As
filed with the Securities and Exchange Commission on July 15, 2020
Registration
Statement No. 333-
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM S-3
REGISTRATION
STATEMENT
UNDER
THE
SECURITIES ACT OF 1933
Elite
Pharmaceuticals, Inc.
(Exact
name of Registrant as specified in its charter)
|
Nevada
|
|
22-3542636
|
(State
or other jurisdiction of
incorporation or organization)
|
|
(I.R.S.
Employer
Identification No.)
|
165
Ludlow Avenue
Northvale,
NJ 07647
(201)
750-2646
(Address,
including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Nasrat
Hakim
President &
Chief Executive Officer
Elite
Pharmaceuticals, Inc.
165
Ludlow Avenue
Northvale,
NJ 07647
(201)
750-2646
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies
to:
Richard
Feiner, Esq.
Wall
Street Plaza
88
Pine Street, 22nd Floor
New
York, NY 10005
Telephone:
(646) 822-1170
Facsimile:
(917) 720-0863
Approximate
date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If
the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans,
please check the following box. ☐
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415
under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans,
check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities
Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration
statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following
box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall
become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following
box. ☐
If
this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register
additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the
following box. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer
|
☐
|
Accelerated filer
|
☐
|
|
Non-accelerated
filer
|
☐
|
Smaller reporting company
|
☒
|
|
|
|
Emerging growth company
|
☐
|
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for
complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities
Act. ☐
CALCULATION
OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered
|
|
Amount to be
Registered (1)
|
|
|
Proposed
Maximum
Aggregate
Offering
Price Per
Share (2)
|
|
|
Proposed
Maximum
Aggregate
Offering
Price
|
|
|
Amount of
Registration
Fee
|
|
|
Common stock
|
|
|
262,500,000
|
|
|
$
|
0.08
|
|
|
$
|
21,000,000
|
|
|
$
|
2,725.80
|
(3)
|
|
|
(1)
|
Pursuant
to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), this registration statement shall
be deemed to cover the additional securities of the same class as the securities covered by this registration statement issued
or issuable prior to completion of the distribution of the securities covered by this registration statement as a result of a
split of, or a stock dividend on, the registered securities.
|
|
|
(2)
|
Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act.
|
|
|
(3)
|
In
accordance with Rule 457(p) under the Securities Act, $1,453 of the $2,725.80 filing fee due for this registration statement has
been offset by the filing fees associated with all of the unsold securities under the Registration Statement on Form S-3 (Registration
No. 333-217866), filed by the registrant with the Securities and Exchange Commission on May 10, 2017.
|
The
registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until
the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become
effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective
on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement
filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it
is not soliciting an offer to buy these securities in any state or jurisdiction where the offer or sale is not permitted.
SUBJECT
TO COMPLETION, DATED JULY 15, 2020
PROSPECTUS
ELITE
PHARMACEUTICALS, INC.
262,500,000
Shares of Common Stock
This
prospectus relates to the sale of up to 262,500,000 shares of our common stock by Lincoln Park Capital Fund, LLC, or Lincoln Park
or the selling stockholder.
The
shares of common stock being offered by the selling stockholder have been or may be issued to Lincoln Park pursuant to the purchase
agreement, dated July 8, 2020, that we entered into with Lincoln Park (the “Purchase Agreement”). See “The
Lincoln Park Transaction” for a description of the purchase agreement and “Selling Stockholder” for additional
information regarding Lincoln Park. We are not selling any securities under this prospectus and will not receive any of the proceeds
from the sale of shares of common stock by the selling stockholder. As consideration for Lincoln Park’s irrevocable commitment
to purchase common stock upon the terms of and subject to satisfaction of the conditions set forth in the Purchase Agreement,
upon execution of the Purchase Agreement, we issued to Lincoln Park 5,975,857 shares of common stock as commitment shares. We
have agreed to issue up to 5,975,857 additional shares of common stock as additional commitment shares, on a pro rata basis at
such times during the term of the Purchase Agreement as we may direct Lincoln Park to purchase shares of common stock under the
Purchase Agreement.
The
selling stockholder may sell or otherwise dispose of the shares of common stock described in this prospectus in a number of different
ways and at varying prices. See “Plan of Distribution” for more information about how the selling stockholder may
sell or otherwise dispose of the shares of common stock being registered pursuant to this prospectus. The selling stockholder
is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended (the “Securities
Act”).
The
selling stockholder will pay all brokerage fees and commissions and similar expenses. We will pay the expenses (except brokerage
fees and commissions and similar expenses) incurred in registering the shares, including legal and accounting fees. See “Plan
of Distribution”.
Our
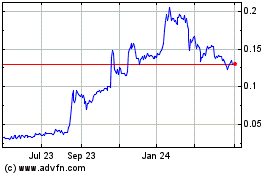
common stock is dually quoted on the OTCBB and the OTCQB. On July 13, 2020, the last reported sale price of our common stock on
the OTCQB was $0.078 per share.
You
should read this prospectus, together with additional information described under the headings “Incorporation of Certain
Information by Reference” and “Where You Can Find More Information,” carefully before you invest in any of our
securities.
Investing
in our securities involves a high degree of risk. These risks are described in the “Risk Factors” section on page
4 of this prospectus. You should also consider the risk factors described or referred to in any documents incorporated by reference
in this prospectus, before investing in these securities.
Neither
the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or
passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
This
prospectus is dated , 2020
ELITE
PHARMACEUTICALS, INC.
TABLE
OF CONTENTS
We
have not, and the selling stockholder has not, authorized anyone to provide you with information different from that contained
or incorporated by reference in this prospectus or in any supplement to this prospectus or free writing prospectus, and neither
we nor the selling stockholder takes any responsibility for any other information that others may give you. This prospectus is
not an offer to sell, nor is it a solicitation of an offer to buy, the securities in any jurisdiction where the offer or sale
is not permitted. You should not assume that the information contained in this prospectus or any prospectus supplement or free
writing prospectus is accurate as of any date other than the date on the front cover of those documents, or that the information
contained in any document incorporated by reference is accurate as of any date other than the date of the document incorporated
by reference, regardless of the time of delivery of this prospectus or any sale of a security. Our business, financial condition,
results of operations and prospects may have changed since those dates.
This
prospectus relates to the offering of our common stock. Before buying any of our common stock, you should carefully read this
prospectus, any supplement to this prospectus, the information and documents incorporated herein by reference and the additional
information under the heading “Where You Can Find More Information” and “Information Incorporated by Reference.”
These documents contain important information that you should consider when making your investment decision.
References
to the “Company,” “Elite,” “we,” “our” and “us” in this prospectus
are to Elite Pharmaceuticals, Inc. and its consolidated subsidiaries, unless the context otherwise requires. This document includes
trade names and trademarks of other companies. All such trade names and trademarks appearing in this document are the property
of their respective holders.
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
prospectus and the documents we have incorporated by reference contain “forward-looking statements” within the meaning
of Section 27A of the Securities and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results,
performance or achievements, or industry results, to be materially different from any future results, performance or achievements
expressed or implied by such forward-looking statements. When used in this report, statements that are not statements of current
or historical fact are forward-looking statements, and include, without limitation, estimated future results of operations, estimates
of future revenues, future expenses, future net income and future net income per share, as well as statements regarding future
financing activities, the impact of the novel strain of coronavirus referred to as COVID-19 on the health and welfare of our employees
and on our business, including any response to COVID-19 such as anticipated return to historical purchasing decisions by customers,
the economic impact of COVID-19, changes in consumer spending, decisions to engage in certain medical procedures, future governmental
orders that could impact our operations and the ability of our manufacturing facilities and suppliers to fulfill their obligations
to us, and any other statements that refer to our expected, estimated or anticipated future results. Without limiting the foregoing,
the words “plan,” “intend,” “may,” “will,” “expect,” “believe,”
“could,” “would,” “continue,” “pursue,” “anticipate,” “estimate,”
“forecast,” “contemplate,” “envisage,” “project” or “continue” or
the negative other variations thereof, or similar expressions or other variations or comparable terminology are intended to identify
such forward-looking statements. All statements other than statements of historical fact included and incorporated by reference
in this prospectus regarding our financial position, business strategy and plans or objectives for future operations are forward-looking
statements. Without limiting the broader description of forward-looking statements above, we specifically note, without limitation,
that statements regarding the preliminary nature of the clinical program results and the potential for further product development,
that involve known and unknown risks, delays, uncertainties and other factors not under our control, the requirement of substantial
future testing, clinical trials, regulatory reviews and approvals by the Food and Drug Administration and other regulatory authorities
prior and subsequent to the commercialization of products under development and those currently related to commercial operations,
our ability to fund all of our activities and our ability to manufacture and sell any products, gain market acceptance earn a
profit from sales or licenses of any drugs or our ability to discover new drugs in the future are all forward-looking in nature.
In
addition, because these statements reflect our current views concerning future events, these forward-looking statements involve
risks and uncertainties including, without limitation, the risks related to the impact of COVID-19 (such as, without limitation,
the scope and duration of the pandemic and the resulting economic crisis and levels of unemployment, governmental actions and
restrictive measures implemented in response, material delays and cancellations of certain medical procedures, potential manufacturing
and supply chain disruptions and other potential impacts to the business as a result of COVID-19) and the other risks and uncertainties
more fully described under the caption “Risk Factors”, including disclosures incorporated therein by reference. These
risks and uncertainties, many of which are outside of our control, and any other risks and uncertainties that we are not currently
able to predict or identify, individually or in the aggregate, could have a material adverse effect on our business, financial
condition, results of operations and cash flows and could cause our actual results to differ materially and adversely from those
expressed in forward-looking statements contained or incorporated by reference in this document.
We
do not undertake any obligation to update our forward-looking statements after the date of this prospectus for any reason, even
if new information becomes available or other events occur in the future, except as may be required under applicable securities
law. You are advised to consult any further disclosures we make on related subjects in our reports filed with the Securities and
Exchange Commission. You are notified and should understand that it is not possible to predict or identify all such factors and
consequently should not consider this to be a complete, all-inclusive discussion of all potential risks or uncertainties.
In
light of these risks, uncertainties and assumptions, you are cautioned not to place undue reliance on forward-looking statements,
which are inherently unreliable and speak only as of the date of this prospectus, any free writing prospectus, or any document
incorporated by reference in this prospectus. When considering forward-looking statements, you should keep in mind the cautionary
statements in this prospectus or any free writing prospectus, and the documents incorporated by reference in this prospectus.
We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements,
whether as a result of new information, future events or otherwise, except as required by law. In light of these risks, uncertainties
and assumptions, the forward-looking events discussed in or incorporated by reference in this prospectus or any accompanying prospectus
supplement or free writing prospectus might not occur.
PROSPECTUS
SUMMARY
This
summary highlights selected information about Elite Pharmaceuticals, Inc. and this offering of common stock. This summary does
not contain all of the information that may be important to you in making an investment decision. For a more complete understanding
of Elite Pharmaceuticals, Inc. you should read carefully this entire prospectus, including the “Risk Factors” section
and the other documents we refer to and incorporate by reference. Unless otherwise indicated, “common stock” means
our common stock, par value $0.001 per share.
About
Us
We
are a specialty pharmaceutical company principally engaged in the development and manufacture of oral, controlled-release products,
using proprietary know-how and technology, particularly as it relates to abuse resistant products and the manufacture of generic
pharmaceuticals. Our strategy includes improving off-patent drug products for life cycle management, developing generic versions
of controlled-release drug products with high barriers to entry and the development of branded and generic products that utilize
our proprietary and patented abuse resistance technologies.
