
BIG MEDICAL
COMPANIES ARE INVESTING IN ELECTROCEUTICALS – A BREAKTHROUGH IN
THERAPEUTICS
June 15,
2021 -- InvestorsHub NewsWire -- via BioResearch Alert
--
- Time Magazine
published, "Why It's
Time to Take Electrified Medicine (Electroceuticals)
Seriously."
- Scientific
American
published, "Electroceuticals
– Nerve stimulating therapies could soon replace drugs for many
chronic conditions."
- GlaxoSmithKline, Microsoft,
Medtronic, Boston Scientific, Abbott Labs, Sonova are
also developing "Electroceuticals" to treat a broad spectrum of
diseases that are now principally treated with
pharmaceuticals.
- GlaxoSmithKline
committed
$715 million to developing
"Electroceuticals."
-
National Institutes of Health (NIH) announced a fund
of $248
million to
be distributed to public and private research communities through
Stimulating Peripheral Activity to Relieve Conditions
(SPARC)
to develop future "electroceuticals."
- Health Canada
cleared CELL MEDX's eBalance® Microcurrent
"Electroceutical"
as a Class II
Medical Device System for Professional and Home Use for the
treatment of pain and general relaxation.
- CELL MEDX
shared early but promising results
that warrant further studies and development of their
'Electroceutical' technology in the hopes of treating a broad
spectrum of medical indications including pain, diabetes, wound
healing and high blood pressure.

"Electroceuticals"
May Hold
New Potential to Treat Disease
Do you
remember when you first heard the words, "biotech" or "recombinant
DNA technology" and learned about the visionary companies looking
to take these futuristic inventions public? This was so
cutting-edge that only
those with drive, vision and grit could have imagined this
future.
It was those
early pioneers that paved the way for the biotech revolution with
their dedication to pursuing groundbreaking science.
Pharmaceuticals
are the primary tool conventional medicine uses to treat most
medical conditions, but there is a growing movement by some large
pharmaceutical companies to learn more about treating certain
disease states with "Electroceuticals" that send
microcurrents
of electricity throughout the body with the intention of correcting
electrical abnormalities or blocked electron flows.
Mounting Evidence: "Electroceuticals" — the Future of
Medicine
There is
mounting evidence that "Electroceuticals" may be a
new way to
treat disease by correcting impaired electron flow that could be
disease related. If successful, there is a potential for a new era
of medicine to unfold without the use of chemicals and their side
effects.
Every cell in our body uses
electricity or electron flow to
communicate and to stay healthy. A disruption
in these electrical currents and electric states can lead to
illness or disease.
In
order for the heart to pump, cells must generate electrical
currents that allow the heart muscle to contract at the right time.
Doctors can even observe these electrical pulses in the heart using
a machine, called an electrocardiogram or ECG.
Irregular
electrical
currents can prevent heart muscles from contracting correctly,
leading to a heart attack. This is just one example showing the
important role of electricity in health and disease.
Correcting the
way cells work and how they signal other cells is
the science
behind "Electroceuticals" and it looks to be big
business.

CELL MEDX Corp. (CMXC: OTC) is an early-stage biotech
company focusing on the discovery, development and
commercialization of therapeutic microcurrent "Electroceuticals"
using
their
eBalance® Technology.
Both
the
eBalance®
Home System and
eBalance®
Pro System
are Health
Canada cleared Class II Medical Device Systems for the treatment of
pain and general relaxation.
CELL MEDX
plans to file their FDA 510(k) submission for their
eBalance® Systems in 2021 — followed
by potential clinical trials for additional indications.
CELL MEDX clearly has great
momentum — why keep going if they already have Health Canada
clearance today and potentially FDA premarket
clearance
later this year?
Both the Health Canada
clearance and FDA submissions are for the use of their
eBalance® Systems for the treatment
of pain
— but there may
be potential to expand that indication based on some of their early
stage
R&D.
CELL MEDX
completed a Health Canada Approved Open Label, 3-Month Evaluation
Study
Observational Clinical Trial in 30 Patients
with Type I and Type II Diabetes. The observational clinical trial
was completed by Hamilton Medical Research Group in Hamilton,
Ontario. The results from that trial were promising for
their eBalance®
technology
"Electroceutical."

