Bayer, Orion Get FDA OK for Nubeqa in Nonmetastatic Castration-Resistant Prostate Cancer
July 31 2019 - 6:32AM
Dow Jones News
By Colin Kellaher
Germany's Bayer AG (BAYN.XE) and Finland's Orion Oyj (ORNBV.HE)
said the U.S. Food and Drug Administration approved Nubeqa, an
androgen receptor inhibitor jointly developed by the pharmaceutical
companies to treat men with nonmetastatic castration-resistant
prostate cancer.
Bayer and Orion said the approval is based on a Phase 3 study in
which Nubeqa plus androgen deprivation therapy showed a highly
significant improvement in the primary efficacy endpoint of
metastasis-free survival.
Nubeqa was approved under the FDA's priority-review designation.
The agency grants priority review to medicines that have the
potential to provide significant improvements in the treatment of a
serious disease, and the designation shortens the review period to
six months from the standard 10 months.
The companies said Bayer has filed for approval of Nubeqa in the
European Union and Japan, and with other health authorities.
Write to Colin Kellaher at colin.kellaher@wsj.com
(END) Dow Jones Newswires
July 31, 2019 06:17 ET (10:17 GMT)
Copyright (c) 2019 Dow Jones & Company, Inc.
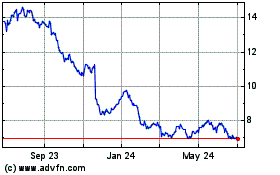
Bayer Aktiengesellschaft (PK) (USOTC:BAYRY)
Historical Stock Chart
From Mar 2024 to Apr 2024
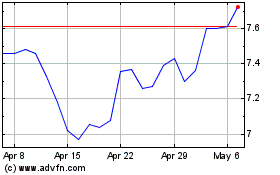
Bayer Aktiengesellschaft (PK) (USOTC:BAYRY)
Historical Stock Chart
From Apr 2023 to Apr 2024