washington, D.C. 20549. You can request copies
of these documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for further
information on the public reference rooms. Our SEC filings are also available to the public at no cost from the SEC’s website
at
http://www.sec.gov
.
INCORPORATION OF DOCUMENTS BY REFERENCE
We have filed a registration statement on Form S-3
with the Securities and Exchange Commission under the Securities Act. This prospectus is part of the registration statement but
the registration statement includes and incorporates by reference additional information and exhibits. The Securities and Exchange
Commission permits us to “incorporate by reference” the information contained in documents we file with the Securities
and Exchange Commission, which means that we can disclose important information to you by referring you to those documents rather
than by including them in this prospectus. Information that is incorporated by reference is considered to be part of this prospectus
and you should read it with the same care that you read this prospectus. Information that we file later with the Securities and
Exchange Commission will automatically update and supersede the information that is either contained, or incorporated by reference,
in this prospectus, and will be considered to be a part of this prospectus from the date those documents are filed. We have filed
with the Securities and E
xchange Commission, and incorporate by reference
in this prospectus:
|
|
☐
|
Annual
Report on Form 10-K for the year ended March 31, 2014, filed on June 23, 2014; and
|
|
|
☐
|
The
description of our common stock contained in our Form 8-A filed on December 11, 2013.
|
We also incorporate by reference all additional
documents that we file with the Securities and Exchange Commission under the terms of Sections 13(a), 13(c), 14 or 15(d) of the
Exchange Act that are made after the initial filing date of the registration statement of which this prospectus is a part until
the offering of the particular securities covered by a prospectus supplement or term sheet has been completed. We are not, however,
incorporating, in each case, any documents or information that we are deemed to furnish and not file in accordance with Securities
and Exchange Commission rules.
You may request, and we will provide you with, a
copy of these filings, at no cost, by calling us at (858) 997-2400 or by writing to us at the following address:
Adamis Pharmaceuticals Corporation
11682 El Camino Real, Suite 300
San Diego, CA 92130
Attn: Corporate Secretary
4,285,715
Shares
of Common Stock

|
PROSPECTUS
SUPPLEMENT
|
|
Sole
Book-Running Manager
RAYMOND
JAMES
Co-Manager
MAXIM GROUP
LLC
|
April 21, 2017
Adamis Pharmaceuticals (NASDAQ:ADMP)
Historical Stock Chart
From Mar 2024 to Apr 2024
Adamis Pharmaceuticals (NASDAQ:ADMP)
Historical Stock Chart
From Apr 2023 to Apr 2024