We
occupy manufacturing, warehouse, laboratory and office space at 165 Ludlow Avenue and 135 Ludlow Avenue in Northvale, NJ (the
“Northvale Facility”). The Northvale Facility operates under Current Good Manufacturing Practice (“cGMP”)
and is a United States Drug Enforcement Agency (“DEA”) registered facility for research, development and manufacturing.
We
focus our efforts on the following areas: (i) manufacturing of a line of generic pharmaceutical products with approved Abbreviated
New Drug Applications (“ANDAs”); (ii) development of additional generic pharmaceutical products; (iii) development
of the other products in our pipeline including the products with our partners; (iv) commercial exploitation of our products either
by license and the collection of royalties, or through the manufacture of our formulations; and (v) development of new products
and the expansion of our licensing agreements with other pharmaceutical companies, including co-development projects, joint ventures
and other collaborations.
We
believe that our business strategy enables us to reduce its risk by having a diverse product portfolio that includes both branded
and generic products in various therapeutic categories and to build collaborations and establish licensing agreements with companies
with greater resources thereby allowing us to share costs of development and improve cash-flow.
For
additional information as to our business, properties and financial condition, please refer to the documents cited in “Where
You Can Find More Information.”
The
Purchase Agreement with Lincoln Park
On
July 8, 2020, we entered into a purchase agreement with Lincoln Park, which we refer to in this prospectus as the Purchase Agreement,
pursuant to which Lincoln Park has agreed to purchase from us up to an aggregate of $25,000,000 of our common stock (subject to
certain limitations) from time to time over the term of the Purchase Agreement. Also on July 8, 2020, we entered into a registration
rights agreement with Lincoln Park, which we refer to in this prospectus as the Registration Rights Agreement, pursuant to which
we filed with the Securities and Exchange Commission (the “SEC”) the registration statement that includes this prospectus
to register for resale under the Securities Act of 1933, as amended, or the Securities Act, the shares of common stock that have
been or may be issued to Lincoln Park under the Purchase Agreement.
This
prospectus covers the resale by the selling stockholder of 262,500,000 shares of our common stock, comprised of: (i) 5,975,857
shares that we already issued to Lincoln Park as a commitment fee for making the commitment under the Purchase Agreement, which
we refer to as the “initial commitment shares,” (ii) an additional 250,548,286 shares we have reserved for issuance
to Lincoln Park in the future under the Purchase Agreement, if and when we sell shares to Lincoln Park under the Purchase Agreement;
and (iii) up to an additional 5,975,857 shares as “additional commitment shares,” on a pro rata basis at such times
during the term of the Purchase Agreement as we may direct Lincoln Park to purchase shares under the Purchase Agreement.
Other
than the 5,975,857 initial commitment shares that we have already issued to Lincoln Park pursuant to the terms of the Purchase
Agreement, we have not issued any other shares of common stock to Lincoln Park under the Purchase Agreement. We do not have the
right to commence any sales of our common stock to Lincoln Park under the Purchase Agreement until all of the conditions set forth
in the Purchase Agreement have been satisfied, including that the SEC has declared effective the registration statement that includes
this prospectus registering the shares being issued and sold to Lincoln Park, which we refer to in this prospectus as the Commencement.
Thereafter, we may, from time to time and at our sole discretion for a period of 36-months, on any business day that we select,
direct Lincoln Park to purchase up to 500,000 shares, which amounts may be increased depending on the market price of our common
stock at the time of sale, which we refer to in this prospectus as “regular purchases.” In addition, at our discretion,
Lincoln Park has committed to purchase other “accelerated amounts” and/or “additional accelerated amounts”
under certain circumstances. We will control the timing and amount of any sales of our common stock to Lincoln Park. The purchase
price of the shares that may be sold to Lincoln Park in regular purchases under the Purchase Agreement will be based on an agreed
upon fixed discount to the market price of our common stock immediately preceding the time of sale as computed under the Purchase
Agreement. The purchase price per share will be equitably adjusted for any reorganization, recapitalization, non-cash dividend,
stock split, or other similar transaction occurring during the business days used to compute such price. We may at any time in
our sole discretion terminate the Purchase Agreement without fee, penalty or cost upon one business day notice. There are no restrictions
on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement
or Registration Rights Agreement, other than a prohibition on our entering into certain types of transactions that are defined
in the Purchase Agreement as “Variable Rate Transactions.” Lincoln Park may not assign or transfer its rights and
obligations under the Purchase Agreement.
As
of July 13, 2020, there were 846,954,821 shares of our common stock outstanding, of which 827,309,769 shares were held by non-affiliates,
including the 5,975,857 initial commitment shares that we have already issued to Lincoln Park under the Purchase Agreement. Although
the Purchase Agreement provides that we may sell up to an aggregate of $25,000,000 of our common stock to Lincoln Park, only 262,500,000
shares of our common stock are being offered under this prospectus, which represents the 5,975,857 initial commitment shares that
we have already issued to Lincoln Park under the Purchase Agreement, the 5,975,857 additional commitment shares which we may issue
to Lincoln Park in the future under the Purchase Agreement, if and when we sell shares to Lincoln Park under the Purchase Agreement,
and 250,548,286 additional shares of common stock that we may issue and sell to Lincoln Park as “purchase shares”
in the future under the Purchase Agreement, if and when we sell shares to Lincoln Park under the Purchase Agreement. Depending
on the market prices of our common stock at the time we elect to issue and sell shares to Lincoln Park under the Purchase Agreement,
we may need to register for resale under the Securities Act additional shares of our common stock in order to receive aggregate
gross proceeds equal to the $25,000,000 total commitment available to us under the Purchase Agreement. If all of the 262,500,000
shares offered by Lincoln Park under this prospectus were issued and outstanding as of the date hereof, such shares would represent
approximately 24% of the total number of shares of our common stock outstanding and approximately 24% of the total number of outstanding
shares held by non-affiliates, in each case as of the date hereof. If we elect to issue and sell more than the 262,500,000 shares
offered under this prospectus to Lincoln Park, which we have the right, but not the obligation, to do, we must first register
for resale under the Securities Act any such additional shares, which could cause additional substantial dilution to our stockholders.
The number of shares ultimately offered for resale by Lincoln Park is dependent upon the number of shares we sell to Lincoln Park
under the Purchase Agreement.
The
Purchase Agreement also prohibits us from directing Lincoln Park to purchase any shares of common stock if those shares, when
aggregated with all other shares of our common stock then beneficially owned by Lincoln Park and its affiliates, would result
in Lincoln Park and its affiliates having beneficial ownership, at any single point in time, of more than 4.99% of the then total
outstanding shares of our common stock, as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 thereunder,
which limitation we refer to as the “beneficial ownership cap.”
Issuances
of our common stock in this offering will not affect the rights or privileges of our existing stockholders, except that the economic
and voting interests of each of our existing stockholders will be diluted as a result of any such issuance. Although the number
of shares of common stock that our existing stockholders own will not decrease, the shares owned by our existing stockholders
will represent a smaller percentage of our total outstanding shares after any such issuance to Lincoln Park.
Summary
of the Offering
|
Common
stock offered by the selling stockholder
|
262,500,000
shares consisting of:
●
5,975,857 commitment shares issued to Lincoln Park upon the execution of the Purchase Agreement;
●
up to 5,975,857 additional commitment shares, on a pro rata basis at such times during the term of the Purchase Agreement
as we may direct Lincoln Park to purchase shares under the Purchase Agreement; and
●
up to 250,548,286 purchase shares that we may sell to Lincoln Park under the Purchase Agreement.
|
|
|
|
|
Common
stock outstanding
|
846,954,821
shares of common stock.
|
|
Use
of proceeds
|
We
will receive no proceeds from the sale of shares of common stock by Lincoln Park in this offering. We may receive up
to $25,000,000 aggregate gross proceeds under the Purchase Agreement from any sales we make to Lincoln Park pursuant to the
Purchase Agreement. Any proceeds that we receive from such sales are anticipated to be used for research and product development,
general corporate purposes and working capital requirements. See “Use of Proceeds.”
|
|
|
|
|
OTCQB
symbol
|
“ELTP”
|
|
|
|
|
Risk
Factors
|
See
“Risk Factors” beginning on page 4 of this prospectus and the other information included in, or incorporated by
reference into, this prospectus for a discussion of certain factors you should carefully consider before deciding to invest
in our securities.
|
The
number of shares of common stock is based on 846,954,821 shares outstanding as of July 13, 2020, and excludes:
|
|
●
|
5,375,000
shares of common stock issuable upon the exercise of options outstanding as of July 13,
2020;
|
|
|
●
|
79,008,661
shares of common stock issuable upon exercise of warrants held by Nasrat Hakim and outstanding
as of July 13, 2020;
|
|
|
●
|
158,017,321
shares of common stock issuable upon conversion of Series J Convertible Preferred Stock
owned by Nasrat Hakim as of July 13, 2020;
|
|
|
●
|
2,750,000
additional shares of common stock reserved for future issuance under our 2014 Equity
Incentive Plan; and
|
|
|
●
|
31,074,817
shares of common stock due and owing Directors, employees and consultants as of July
13, 2020.
|
RISK
FACTORS
An
investment in our common stock involves a high degree of risk. Before making an investment decision, you should carefully
consider the risks described below, as well as the risks described under the caption “Risk Factors” in our Annual
Report on Form 10-K
for the year ended March 31, 2020 and in the other filings we make with the SEC pursuant to Sections 13(a), 13(c), 14 or
15(d) of the Exchange Act, which we have incorporated herein by reference. Our business, financial condition, results of
operations and cash flows could be materially adversely affected by any of these risks, and the market or trading price of
our common stock could decline due to any of these risks. In addition, please read “Cautionary Note Regarding
Forward-Looking Statements” in this prospectus, where we describe additional uncertainties associated with our business
and the forward-looking statements included or incorporated by reference in this prospectus. Please note that additional
risks not presently known to us or that we currently deem immaterial may also impair our business and
operations.
Risks
Related to this Offering and Ownership of Our Common Stock
The
sale or issuance of our common stock to Lincoln Park may cause dilution and the sale of the shares of common stock acquired by
Lincoln Park, or the perception that such sales may occur, could cause the price of our common stock to fall.
On July 8, 2020, we entered into the Purchase
Agreement with Lincoln Park, pursuant to which (i) we issued 5,975,857 initial commitment shares to Lincoln Park as a partial
commitment fee payment for making its irrevocable commitment under the Purchase Agreement, (ii) Lincoln Park has committed to
purchase up to an additional $25,000,000 of our common stock; and (iii) we may issue to Lincoln Park up to an additional 5,975,857
shares as additional commitment shares, on a pro rata basis at such times during the term of the Purchase Agreement as we may
direct Lincoln Park to purchase shares under the Purchase Agreement.
The
shares of our common stock that may be issued under the Purchase Agreement may be sold by us to Lincoln Park at our discretion
from time to time over a 36-month period commencing after the satisfaction of certain conditions set forth in the Purchase Agreement,
including that the SEC has declared effective the registration statement on Form S-3 that includes this prospectus. The purchase
price for the shares that we may sell to Lincoln Park under the Purchase Agreement will fluctuate based on the price of our common
stock. Depending on market liquidity at the time, sales of such shares may cause the trading price of our common stock to fall.
We
generally have the right to control the timing and amount of any future sales of our shares to Lincoln Park. Additional sales
of our common stock, if any, to Lincoln Park will depend upon market conditions and other factors to be determined by us. We may
ultimately decide to sell to Lincoln Park all, some or none of the additional shares of our common stock that may be available
for us to sell pursuant to the Purchase Agreement. If and when we do sell additional shares to Lincoln Park, after Lincoln Park
has acquired the shares, Lincoln Park may resell all, some or none of those shares at any time or from time to time in its discretion.
Therefore, sales to Lincoln Park by us could result in substantial dilution to the interests of other holders of our common stock.
