- Coming up on June 10th, 2014, Orexigen Therapeutics expects a Food and Drug Administration (FDA) for it’s weight loss drug NB32 (Contrave).
- Contrave was initially rejected by the FDA due to a single issue cardiovascular safety concern and requested the company engage a study to provide data to address the concern.
- Orexigen’s “Light Study” was successfully completed on 8900 patients and showed Contrave did not increase the risk of major adverse cardiovascular events (MACE).
- Due to both the FDA Special Protocol Assessment (SPA) designation and the positive Light Study, it’s virtually certain that Contrave will win FDA approval on June 10th.
- Because Contrave has safety data that other weight loss drugs do not have, notwithstanding excellent efficacy, we believe it will be the drug of choice prescribed by doctors for patients.
The prediction of the FDA approving Contrave is an easy one. If Orexigen (OREX) has followed the agreed protocols in the SPA and the company has not deviated from the agreement, the FDA is bound to issue an approval.
The FDA decision for Contrave is expected on June 10th, so about two weeks from now. With overall market conditions improving and the focus on additional stimulus from the Bank of Japan and the European Central Bank meeting in early June, we expect to see biotech catalyst traders come onboard here and drive the stock price up considerably over this time frame.
- Contrave efficacy
In clinical trials in excess of 4,500 people, 53% of those taking Contrave and 21% of those taking placebo lost five percent or more of their body weight over the twelve month trial duration, with many patients seeing improvements in cholesterol levels and blood sugar control. For those who actually went on a proper diet and properly exercised, the results were predictably better.
Once Contrave is approved, it will be marketed by Takeda Pharma (OTCPK:TKPHF), a large Japanese company worth in excess of $35B. We feel because of the extensive safety studies Orexigen has engaged in and the SPA agreement, it will become the weight loss drug choice of doctors. Doctors want to feel comfortable knowing they are prescribing drugs that are proven safe for patients. Cardiovascular issues seems to be the number one concern with these weight loss drugs. Belviq and Qsymia are unknowns in this regard while Contrave has the positive safety data from the Light Study.
We feel this data will tip the scripts in favor of Contrave in a large way.
It’s worth mentioning tone very important factor with Contrave over Belviq and Qsymia that should be noted; with the light study in hand, Contrave has the necessary Cardiovascular safety data needed European approval. This where both Vivus and Arena failed — their safety data was not good enough for approval in Europe.
The European approval decision for Contrave is expected in late August or early September. This is yet an additional price driver for Orexigen.
Additionally, Orexigen has another potential near-term catalyst that should be considered in terms of short term stock price appreciation, notwithstanding the upcoming FDA approval date for Contrave on June 10th;
Orexigen is developing another weight loss drug called Empatic, which is a fixed-dose combination of a proprietary formulation of zonisamide sustained release (SR) and bupropion SR.
In a Phase IIB clinical trial of Empatic 360 mg, patients completing 24 weeks of therapy, lost 9.9% of their baseline body weight, or 22 pounds, compared to 1.7% for placebo patients (p<0.001). Additionally, 82.6% of these patients lost at least 5% of their baseline body weight and 47.7% lost at least 10% of their baseline body weight compared to 18.9% and 5.7% of placebo patients, respectively (p<0.001 for both).
Orexigen has been waiting to engage a Phase III trial of Empatic, as the Phase II clinical has been completed as shown above. We believe that once Contrave is approved June 10th that Orexigen is likely to gain a partner for Empatic which should add additional upside to Orexigen’s stock price.
- Chart
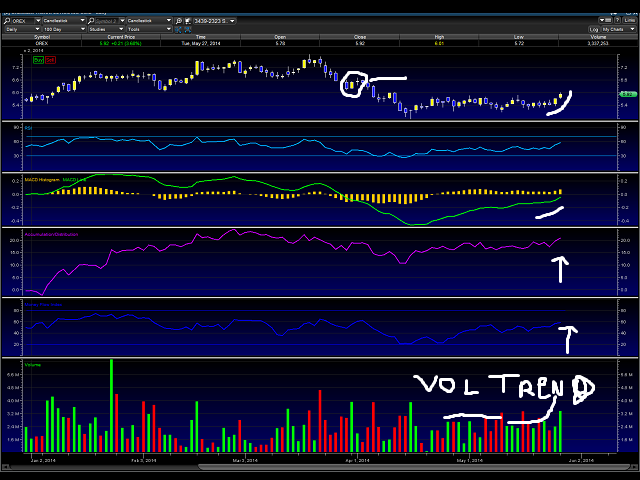
We can see above, that the volume trend is increasing, along with a new wave forming off of a tight wedge. With market conditions improving with the $IBB closing over $240 in its’ last trading session, we see strong upside with the $OREX trade. I have marked where I believe the stock is headed to — around $7 a share in the next few trading sessions.
- Conclusion
As mentioned prior, with overall market conditions improving and the focus on additional stimulus from the Bank of Japan and the European Central Bank meeting in early June, we expect to see biotech catalyst traders come onboard here and drive the stock price upwards. Also mentioned is Orexigen’s compliance with the SPA designation from the FDA. The company was asked to engage a cardiovascular study and went above and beyond the request with an 8900 person study that proved Contrave to be safe in this regard.
Contrave has a unique advantage over both Arena’s (ARNA) Belviq and Vivus’s (VVUS) Qsymia with this extensive and large cardiovascular study. We feel this will give most doctors the confidence in prescribing Contrave.
Also, it’s likely that Orexigen’s Empatic gains a larger pharma partner soon after the likely Contrave approval, which would serve as an additional stock price catalyst.
Additionally, the iShares Nasdaq Biotechnology (IBB), the benchmark index used to track biotech, is on the verge of breaking through its resistance level of $235. The IBB saw a good deal of technical damage done over the last few weeks due to geopolitical risks and uncertain Federal Reserve policy. With a “risk on” trade coming back to the market, we feel both biotech and small cap names that have been beaten down will lead a rally moving forward into the summer.
On the insider transactions, it’s worth noting that Orexigen board of director Brian Dovey executed an option to receive 4,691,470 sharesin April, of which he disposed (sold) only 886 shares. This shows us a good deal of insider confidence in the company’s chances in our opinion. Mr. Dovey currently holds 7,580,065 shares of Orexigen.
We think Orexigen will ultimately be a successful company and that Contrave will lead the weight loss drug market. Considering the factors mentioned in this article, our one year price target opinion on Orexigen is $12. We strongly feel with the short term catalysts in focus, the stock can reach a price of $7+.
The risks we see with Orexigen are more concerned with downside pressure to the stock caused by geopolitical and stimulus concerns from Japan and Europe. Additional concerns from these would mean the IBB and small caps in general would take a hit again, and could cause Orexigen shares to “run down” before June 10th. As mentioned, we feel these risks are behind the market at least for now.






