CAN
A DISINFECTANT
BE POWERFUL
AND STILL
BE
GENTLE
AND
SAFE?
June 29, 2020
-- InvestorsHub NewsWire -- via BioResearchAlert
-
Conventional
disinfectants such as chlorine, bleach,
alcohol, ammonia or peroxide may be effective against
pathogens
but
they often
have limited use
because they can also be harmful to humans or the
environment
-
BioLargo
(BLGO: OTCQB) features
its
patented,
colorless,
non-staining
non-toxic
iodine
technology -
proven
to be a
powerful disinfectant against a broad spectrum of viruses,
bacteria, fungi,
and
biofilm,
including
SARS-CoV-2.
It
targets both environmental and medical markets
-
BioLargo's
medical
subsidiary is launching
Clyraguard,
a
hospital grade personal protection spray for PPE,
including
facemasks,
that is
safe on skin.
Clyraguard
is
recently
FDA
registered
and
proven safe and effective at 99.999%.
-
The Galveston
National Laboratory at
the University of Texas Medical Branch,
tested
and found BioLargo's
CupriDyne for
environmental
use
and
ClyraGuard
for
medical
use
effective
for
complete
inactivation of SARS-CoV-2
-
BioLargo
also
has other potential
blockbuster products for water treatment (including PFAS removal),
environmental remediation and odor and VOC control that are either in early stages
of commercialization or close to
commercialization
-
BioResearchAlert
believes
BioLargo's
new ultra-safe
disinfection technology has the potential to become the new
standard in a $16
billion market.
BioLargo
has a
market cap
in the
$25 million
range (recent
price $0.14), its fundamental value is
low
Summary
The COVID-19
pandemic has caused demand for disinfection
products to skyrocket to the point where
suppliers are struggling to meet demand. The problem is compounded by the fact
that bleach
disinfectants are
the most commonly used products in spite of CDC warnings
that
even "low level
exposure to chlorine (which is the active ingredient in bleach) can
result in nose, throat, and eye irritation. At higher levels,
breathing chlorine gas may result in changes in breathing rate and
coughing, and damage to the lungs. Additional symptoms of exposure
to chlorine can be severe. Workers may be harmed from exposure to
chlorine. The level of exposure depends upon the dose, duration,
and work being done."
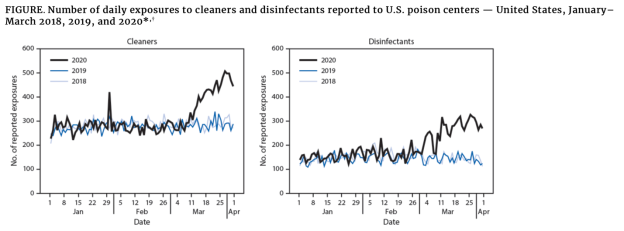
The
CDC reports calls
to poison centers due to cleaning and disinfectant exposures
increased by 20% in early 2020. In line with toxicity claims,
the CDC reports that during January - March 2020, poison centers
received 45,550 exposure calls related to cleaners (28,158) and
disinfectants (17,392), representing overall increases of 20.4% and
16.4% from January - March 2019 (37,822).
The FDA
recently
advised consumers to avoid 9 different
brands of hand sanitizers because of their potential harmful
effects from methanol that is wood alcohol.
Bloomberg
recently
published, "Rush to
Disinfect U.S. Offices Has Some Health Experts
Worried."

"Businesses
across the U.S. have begun intensive Covid-19 disinfection
regimens, exposing returning workers and consumers to some
chemicals that are largely untested for human health, a development
that's alarming health and environmental safety experts.
The rush to disinfect is well-intentioned. Executives want to
protect employees while abiding by U.S. Centers for Disease Control
& Prevention guidelines (and to avoid liability). Pre-pandemic,
corporate cleaning staffs typically "freshened" lobbies every three
hours, sanitized restrooms every four hours and cleaned other areas
at night, said Rich
Feczko,
national director of systems, standards and innovation at
Crothall
Healthcare, which cleans hundreds of hospitals.