Additionally, the sale of a substantial number of shares of our common stock to Lincoln Park, or the anticipation of such sales,
could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we
might otherwise wish to effect sales.
We
may require additional financing to sustain our operations, without which we may not be able to continue operations, and the terms
of subsequent financings may adversely impact our stockholders.
We
may direct Lincoln Park to purchase up to $25,000,000 worth of shares of our common stock under our agreement over a 36-month
period generally in amounts up to 500,000 shares of our common stock, which may be increased to up to 900,000 shares of our common
stock depending on the market price of our common stock at the time of sale, and, in each case, subject to a maximum limit of
$1,000,000 per purchase, on any single business day (such share amounts being subject to adjustment for any reorganization, recapitalization,
non-cash dividend, stock split, reverse stock split or other similar transaction as provided in the Purchase Agreement). We also
may direct Lincoln Park to purchase additional shares pursuant to Accelerated Purchases (see “The Lincoln Park Transaction”).
Assuming a purchase price of $0.078 per share (the closing sale price of the common stock on July 13, 2020) and the purchase by
Lincoln Park of 500,000 purchase shares, proceeds to us would only be $39,000.
The
extent we rely on Lincoln Park as a source of funding will depend on a number of factors including the prevailing market price
of our common stock and the extent to which we are able to secure working capital from other sources. If obtaining sufficient
funding from Lincoln Park were to prove unavailable or prohibitively dilutive, we will need to secure another source of funding
in order to satisfy our working capital needs. Even if we sell all $25,000,000 under the Purchase Agreement to Lincoln Park, we
may still need additional capital to finance our future business plans and working capital needs, and we may have to raise funds
through the issuance of equity or debt securities. Depending on the type and the terms of any financing we pursue, stockholders’
rights and the value of their investment in our common stock could be reduced. A financing could involve one or more types of
securities including common stock, preferred stock, convertible debt or warrants to acquire common stock. These securities could
be issued at or below the then prevailing market price for our common stock. In addition, if we issue secured debt securities,
the holders of the debt would have a claim to our assets that would be prior to the rights of stockholders until the debt is paid.
Interest on these debt securities would increase costs and negatively impact operating results. If the issuance of new securities
results in diminished rights to holders of our common stock, the market price of our common stock could be negatively impacted.
Should
the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the
consequences could be a material adverse effect on our business, operating results, financial condition and prospects.
Our
management will have broad discretion over the use of the net proceeds from our sale of shares of common stock to Lincoln Park,
you may not agree with how we use the proceeds and the proceeds may not be invested successfully.
Our
management will have broad discretion as to the use of the net proceeds from our sale of shares of common stock to Lincoln Park,
and we could use them for purposes other than those contemplated at the time of commencement of this offering. Accordingly, you
will be relying on the judgment of our management with regard to the use of those net proceeds, and you will not have the opportunity,
as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that, pending
their use, we may invest those net proceeds in a way that does not yield a favorable, or any, return for us. The failure of our
management to use such funds effectively could have a material adverse effect on our business, financial condition, operating
results and cash flows.
The
Company has no history of paying dividends on its common stock, and we do not anticipate paying dividends in the foreseeable
future.
The
Company has not previously paid dividends on its common stock. We currently anticipate that we will retain all of our available
cash, if any, for use as working capital and for other general corporate purposes. Any payment of future dividends will be at
the discretion of our Board of Directors and will depend upon, among other things, our earnings, financial condition, capital
requirements, level of indebtedness, statutory and contractual restrictions applicable to the payment of dividends and other considerations
that our Board of Directors deems relevant. Investors must rely on sales of their common stock after price appreciation, which
may never occur, as the only way to realize a return on their investment.
THE
LINCOLN PARK TRANSACTION
General
On
July 8, 2020, we entered into a purchase agreement with Lincoln Park, which we refer to in this prospectus as the Purchase Agreement,
pursuant to which Lincoln Park has agreed to purchase from us up to an aggregate of $25,000,000 of our common stock (subject to
certain limitations) from time to time over the term of the Purchase Agreement. Also on July 8, 2020, we entered into a registration
rights agreement with Lincoln Park, which we refer to in this prospectus as the Registration Rights Agreement, pursuant to which
we have filed with the SEC the registration statement that includes this prospectus to register for resale under the Securities
Act the shares of common stock that have been or may be issued to Lincoln Park under the Purchase Agreement.
This prospectus covers the resale by the
selling stockholder of 262,500,000 shares of our common stock, comprised of: (i) 5,975,857 initial commitment shares that we
already issued to Lincoln Park as partial payment of a commitment fee for making its irrevocable commitment under the
Purchase Agreement, (ii) an additional 250,548,286 purchase shares we have reserved for issuance and sale to Lincoln Park in
the future under the Purchase Agreement, if and when we decide to sell shares to Lincoln Park under the Purchase Agreement;
and (iii) up to an additional 5,975,857 shares as additional commitment shares, on a pro rata basis at such times during the
term of the Purchase Agreement as we may direct Lincoln Park to purchase shares under the Purchase Agreement.
Other
than the 5,975,857 initial commitment shares, we have not issued any common stock to Lincoln Park under the Purchase Agreement.
We do not have the right to commence any sales of our common stock to Lincoln Park under the Purchase Agreement until all of the
conditions set forth in the Purchase Agreement have been satisfied, including that the SEC has declared effective the registration
statement that includes this prospectus registering the shares that will be issued and sold to Lincoln Park, which we refer to
in this prospectus as the Commencement. Thereafter, we may, from time to time and at our sole discretion for a period of 36-months,
on any business day that we select, direct Lincoln Park to purchase up to 500,000 shares of common stock, which amounts may be
increased depending on the market price of our common stock at the time of sale, which we refer to in this prospectus as “regular
purchases.” In addition, at our discretion, Lincoln Park has committed to purchase other “accelerated amounts”
and/or “additional accelerated amounts” under certain circumstances. We will control the timing and amount of any
sales of our common stock to Lincoln Park. The purchase price of the shares that may be sold to Lincoln Park in regular purchases
under the Purchase Agreement will be based on an agreed upon fixed discount to the market price of our common stock immediately
preceding the time of sale as computed under the Purchase Agreement. The purchase price per share will be equitably adjusted for
any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the business
days used to compute such price. We may at any time in our sole discretion terminate the Purchase Agreement without fee, penalty
or cost upon one business day notice. There are no restrictions on future financings, rights of first refusal, participation
rights, penalties or liquidated damages in the Purchase Agreement or Registration Rights Agreement, other than a prohibition on
our entering into certain types of transactions that are defined in the Purchase Agreement as “Variable Rate Transactions.”
Lincoln Park may not assign or transfer its rights and obligations under the Purchase Agreement.
As
of July 13, 2020, there were 846,954,821 shares of our common stock outstanding, of which 827,309,769 shares were held by non-affiliates,
including the 5,975,857 shares that we have already issued to Lincoln Park under the Purchase Agreement and additional shares
owned by Lincoln Park. Although the Purchase Agreement provides that we may sell up to an aggregate of $25,000,000 of our common
stock to Lincoln Park, only 262,500,000 shares of our common stock are being offered under this prospectus, which represents the
5,975,857 shares that we have already issued to Lincoln Park under the Purchase Agreement and additional shares which may be issued
to Lincoln Park in the future under the Purchase Agreement, if and when we sell shares to Lincoln Park under the Purchase Agreement,
including additional commitment shares. Depending on the market prices of our common stock at the time we elect to issue and sell
shares to Lincoln Park under the Purchase Agreement, we may need to register for resale under the Securities Act additional shares
of our common stock in order to receive aggregate gross proceeds equal to the $25,000,000 total commitment available to us under
the Purchase Agreement. If all of the 262,500,000 shares offered by Lincoln Park under this prospectus were issued and outstanding
as of the date hereof, such shares would represent approximately 24% of the total number of shares of our common stock outstanding
and approximately 24% of the total number of outstanding shares held by non-affiliates, in each case as of the date hereof. If
we elect to issue and sell more than the 262,500,000 shares offered under this prospectus to Lincoln Park, which we have the right,
but not the obligation, to do, we must first register for resale under the Securities Act any such additional shares, which could
cause additional substantial dilution to our stockholders. The number of shares ultimately offered for resale by Lincoln Park
is dependent upon the number of shares we sell to Lincoln Park under the Purchase Agreement.
The
Purchase Agreement also prohibits us from directing Lincoln Park to purchase any shares of common stock if those shares, when
aggregated with all other shares of our common stock then beneficially owned by Lincoln Park and its affiliates, would result
in Lincoln Park and its affiliates having beneficial ownership, at any single point in time, of more than 4.99% of the then total
outstanding shares of our common stock, as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 thereunder,
which limitation we refer to as the beneficial ownership cap.
Issuances
of our common stock in this offering will not affect the rights or privileges of our existing stockholders, except that the economic
and voting interests of each of our existing stockholders will be diluted as a result of any such issuance. Although the number
of shares of common stock that our existing stockholders own will not decrease, the shares owned by our existing stockholders
will represent a smaller percentage of our total outstanding shares after any such issuance to Lincoln Park.
We
previously sold shares to Lincoln Park pursuant to a prior purchase agreement that expired on July 1, 2020.
Purchase
of Shares Under the Purchase Agreement
Under
the Purchase Agreement, upon Commencement, on any business day that we select, we may direct Lincoln Park to purchase up to 500,000
shares of our common stock in a regular purchase on such business day, which is referred to as a Regular Purchase in this prospectus,
provided, however, that (i) the Regular Purchase may be increased to up to 600,000 shares, provided that the closing sale price
of the common stock is not below $0.15 on the purchase date; (ii) the Regular Purchase may be increased to up to 700,000 shares,
provided that the closing sale price of the common stock is not below $0.20 on the purchase date; (iii) the Regular Purchase may
be increased to up to 800,000 shares, provided that the closing sale price of the common stock is not below $0.25 on the purchase
date; and (iv) the Regular Purchase may be increased to up to 900,000 shares, provided that the closing sale price of the common
stock is not below $0.30 on the purchase date. In each case, Lincoln Park’s maximum dollar commitment in any single Regular
Purchase may not exceed $1,000,000. The Regular Purchase Share Limit is subject to proportionate adjustment in the event of a
reorganization, recapitalization, non-cash dividend, stock split or other similar transaction; provided, that if after giving
effect to such full proportionate adjustment, the adjusted Regular Purchase Share Limit would preclude us from requiring Lincoln
Park to purchase common stock at an aggregate purchase price equal to or greater than $100,000 in any single Regular Purchase,
then the Regular Purchase Share Limit will not be fully adjusted, but rather the Regular Purchase Share Limit for such Regular
Purchase shall be adjusted as specified in the Purchase Agreement, such that, after giving effect to such adjustment, the Regular
Purchase Share Limit will be equal to (or as close as can be derived from such adjustment without exceeding) $100,000.