The trial was
conducted in accordance with the ethical principles of Good
Clinical Practice, per the ICH Harmonized Tripartite Guidelines.
The trial was reviewed and approved by the Research Ethics Board
(REB);
and received regulatory approval from Health Canada to perform
investigational testing prior to implementation.
Once the
3-Month Observational
Trial was finalized, the researchers concluded: "there were several
encouraging trends in HbA1c, and secondary efficacy endpoints
assessing insulin resistance, insulin sensitivity, blood pressure
and kidney function... which warrant further
exploration."
While CELL
MEDX's eBalance®
technology showed real results in real patients in diabetes, blood
pressure and kidney function, the Company brought their medical
device systems to market first in Canada for the indications of the
treatment of pain and general
relaxation — with an eye to expanding those indications in
future.
What's next
for this Company? CELL MEDX plans to file their FDA 510(k)
submission in 2021 then turn their attention back to their clinical
trials to potentially broaden the scope of those
approvals. And, of course, continue their research to bring new
"Electroceuticals" to market.
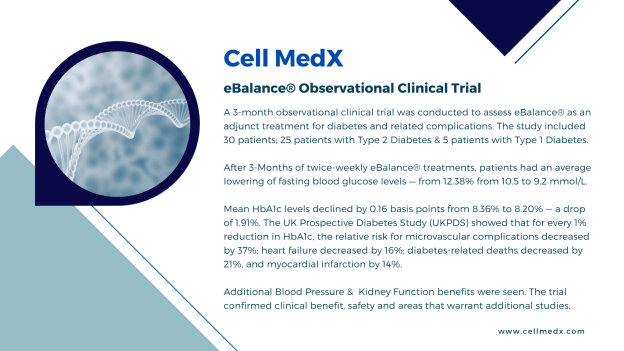
CELL MEDX
Corp.
provided additional information on its
observational clinical trial with diabetes and related
conditions.
Diabetes:
Efficacy Results
Primary
Efficacy Endpoint: On average, the mean Hemoglobin
A1c (HbA1c) decreased by 0.16% ±0.82 following the treatment
intervention at the end of the study compared to baseline
HbA1c.
Type 1 diabetes is an
auto-immune disorder which causes the pancreas to produce little or
no insulin
and leads to high blood glucose levels. Type 2 diabetes occurs as a
result of decreased insulin effectiveness or production which also
leads to high blood glucose levels. During the Trial, the
effectiveness of the Company's eBalance® therapy as an
adjunct
treatment for diabetes and related complications in Type 1 and Type
2 diabetics was assessed over 3 months.
In non-diabetics, insulin
rises sharply after a meal, attaching to a receptor on the cell
surface, allowing glucose to rapidly enter the cell. In
Type 2 diabetes,
insulin is less able to stimulate the entry of glucose, leading to
high blood glucose levels and sustained elevated levels of
insulin.
When hemoglobin in the red
blood cells combines with glucose, it is referred to as glycated
hemoglobin or HbA1c. Measuring HbA1c
provides an estimate of average glucose levels over
a
8 to 12 week
period — the life span of red blood cells.
The aim of this observational
study was to measure fasting blood glucose and changes in
glycosylated hemoglobin levels in type 1 and type 2 diabetes
mellitus patients following 3-months of eBalance® treatment.
Plasma insulin declined from
167.93 pmol/L to 86.38
pmol/L — a 48.6% decrease and
mean change of -78.50 from baseline. The changes in plasma insulin
results indicate that, on average, the
blood glucose uptake was increased and that less insulin was
required to achieve that uptake.
Average fasting blood glucose
levels also declined — from 12.38% from 10.5 to 9.2 mmol/L.
Significantly, mean HbA1c levels declined by 0.16 basis points from 8.36%
to 8.20% — a drop of 1.91%.
To give you a sense of how
significant a 1% drop in HbA1c is for patients, we can look to the
UK Prospective Diabetes Study (UKPDS) — one of the largest diabetes
clinical trials ever conducted.
The UK Prospective Diabetes
Study
(UKPDS) showed that for every 1%
reduction in HbA1c, the relative risk for: microvascular
complications decreased by 37%; heart failure decreased by 16%;
diabetes-related deaths decreased by 21%; and,
myocardial
infarction by 14%.
Future studies evaluating the
role of eBalance® technology as an adjunct
therapy for diabetes can use this HbA1c data to design
double-blind,
placebo
controlled trials adequately powered to
detect changes in HbA1c between placebo and treatment groups and
assess the use of eBalance® microcurrent therapy as a
potential treatment for diabetes.
Blood
pressure: Efficacy Results
On average, there was a trend
towards a decrease in blood pressure, as measured by systolic and
diastolic blood pressure compared to baseline.
Interestingly, the mean
change from baseline in both systolic and diastolic blood pressure
decreased gradually from weeks 1-7, plateauing
and stabilizing from week 7 to the end of the study at week
11.
After 3-months of treatments,
systolic pressure, the higher amount
of pressure in the arteries during the contraction of the heart
muscle, declined by 9.6% from 142 to 128 millimeters of mercury
(mm Hg) and stabilized at the lower
lever through to the end of the study.
During the same period,
diastolic pressure, the lower pressure number in the arteries when
the heart muscle is between beats, declined by 10.4% from
78 to 70 mm Hg
and also remained at the lower level. The Company has been
encouraged to undertake further studies on subjects with higher
blood pressures to determine if a proportional effect is
obtained.
If this does occur, a 10%
decrease in blood pressure for individuals at risk
from high blood pressure could be very beneficial without the side
effects of medications.
Pain and
numbness: Efficacy Results
Neuropathy is nerve damage
that can occur with diabetes as a result of high blood glucose
levels and
high blood pressure. The damage most often affects the extremities
and causes pain, tingling or numbness in the hands, arms, legs and
feet. Only two subjects suffered from pain at the beginning of
the Trial and both reported feeling
either less pain or reduced coldness or numbness
in their extremities. Future studies will need to be conducted with
a larger number of subjects experiencing pain
and loss of feeling to determine true efficacy in
these
areas.
Kidney
function (Nephropathy): Efficacy Results
On average, there was a trend
towards a decrease in one marker assessing kidney
function,
which was eGFR compared to baseline.
Nephropathy is damage caused
to the small blood vessels in the kidneys by high blood glucose
levels and high blood pressure that prevents them from functioning
properly or even causes them to fail completely.
When the blood vessels in the
kidneys are injured, the kidneys cannot
clean the blood properly. The body will retain more water and salt
than it should, which can result in weight gain and edema. The
decrease in eGFR (estimated glomerular filtration rate) observed in
the Trial and a reduction in edema seen in the
Company's
research and development testing may warrant further
investigation to assess the effect of eBalance® treatments on kidney
function.
Study
Conclusions: The 3-month observational
clinical trial with diabetes and related conditions confirmed
clinical benefit, safety and areas that warrant additional
investigation.