That pace has now accelerated. "Our frequencies have ramped up in
public places like lobbies and elevators to 6-8 times per day,"
said
Feczko.
Restrooms are cleaned every two hours. "Before the pandemic,
clients were happy if their trash was emptied and vacuum marks were
in the plush carpet," said Jill Frey, owner of Ohio-based
Cummins
Facility Services. Now, customers ask for sanitization (reducing
pathogens on a surface) and disinfection (killing all
pathogens).
"This is a hazardous proposition," said Dr.
Claudia Miller,
an immunologist, allergist and co-author of Chemical
Exposures: Low Levels and High Stakes.
"Cleaners tend to go in with hugely toxic chemicals. We're creating
another problem for a whole group of people, and I'm not sure we're
actually controlling infections.
Cleaning companies are selecting disinfectants from hundreds
on List
N,
the month-old compendium of products approved by the Environmental
Protection Agency to kill the novel coronavirus. Those chemicals
have passed tests to show they're effective against the pathogen,
but "this doesn't mean that they have been approved because they're
considered safe with regard to human health," said exposure
scientist Lesliam
Quirós-Alcalá,
an assistant professor at Johns Hopkins Bloomberg School of Public
Health."
Key Investor
Takeaway
What would it mean
to the $16 billion disinfection
industry if there was a powerful disinfectant that would
inactivate
viruses like
SARS-CoV-2, and destroy harmful bacteria, fungi and
biofilm, but that was truly safe and gentle and did not harm
healthy tissue such as skin, nose, throat,
eyes, lungs or
mucous membranes? If it was competitively
priced, marketed effectively,
and well
capitalized, it seems that in time,
it
could have the
potential to become a new standard for disinfection.
BioLargo's
subsidiaries
and their effective, safe and gentle products are just being
discovered
Like chlorine or
bleach,
iodine
has long
been
known
as
a powerful
disinfecting
tool against
viruses, bacteria and fungi, but its widespread adoption was
limited because until now, traditional iodine
products were often toxic and create problematic staining. A paper
titled, "Iodine:
A Forgotten Weapon Against Influenza Viruses" was
published in Thyroid Science and describes how effective iodine has
been against Influenza such as the great Spanish Flu Pandemic of
1918.


While it
subsidiary is launching its FDA registered Clyraguard,
BioLargo
has
also
reported
that it intends to pursue EPA
registration on
fast-track basis if available,
or
traditional filing if required for
CupriDyne.
Leading
Suppliers of Disinfectants
The EPA
lists a
large number of disinfectant products for use against SARS-CoV-2
and the following companies are leading suppliers:
Proctor &
Gamble (PG:
NYSE)
3 M Company
(MMM:
NYSE)
Kimberly
Clark (KMB:
NYSE)
Ecolab
(ECL:
NYSE)
Clorox
Company (CLX:
NYSE)
DOW, Inc.
(DOW:
NYSE)
Conclusion
BioResearchAlert
believes
BioLargo
is worthy of
a serious look by
investors for several
reasons.
-
Disinfection:
Its effective, safe
and gentle disinfection products are just
launching into an unprecedented global market at time where demand and
awareness are peaked which highlight the potential for very large
blockbuster sales
-
Multiple Commercial
Opportunities: Its portfolio of
products
include already-commercial odor and VOC control products
and
environmental
engineering
and remediation with a growing
revenue base
-
New Solutions
Coming Soon: Its PFAS removal and advanced
water treatment have near-term substantial
promise
-
Undervalued:
With an
exceptionally undervalued market cap of roughly $25 million, and with a runway
of blockbuster products that are in early stages of
commercialization, now is the perfect time to add
BioLargo
to a
portfolio.
SOURCE: BioResearchAlert