The
purchase price per share for each such Regular Purchase will be equal to 97% of the lower of:
|
|
●
|
the
lowest sale price for our common stock on the purchase date of such shares; and
|
|
|
●
|
the
arithmetic average of the three lowest closing sale prices for our common stock during
the 10 consecutive business days ending on the business day immediately preceding the
purchase date of such shares.
|
In
addition to Regular Purchases described above, we may also direct Lincoln Park, on any business day on which we have properly
submitted a Regular Purchase notice directing Lincoln Park to purchase the maximum number of shares of our common stock that we
are then permitted to include in a single Regular Purchase notice and the closing price of our common stock on such business day
is not less than $0.03 per share (subject to adjustment for any reorganization, recapitalization, non-cash dividend, stock split,
reverse stock split or other similar transaction as provided in the Purchase Agreement), to purchase an additional amount of our
common stock, which we refer to as an Accelerated Purchase, not to exceed the lesser of:
|
|
●
|
30%
of the aggregate shares of our common stock traded during all or, if certain trading
volume or market price thresholds specified in the Purchase Agreement are crossed on
the applicable Accelerated Purchase date, which is defined as the next business day following
the purchase date for the corresponding Regular Purchase, the portion of the normal trading
hours on the applicable Accelerated Purchase date prior to such time that any one of
such thresholds is crossed, which period of time on the applicable Accelerated Purchase
date we refer to as the Accelerated Purchase Measurement Period; and
|
|
|
●
|
four
times the number of purchase shares purchased pursuant to the corresponding Regular Purchase.
|
The
purchase price per share for each such Accelerated Purchase will be equal to the lower of:
|
|
●
|
97%
of the volume weighted average price of our common stock during the Accelerated Purchase
Measurement Period on the applicable Accelerated Purchase date; and
|
|
|
●
|
the
closing sale price of our common stock on the applicable Accelerated Purchase date; but
in no event would the purchase price for such Accelerated Purchase be less than the applicable
minimum Accelerated Purchase threshold price established in accordance with the Purchase
Agreement.
|
We
may also direct Lincoln Park, not later than 1:00 p.m., Eastern time, on a business day on which an Accelerated Purchase has been
completed and all of the shares to be purchased thereunder (and under the corresponding Regular Purchase) have been properly delivered
to Lincoln Park in accordance with the Purchase Agreement prior to such time on such business day, and provided that the closing
price of our common stock on the business day immediately preceding such business day is not less than $0.03 per share (subject
to adjustment for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction
as provided in the Purchase Agreement), to purchase an additional amount of our common stock, which we refer to as an Additional
Accelerated Purchase, of up to the lesser of:
|
|
●
|
30%
of the aggregate shares of our common stock traded during a certain portion of the normal
trading hours on such Accelerated Purchase date as determined in accordance with the
Purchase Agreement, which period of time we refer to as the Additional Accelerated Purchase
Measurement Period; and
|
|
|
●
|
four
times the number of purchase shares purchased pursuant to the Regular Purchase corresponding
to the Accelerated Purchase that was completed on such Accelerated Purchase date on which
an Additional Accelerated Purchase notice was properly received.
|
We
may, in our sole discretion, submit multiple Additional Accelerated Purchase notices to Lincoln Park prior to 1:00 p.m., Eastern
time, on a single Accelerated Purchase date, provided that all prior Accelerated Purchases and Additional Accelerated Purchases
(including those that have occurred earlier on the same day) have been completed and all of the shares to be purchased thereunder
(and under the corresponding Regular Purchase) have been properly delivered to Lincoln Park in accordance with the Purchase Agreement
and the closing sale price of our common stock on the business day immediately preceding the delivery of multiple Additional Accelerated
Purchase notices is greater than $0.03.
The
purchase price per share for each such Additional Accelerated Purchase will be equal to the lower of:
|
|
●
|
97%
of the volume weighted average price of our common stock during the applicable Additional
Accelerated Purchase Measurement Period on the applicable Additional Accelerated Purchase
date; and
|
|
|
●
|
the
closing sale price of our common stock on the applicable Additional Accelerated Purchase
date; but in no event would the purchase price for such Additional Accelerated Purchase
be less than the applicable minimum Additional Accelerated Purchase threshold price established
in accordance with the Purchase Agreement.
|
In
the case of the Initial Purchase, Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases, the purchase
price per share will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock
split or other similar transaction occurring during the business days used to compute the purchase price.
Other
than as described above, there are no trading volume requirements or restrictions under the Purchase Agreement, and we will
control the timing and amount of any sales of our common stock to Lincoln Park.
Events
of Default
Events
of default under the Purchase Agreement include the following:
|
|
●
|
the
effectiveness of the registration statement of which this prospectus forms a part lapses
for any reason (including, without limitation, the issuance of a stop order), or any
required prospectus supplement and accompanying prospectus are unavailable for the resale
by Lincoln Park of our common stock offered hereby, and such lapse or unavailability
continues for a period of 10 consecutive business days or for more than an aggregate
of 30 business days in any 365-day period;
|
|
|
●
|
suspension
by our principal market of our common stock from trading for a period of one business
day;
|
|
|
●
|
the
delisting of our common stock from the OTCQB operated by the OTC Markets Group, Inc.
(or nationally recognized successor thereto), provided, however, that our common stock
is not immediately thereafter trading on the New York Stock Exchange, The NASDAQ Capital
Market, The NASDAQ Global Market, The NASDAQ Global Select Market, the NYSE American,
the NYSE Arca, the OTC Bulletin Board or the OTCQX operated by the OTC Markets Group,
Inc. (or nationally recognized successor to any of the foregoing);
|
|
|
●
|
the
failure of our transfer agent to issue to Lincoln Park shares of our common stock within
two business days after the applicable date on which Lincoln Park is entitled to receive
such shares;
|
|
|
●
|
any
breach of the representations or warranties or covenants contained in the Purchase Agreement
or Registration Rights Agreement that has or could have a material adverse effect on
us and, in the case of a breach of a covenant that is reasonably curable, that is not
cured within five business days;
|
|
|
●
|
any
voluntary or involuntary participation or threatened participation in insolvency or bankruptcy
proceedings by or against us; or
|
|
|
●
|
if
at any time we are not eligible to transfer our common stock electronically.
|
Lincoln
Park does not have the right to terminate the Purchase Agreement upon any of the events of default set forth above. During an
event of default, all of which are outside of Lincoln Park’s control, we may not direct Lincoln Park to purchase any shares
of our common stock under the Purchase Agreement.
Our
Termination Rights
We
have the unconditional right, at any time, for any reason and without any payment or liability to us, to give notice to Lincoln
Park to terminate the Purchase Agreement. In the event of bankruptcy proceedings instituted by us, the Purchase Agreement will
automatically terminate. In the event of bankruptcy proceedings instituted against us, the Purchase Agreement will terminate if
the proceedings are not discharged within 90 days.
No Short-Selling
or Hedging by Lincoln Park
Lincoln
Park has agreed that neither it nor any of its affiliates shall engage in any direct or indirect short-selling or hedging of our
common stock during any time prior to the termination of the Purchase Agreement.
Prohibitions
on Variable Rate Transactions
There
are no restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages
in the Purchase Agreement or Registration Rights Agreement other than a prohibition on entering into a “Variable Rate Transaction,”
as defined in the Purchase Agreement.
Effect
of Performance of the Purchase Agreement on Our Stockholders
All
262,500,000 shares registered in this offering which have been or may be issued or sold by us to Lincoln Park under the Purchase
Agreement are expected to be freely tradable. It is anticipated that shares registered in this offering will be sold over a period
of up to 36-months commencing on the date that the registration statement including this prospectus becomes effective. The sale
by Lincoln Park of a significant amount of shares registered in this offering at any given time could cause the market price of
our common stock to decline and to be highly volatile. Sales of our common stock to Lincoln Park, if any, will depend upon market
conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park all, some or none of the
additional shares of our common stock that may be available for us to sell pursuant to the Purchase Agreement. If and when we
do sell shares to Lincoln Park, after Lincoln Park has acquired the shares, Lincoln Park may resell all, some or none of those
shares at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us under the Purchase Agreement
may result in substantial dilution to the interests of other holders of our common stock. In addition, if we sell a substantial
number of shares to Lincoln Park under the Purchase Agreement, or if investors expect that we will do so, the actual sales of
shares or the mere existence of our arrangement with Lincoln Park may make it more difficult for us to sell equity or equity-related
securities in the future at a time and at a price that we might otherwise wish to effect such sales. However, we have the right
to control the timing and amount of any additional sales of our shares to Lincoln Park and the Purchase Agreement may be terminated
by us at any time at our discretion without any cost to us.
Pursuant
to the terms of the Purchase Agreement, we have the right, but not the obligation, to direct Lincoln Park to purchase up to $25,000,000
of our common stock. Depending on the price per share at which we sell our common stock to Lincoln Park pursuant to the Purchase
Agreement, we may need to sell to Lincoln Park under the Purchase Agreement more shares of our common stock than are offered under
this prospectus in order to receive aggregate gross proceeds equal to the $25,000,000 total commitment available to us under the
Purchase Agreement. If we choose to do so, we must first register for resale under the Securities Act such additional shares of
our common stock, which could cause additional substantial dilution to our stockholders. The number of shares ultimately offered
for resale by Lincoln Park under this prospectus is dependent upon the number of shares we direct Lincoln Park to purchase under
the Purchase Agreement.
The
Purchase Agreement prohibits us from issuing or selling to Lincoln Park under the Purchase Agreement any shares of our common
stock if those shares, when aggregated with all other shares of our common stock then beneficially owned by Lincoln Park and its
affiliates, would exceed the beneficial ownership cap.
The
following table sets forth the amount of gross proceeds we would receive from Lincoln Park from our sale of shares to Lincoln
Park under the Purchase Agreement at varying purchase prices:
Assumed
Average
Purchase
Price Per
Share
|
|
|
Number of Registered
Shares to be Issued if
Full Purchase(1)
|
|
|
Percentage of Outstanding
Shares After Giving Effect to
the Issuance to Lincoln
Park(2)
|
|
|
Proceeds from the Sale of Shares
to Lincoln Park Under the
Purchase Agreement(1)
|
|
|
$
|
0.05
|
|
|
|
262,500,000
|
|
|
|
23
|
%
|
|
$
|
12,674,723
|
|
|
$
|
0.078
|
(3)
|
|
|
262,500,000
|
|
|
|
24
|
%
|
|
$
|
19,642,652
|
|
|
$
|
0.10
|
|
|
|
261,951,714
|
|
|
|
24
|
%
|
|
$
|
25,000,000
|
|
|
$
|
0.15
|
|
|
|
178,618,381
|
|
|
|
17
|
%
|
|
$
|
25,000,000
|
|
|
$
|
0.20
|
|
|
|
136,951,714
|
|
|
|
14
|
%
|
|
$
|
25,000,000
|
|
|
1.
|
Although
the Purchase Agreement provides that we may sell up to $25,000,000 of our common stock
to Lincoln Park, we are only registering 262,500,000 shares under this prospectus, including
5,975,857 initial commitment shares that we already issued to Lincoln Park as a partial
commitment fee under the Purchase Agreement and up to an additional 5,975,857 shares
we may issue as additional commitment shares, on a pro rata basis at such times during
the term of the Purchase Agreement as we may direct Lincoln Park to purchase shares under
the Purchase Agreement, which may or may not cover all the shares we ultimately sell
to Lincoln Park under the Purchase Agreement, depending on the purchase price per share.
We have not and will not receive any proceeds from the issuance of the initial commitment
shares or any additional commitment shares to Lincoln Park.
|
|
2.
|
The
denominator is based on 846,954,821 shares outstanding as of July 13, 2020, adjusted
to include the number of shares set forth in the adjacent column which we would have
sold to Lincoln Park, assuming the purchase price in the adjacent column. The numerator
is based on the number of shares issuable under the Purchase Agreement at the corresponding
assumed purchase price set forth in the adjacent column. The table does not give effect
to the prohibition contained in the Purchase Agreement that prevents us from selling
and issuing to Lincoln Park shares such that, after giving effect to such sale and issuance,
Lincoln Park and its affiliates would beneficially own more than 4.99% of the then outstanding
shares of our common stock.
|
|
3.
|
The
closing sale price of our shares on July 13, 2020.
|
USE
OF PROCEEDS
This
prospectus relates to shares of our common stock that may be offered and sold from time to time by Lincoln Park. We will receive
no proceeds from the sale of shares of common stock by Lincoln Park in this offering. We may receive up to $25,000,000 aggregate
gross proceeds under the Purchase Agreement from any sales we make to Lincoln Park pursuant to the Purchase Agreement after the
date of this prospectus. We estimate that the net proceeds to us from the sale of our common stock to Lincoln Park pursuant to
the Purchase Agreement would be up to $24.974 million over an approximately 36-month period, assuming that we sell the full amount
of our common stock that we have the right, but not the obligation, to sell to Lincoln Park under the Purchase Agreement, and
after other estimated fees and expenses. See “Plan of Distribution” elsewhere in this prospectus for more information.