Electroceuticals — The Next
Billion Dollar Bioelectric Market
Electroceuticals are an
exciting
new area of therapeutics being pioneered by a growing list of
companies. This rapidly growing market is projected to reach USD
33.14 billion by 2025, growing at a CAGR of 7.67% according
to
Kenneth Research.
As
Time Magazine speculates, "Even with the
still rudimentary efforts at stimulating some of the larger nerves
in the body to treat, for example, headaches and chronic pain,
financial analysts expect the market to reach $7 billion by
2025."
CELL MEDX already has
clearance for their "Electroceutical" in the
treatment of pain today. The market
will watch with interest as
CELL MEDX
and other
"Electroceutical" trail blazers — bold, forward-thinking companies
— bring groundbreaking solutions to a new field of
therapeutics.
"Electroceuticals" — An
exciting growing market at the vanguard of cutting-edge
science.

Disclaimer:
This report is for
information purposes only and is neither a solicitation or
recommendation to buy nor an offer to sell securities. Information,
opinions, and analysis contained herein are based on sources
believed to be reliable, but no representation,
expressed
or implied, is made as to its accuracy, completeness or
correctness. The opinions contained herein reflect our current
judgment and are subject to change without notice.
Cell MedX Corp. accepts no liability
for any losses arising from an investor's reliance on the use of
this material. Richard Cavalli and Howard Isaacs
("Bioresearchalert")
have been compensated by the Company. Richard Cavalli is
compensated $5,000 USD cash per month for a period of 3-months with
the option to renew. Howard Isaacs is compensated $5,000 USD
cash per month or 25,000 Restricted Common Shares (RCS). At the
time of publishing, neither Mr. Cavalli nor Mr. Isaacs holds shares
of this stock. Mr. Cavalli and Mr. Isaacs may purchase and sell
common shares of this stock in the open market at any time
without notice.
Certain information included
herein is forward-looking within the context of the Private
Securities Litigation Reform Act of 1995, including, but not
limited to, statements concerning manufacturing, marketing,
growth,
and expansion. The words "may", "would," "will," "expect,"
"estimate," "anticipate," "believe," "intend," " project," and similar
expressions and variations thereof are intended to identify
forward-looking statements. Such forward-looking information
involves
important risks and uncertainties that could affect actual results
and cause them to differ materially from expectations expressed
herein. Cell MedX Corp. does not set price
targets on securities. Never invest into a stock discussed on this
website or
in this email alert unless you can afford to lose your entire
investment. This report has not been reviewed by the FDA or Health
Canada.
SOURCE: BioResearch Alert