We
intend to use any net proceeds that we receive under the Purchase Agreement for research and product development, general corporate
purposes and working capital requirements. It is possible that no shares will be issued under the Purchase Agreement.
MARKET
FOR COMMON STOCK AND DIVIDEND POLICY
Our
common stock is traded in the over-the-counter market and quoted on the OTCQB and on the OTC Bulletin Board under the symbol “ELTP.”
The last reported sale price of our common stock on July 13, 2020 on the OTCQB was $0.078 per share. As of July 13, 2020, there
were 124 holders of record of our common stock.
We
have never declared or paid any cash dividend on our common stock, nor do we currently intend to pay any cash dividend on our
common stock in the foreseeable future. We expect to retain our earnings, if any, for the growth and development of our business.
SELLING
STOCKHOLDER
This
prospectus relates to the possible resale by the selling stockholder, Lincoln Park, of shares of our common stock that have been
or may be issued to Lincoln Park pursuant to the Purchase Agreement. We are filing the registration statement of which this
prospectus forms a part pursuant to the provisions of the Registration Rights Agreement, which we entered into with Lincoln Park
on July 8, 2020, concurrently with our execution of the Purchase Agreement, in which we agreed to provide certain registration
rights with respect to sales by Lincoln Park of the shares of our common stock that have been or may be issued to Lincoln Park
under the Purchase Agreement.
Lincoln
Park, as the selling stockholder, may, from time to time, offer and sell pursuant to this prospectus any or all of the shares
that we may issue to Lincoln Park from time to time at our discretion under the Purchase Agreement. The “selling stockholder”
may sell some, all or none of its shares. We do not know how long the selling stockholder will hold the shares before selling
them, and we currently have no agreements, arrangements or understandings with the selling stockholder regarding the sale
of any of the shares.
The
following table presents information regarding the selling stockholder and the shares that it may offer and sell from time to
time under this prospectus. The table is prepared based on information supplied to us by the selling stockholder, and reflects
its holdings as of July 13, 2020. Neither Lincoln Park nor any of its affiliates has held a position or office, or had any
other material relationship, with us or any of our predecessors or affiliates. Beneficial ownership is determined in accordance
with Section 13(d) of the Exchange Act and Rule 13d-3 thereunder. The percentage of shares beneficially owned prior to the
offering is based on 846,954,821 shares of our common stock actually outstanding as of July 13, 2020.
|
|
|
Shares Beneficially
Owned Prior to Offering
|
|
|
Number of Shares
|
|
|
Shares Beneficially
Owned After Offering(1)
|
|
|
Name
|
|
Number
|
|
|
%
|
|
|
Being Offered
|
|
|
Number
|
|
|
%
|
|
|
Lincoln Park Capital Fund, LLC(2)
|
|
|
5,975,857
|
(3)
|
|
|
*
|
(4)
|
|
|
262,500,000
|
|
|
|
0
|
|
|
|
*
|
(5)
|
|
(1)
|
Assumes
the sale of all shares of common stock registered pursuant to this prospectus, although
the selling stockholder is under no obligation known to us to sell any shares of
common stock at this time.
|
|
(2)
|
Josh
Scheinfeld and Jonathan Cope, the Managing Members of Lincoln Park Capital, LLC, the
manager of Lincoln Park Capital Fund, LLC, are deemed to be beneficial owners of all
of the shares of common stock owned by Lincoln Park Capital Fund, LLC. Messrs. Cope and
Scheinfeld have shared voting and investment power over the shares being offered under
the prospectus filed with the SEC in connection with the transactions contemplated under
the Purchase Agreement. Lincoln Park Capital, LLC is not a licensed broker dealer or
an affiliate of a licensed broker dealer.
|
|
(3)
|
Consists
of 5,975,857 initial commitment shares we issued to Lincoln Park upon the execution of
the Purchase Agreement. Excludes (i) up to 250,548,286 purchase shares being registered
hereunder because the issuance and sale of such shares to Lincoln Park is solely at our
discretion and is subject to certain conditions precedent, the satisfaction of all of
which are outside of Lincoln Park’s control, including the registration statement
on Form S-3 of which this prospectus is a part becoming and remaining effective under
the Securities Act and (ii) up to 5,975,857 additional commitment shares because the
issuance of such shares to Lincoln Park would only occur on a pro rata basis at such
times during the term of the Purchase Agreement as we may direct Lincoln Park to purchase
shares under the Purchase Agreement. Furthermore, under the terms of the Purchase Agreement,
issuances and sales of shares of our common stock to Lincoln Park are subject to certain
limitations on the amounts we may sell to Lincoln Park at any time, including the beneficial
ownership cap. See the description under the heading “Lincoln Park Transaction”
for more information about the Purchase Agreement.
|
|
(4)
|
Calculated
by dividing (1) the total number of shares beneficially owned by the selling stockholder
on July 13, 2020, which pursuant to Rule 13d-3 under the Exchange Act solely consists
of (a) the 5,975,857 shares of common stock held by Lincoln Park and (b) excludes 250,548,286
shares of common stock being registered hereunder because the issuance and sale of such
shares to Lincoln Park is solely at our discretion and is subject to certain conditions
precedent, the satisfaction of all of which are outside of Lincoln Park’s control,
including the registration statement on Form S-3 of which this prospectus is a part becoming
and remaining effective under the Securities Act and (b) up to 5,975,857 additional commitment
shares because the issuance of such shares to Lincoln Park would only occur on a pro
rata basis at such times during the term of the Purchase Agreement as we may direct Lincoln
Park to purchase shares under the Purchase Agreement, by (2) the number of shares of
our common stock outstanding as of July 13, 2020.
|
|
(5)
|
Calculated
by dividing (i) the total number of shares beneficially owned by the selling stockholder
on July 13, 2020 (which pursuant to Rule 13d-3 under the Exchange Act consists of the
5,975,857 initial commitment shares held by Lincoln Park, assuming all shares of common
stock registered hereunder have been resold by (ii) the number of shares of our common
stock outstanding as of July 13, 2020, as adjusted to include the 256,524,143 shares
which may be sold or issued to Lincoln Park as additional commitment shares hereunder
in connection with the Purchase Agreement.
|
PLAN
OF DISTRIBUTION
The
common stock offered by this prospectus is being offered by the selling stockholder, Lincoln Park. The common stock may
be sold or distributed from time to time by the selling stockholder directly to one or more purchasers or through brokers, dealers,
or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing
market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the common stock offered by this prospectus
could be effected in one or more of the following methods:
|
|
●
|
ordinary
brokers’ transactions;
|
|
|
●
|
transactions
involving cross or block trades;
|
|
|
●
|
through
brokers, dealers, or underwriters who may act solely as agents;
|
|
|
●
|
“at
the market” into an existing market for the common stock;
|
|
|
●
|
in
other ways not involving market makers or established business markets, including direct
sales to purchasers or sales effected through agents;
|
|
|
●
|
in
privately negotiated transactions; or
|
|
|
●
|
any
combination of the foregoing.
|
In
order to comply with the securities laws of certain states, if applicable, the shares may be sold only through registered or licensed
brokers or dealers. In addition, in certain states, the shares may not be sold unless they have been registered or qualified for
sale in the state or an exemption from the state’s registration or qualification requirement is available and complied with.
Lincoln
Park is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
Lincoln
Park has informed us that it intends to use an unaffiliated broker-dealer to effectuate all sales, if any, of the common stock
that it may purchase from us pursuant to the Purchase Agreement. Such sales will be made at prices and at terms then prevailing
or at prices related to the then current market price. Each such unaffiliated broker-dealer will be an underwriter within
the meaning of Section 2(a)(11) of the Securities Act. Lincoln Park has informed us that each such broker-dealer will receive
commissions from Lincoln Park that will not exceed customary brokerage commissions.
Brokers,
dealers, underwriters or agents participating in the distribution of the shares offered by this prospectus may receive compensation
in the form of commissions, discounts, or concessions from the purchasers, for whom the broker-dealers may act as agent, of the
common stock sold by Lincoln Park through this prospectus. The compensation paid to any such particular broker-dealer by any such
purchasers of common stock sold by Lincoln Park may be less than or in excess of customary commissions. Neither we nor Lincoln
Park can presently estimate the amount of compensation that any agent will receive from any purchasers of common stock sold by
Lincoln Park.
We
know of no existing arrangements between Lincoln Park or any other stockholder, broker, dealer, underwriter or agent relating
to the sale or distribution of the shares offered by this prospectus.
We
may from time to time file with the SEC one or more supplements to this prospectus or amendments to the registration statement
of which this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and
when required under the Securities Act, to disclose certain information relating to a particular sale of shares offered by this
prospectus by the selling stockholder, including the names of any brokers, dealers, underwriters or agents participating in the
distribution of such shares by the selling stockholder, any compensation paid by Lincoln Park to any such brokers, dealers, underwriters
or agents, and any other required information.
We
will pay the expenses incident to the registration under the Securities Act of the offer and sale of the shares covered by this
prospectus by Lincoln Park. We have agreed to indemnify Lincoln Park and certain other persons against certain liabilities
in connection with the offering of shares of common stock offered hereby, including liabilities arising under the Securities Act
or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Lincoln
Park has agreed to indemnify us against liabilities under the Securities Act that may arise from certain written information furnished
to us by Lincoln Park specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required
to be paid in respect of such liabilities.
Lincoln
Park has represented to us that at no time prior to the Purchase Agreement has Lincoln Park or its agents, representatives
or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined
in Rule 200 of Regulation SHO of the Exchange Act) of our common stock or any hedging transaction, which establishes a net short
position with respect to our common stock. Lincoln Park agreed that during the term of the Purchase Agreement, it, its agents,
representatives or affiliates will not enter into or effect, directly or indirectly, any of the foregoing transactions.
We
have advised Lincoln Park that it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions,
Regulation M precludes the selling stockholder, any affiliated purchasers, and any broker-dealer or other person who participates
in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which
is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases
made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing
may affect the marketability of the securities offered by this prospectus.
This
offering will terminate on the date that all shares offered by this prospectus have been sold by Lincoln Park.
Our
common stock is quoted on the OTCQB and on the OTC Bulletin Board under the symbol “ELTP”.
DESCRIPTION
OF COMMON STOCK
Our
articles of incorporation authorize us to issue 1,445,000,000 shares of common stock, par value $0.001 per share. As of July 13,
2020, there were 846,954,821 shares of our common stock issued and outstanding, all of which are fully paid and non-assessable.
As of July 13, 2020, there were 5,510,000 shares of common stock issuable upon the exercise of outstanding options, 79,008,661
shares of common stock issuable upon exercise of outstanding warrants owned by Nasrat Hakim; 158,017,321 shares of common stock
issuable upon conversion of Series J Convertible Preferred Stock owned by Nasrat Hakim; 2,750,000 additional shares of common
stock reserved for future issuance under our 2014 Equity Incentive Plan and 31,074,817 shares of common stock due and owing to
Directors, employees and consultants.
Voting
Rights
Holders
of our common stock are entitled to one vote per share in the election of directors and on all other matters on which stockholders
are entitled or permitted to vote, and vote along with the holders of our Series J Preferred Stock, which preferred stock entitles
the holder of record thereof to the number of votes equal to the number of shares of common stock into which such share of Series
J Preferred Stock is convertible (approximately 158,017,321 votes) on all matters brought before our stockholders. Holders of
our common stock are not entitled to cumulative voting rights.
Dividend
Rights
Subject
to the terms of any then outstanding series of preferred stock, the holders of our common stock are entitled to dividends in the
amounts and at times as may be declared by our board of directors out of funds legally available therefor.
Liquidation
Rights
Upon
liquidation or dissolution, holders of our common stock are entitled to share ratably in all net assets available, if any, for
distribution to stockholders after we have paid, or provided for payment of, all of our debts and liabilities, and after payment
of any liquidation preferences to holders of any then outstanding shares of preferred stock.
Other
Matters
Holders
of our common stock have no redemption, conversion or preemptive rights. There are no sinking fund provisions applicable to our
common stock. The rights, preferences and privileges of the holders of our common stock are subject to the rights of the holders
of shares of any series of outstanding preferred stock and preferred stock that we may issue in the future.
Anti-Takeover
Effects of Provisions of Nevada Law, Our Articles of Incorporation,
Our
Bylaws and Our Stockholders’ Rights Plan
Nevada
Control Share Law
We
may be, or in the future we may become, subject to Nevada’s control share law. A corporation is subject to Nevada’s
control share law if it has more than 200 stockholders, at least 100 of whom are stockholders of record and residents of Nevada,
and if the corporation does business in Nevada, including through an affiliated corporation. This control share law may have the
effect of discouraging corporate takeovers. As of the date of this prospectus, we have less than 100 stockholders of record who
are residents of Nevada.
The
control share law focuses on the acquisition of a “controlling interest,” which means the ownership of outstanding
voting shares that would be sufficient, but for the operation of the control share law, to enable the acquiring person to exercise
the following proportions of the voting power of the corporation in the election of directors: (1) one-fifth or more but less
than one-third; (2) one-third or more but less than a majority; or (3) a majority or more. The ability to exercise this voting
power may be direct or indirect, as well as individual or in association with others.
The
effect of the control share law is that an acquiring person, and those acting in association with that person, will obtain only
such voting rights in the control shares as are conferred by a resolution of the stockholders of the corporation, approved at
a special or annual meeting of stockholders. The control share law contemplates that voting rights will be considered only once
by the other stockholders. Thus, there is no authority to take away voting rights from the control shares of an acquiring person
once those rights have been approved. If the stockholders do not grant voting rights to the control shares acquired by an acquiring
person, those shares do not become permanent non-voting shares. The acquiring person is free to sell the shares to others. If
the buyer or buyers of those shares themselves do not acquire a controlling interest, the shares are not governed by the control
share law.
If
control shares are accorded full voting rights and the acquiring person has acquired control shares with a majority or more of
the voting power, a stockholder of record, other than the acquiring person, who did not vote in favor of approval of voting rights,
is entitled to demand fair value for such stockholder’s shares.
In
addition to the control share law, Nevada has a business combination law, which prohibits certain business combinations between
Nevada publicly traded corporations and “interested stockholders” for two years after the interested stockholder first
becomes an interested stockholder, unless the corporation’s board of directors approves the combination in advance. For
purposes of Nevada law, an interested stockholder is any person who is: (a) the beneficial owner, directly or indirectly, of 10%
or more of the voting power of the outstanding voting shares of the corporation, or (b) an affiliate or associate of the corporation
and at any time within the previous two years was the beneficial owner, directly or indirectly, of 10% or more of the voting power
of the then-outstanding shares of the corporation. The definition of “business combination” contained in the statute
is sufficiently broad to cover virtually any kind of transaction that would allow a potential acquirer to use the corporation’s
assets to finance the acquisition or otherwise to benefit its own interests rather than the interests of the corporation and its
other stockholders.
The
effect of Nevada’s business combination law is to potentially discourage parties interested in taking control of the Company
from doing so if it cannot obtain the approval of our board of directors.
Articles
of Incorporation and Bylaws
Our
Articles of Incorporation and/or Bylaws provide that:
|
|
●
|
our
Bylaws may be amended or repealed by our board of directors or our stockholders;
|
|
|
●
|
our
board of directors is authorized to issue, without stockholder approval, preferred stock,
the rights of which will be determined at the discretion of our board of directors and
that, if issued, could operate as a “poison pill” to dilute the stock ownership
of a potential hostile acquirer to prevent an acquisition that our board of directors
does not approve;
|
|
|
●
|
our
Board of directors is classified into three separate classes of directors with each respective
class serving a three-year term;
|
|
|
●
|
our
stockholders do not have cumulative voting rights, and therefore stockholders holding
a majority of the voting stock outstanding will be able to elect all of our directors;
and
|
|
|
●
|
our
stockholders must comply with advance notice provisions to bring business before or nominate
directors for election at a stockholder meeting.
|
Stockholder
Rights Plan
On
November 15, 2013, we entered into a Stockholder Rights Plan and, under the Rights Plan, our board of directors declared a dividend
distribution of one Right for each outstanding share of our common stock and one right for each share of common stock into which
any of our outstanding Preferred Stock is convertible, to stockholders of record at the close of business on that date. Each Right
entitles the registered holder to purchase from us one “Unit” consisting of one one-millionth (1/1,000,000) of a share
of Series H Junior Participating preferred stock, at a purchase price of $2.10 per Unit, subject to adjustment, and may be redeemed
prior to November 15, 2023, the expiration date, at $0.000001 per Right, unless earlier redeemed by us. The Rights generally are
not transferable apart from the common stock and will not be exercisable unless and until a person or group acquires or commences
a tender or exchange offer to acquire, beneficial ownership of 15% or more of our common stock. However, for Mr. Hakim, our Chief
Executive Officer, the Rights Plan’s 15% threshold excludes shares beneficially owned by him as of November 15, 2013 and
all shares issuable to him pursuant to his employment agreement and the Mikah Note.
The
description and terms of the Rights are set forth in the Rights Agreement. The foregoing description of the Rights and the Rights
Agreement are qualified in their entire by reference to the disclosure in our Registration Statement on Form 8-A12G and
the Rights Agreement filed therewith, filed with the SEC on November 15, 2013, with such filing and exhibit being
herein incorporated by reference.
Potential
Effects of Authorized but Unissued Stock
We
have shares of common stock and preferred stock available for future issuance without stockholder approval. We may utilize these
additional shares for a variety of corporate purposes, including future public offerings to raise additional capital, to facilitate
corporate acquisitions or payment as a dividend on the capital stock.
The
existence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons
friendly to current management or to issue preferred stock with terms that could render more difficult or discourage a third-party
attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise, thereby protecting the continuity
of our management. In addition, our board of directors has the discretion to determine designations, rights, preferences, privileges
and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences
of each series of preferred stock, all to the fullest extent permissible under Nevada Law and subject to any limitations set forth
in our Articles of Incorporation. The purpose of authorizing our board of directors to issue preferred stock and to determine
the rights and preferences applicable to such preferred stock is to eliminate delays associated with a stockholder vote on specific
issuances. The issuance of preferred stock, while providing desirable flexibility in connection with possible financings, acquisitions
and other corporate purposes, could have the effect of making it more difficult for a third-party to acquire, or could discourage
a third-party from acquiring, a majority of our outstanding voting stock.
American
Stock Transfer & Trust Company, LLC is the transfer agent and registrar for our common stock.
Our
common stock is quoted on the OTCQB and on the OTC Bulletin Board under the symbol “ELTP”.
LEGAL
MATTERS
The
validity of the securities offered by this prospectus has been passed upon for us by Richard Feiner, Esq., New York, New York.
EXPERTS
The
consolidated financial statements of Elite Pharmaceuticals, Inc. for the fiscal years ended March 31, 2020 and the two years then
ended incorporated in this prospectus by reference from Elite Pharmaceuticals, Inc.’s Annual Report on Form 10-K for the
year ended March 31, 2020 have been audited by Buchbinder Tunick & Company LLP, an independent registered public accounting
firm, as stated in their report, which is incorporated herein by reference. Such consolidated financial statements have
been so incorporated in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE
YOU CAN FIND MORE INFORMATION
This
prospectus forms part of a registration statement on Form S-3 filed by us with the SEC under the Securities Act. As permitted
by the SEC, this prospectus does not contain all the information set forth in the registration statement filed with the SEC. For
a more complete understanding of this offering, you should refer to the complete registration statement, including the exhibits
thereto, on Form S-3 that may be obtained as described below. Statements contained or incorporated by reference in this prospectus
or any prospectus supplement about the contents of any contract or other document are not necessarily complete. If we have filed
any contract or other document as an exhibit to the registration statement or any other document incorporated by reference in
the registration statement of which this prospectus forms a part, you should read the exhibit for a more complete understanding
of the document or matter involved. Each statement regarding a contract or other document is qualified in its entirety by reference
to the actual document.
We
file annual, quarterly and special reports, proxy statements and other information with the SEC. Our SEC filings are available
to the public from commercial retrieval services and at the website maintained by the SEC at www.sec.gov. The reports and
other information filed by us with the SEC are also available at our website. The address of the Company’s website is http://www.Elitepharma.com.
Information contained on our website or that can be accessed through our website is not incorporated by reference into this prospectus.
INFORMATION
INCORPORATED BY REFERENCE
The
SEC allows us to incorporate information into this prospectus “by reference,” which means that we can disclose important
information to you by referring you to another document that we file separately with the SEC. The information incorporated by
reference is deemed to be part of this prospectus, except for any information superseded by information contained directly in
this prospectus. These documents contain important information about Elite and its financial condition, business and results.
We
are incorporating by reference the filings listed below and any additional documents that we may file with the SEC pursuant to
Section 13(a), 13(c), 14 or 15(d) of the Exchange Act on or after the date we file this prospectus and prior to the termination
of the offering, except we are not incorporating by reference any information furnished (but not filed) under Item 2.02 or Item
7.01 of any Current Report on Form 8-K and corresponding information furnished under Item 9.01 as an exhibit thereto: [hyperlink
all of the following]
|
|
●
|
our
Annual Report on Form 10-K for the fiscal year ended March 31, 2020, filed with the SEC on June 29, 2020;
|
|
|
●
|
the description of
our common stock contained in our Form
8-A filed on February 16, 2000, including any amendments or reports filed for the purpose of updating the
description.
|
We
will provide, without charge, to each person to whom a copy of this prospectus has been delivered, including any beneficial owner,
a copy of any and all of the documents referred to herein that are summarized in this prospectus, if such person makes a written
or oral request directed to:
Elite
Pharmaceuticals, Inc.
165
Ludlow Avenue
Northvale,
NJ 07647
Attn:
Corporate Secretary
(201)
750-2646
262,500,000
Shares
ELITE
PHARMACEUTICALS, INC.
Common
Stock
PROSPECTUS
,
2020
PART
II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
14. Other Expenses of Issuance and Distribution
The
following table sets forth the costs and expenses payable by the Company in connection with the registration and sale of the securities
being registered. All amounts are estimated except the Securities and Exchange Commission registration fee.
|
SEC filing fee
|
|
$
|
2,726
|
|
|
Legal fees and expenses
|
|
$
|
10,000
|
|
|
Printing fees
|
|
$
|
5,000
|
|
|
Accounting fees and expenses
|
|
$
|
5,000
|
|
|
Miscellaneous fees and expenses
|
|
$
|
3,274
|
|
|
Total
|
|
$
|
26,000
|
|
Item
15. Indemnification of Directors and Officers
Our
directors and officers are indemnified by our articles of incorporation and bylaws to the fullest extent legally permissible under
the laws of Nevada against all expenses, liability and loss, reasonably incurred by them in connection with the defense of any
action, suit or proceeding in which they are a party by reason of being or having been directors or officers of the Company. Unless
our Board determines by a majority vote of a quorum of disinterested directors that, based upon the facts known, such person acted
in bad faith and in a manner that such person did not believe to be in or not opposed to our best interest (or, with respect to
any criminal proceeding, that such person believed or had reasonable cause to believe his conduct was unlawful), costs, charges
and expenses (including attorneys' fees) incurred by such person in defending a civil or criminal proceeding shall be paid by
the Company in advance upon receipt of an undertaking to repay all amounts advanced if it is ultimately determined that the person
is not entitled to be indemnified by the Company as authorized by the bylaws, and upon satisfaction of other conditions required
by current or future legislation. Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended,
may be permitted to such directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have
been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed
in the Securities Act and is, therefore, unenforceable.
In
the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid
by such director, officer or controlling person in the successful defense of any action, suit or proceeding, is asserted by such
director, officer or controlling person in connection with the securities being registered, we may, unless in the opinion of counsel
the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such
indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication
of such issue.
We
maintain a policy of directors and officers liability insurance which reimburses us for expenses which we may incur in connection
with the foregoing indemnity provisions and which may provide direct indemnification to directors and officers where we are unable
to do so.
Item
16. Exhibits
The
exhibits listed in the index below are filed as part of this report.
|
3.1(d)
|
|
Certificate of Designations of the Series I Convertible Preferred Stock as filed with the Secretary of State of the State of Nevada on February 6, 2014, incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K, dated February 6, 2014 and filed with the SEC on February 7, 2014.
|
|
|
|
|
|
3.1(e)
|
|
Certificate of Designations of the Series J Convertible Preferred Stock as filed with the Secretary of State of the State of Nevada on May 3, 2017, incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K, dated April 28, 2017 and filed with the SEC on April 28, 2017.
|
|
|
|
|
|
3.1(f)
|
|
Certificate of Amendment to Articles of Incorporation, incorporated by reference to Exhibit 3.1 to the Current Report on Form 8-K, dated June 24, 2020 and filed with the SEC on June 24, 2020.
|
|
|
|
|
|
3.2(a)
|
|
Amended and Restated By-Laws of the Company, incorporated by reference to Exhibit 3.2 to the Current Report on Form 8-K dated April 23, 2020 and filed with the SEC on April 23, 2020.
|
|
|
|
|
|
4.1
|
|
Form of specimen certificate for Series G Convertible Preferred Stock of the Company, incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K, dated April 18, 2013 and filed with the SEC on April 22, 2013.
|
|
|
|
|
|
4.2
|
|
Form of specimen certificate for Series I Convertible Preferred Stock of the Company, incorporated by reference to Exhibit 4.2 to the Current Report on Form 8-K, dated February 6, 2014 and filed with the SEC on February 7, 2014.
|
|
|
|
|
|
4.3
|
|
Rights Agreement, dated as of November 15, 2013, between the Company and American Stock Transfer & Trust Company, LLC., incorporated by reference to Exhibit 1 to the Registration Statement on Form 8-A filed with the SEC on November 15, 2013.
|
|
|
|
|
|
4.4
|
|
Form of Series H Preferred Stock Certificate, incorporated by reference to Exhibit 1 to the Registration Statement on Form 8-A filed with the SEC on November 15, 2013.
|
|
|
|
|
|
4.5
|
|
Warrant to purchase shares of Common Stock issued to Nasrat Hakim dated April 28, 2017 incorporated by reference to Exhibit 4.1 to the Current Report on Form 8-K, dated April 28, 2017, and filed with the SEC on April 28, 2017.
|
|
|
|
|
|
5.1
|
|
Opinion of Richard Feiner, Esq.*
|
|
|
|
|
|
10.1
|
|
Elite Pharmaceuticals, Inc. 2014 Equity Incentive Plan, incorporated by reference to Appendix B to the Company’s Definitive Proxy Statement for its Annual Meeting of Shareholders, filed with the SEC on April 3, 2014.
|
|
|
|
|
|
10.2
|
|
Form of Confidentiality Agreement (corporate), incorporated by reference to Exhibit 10.7 to the Form SB-2.
|
|
|
|
|
|
10.3
|
|
Form of Confidentiality Agreement (employee), incorporated by reference to Exhibit 10.8 to the Form SB-2.
|
|
|
|
|
|
10.4
|
|
Loan Agreement, dated as of August 15, 2005, between New Jersey Economic Development Authority (“NJEDA”) and the Company, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated August 31, 2005 and filed with the SEC on September 6, 2005.
|
|
|
|
|
|
10.5
|
|
Series A Note in the aggregate principal amount of $3,660,000.00 payable to the order of the NJEDA, incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, dated August 31, 2005 and filed with the SEC on September 6, 2005.
|
|
|
|
|
|
10.6
|
|
Series B Note in the aggregate principal amount of $495,000.00 payable to the order of the NJEDA, incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K, dated August 31, 2005 and filed with the SEC on September 6, 2005.
|
|
|
|
|
|
10.7
|
|
Mortgage from the Company to the NJEDA, incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K, dated August 31, 2005 and filed with the SEC on September 6, 2005.
|
|
|
|
|
|
10.8
|
|
Indenture between NJEDA and the Bank of New York as Trustee, dated as of August 15, 2005, incorporated by reference to Exhibit 10.5 to the Current Report on Form 8-K, dated August 31, 2005 and filed with the SEC on September 6, 2005.
|
|
|
|
|
|
10.9
|
|
Strategic Alliance Agreement, dated as of March 18, 2009, by and among the Company, Epic Pharma, LLC and Epic Investments, LLC, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated March 18, 2009 and filed with the SEC on March 23, 2009.
|
|
10.10
|
|
Amendment to Strategic Alliance Agreement, dated as of April 30, 2009, by and among the Company, Epic Pharma, LLC and Epic Investments, LLC, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated April 30, 2009 and filed with the SEC on May 6, 2009.
|
|
|
|
|
|
10.11
|
|
Second Amendment to Strategic Alliance Agreement, dated as of June 1, 2009, by and among the Company, Epic Pharma, LLC and Epic Investments, LLC, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated June 1, 2009, and filed with the SEC on June 5, 2009.
|
|
|
|
|
|
10.12
|
|
Third Amendment to Strategic Alliance Agreement, dated as of Aug 18, 2009, by and among the Company, Epic Pharma LLC and Epic Investments, LLC, incorporated by reference to Exhibit 10.3 to the Quarterly Report on Form 10-Q, for the period ending June 30, 2009 and filed with the SEC on August 19, 2009.
|
|
|
|
|
|
10.13
|
|
Employment Agreement, dated as of November 13, 2009, by and between the Company and Carter J. Ward, incorporated by reference to Exhibit 10.2 to the Quarterly Report on Form 10-Q, for the period ending September 30, 2009 and filed with the SEC on November 16, 2009.
|
|
|
|
|
|
10.14
|
|
Elite Pharmaceuticals Inc. 2009 Equity Incentive Plan, as adopted November 24, 2009, incorporated by reference to Exhibit 10.1 to the Registration Statement Under the Securities Act of 1933 on Form S-8, dated December 18, 2009 and filed with the SEC on December 22, 2009.
|
|
|
|
|
|
10.15
|
|
License Agreement, dated as of September 10, 2010, by and among Precision Dose Inc. and the Company, incorporated by reference to Exhibit 10.8 to the Quarterly Report on Form 10-Q, for the period ended September 30, 2010 and filed with the SEC on November 15, 2010 (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.16
|
|
Manufacturing and Supply Agreement, dated as of September 10, 2010, by and among Precision Dose Inc. and the Company, incorporated by reference to Exhibit 10.9 to the Quarterly Report on Form 10-Q, for the period ended September 30, 2010 and filed with the SEC on November 15, 2010 (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.17
|
|
August 1, 2013 Employment Agreement with Nasrat Hakim, incorporated by reference to Exhibit 10.4 to the Current Report on Form 8-K, dated August 1, 2013 and filed with the SEC on August 5, 2013.
|
|
|
|
|
|
10.18
|
|
August 1, 2013 Mikah LLC Asset Purchase Agreement, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K/A, dated August 1, 2013 and filed with the SEC on August 30, 2018. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.19
|
|
August 1, 2013 Secured Convertible Note from the Company to Mikah Pharma LLC., incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, dated August 1, 2013 and filed with the SEC on August 5, 2013.
|
|
|
|
|
|
10.20
|
|
August 1, 2013 Security Agreement from the Company to Mikah Pharma LLC., incorporated by reference to Exhibit 10.3 to the Current Report on Form 8-K, dated August 1, 2013 and filed with the SEC on August 5, 2013.
|
|
|
|
|
|
10.21
|
|
October 15, 2013 Hakim Credit Line Agreement, incorporated by reference to Exhibit 10.16 to the Quarterly Report on Form 10-Q for the period ended September 30, 2013.
|
|
|
|
|
|
10.22
|
|
October 2, 2013 Manufacturing and Licensing Agreement with Epic Pharma LLC, incorporated by reference to Exhibit 10.17 to the Amended Quarterly Report on Form 10-Q/A for the period ended September 30, 2013 and filed with the SEC on April 25, 2014. Confidential Treatment granted with respect to portions of the Agreement.
|
|
|
|
|
|
10.23
|
|
February 7, 2014 Amendment to Secured Convertible Note from the Company to Mikah, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated February 7, 2014 and filed with the SEC on February 7, 2014.
|
|
|
|
|
|
10.24
|
|
Employment Agreement with Dr. G. Kenneth Smith, dated October 20, 2014, incorporated by reference to Exhibit 10.82 to the Quarterly Report on Form 10-Q for the period ended September 30, 2014 and filed with the SEC on November 14, 2014.
|
|
|
|
|
|
10.25
|
|
January 28, 2015 First Amendment to the Loan Agreement between Nasrat Hakim and Elite Pharmaceuticals dated October 15, 2013, incorporated by reference to Exhibit 10.83 to the Quarterly Report on Form 10-Q for the period ended December 31, 2014 and filed with the SEC on February 17, 2015.
|
|
|
|
|
|
10.26
|
|
January 28, 2015 Termination of Development and License Agreement for Mikah-001 between Elite Pharmaceuticals, Inc. and Mikah Pharma LLC and Transfer of Payment, incorporated by reference to Exhibit 10.84 to the Quarterly Report on Form 10-Q for the period ended December 31, 2014 and filed with the SEC on February 17, 2015.
|
|
10.27
|
|
June 4, 2015 License Agreement with Epic Pharma LLC, incorporated by reference to Exhibit 10.85 to Amendment No. 1 to the Annual Report on Form 10-K for the fiscal year ended March 31, 2015 and filed with the SEC on July 11, 2016. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.28
|
|
Amendment No. 1 to Hakim Employment Agreement, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K filed with the SEC on January 29, 2016.
|
|
|
|
|
|
10.29
|
|
August 24, 2016 Master Development and License Agreement between Elite and SunGen Pharma LLC. incorporated by reference to Exhibit 10.44 to the Quarterly Report on Form 10-Q for the period ended September 30, 2016 and filed with the SEC on November 9, 2016. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.30
|
|
Purchase Agreement between the Company and Lincoln Park Capital LLC dated May 1, 2017, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated May 2, 2017 and filed with the SEC on May 2, 2017.
|
|
|
|
|
|
10.31
|
|
Registration Rights Agreement between the Company and Lincoln Park Capital LLC dated May 1, 2017, incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, dated May 2, 2017 and filed with the SEC on May 2, 2017.
|
|
|
|
|
|
10.32
|
|
April 28, 2017 Exchange Agreement between the Company and Nasrat Hakim, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated April 28, 2017 and filed with the SEC on April 28. 2017.
|
|
|
|
|
|
10.33
|
|
May 2017 Trimipramine Acquisition Agreement from Mikah Pharma, incorporated by reference to Exhibit 10.50 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017.
|
|
|
|
|
|
10.34
|
|
May 2017 Secured Promissory Note from the Company to Mikah Pharma, incorporated by reference to Exhibit 10.51 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017.
|
|
|
|
|
|
10.35
|
|
May 2017 Security Agreement between the Company to Mikah Pharma, incorporated by reference to Exhibit 10.52 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017.
|
|
|
|
|
|
10.36
|
|
May 2017 Assignment of Supply and Distribution Agreement between Dr. Reddy’s Laboratories and Mikah Pharma, incorporated by reference to Exhibit 10.53 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017.
|
|
|
|
|
|
10.37
|
|
May 2017 Assignment of Manufacturing and Supply Agreement between Epic and Mikah Pharma, incorporated by reference to Exhibit 10.54 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017.
|
|
|
|
|
|
10.38
|
|
Supply and Distribution Agreement between Dr. Reddy’s Laboratories and Mikah Pharma, incorporated by reference to Exhibit 10.55 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.39
|
|
Manufacturing and Supply Agreement between Epic and Mikah Pharma, incorporated by reference to Exhibit 10.56 to the Annual Report on Form 10-K, for the period ended March 31, 2017 and filed with the SEC on June 14, 2017. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.40
|
|
Master Development and License Agreement For Products Between Elite Pharmaceuticals, Inc. And SunGen dated July 6, 2017, incorporated by reference to Exhibit 10.57 to the Quarterly Report on Form 10-Q for the period ended June 30, 2017 and filed with the SEC on August 9, 2017. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.41
|
|
First Amendment to Master Development And License Agreement For Products Between Elite Pharmaceuticals, Inc. and SunGen Pharma, LLC, incorporated by reference to Exhibit 10.59 to the Quarterly Report on Form 10-Q for the period ended June 30, 2017 and filed with the SEC on August 9, 2017. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.42
|
|
Second Amendment to Master Development And License Agreement For Products Between Elite Pharmaceuticals, Inc. and SunGen Pharma, LLC, incorporated by reference to Exhibit 10.58 to the Quarterly Report on Form 10-Q for the period ended June 30, 2017 and filed with the SEC on August 9, 2017. (Confidential Treatment granted with respect to portions of the Agreement).
|
|
10.43
|
|
May 22, 2018 License, Manufacturing and Supply Agreement with Glenmark Pharmaceuticals Inc. USA, incorporated by reference to Exhibit 10.60 to the Annual Report on Form 10-K for the fiscal year ended March 31, 2018 and filed with the SEC on June 14, 2018. (Confidential treatment granted with respect to portions of the Agreement).
|
|
|
|
|
|
10.44
|
|
August 1, 2018 Amendment to the Glenmark Pharmaceuticals Inc. USA License, Supply and Distribution Agreement, incorporated by reference to Exhibit 10.44 to the Quarterly Report on Form 10-Q, for the period ended December 31, 2019 and filed with the SEC on February 10, 2020. (Portions of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.45
|
|
License, Supply And Distribution Agreement effective March 6, 2019 by and between Elite Pharmaceuticals, Inc., and Elite Laboratories, Inc. and Lannett Company, Inc., USA, incorporated by reference to Exhibit 10.45 to the Quarterly Report on Form 10-Q, for the period ended December 31, 2019 and filed with the SEC on February 10, 2020. (Portions of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.46
|
|
License, Supply and Distribution Agreement effective April 9, 2019 by and between Elite Pharmaceuticals, Inc., and Elite Laboratories, Inc. and Lannett Company, Inc., USA, incorporated by reference to Exhibit 10.49 to the Annual Report on Form 10-K for the period ended March 31, 2019 and filed with the SEC on June 21, 2019 (portions of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.47
|
|
License, Supply and Distribution Agreement effective March 6, 2019 by and between Elite Pharmaceuticals, Inc., and Elite Laboratories, Inc. and Lannett Company, Inc., USA, incorporated by reference to Exhibit 10.50 to the Annual Report on Form 10-K for the period ended March 31, 2019 and filed with the SEC on June 21, 2019 (portions of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.48
|
|
Development Agreement effective December 3, 2018 by and between Mikah Pharma LLC and Elite Laboratories, Inc., incorporated by reference to Exhibit 10.51 to the Annual Report on Form 10-K for the period ended March 31, 2019 and filed with the SEC on June 21, 2019 (portions of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.49
|
|
Asset Purchase Agreement dated November 13, 2019 by and between the Company and Nostrum Laboratories Inc. , incorporated by reference to Exhibit 10.49 to the Quarterly Report on Form 10-Q, for the period ended December 31, 2019 and filed with the SEC on February 10, 2020.
|
|
|
|
|
|
10.50
|
|
January 2, 2020 Amendment to the Glenmark Pharmaceuticals Inc. USA License, Supply and Distribution Agreement, incorporated by reference to Exhibit 10.50 to the Quarterly Report on Form 10-Q, for the period ended December 31, 2019 and filed with the SEC on February 10, 2020. (Portions of this Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.51
|
|
Asset Purchase Agreement executed January 16, 2020 by and between the Company and Nostrum Laboratories Inc., incorporated by reference to Exhibit 10.49 to the Quarterly Report on Form 10-Q, for the period ended December 31, 2019 and filed with the SEC on February 10, 2020.
|
|
|
|
|
|
10.52
|
|
Employment Agreement with Douglas Plassche, incorporated by reference to
Exhibit 10.52 to the Annual Report on Form 10-K for the period ended March 31, 2020 and filed with the SEC on June 29, 2020.
|
|
|
|
|
|
10.53
|
|
June 21, 2019 Retention Agreement with Douglas Plassche, incorporated
by reference to Exhibit 10.53 to the Annual Report on Form 10-K for the period ended March 31, 2020 and filed with the SEC on June
29, 2020.
|
|
|
|
|
|
10.54
|
|
July 29, 2019 Amendment To The License, Supply And Distribution Agreement
Between Elite Pharmaceuticals, Inc./Elite Laboratories, Inc. And Lannett Company, Inc., incorporated by reference to Exhibit 10.54
to the Annual Report on Form 10-K for the period ended March 31, 2020 and filed with the SEC on June 29, 2020 (portions of this
Agreement have been redacted in compliance with Regulation S-K Item 601(b)(10)).
|
|
|
|
|
|
10.55
|
|
Purchase Agreement, dated July 8, 2020, by and between the Company and Lincoln Park Capital Fund, LLC, incorporated by reference to Exhibit 10.1 to the Current Report on Form 8-K, dated July 9, 2020 and filed with the SEC on July 9, 2020.
|
|
|
|
|
|
10.56
|
|
Registration Rights Agreement, dated July 8, 2020, by and between the Company and Lincoln Park Capital Fund, LLC, incorporated by reference to Exhibit 10.2 to the Current Report on Form 8-K, dated July 9, 2020 and filed with the SEC on July 9, 2020.
|
|
|
|
|
|
21
|
|
Subsidiaries of the Company, incorporated by reference to Exhibit 21 to the Annual Report on Form 10-K, for the period ended March 31, 2019 and filed with the SEC on June 21, 2019.
|
|
|
|
|
|
23.1
|
|
Consent of Buchbinder Tunick & Company LLP, Independent Registered Public Accounting Firm.*
|
|
|
|
|
|
23.2
|
|
Consent of Richard Feiner, Esq. (included in Exhibit 5.1).*
|
|
|
|
|
|
24.1
|
|
Power of Attorney (included on signature page).*
|
Item 17.
Undertakings
a. The
undersigned registrant hereby undertakes:
1. To
file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To
include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
ii. To
reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent
post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set
forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if
the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high
end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b)
if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price
set forth in the “Calculation of Registration Fee” table in the effective registration statement.
iii. To
include any material information with respect to the plan of distribution not previously disclosed in the registration statement
or any material change to such information in the registration statement;
Provided
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to be
included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant
pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration
statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
2. That,
for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be
a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
3. To
remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at
the termination of the offering.
4. That,
for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
i. Each
prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the
date the filed prospectus was deemed part of and included in the registration statement; and
ii. Each
prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance
on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information
required by section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of
the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of
securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any
person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement
relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities
at that time shall be deemed to be the initial bona fide offering thereof. Provided, however , that no statement made in a registration
statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by
reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with
a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement
or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date.
5. That,
for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution
of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant
pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if
the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant
will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
i. Any
preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule
424;
ii. Any
free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to
by the undersigned registrant;
iii. The
portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant
or its securities provided by or on behalf of the undersigned registrant; and
iv. Any
other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
b. The
undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each
filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934
(and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities
Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration
statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the
initial bona fide offering thereof.
c. Insofar
as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the provisions described in Item 15 above, or otherwise, the registrant has been advised
that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred
or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding)
is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant
will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed
by the final adjudication of such issue.
The
undersigned registrant also hereby undertakes that:
(a)
For the purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus
filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant
pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement
as of the time it was declared effective.
(b)
For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form
of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering
of such securities at that time shall be deemed to be the initial bona fide offering thereof.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it
meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf
by the undersigned, thereunto duly authorized in the city of Northvale, State of New Jersey, on July 15, 2020.
|
|
ELITE PHARMACEUTICALS, INC.
|
|
|
(Registrant)
|
|
|
|
|
|
|
By:
|
/s/
Nasrat Hakim
|
|
|
|
Nasrat
Hakim,
|
|
|
|
Chief
Executive Officer
|
POWER
OF ATTORNEY
KNOW
ALL MEN BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Nasrat Hakim, as
his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution for him in any and all capacities,
to sign any or all amendments or post-effective amendments to this Registration Statement, or any Registration Statement for the
same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933, as amended, and to
file the same, with exhibits hereto and other documents in connection therewith or in connection with the registration of the
securities under the Securities Act of 1933, as amended, with the Securities and Exchange Commission, granting unto such attorney-in-fact
and agent full power and authority to do and perform each and every act and thing requisite and necessary in connection with such
matters and hereby ratifying and confirming all that such attorney-in-fact and agent or his substitute may do or cause to be done
by virtue hereof.
Pursuant
to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the
capacities and on the dates indicated below.
|
Signature
|
|
Title
|
|
Date
|
|
|
|
|
|
|
|
/s/
Nasrat Hakim
|
|
Chief
Executive Officer
|
|
|
|
Nasrat
Hakim
|
|
(Principal
Executive) and Director
|
|
July
15, 2020
|
|
|
|
|
|
|
|
/s/
Carter Ward
|
|
Chief
Financial Officer (Principal Financial Officer),
|
|
|
|
Carter
Ward
|
|
Treasurer,
Secretary and Chief Accounting Officer
|
|
July
15, 2020
|
|
|
|
|
|
|
|
/s/
Barry Dash
|
|
Director
|
|
|
|
Barry
Dash
|
|
|
|
July
15, 2020
|
|
|
|
|
|
|
|
/s/
Davis Caskey
|
|
Director
|
|
|
|
Davis
Caskey
|
|
|
|
July
15, 2020
|
|
|
|
|
|
|
|
/s/
Jeffrey Whitnell
|
|
Director
|
|
|
|
Jeffrey
Whitnell
|
|
|
|
July
15, 2020
|
Elite
Pharmaceuticals, Inc.
Registration
Statement on Form S-3
Index
to Exhibits
II-9
Elite Pharmaceuticals (QB) (USOTC:ELTP)
Historical Stock Chart
From Mar 2024 to Apr 2024
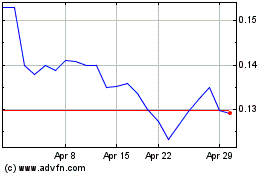
Elite Pharmaceuticals (QB) (USOTC:ELTP)
Historical Stock Chart
From Apr 2023 to Apr 2024